Abstract
The phenomenon of aging is an intrinsic feature of life. Accordingly, the possibility to manipulate it has fascinated humans likely since time immemorial. Recent evidence is shaping a picture where low caloric regimes and exercise may improve healthy senescence, and several pharmacological strategies have been suggested to counteract aging. Surprisingly, the most effective interventions proposed to date converge on only a few cellular processes, in particular nutrient signaling, mitochondrial efficiency, proteostasis, and autophagy. Here, we critically examine drugs and behaviors to which life- or healthspan-extending properties have been ascribed and discuss the underlying molecular mechanisms.
Keywords: Aging, caloric restriction, exercise, rapamycin, metformin, resveratrol, spermidine
Introduction
An organism’s lifespan is inevitably accompanied by the aging process, which involves functional decline, steady increase of a plethora of chronic diseases and ultimately death. Thus, it has been an ongoing dream of mankind to improve health span and extend life. In the last century, developed countries have profited from medical advances and improvements in public healthcare systems and better living conditions derived from their socioeconomic power to achieve a remarkable increase in life expectancy. The current US population reports estimate that the percentage of people aged 65 years and older in the USA will increase from 13% in 2010 to 19.3% in 2030. However, according to the WHO, age itself remains the greatest risk factor for all major life-threatening disorders, and the number of people suffering from age-related diseases is anticipated to almost double over the next two decades. The fact that healthspan has not increased at the same pace as lifespan is a source of grave concern (Mercken et al., 2012). Indeed, in 2009–2010 almost 70% of the adult population in the USA was obese or overweight and one in every three adults suffered from hypertension (NCHS, 2012). Both, obesity and hypertension represent major risk factors for stroke and cardiovascular disease. Although weight loss and increase in physical activity are generally prescribed to avoid such age-associated diseases, only a small percentage of people have the discipline to change their lifestyle accordingly (Wing and Phelan, 2005). The prevalence of age-related pathologies represents major psychological and social impediments as well as an economic burden that urgently needs appropriate interventions.
Dietary regimens and drugs that can slow the aging process continue to raise interest among the general public as well as the scientific and medical communities. Besides the direct applicability of such interventions to meet the need for improving health and longevity, they help provide valuable insights for basic research. Indeed, the molecular mechanisms underlying aging-associated defects appear to be interconnected and affect the same pathways as those responsible for diseases such as cancer, cardiovascular and neurodegenerative disorders. Therefore, efforts aimed at identifying extrinsic solutions to slow aging require a two-pronged approach: greater understanding of the molecular mechanisms involved in the aging process and application of this knowledge in a practical manner. Unfortunately, only a few proposed aging interventions have received good press, the reasons ranging from their intrinsic complexities to the multifactorial causes underlying aging. One of the problems in aging research is the relatively low reproducibility of experimental findings due to the simple fact that aging is a long-lasting process. A long experiment is more prone to develop artifacts that are difficult to control, hence increasing the possibilities and time windows for experimental discrepancies. Furthermore, it is our opinion that some inconsistencies in the field arise from over-interpretation of lifespan-shortening scenarios associated with aging, where, in many cases, the reduced lifespan may have rather resulted from collateral damage.
To consolidate the core of reliable advances in anti-aging research, the present review considers only pharmacological interventions, regimens and genetic manipulations that meet three highly selective criteria: (i) promotion of healthspan and/or life extension; (ii) validation in at least three model organisms; and (iii) confirmation by at least three different laboratories. There are only a few proposed aging interventions that have met such stringent criteria: fasting regimens, caloric restriction, exercise, and the use of low molecular weight compounds, including spermidine, metformin, resveratrol and rapamycin (Table 1). From a molecular point of view, these interventions may act through epigenetic mechanisms (histone acetylation/methylation), the insulin/TOR pathway, the Ras signaling pathway, mitochondrial function, proteostasis, autophagy, and stress resistance. This review summarizes the current knowledge on these promising approaches and critically evaluates their applicability as anti-aging interventions.
Table 1.
Positive and negative effects of lifestyle and pharmacological interventions on humans; where no direct data is available for humans, corresponding effects on mice are listed.
| Treatment | Positive effects | Negative effects |
|---|---|---|
| Caloric restriction | Decreased body fat, blood pressure, resting heart rate and improved lipid profile | Danger of malnutrition (e.g. neurologic deficits, lowered fertility and libido, wound healing problems, amenorrhea, osteoporosis, decreased potential to combat infections) |
| Fasting strategies | Longer lifespan; decreased hypertension and of other features of metabolic syndrome; improved verbal memory loss in the aged and overweight; weight loss in the obese | Limited if not integrated with health- associated diets; might be harmful in children, underweight people and during pregnancy as well as in some disease states |
| Exercise | Prevents cardiovascular diseases, diabetes, osteoporosis, sarcopenia and depression; prolongs independent living by the elderly | Excessive exercise in the elderly is correlated to mortality |
| Resveratrol | (in mice) prevents oxidative stress in the aging heart, neurodegeneration and diabetes | Lifespan effects controversial |
| Rapamycin | (in mice) Extends lifespan; exerts anti- proliferative effects | Potent immunosuppressive properties; long-term administration has adverse effects (e.g. impaired wound healing, proteinuria, or pneumonitis) |
| Spermidine | (in mice) Extends lifespan; inhibits neurodegeneration | |
| Metformin | (in mice) decreases hepatic gluconeogenesis and increases insulin sensitivity; lifespan extension |
Why do we age?
The more complex a problem, the more important it may be to ask simple questions. So, why do we age? Actually, we do hold the capacity for immortality. The molecular clock in our germ line stem cells, which sustain gamete production, is kept at zero as evidenced by the fact that our offspring are not born with the father’s or mother’s age. Then, why do somatic cells age? Firstly, the maintenance of repair activity in all our cells represents a vast energetic demand and, secondly, the pressure of recombination and dying of generations allows organisms to adapt to changing environments (e.g., ice ages). This implies that aging may be an atavistic, adaptive and altruistic program, by which single cells or organisms eventually die for the benefit of the whole population in a highly coordinated (programmatic) fashion. In that case, groups harboring individual cells or organisms capable of dying in times of stress (e.g., nutrient deprivation, starvation) would have a selective evolutionary advantage over groups in which every single cell or organism is selfish. In fact, one of Darwin’s most famous theories is that selection can work at the group level. However, most evolutionary biologists reject this idea. For instance, Maynard Smith argued that if groups would altruistically inhibit the generation of offspring, an egoist would infect and take advantage. August Weismann maintained that an individual’s demise would depend solely on the fulfillment of its contribution to the species (sufficient reproductive activity) and not to the group. Further, aging as an adaptive program has been questioned because this process seldom occurs in nature, where most individuals die at relatively young age due to predators or infection. However, aging may start early in life. For instance, several lines of evidence indicate that the general physical fitness already of a 30-year old person is less than that of a 20-year old.
The last decade has provided evidence for the existence of group selection. Within chronologically aged yeast cultures, single yeast cells induce programmed cell death (PCD) as a result of the accumulation of oxygen radicals and metacaspase activation (Fabrizio et al., 2004; Herker et al., 2004). Genetic disruption of PCD-mediated cell removal improves short-term survival of aged cultures; however, the surviving cells lose their ability to re-grow, indicating an accumulation of damaged cells in the absence of PCD (Fabrizio et al., 2004; Herker et al., 2004). Hence, the demise of single individuals in an aged population has a cleaning function that allows only the fittest to survive. This evolutionary advantage can be simulated in a competition experiment carried out between PCD-functional and –defective yeast populations that mimic the encounter of a group containing altruistic individuals with one comprising only the egoistic kind. Strains with dysfunctional PCD have an initial advantage but are eventually overgrown and outcompeted by wild type cells (Fabrizio et al., 2004; Herker et al., 2004). Features like superoxide levels and regrowth frequency are connected to PCD, an adaptive mechanism required for programmed aging through selective advantage at least in yeast (Fabrizio et al., 2004; Herker et al., 2004). Many observations argue that programmed aging (sometimes referred to as a pseudo-program) also occurs in higher eukaryotes, for example, the rapid aging after the flowering of annual plants or the accelerated aging (progeria) of Pacific salmon after spawning. As to its possibility as a selective advantage in human populations, Darwin stated in his famous work “The Descent of Man”, “There can be no doubt that a tribe including many members who… were always ready to aid one another, and to sacrifice themselves for the common good would be victorious over most other tribes; and this would be natural selection.” The existence of some programmatic features during aging is a conditio sine qua non for a therapeutic approach to combat aging.
Behavioral anti-aging interventions
Caloric restriction
The reduction in the intake of calories without malnutrition is defined as caloric restriction (CR). Such reduction ideally corresponds to a decrease of approximately 30% of calories per day, at least in mice. In humans, there exists some indication that a CR of around 15% may be most favorable against mortality during aging (Willcox and Willcox, 2014). CR reduces the release of growth factors like growth hormone, insulin and insulin-like growth factor 1 (IGF1), which have been shown to accelerate aging and increase mortality in many organisms (Fontana et al., 2010). Long before knowing about the pro-aging effects of growth factors, the connection between lower caloric intake and prolonged lifespan was already described almost a hundred years ago. Since then, CR has been shown to prolong mean and maximum lifespan in dogs, rodents, worms, flies, yeasts, and prokaryotes (Fontana et al., 2010). In unicellular organisms, nutrient deprivation triggers lifespan extension to probably maximize the chances of reproduction in the event of future exposure to a nutrient-rich environment. Importantly, CR-mediated longevity in eukaryotes seems to be governed by a set of conserved nutrient signaling pathways (Fontana et al., 2010). Among them are the insulin and the TOR/S6K pathways, whose inhibition upon CR eventually confer stress resistance and promote survival during aging. Stress-related transcription factors represent the common effectors in yeast, flies, worms, and mammals (Fontana et al., 2010). Moreover, the histone deacetylase SIR2 (the founding member of the sirtuin family) has been linked to CR-mediated anti-aging effects in yeast mother cells, flies, and mammals (Haigis and Guarente, 2006). Sirtuin-mediated protein acetylation control might thereby not be restricted to chromatin packaging and transcriptional modulation. For instance, recent evidence points toward a role of the mitochondrial sirtuin SIRT3 in the prevention of age-related diseases, possibly through modulation of the mitochondrial acetyl proteome (Hebert et al., 2013). Of note, in rodents, moderate CR (8%) positively affects median (not maximal) lifespan, but a more severe (30%) dietary restriction increases lifespan by up to 50%, partly by delaying the emergence of chronic diseases (Anderson et al., 2009). Interestingly, a recent report showed that mice with transgenic overexpression of fibroblast growth factor 21 (FGF21), a fasting hormone, can extend life span when maintained on an ad libitum diet (Zhang et al., 2012).
The beneficial effects of CR may represent an adaptive response forged during evolution to overcome short-time famine conditions. In that case, the quantitative impact of lifespan extension relative to the total, natural lifespan of an organism might be more prominent in short-lived species (like mice) than in long-lived ones (like primates). Two studies have addressed the effects of CR on non-human primates, but with different outcomes. One study observed prolonged life (Colman et al., 2009) while the other did not (Mattison et al., 2012). Many parameters (including husbandry and diet composition) may strongly influence the positive effects of CR. It would appear that the high sugar (sucrose) concentration contained in the ad libitum diet (control group) of the UW study (Colman et al., 2009) led to a shortened lifespan within the cohort of control monkeys, thus accounting for the lifespan extension when animals were fed a CR diet. In contrast, the healthier ad libitum diet in the NIA study (Mattison et al., 2012) allowed for longer lifespan in control animals without conferring additional benefit from CR. Hence, a healthy diet might be comparable to a bad diet (high sugar) plus CR. However, both studies point to the fact that CR increases healthspan in monkeys by reducing the risk for diabetes, cardiovascular disease, and cancer.
One of the best correlations between CR and improvement in healthspan and prolonged life in humans is the long-lived population in Okinawa, Japan (Mizushima et al., 1997). In comparison to the rest of the Japanese population, Okinawan people usually combine an above-average amount of daily exercise with a below-average food intake (Willcox and Willcox, 2014). However, when Okinawan families moved to Brazil, they adopted a Western lifestyle that impacted both their diet and physical activity, resulting in increased weight gain and a drop in life expectancy of 17 years (Mizushima et al., 1997). CR changes many parameters in the aging human body, including the transcriptome, the hormonal status (in particular the serum concentration of IGF-1 and thyroid hormones), oxidative stress, inflammation, mitochondrial function, and glucose homeostasis (Fontana et al., 2010). Accumulating evidence suggests that protein restriction is necessary and sufficient for depletion of serum IGF1 in humans (Fontana et al., 2010). It remains to be determined which of these factors, if any, are responsible for CR-induced lifespan extension. Epigenetic modifications observed during aging are another possible target of CR, although this is based largely on correlative data. Studies with monozygotic twins suggest the accumulation of epigenetic changes as twins age (e.g., demethylation of 5-methylcytosines in DNA), thus affecting the transcriptome (Fraga et al., 2005).
Nevertheless, sustenance on a severely restricted diet without malnutrition (around 30% CR) is arduous and is linked to chronic low weight and substantial side effects. Studies have shown that prolonged CR may decrease fertility and libido, create wound healing problems, and result in amenorrhea, osteoporosis, and decreased potential to combat infections. Female rats subjected to prolonged CR become infertile in a reversible fashion (Martin et al., 2007), and moderate CR may be harmful to lean human beings (Fontana et al., 2010).
Dietary strategies based on CR
Given the disadvantages of CR, a key question remains. How can CR be a socially and ethically acceptable method to reduce health risks in the general population? A number of fasting regimens are asserting themselves as suitable strategies, among them the alternate day fasting diet (Johnson et al., 2007; Varady et al., 2009), the ‘5:2’ intermittent fasting diet (Harvie et al., 2011), and a 48-hour fast once or twice each month. As opposed to CR, periodic fasting is psychologically more viable, lacks some of the negative side effects and is only accompanied by minimal weight loss (Heilbronn et al., 2005).
Several lines of evidence indicate that fasting regimens exert anti-aging effects. A lower age-associated morbidity and a longer lifespan were observed among Spanish home nursing residents performing alternate day fasting, where residents fasted every other day with unrestricted food supply on the non-fasting day (Mercken et al., 2012). In the Biosphere II project, human volunteers who were living together for 24 months experienced an unforeseen severe CR. As a result, metabolic and hormonal changes, similar to those observed in CR-induced longevity increase in rodents, were detected. These included low circulating glucose, insulin, and glycated hemoglobin levels, as well as a decrease in both cholesterol levels and blood pressure (Walford et al., 2002). Interestingly, food deprivation in adults during World War II in some European countries has been associated with long term health span, but also with detrimental effects. Indeed, the psychological and physical adversities that accompany a war situation make it difficult to accurately interpret this involuntary restriction of caloric intake. Food shortage especially during the intrauterine phase and childhood may be more prone to cause malnutrition and thus result in a vast array of adverse effects. While fasting may prolong life in adult humans, the results with monkeys are still contentious (see above).
Upon fasting, the body undergoes major metabolic changes to meet its energy needs. After depletion of glycogen stores, it activates hepatic and non-hepatic gluconeogenesis (e.g. via glucogenic amino acids or glycerol) as well as production of fat-derived free fatty acids and ketone bodies. For instance, free fatty acids already appear in the bloodstream after fasting for 12–24 hours. The body’s main source of energy then comes from ketolysis, which generates acetyl-CoA, and gluconeogenesis-dependent generation of approximately 80 g of glucose each day, which is mainly consumed by the brain. Interestingly, a ketone ester diet, which seemingly recapitulates fasting energy metabolism, alleviates proteopathic and behavioral deficiencies in a mouse model of Alzheimer’s disease (Kashiwaya et al., 2013).
Based on the available evidence, we hypothesize that repeated fasting and eating cycles – as practiced during periodic fasting regimens – may circumvent the negative side effects of permanent CR. Of note, rats on an alternate day fasting protocol live up to 83% longer than their normally fed counterparts, and a 24-hour fasting period every 4 days is sufficient to promote a significant lifespan extension (Longo and Mattson, 2014). Such an intermittent fasting strategy may even yield positive effects despite overconsumption of calories. Of significance, mice that were fed a high-fat diet in a time-restricted manner, i.e. with regular fasting breaks, had lower inflammatory markers, no fatty liver and were slim in comparison to mice with equivalent total number of calories consumed ad libitum (Hatori et al., 2012). From an evolutionary point of view, this form of feeding pattern may actually reflect mammalian adaptation to food, for example, overeating in times of nutrient abundance (e.g. after a successful hunt) and starvation in between. This is also how native tribes live today, many of which showing no signs of age-associated diseases, such as cancer, neurodegeneration, diabetes, cardiovascular disease, or hypertension (Gurven et al., 2009).
Healthy aging to combat diseases
Fasting
Fasting rituals are part of most human populations and cultures that have prevailed to date, including Buddhism, Christianity, Hinduism, Judaism, and Islam as well as African animistic religions. It may thus be speculated that such routines that periodically limit caloric intake can have health-promoting attributes. Indeed, fasting has been shown to exert a series of beneficial effects on healthspan by minimizing the risk of developing age-related diseases, such as neurodegeneration, cancer, or cardiovascular diseases, in animal models and, possibly, humans (Longo and Mattson, 2014). Periodic fasting dampens the deleterious consequences of Alzheimer’s, Parkinson’s, and Huntington’s diseases as well as frontotemporal dementia in various mouse models, without beneficial effect on amyotrophic lateral sclerosis (Longo and Mattson, 2014). Fasting cycles have been reported to be as effective as chemotherapy against certain tumors in mice. When combined with chemotherapy, a 2-to-3-day fast enhances efficacy of a variety of chemotherapeutic drugs while reducing their negative side effects in mice (Raffaghello et al., 2008). A treatment that combines fasting and chemotherapy rendered 20–60% of mice cancer-free when inoculated with highly aggressive tumors like glioblastoma or pancreatic tumors, which killed 100% of the cohort when treated with chemotherapy alone (Raffaghello et al., 2008). In stark contrast to normal cells, tumor cells never cease to proliferate and require enhanced glucose metabolism to meet their high energy and biosynthetic demands. When faced with starvation, cancer cells simply die.
The single most effective way to lower hypertension is the practice of fasting. A two-week period of medically supervised, water-only fasting resulted in blood pressure readings below 120/80 mm Hg in 82% of subjects with borderline hypertension (Goldhamer et al., 2002). Ten days of water-only fasting normalized blood pressure readings in all hypertensive patients who were taking antihypertensive medication (Goldhamer et al., 2002).
The metabolic syndrome includes a cluster of risk factors, such as high blood pressure, dyslipidemia and insulin resistance, that markedly enhances the risk for coronary artery disease, stroke, and diabetes when combined with obesity. Of note, while extremely obese people (BMI above 35) are at 29% higher risk of premature death, moderately overweight individuals seem to be protected, having lower overall mortality risk as compared to normal weight persons (Flegal et al., 2013). Moderately overweight individuals probably have better protection in times of disease/crisis and improved response against infections and toward wound healing (Flegal et al., 2013). However, the BMI is an insufficient indicator of body composition and percent body fat. For example, a well-trained athlete may have a BMI equivalent to that of an obese individual because of the athlete’s higher muscle mass density. In fact, the waist/hip ratio is a much better indicator for body fat and an excellent predictor of the odds of dying from cardiovascular diseases, with low waist/hip ratio associated with a lower mortality risk (Welborn et al., 2003). Several periodic fasting strategies revert features of metabolic syndrome in overweight and obese subjects, which coincide with increases in insulin sensitivity and lipolytic activity (Harvie et al., 2011; Heilbronn et al., 2005; Varady et al., 2009).
A worldwide sociohistorical turn occurred in 2006 when the number of deaths related to obesity exceeded those linked to starvation. Studies on human twins have convincingly demonstrated that adiposity increases the probability of age-related dementia and Alzheimer’s disease (Xu et al., 2011). A 3-to-4-month period of CR improves verbal memory in overweight women and aged people (Witte et al., 2009) despite the fact that the brain relies mainly on glucose. In line with these observations, it has been demonstrated that fasting facilitates long-term memory formation in Drosophila melanogaster (Hirano et al., 2013). Moreover, alternate day fasting over a period of 2–4 weeks reduces asthma-related symptoms and markers of oxidative stress in overweight people suffering from asthma (Johnson et al., 2007). Marked reduction of metabolic disease markers, such as high blood pressure or levels of circulating IGF1 and C-reactive protein, has been reported in overweight women that were subjected to a 2-day per week fast over 6 months (Harvie et al., 2011). Similarly, aged men on an eating plan involving 2 days of fasting each week lose weight and experienced fewer bouts of depression (Teng et al., 2011). However, the efficacy of most fasting strategies as they relate to medical conditions and/or aging might be limited if they are not integrated with diets that have health-associated benefits, such as the Mediterranean diet or the Okinawa low protein diet. It should also be noted that fasting might even be harmful to certain individuals (e.g., children and underweight people) and in some disease states (e.g., type 1 diabetes or extreme hypertension), where fasting can result in severe health consequences and possible death unless performed under medical supervision.
Exercise
In rodents as in humans, regular exercise can reduce the risk of morbidity and mortality (Holloszy and Schechtman, 1991; Huffman et al., 2011; Pekkanen et al., 1987; Samorajski et al., 1985). Given that cardiovascular diseases are strongly associated with aging in humans (but not in mice), the effects of exercise on human health may be even stronger than in animal models. Indeed, an increase in aerobic exercise in the elderly is associated with favorable outcomes on blood pressure, lipids, glucose tolerance, bone density, and depression (Fleg, 2012). Similar positive results also occur upon episodic fasting. Hence, exercise training can have a beneficial impact against diseases and metabolic disorders that typically accompany aging, notably cardiovascular diseases, diabetes and osteoporosis (Fontana et al., 2010). Consumption of energy-dense, nutrient-poor food along with a sedentary lifestyle has a compounding effect on these disorders (Pereira et al., 2005). The loss of abdominal fat and reduction in the waist/hip ratio (rather than BMI) contribute to the protection against these age-related disorders (Welborn et al., 2003). Moderate or even low levels of exercise (e.g. daily 30-min walks) without weight loss have a positive effect in obese subjects (Laaksonen et al., 2005) by inhibiting the development of the metabolic syndrome (Huffman et al., 2011). Prolonged independent living by the elderly can be achieved through regular physical activity (Nicklas and Brinkley, 2009), but excessive exercise in the elderly is correlated to mortality and needs to be controlled in this population (Lee and Skerrett, 2001). Exercise is the only known treatment that can prevent or even reverse sarcopenia, a chronic disease associated with age-related loss of muscle strength and function (Glass and Roubenoff, 2010).
Contrary to CR, regular exercise fails to extend lifespan (Mercken et al., 2012) despite its clear benefits at improving health-related quality of life. Moreover, the combination of exercise and CR has no additive effect on maximal lifespan in rodents (Holloszy and Schechtman, 1991). Because exercise and CR do reveal distinct molecular signatures (Mercken et al., 2013), it is likely that the integration of regular exercise with CR results in synergistic health-promoting effects. From a socio-evolutionary point of view, this synergy can be quite advantageous. Hunger forces individuals to move and hunt. Alternate day fasting combined with exercise is more beneficial for muscle mass than exercise alone (Sakamoto and Grunewald, 1987). In non-obese subjects, CR combined with exercise has synergistic effects on insulin sensitivity and the lowering of circulating C-reactive protein (Solomon et al., 2010). Still, CR and exercise seem to be mechanistically connected to some extent, since both starvation and exercise induce autophagy through disruption of the BCL-2-beclin-1 complex (He et al., 2012).
Pharmacological anti-aging interventions
Although virtually all obese people know that stable weight loss would reduce their elevated risk for metabolic diseases and enhance their overall odds for survival, only 20% of overweight individuals are able to lose 10% of their weight for a period of at least 1 year (Wing and Phelan, 2005). A similar behavior can be found in most of the population with regard to food choice and exercise, possibly because the acute satisfaction of comfort overrides the potential long-term benefits that lifestyle changes bring. Thus, drugs that may work as CR mimetics have drawn general attention in recent years, with some of them yielding promising results in current anti-aging trials (Cloughesy et al., 2008; Patel et al., 2011).
Resveratrol is a polyphenol that is found in grapes and in red wine. Its potential to promote lifespan was first identified in yeast (Howitz et al., 2003) and has since gained fame because it was suggested to be responsible for the so-called French paradox (French winemakers do not suffer from cardiovascular diseases though enjoying a high-fat diet). In monkeys fed a diet high in sugar and fat, resveratrol attenuated peripheral inflammation in adipose tissue (Jimenez-Gomez et al., 2013), maintained pancreatic homeostasis by preventing β-cell dedifferentiation (Fiori et al., 2013) and improved vascular function, particularly pulse wave velocity (Mattison et al., 2014). Resveratrol supplementation prolongs the lifespan of mice fed a high-fat diet or fed every other day (Baur et al., 2006), but not of mice on regular chow. Resveratrol prolongs the life of flies (Bauer et al., 2004), worms (Baur and Sinclair, 2006), and the replicative lifespan of yeast (Howitz et al., 2003), but not yeast’s chronological lifespan (our unpublished observations). However, recent data claim that the extent of lifespan extension in worms and flies on a resveratrol-supplemented diet may be shorter than previously reported (Burnett et al., 2011). Nevertheless, resveratrol offers protection in models of stress- or age-associated diseases, including chronic overfeeding, insulin resistance, type 2 diabetes, or cardiovascular dysfunction (Baur et al., 2006; Lagouge et al., 2006). The mechanisms behind these effects may rely on the fact that resveratrol mimics some of the metabolic actions of CR, as a series of studies on humans have suggested (Timmers et al., 2012). Resveratrol interacts with many stress-related targets in the cell, including the mammalian NAD+-dependent deacetylase SIRT1 (Baur and Sinclair, 2006; Lagouge et al., 2006), although the resveratrol-SIRT1 interaction may be indirect (Beher et al., 2009). SIRT1 is a member of a family of proteins (sirtuins) that have been linked to longevity in yeast, flies and worms (Haigis and Guarente, 2006; Kaeberlein et al., 1999; Mouchiroud et al., 2013), but the effects and extent in lifespan extension have been challenged (Burnett et al., 2011). Still, recent evidence in mammals suggests a connection between sirtuins and a longevity-promoting function; for example, a study indicates that brain-specific overexpression of SIRT1 is sufficient to promote longevity in mice (Satoh et al., 2013). Overall, it is clear that resveratrol or SIRT1 overexpression prevents several age-associated diseases and pathogenic conditions in mice, including oxidative stress in the aging heart, neurodegeneration, or diabetes (Haigis and Guarente, 2006). Importantly, activation of autophagy by resveratrol is required for lifespan extension in C. elegans (Morselli et al., 2010).
Rapamycin, an inhibitor of the TOR kinase, is a natural product secreted by a soil bacterium originally discovered on Easter Island (also known as Rapa Nui, hence the name rapamycin). Before its introduction in the anti-aging field, rapamycin was already well known as an immunosuppressive drug for the treatment of allograft rejection. Rapamycin is a strong inducer of autophagy and it extends lifespan in all organisms tested so far, including yeast, flies, worms and mice (Bjedov and Partridge, 2011; Kapahi et al., 2004). Middle-aged mice on a diet supplemented with rapamycin survived significantly longer (Harrison et al., 2009); however, reports of the potent immunosuppressive properties of rapamycin suggest that the drug is unsuitable for lifespan extension in humans. Moreover, long-term administration of rapamycin has been found to cause a variety of adverse health effects in patients, including impaired wound healing, anemia, proteinuria, pneumonitis, and hypercholesterolemia. Chronic inhibition of mammalian TOR (mTOR) function by rapamycin promotes insulin resistance and diabetes in laboratory mice (Lamming et al., 2012). It is interesting that intermittent rapamycin feeding, which was recently shown to also increase lifespan in mice, opens up new opportunities to avoid immunosuppression and its potentially detrimental effects on lifespan extension (Anisimov et al., 2011a). Because of its anti-proliferative properties, rapamycin or its analogs (rapalogs) are used as anticancer drugs (Benjamin et al., 2011; Guba et al., 2002).
Spermidine is a naturally occurring polyamine that triggers autophagy and, thereby, lifespan extension when supplemented in the food supply of yeast, flies and worms (Eisenberg et al., 2009). Endogenous spermidine concentrations decrease as the organism age, including in humans with the exception of centenarians (Pucciarelli et al., 2012). Acute injections of spermidine in mice activate autophagy in multiple organs (Morselli et al., 2011) while chronic spermidine feeding promotes increase in healthspan (Eisenberg et al., 2009). The impact of long-term effects of spermidine on autophagy has not been investigated. Spermidine has been found to impede a number of neurological pathologies and induce neuronal autophagy. It inhibits neurodegeneration and alleviates pathogenesis and motor dysfunction in a mouse model for TDP-43 aggregation-induced frontotemporal dementia and amyotrophic lateral sclerosis (Wang et al., 2012). Moreover, age-associated memory loss in flies is reversed by spermidine in an autophagy-dependent fashion (Gupta et al., 2013). In yeast cells, spermidine controls transcription of autophagy-relevant genes through hypoacetylation of several promoter regions (Eisenberg et al., 2009). Intriguingly, the promoter region of ATG7, an essential autophagy gene, remains selectively acetylated, which allows for its transcription in a state of general gene silencing (Eisenberg et al., 2009). Lifespan extension in two short-lived mouse models has been reported after either oral administration of spermidine or the upregulation of bacterial polyamine production in the gut (Matsumoto et al., 2011; Soda et al., 2009). It is possible, however, that factors other than polyamines are secreted by gut bacteria to influence longevity.
Metformin, a biguanide first isolated from the French liliac, is the most prescribed drug for the treatment of type-2 diabetes. Metformin decreases hepatic gluconeogenesis and increases insulin sensitivity (Berstein, 2012). It is a potent, indirect activator of adenosine monophosphate-activated protein kinase (AMPK) (Scarpello and Howlett, 2008), an enzyme involved in cellular and whole-organism energy balance, as well as in glucose and fat metabolism. Interestingly, AMPK is also induced by CR. Upon metformin exposure, AMPK is activated by an increase of the AMP/ATP ratio, thereby inhibiting mTOR, a protein kinase involved in the control of cellular proliferation and tumor growth (Dazert and Hall, 2011). Although the direct target of metformin is not known, it indirectly inhibits complex I of the electron transport chain (El-Mir et al., 2000; Viollet et al., 2011). Therefore, metformin compromises ATP production in mitochondria, leading to an increase of the AMP/ATP ratio without ROS accumulation. The transcription factor SKN-1/Nrf2 is activated upon metformin treatment, resulting in increased expression of antioxidant genes and subsequent protection from oxidative damage (Berstein, 2012). Metformin increases mean and maximum lifespan in different female mouse strains predisposed to high incidence of mammary tumors (Anisimov et al., 2011b; Martin-Montalvo et al., 2013). Recently, we and others have been testing pharmacological interventions that mimic the effects of CR and can delay aging and the incidence of age-related diseases (Anisimov et al., 2011b; Baur et al., 2006; Harrison et al., 2009; Martin-Montalvo et al., 2013). Some metabolic effects of metformin resemble those of CR, even though food intake was increased (Anisimov et al., 2011b; Martin-Montalvo et al., 2013). At a biochemical level, metformin supplementation is associated with inhibition of chronic inflammation and reduction in oxidative damage, two well-known factors that compromise health and lifespan (Martin-Montalvo et al., 2013). There are now several reports demonstrating that the effects of metformin on transcription mimic the gene expression profile of animals on CR through the transcriptional regulatory pathways controlling lifespan extension (Anisimov et al., 2011b; Martin-Montalvo et al., 2013).
Common molecular denominators
Insulin signaling
The first genetic alteration described to exhibit lifespan extension was a mutation in the phosphatidylinositol 3-kinase (PI3K) of C. elegans (Friedman and Johnson, 1988). PI3K is a downstream component of the insulin signaling pathway, and deletion of the nematode’s insulin receptor (daf-2) also results in longevity (Kenyon et al., 1993). The fact that a double knockout mutant showed no additional effect suggested an epistatic interaction between the two mutations (Dorman et al., 1995). Lifespan in yeast, flies, and mice is extended in response to nutrient deprivation or following the disruption and/or genetic down-regulation of insulin and IGF signaling (Holzenberger et al., 2003). PI3Ks phosphorylate phosphatidylinositol-4,5-bisphosphate to generate phosphatidylinositol-3,4,5-trisphosphate, which acts as a second messenger for downstream activation of protein kinase B (AKT/PKB). Deletion of AKT/PKB prolongs life in S. cerevisiae, C. elegans, and Drosophila melanogaster (Fontana et al., 2010). Metazoan TOR, the master regulator of growth and inhibitor of autophagy, is upregulated by the PI3K/AKT/PKB cascade. Of significance, TOR inactivation extends lifespan in yeast (Kaeberlein et al., 2005), flies (Kapahi et al., 2004), worms (Vellai et al., 2003) and mice (Harrison et al., 2009).
IGF1 is the major growth factor in mammals, and high levels of IGF1 can cause rapid aging and cancer (Fontana et al., 2010). Mutations that decrease IGF1 levels or activity cause reduced growth and longer life in mice by up to 65% (Coschigano et al., 2000; Holzenberger et al., 2003). A group of Ecuadorians with an IGF1 signaling deficiency due to mutations in the growth hormone receptor gene exhibits dwarfism and virtually no age-associated diseases, such as cancer or diabetes (Fontana et al., 2010). It is interesting that mutations that extend lifespan also result in energy storage either in the form of glycogen, fat, or both, in virtually all tested models. Indeed, IGF1 deficiency causes greater fat accumulation in humans (Laron and Klinger, 1993). Most anti-aging regimens elicit a drop in circulating IGF1, as evidenced by the fact that a 5-day fasting period lowers IGF1 plasma levels by more than 60% and causes a significant increase in IGFBP-1, one of the principal IGF1-inhibiting proteins (Longo and Mattson, 2014). Protein restriction is required for the reduction of IGF1 levels during CR in humans (Fontana et al., 2010). In rodents, a protein-restricted diet given every other week (without CR) is sufficient to lower circulating IGF1 and increase IGFBP-1 levels, resulting in improved cognitive ability of mice with Alzheimer’s disease-like symptoms (Longo and Mattson, 2014). These findings are in line with recent studies from calorie-restricted humans, which show reduced serum levels of IGF1 only when protein intake is restricted (Fontana et al., 2010). These observations, coupled with the fact that reducing nutrient intake and disrupting TOR are anti-aging interventions, suggest that nutrient signaling has evolved from a primordial starvation response to a molecular mechanism of health and lifespan extension.
Autophagy
Autophagy (from the Greek “self-eating”) is a catabolic process that degrades defective and expandable cellular components for recycling. It ensures that sufficient energy levels are maintained (for instance upon starvation) and, at the same time, eliminates damaged cellular material (Klionsky, 2007; Levine and Kroemer, 2008). CR is a strong inducer of autophagy, which may be an efficient strategy to maintain life under times of scarce resources. This process would enhance the probability of survival until nutrients become available, and thereby allow mating and recombination. In mice, overexpression of Atg5, a gene essential for autophagy, is sufficient to activate autophagy and extend lifespan (Pyo et al., 2013). In nematodes, lifespan is extended either by CR, rapamycin-mediated inhibition of TOR signaling (a key negative regulator of autophagy), or knockdown of the cytoplasmic portion of the p53 ortholog encoded by cep-1, a tumor suppressor and autophagy inhibitor (Jia and Levine, 2007; Tavernarakis et al., 2008). The fact that knocking down genes essential for autophagy abrogates lifespan extension in worms (Jia and Levine, 2007; Tavernarakis et al., 2008) argues for a causal link between autophagy and longevity.
Autophagy and the expression/activity of sirtuins have been functionally linked in the anti-aging effects of CR, which has generated an intense scientific debate (Kaeberlein et al., 2004, 2005; Burnett et al., 2011). However, several lines of evidence suggest a closer connection of autophagy and sirtuins than previously assumed, and it involves the acetylproteome. In fact, both the autophagy inducer spermidine and the sirtuin activator resveratrol create a repressive chromatin state. Spermidine inhibits histone acetyltransferase activity, whereas resveratrol activates counteracting histone deacetylase (sirtuin) activity (Morselli et al., 2010). Moreover, both compounds also seem to affect the acetylproteome outside the nucleus, through deacetylation of the autophagy-related factor Atg5 (Morselli et al., 2011). Indeed, SIRT1 is required for the induction of autophagy in mice (Lee et al., 2008) and in human cells subjected to either resveratrol treatment or nutrient starvation, and in C. elegans under CR (Morselli et al., 2010). Conversely, lifespan extension mediated by SIRT1 overexpression or resveratrol administration is eliminated when beclin 1, a protein required for autophagy induction, is knocked down (Morselli et al., 2010). Even though spermidine and resveratrol promote autophagy through distinct mechanisms, some convergence occurs with regard to the roles of regulators or effectors of autophagy (Morselli et al., 2011). Thus, it appears that autophagy induction may be the result of a combination of transcriptional and cytoplasmic (transcription-independent) events that influence the cellular acetylproteome. In fact, acetyl-CoA controls longevity and autophagy in a panel of model systems (Eisenberg et al., 2014; Mariño et al., 2014): This energy metabolite integrates various nutrition pathways and is the only known donor in acetylation reactions. This suggests that acetyl-CoA may be a sensor, integrator and transducer of both the devastating consequences of overeating and the protective effects of CR.
As an organism ages, more fat accumulates and the capacity to induce autophagy decreases (Singh et al., 2009). Recent research indicates that these two phenomena are causally connected, because the autophagic machinery is also required for the buildup of lipid stores. Thus, autophagy may be constrained by the presence of excess fat, while also participating in lipolysis through the vacuolar breakdown of lipids, a process known as lipoautophagy (Singh et al., 2009). An interesting possibility is that autophagy and lipolysis may also be linked through sirtuins, although this remains to be explored. Nevertheless, there appears to be a connection between fasting (as an inducer of autophagy), sirtuin activation and fat metabolism. For instance, in vivo SIRT1 expression in human adipose tissue is decreased in obese women, but restored upon fasting (Pedersen et al., 2008). The mitochondrial sirtuin paralog SIRT3 stimulates fasting-induced fatty acid oxidation in mice via deacetylation-mediated activation of long chain acyl-CoA dehydrogenase (LCAD) (Hirschey et al., 2010). In addition, resveratrol promotes lipolysis, attenuates lipogenesis, and prolongs the lifespan of mice on a high-fat diet (Baur et al., 2006).
Hormesis
In the 1980s, several toxicologists coined the term hormesis to describe the protective effects conferred by the exposure to low (subtoxic) doses of stressors or even toxins (Calabrese et al., 1987). Indeed, an adaptive stress response elicited by a noxious agent, be it of a chemical, thermal, or radiological nature, renders an organism (or a cell) resistant to a larger and otherwise lethal dose of the same agent (Calabrese et al., 1987). For example, yeast cells that have been exposed to a low dose of reactive oxygen species (ROS) before being challenged with toxic levels of oxidative stress exhibit a marked anti-stress response and delay in subsequent death (Thorpe et al., 2004). During ischemic preconditioning in humans, transient and weak ischemic episodes can protect the brain and the heart against a more severe oxygen deprivation (Martins et al., 2011). Similarly, the lifelong and periodic exposure to various stressors may be important to inhibit or retard the aging process, which can be considered a major stressor (Pan et al., 2011). Consistent with this concept, heat or mild doses of oxidative stress can lead to lifespan extension in C. elegans (Cypser et al., 2006) and, in yeast, subtoxic levels of hydrogen peroxide are even essential for lifespan extension by CR (Mesquita et al., 2010). Concordantly, CR results in the activation of various anti-stress transcription factors, such as Rim15, Gis1, and Msn2/Msn4 in yeast and FOXO in mammals, to enhance the expression of a panel of various ROS-scavenging factors and heat shock proteins. Moreover, resveratrol triggers the ER stress response pathways (Wang et al., 2011) and rapamycin elicits an oxidative stress response in stem cells (Kofman et al., 2012). One can conclude that the anti-aging effects of periodic fasting and treatment with resveratrol, rapamycin or spermidine are based, at least in part, on hormesis.
Mitochondrial function
More than six decades ago, oxidative stress was proposed to be a causal trigger for aging (Harman, 1956). Harman speculated that mitochondria are the source of excess ROS during aging and hence represent the central organelles in aging progression (Harman, 1956). Although the oxidative radical theory of aging is still controversial, the concept of ROS being the driving force behind the detrimental effects of aging remains a hot topic, in part due to the consensus that mitochondrial alterations play a key regulatory role in aging. A number of nuclear encoded mitochondrial proteins, including those residing in Complex I and IV of the electron transport chain, are required for lifespan extension during CR in flies (Zid et al., 2009). The expression of these mitochondrial genes is regulated at the translational level by the translation repressor d4E-BP, a protein required for maximal lifespan extension upon CR. It appears that upregulation of active d4E-BP, normally inhibited by TOR in response to nutrients, is sufficient to extend lifespan of flies on a rich diet (Zid et al., 2009). This mechanism might be a means to mediate a metabolic shift towards increased mitochondrial capacity when nutrients are limited and lifespan needs to be prolonged (Zid et al., 2009). From a hormetic point of view, the generation of constant but low doses of ROS by mitochondria may enable cells to adapt to ROS-inflicted damage and thus to survive higher ROS levels.
Mitochondrial respiratory thresholds regulate yeast chronological lifespan and its CR-mediated extension, probably by increasing stress resistance (Ocampo et al., 2012). An important element of stress resistance is the optimal accumulation and mobilization of nutrient stores. In fact, mitochondria isolated from yeast on CR show elevated respiration, increased ROS levels and an enhanced antioxidant defense system (Sharma et al., 2011), indicating that CR may elicit mitohormetic effects with resultant life extension. In line with these results, transgenic mice overexpressing human catalase targeted solely to the mitochondria (not to the peroxisome or the nucleus) live longer and have less cardiac pathology (Schriner et al., 2005). Paradoxically, it is the impairment of mitochondrial function and associated lower ATP levels that prolongs lifespan in worms (Dillin et al., 2002; Lee et al., 2003). Mutations in the mitochondrial leucyl-tRNA synthetase gene (lrs-2) disturb mitochondrial function, thus leading to lower ATP content, impaired oxygen consumption, and longer lifespan (Lee et al., 2003). Taken together, these results indicate a key modulatory role of mitochondria during aging.
Conclusion
The purpose of aging research is the identification of interventions that may avoid or ameliorate the ravages of time. In other words, the quest is for healthy aging, where improved longevity is coupled to a corresponding healthspan extension. Unfortunately, numerous claims about substances or behaviors predicted to hold such benefits have been proven erroneous. In recent years, however, fasting and exercise regimens, and a number of small molecules have been shown to prolong life and/or sustain health late in life in model organisms as different as yeast and monkeys. All these interventions are compatible with the concept of programmed aging and, thus, amenable to interventions aimed at modulating molecular components and pathways responsible for the aging process. Autophagy up-regulation, improved stress resistance and mitochondrial efficiency are among the cellular functions needed to hold off features associated with accelerated aging, such as free radical generation, excess caloric intake, chronic hyperglycemia, and fat accumulation. More research is needed to gain further insights into the long-term effects of these interventions alone and in combination, and clarify their applicability to humans. The current epidemics of obesity, diabetes and related disorders constitute major impediments for healthy aging. It is only by extending the healthy human lifespan that we will truly meet the premise of the Roman poet Cicero: “No one is so old as to think that he may not live a year.”
Figure 1. Health-promoting interventions against age-related diseases.
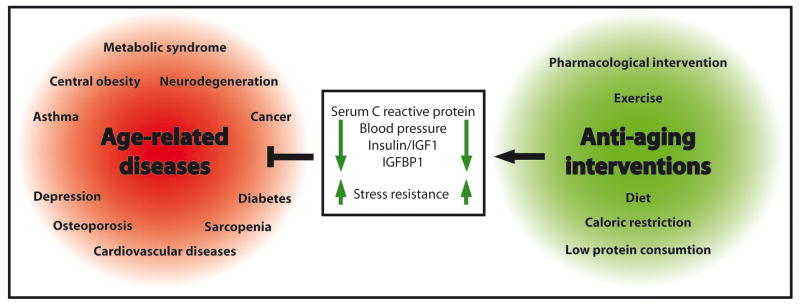
Three distinct approaches are currently being tested as feasible strategies to counteract a series of disorders, whose risk of emergence is directly linked to increased age: (i) specific anti-aging drugs, (ii) fasting, which seems best associated to a beneficial diet that includes low protein consumption, and (iii) periodic exercise. All of them have been shown to reduce the risk of age-related diseases in mammalian models, including neurodegeneration and cardiovascular afflictions, among others.
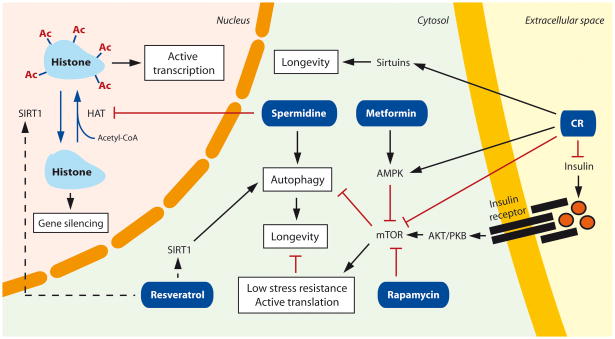
Figure 2. Molecular targets for caloric restriction and pharmacological interventions against premature aging.
Caloric restriction promotes longevity through sirtuin activation as well as through inhibition of the insulin and the mTOR pathways, which both lead to stress resistance and autophagy activation. Resveratrol, rapamycin, and spermidine exert autophagy-dependent anti-aging mechanisms that are exerted both at the cytosolic and nuclear levels. Whether resveratrol directly interacts with the sirtuin family member SIRT1 remains controversial. Metformin promotes AMPK activity and prevents oxidative damage. Ac: acetyl residue; HAT: histone acetyl transferase; AMPK: adenosine monophosphate-activated protein kinase; AKT/PKB: protein kinase B; CR: caloric restriction.
Acknowledgments
FM and DC-G are grateful to the Austrian Science Fund FWF (SFB-LIPOTOX F3007, F3012, P23490-B12, P24381-B20, W1226-B18) and the European Commission for project APOSYS. RdC and MB were funded by the Intramural Research Program of the National Institute on Aging, National Institutes of Health. MNH acknowledges the Swiss National Science Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson RM, Shanmuganayagam D, Weindruch R. Caloric restriction and aging: studies in mice and monkeys. Toxicol Pathol. 2009;37:47–51. doi: 10.1177/0192623308329476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Rosenfeld SV, Blagosklonny MV. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011a;10:4230–4236. doi: 10.4161/cc.10.24.18486. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Berstein LM, Popovich IG, Zabezhinski MA, Egormin PA, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Kovalenko IG, et al. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY) 2011b;3:148–157. doi: 10.18632/aging.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer JH, Goupil S, Garber GB, Helfand SL. An accelerated assay for the identification of lifespan-extending interventions in Drosophila melanogaster. Proc Natl Acad Sci USA. 2004;101:12980–12985. doi: 10.1073/pnas.0403493101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, Wang M. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10:868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- Berstein LM. Metformin in obesity, cancer and aging: addressing controversies. Aging (Albany NY) 2012;4:320–329. doi: 10.18632/aging.100455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjedov I, Partridge L. A longer and healthier life with TOR down-regulation: genetics and drugs. Biochem Soc Trans. 2011;39:460–465. doi: 10.1042/BST0390460. [DOI] [PubMed] [Google Scholar]
- Burnett C, Valentini S, Cabreiro F, Goss M, Somogyvári M, Piper MD, Hoddinott M, Sutphin GL, Leko V, McElwee JJ, et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477:482–485. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, McCarthy ME, Kenyon E. The occurrence of chemically induced hormesis. Health Phys. 1987;52:531–541. doi: 10.1097/00004032-198705000-00002. [DOI] [PubMed] [Google Scholar]
- Cloughesy TF, Yoshimoto K, Nghiemphu P, Brown K, Dang J, Zhu S, Hsueh T, Chen Y, Wang W, Youngkin D, et al. Antitumor Activity of Rapamycin in a Phase I Trial for Patients with Recurrent PTEN-Deficient Glioblastoma. PLoS Med. 2008;5:e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141:2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- Cypser JR, Tedesco P, Johnson TE. Hormesis and aging in Caenorhabditis elegans. Exp Gerontol. 2006;41:935–939. doi: 10.1016/j.exger.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazert E, Hall MN. mTOR signaling in disease. Curr Opin Cell Biol. 2011;23:744–755. doi: 10.1016/j.ceb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Rates of behavior and aging specified by mitochondrial function during development. Science. 2002;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- Dorman JB, Albinder B, Shroyer T, Kenyon C. The age-1 and daf-2 genes function in a common pathway to control the lifespan of Caenorhabditis elegans. Genetics. 1995;141:1399–1406. doi: 10.1093/genetics/141.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- Eisenberg T, Schroeder S, Andryushkova A, Pendl T, Küttner V, Bhukel A, Mariño G, Pietrocola F, Harger A, Zimmermann A, et al. Nucleocytosolic Depletion of the Energy Metabolite Acetyl-Coenzyme A Stimulates Autophagy and Prolongs Lifespan. Cell Metabolism. 2014;19:431–444. doi: 10.1016/j.cmet.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mir MY, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Battistella L, Vardavas R, Gattazzo C, Liou LL, Diaspro A, Dossen JW, Gralla EB, Longo VD. Superoxide is a mediator of an altruistic aging program in Saccharomyces cerevisiae. J Cell Biol. 2004;166:1055–1067. doi: 10.1083/jcb.200404002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori JL, Shin YK, Kim W, Krzysik-Walker SM, Gonzalez-Mariscal I, Carlson OD, Sanghvi M, Moaddel R, Farhang K, Gadkaree SK, et al. Resveratrol Prevents beta-cell Dedifferentiation in Non-Human Primates Given a High Fat/ High Sugar Diet. Diabetes. 2013;24 doi: 10.2337/db13-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleg JL. Aerobic exercise in the elderly: a key to successful aging. Discov Med. 2012;13:223–228. [PubMed] [Google Scholar]
- Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suñer D, Cigudosa JC, Urioste M, Benitez J, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci USA. 2005;102:10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988;118:75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass D, Roubenoff R. Recent advances in the biology and therapy of muscle wasting. Ann N Y Acad Sci. 2010;1211:25–36. doi: 10.1111/j.1749-6632.2010.05809.x. [DOI] [PubMed] [Google Scholar]
- Goldhamer AC, Lisle DJ, Sultana P, Anderson SV, Parpia B, Hughes B, Campbell TC. Medically supervised water-only fasting in the treatment of borderline hypertension. J Altern Complement Med. 2002;8:643–650. doi: 10.1089/107555302320825165. [DOI] [PubMed] [Google Scholar]
- Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- Gupta VK, Scheunemann L, Eisenberg T, Mertel S, Bhukel A, Koemans TS, Kramer JM, Liu KSY, Schroeder S, Stunnenberg HG, et al. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat Neurosci. 2013;16:1453–1460. doi: 10.1038/nn.3512. [DOI] [PubMed] [Google Scholar]
- Gurven M, Kaplan H, Winking J, Eid Rodriguez D, Vasunilashorn S, Kim JK, Finch C, Crimmins E. Inflammation and infection do not promote arterial aging and cardiovascular disease risk factors among lean horticulturalists. PLoS ONE. 2009;4:e6590. doi: 10.1371/journal.pone.0006590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond) 2011;35:714–727. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JAJ, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert AS, Dittenhafer-Reed KE, Yu W, Bailey DJ, Selen ES, Boersma MD, Carson JJ, Tonelli M, Balloon AJ, Higbee AJ, et al. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol Cell. 2013;49:186–199. doi: 10.1016/j.molcel.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn LK, Smith SR, Martin CK, Anton SD, Ravussin E. Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am J Clin Nutr. 2005;81:69–73. doi: 10.1093/ajcn/81.1.69. [DOI] [PubMed] [Google Scholar]
- Herker E, Jungwirth H, Lehmann KA, Maldener C, Fröhlich KU, Wissing S, Büttner S, Fehr M, Sigrist S, Madeo F. Chronological aging leads to apoptosis in yeast. J Cell Biol. 2004;164:501–507. doi: 10.1083/jcb.200310014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y, Masuda T, Naganos S, Matsuno M, Ueno K, Miyashita T, Horiuchi J, Saitoe M. Fasting launches CRTC to facilitate long-term memory formation in Drosophila. Science. 2013;339:443–446. doi: 10.1126/science.1227170. [DOI] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy JO, Schechtman KB. Interaction between exercise and food restriction: effects on longevity of male rats. J Appl Physiol. 1991;70:1529–1535. doi: 10.1152/jappl.1991.70.4.1529. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Géloën A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Huffman KM, Slentz CA, Bateman LA, Thompson D, Muehlbauer MJ, Bain JR, Stevens RD, Wenner BR, Kraus VB, Newgard CB, et al. Exercise-induced changes in metabolic intermediates, hormones, and inflammatory markers associated with improvements in insulin sensitivity. Diabetes Care. 2011;34:174–176. doi: 10.2337/dc10-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia K, Levine B. Autophagy is required for dietary restriction-mediated life span extension in C. elegans. Autophagy. 2007;3:597–599. doi: 10.4161/auto.4989. [DOI] [PubMed] [Google Scholar]
- Jimenez-Gomez Y, Mattison JA, Pearson KJ, Martin-Montalvo A, Palacios HH, Sossong AM, Ward TM, Younts CM, Lewis K, Allard JS, et al. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell metabolism. 2013;18:533–545. doi: 10.1016/j.cmet.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Telljohann R, Tellejohan R, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Kirkland KT, Fields S, Kennedy BK. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2004;2:E296. doi: 10.1371/journal.pbio.0020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Powers RW, 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwaya Y, Bergman C, Lee JH, Wan R, King MT, Mughal MR, Okun E, Clarke K, Mattson MP, Veech RL. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2013;34:1530–1539. doi: 10.1016/j.neurobiolaging.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- Kofman AE, McGraw MR, Payne CJ. Rapamycin increases oxidative stress response gene expression in adult stem cells. Aging (Albany NY) 2012;4:279–289. doi: 10.18632/aging.100451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laaksonen DE, Lindström J, Lakka TA, Eriksson JG, Niskanen L, Wikström K, Aunola S, Keinänen-Kiukaanniemi S, Laakso M, Valle TT, et al. Physical activity in the prevention of type 2 diabetes: the Finnish diabetes prevention study. Diabetes. 2005;54:158–165. doi: 10.2337/diabetes.54.1.158. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laron Z, Klinger B. Body fat in Laron syndrome patients: effect of insulin-like growth factor I treatment. Horm Res. 1993;40:16–22. doi: 10.1159/000183762. [DOI] [PubMed] [Google Scholar]
- Lee IM, Skerrett PJ. Physical activity and all-cause mortality: what is the dose-response relation? Med Sci Sports Exerc. 2001;33:S459–471. doi: 10.1097/00005768-200106001-00016. discussion S493–494. [DOI] [PubMed] [Google Scholar]
- Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci USA. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Lee RYN, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat Genet. 2003;33:40–48. doi: 10.1038/ng1056. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Mattson MP. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab. 2014;19:181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariño G, Pietrocola F, Eisenberg T, Kong Y, Malik SA, Andryushkova A, Schroeder S, Pendl T, Harger A, Niso-Santano M, et al. Regulation of Autophagy by Cytosolic Acetyl-Coenzyme A. Molecular Cell. 2014;53:710–725. doi: 10.1016/j.molcel.2014.01.016. [DOI] [PubMed] [Google Scholar]
- Martin B, Pearson M, Kebejian L, Golden E, Keselman A, Bender M, Carlson O, Egan J, Ladenheim B, Cadet JL, et al. Sex-dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Endocrinology. 2007;148:4318–4333. doi: 10.1210/en.2007-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins I, Galluzzi L, Kroemer G. Hormesis, cell death and aging. Aging (Albany NY) 2011;3:821–828. doi: 10.18632/aging.100380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Kurihara S, Kibe R, Ashida H, Benno Y. Longevity in mice is promoted by probiotic-induced suppression of colonic senescence dependent on upregulation of gut bacterial polyamine production. PLoS ONE. 2011;6:e23652. doi: 10.1371/journal.pone.0023652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Wang M, Bernier M, Zhang J, Park SS, Maudsley S, An SS, Santhanam L, Martin B, Faulkner S. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab. 2014 doi: 10.1016/j.cmet.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev. 2012;11:390–398. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken EM, Crosby SD, Lamming DW, JeBailey L, Krzysik-Walker S, Villareal DT, Capri M, Franceschi C, Zhang Y, Becker K, et al. Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile. Aging Cell. 2013;12:645–651. doi: 10.1111/acel.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita A, Weinberger M, Silva A, Sampaio-Marques B, Almeida B, Leão C, Costa V, Rodrigues F, Burhans WC, Ludovico P. Caloric restriction or catalase inactivation extends yeast chronological lifespan by inducing H2O2 and superoxide dismutase activity. Proc Natl Acad Sci USA. 2010;107:15123–15128. doi: 10.1073/pnas.1004432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima S, Moriguchi EH, Ishikawa P, Hekman P, Nara Y, Mimura G, Moriguchi Y, Yamori Y. Fish intake and cardiovascular risk among middle-aged Japanese in Japan and Brazil. J Cardiovasc Risk. 1997;4:191–199. [PubMed] [Google Scholar]
- Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, Criollo A, Galluzzi L, Malik SA, Vitale I, et al. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1:e10. doi: 10.1038/cddis.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morselli E, Mariño G, Bennetzen MV, Eisenberg T, Megalou E, Schroeder S, Cabrera S, Bénit P, Rustin P, Criollo A, et al. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol. 2011;192:615–629. doi: 10.1083/jcb.201008167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Cantó C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, et al. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell. 2013;154:430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCHS. Health, United States, 2011: With Special Feature on Socioeconomic Status and Health. 2012 [PubMed] [Google Scholar]
- Nicklas BJ, Brinkley TE. Exercise training as a treatment for chronic inflammation in the elderly. Exerc Sport Sci Rev. 2009;37:165–170. doi: 10.1097/JES.0b013e3181b7b3d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocampo A, Liu J, Schroeder EA, Shadel GS, Barrientos A. Mitochondrial respiratory thresholds regulate yeast chronological life span and its extension by caloric restriction. Cell Metab. 2012;16:55–67. doi: 10.1016/j.cmet.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Schroeder EA, Ocampo A, Barrientos A, Shadel GS. Regulation of yeast chronological life span by TORC1 via adaptive mitochondrial ROS signaling. Cell Metab. 2011;13:668–678. doi: 10.1016/j.cmet.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KR, Scott E, Brown VA, Gescher AJ, Steward WP, Brown K. Clinical trials of resveratrol. Ann N Y Acad Sci. 2011;1215:161–169. doi: 10.1111/j.1749-6632.2010.05853.x. [DOI] [PubMed] [Google Scholar]
- Pedersen SB, Ølholm J, Paulsen SK, Bennetzen MF, Richelsen B. Low Sirt1 expression, which is upregulated by fasting, in human adipose tissue from obese women. Int J Obes (Lond) 2008;32:1250–1255. doi: 10.1038/ijo.2008.78. [DOI] [PubMed] [Google Scholar]
- Pekkanen J, Marti B, Nissinen A, Tuomilehto J, Punsar S, Karvonen MJ. Reduction of premature mortality by high physical activity: a 20-year follow-up of middle-aged Finnish men. Lancet. 1987;1:1473–1477. doi: 10.1016/s0140-6736(87)92218-5. [DOI] [PubMed] [Google Scholar]
- Pereira MA, Kartashov AI, Ebbeling CB, Van Horn L, Slattery ML, Jacobs DR, Jr, Ludwig DS. Fast-food habits, weight gain, and insulin resistance (the CARDIA study): 15-year prospective analysis. Lancet. 2005;365:36–42. doi: 10.1016/S0140-6736(04)17663-0. [DOI] [PubMed] [Google Scholar]
- Pucciarelli S, Moreschini B, Micozzi D, De Fronzo GS, Carpi FM, Polzonetti V, Vincenzetti S, Mignini F, Napolioni V. Spermidine and spermine are enriched in whole blood of nona/centenarians. Rejuvenation Res. 2012;15:590–595. doi: 10.1089/rej.2012.1349. [DOI] [PubMed] [Google Scholar]
- Pyo JO, Yoo SM, Ahn HH, Nah J, Hong SH, Kam TI, Jung S, Jung YK. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat Commun. 2013;4:2300. doi: 10.1038/ncomms3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, Longo VD. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci USA. 2008;105:8215–8220. doi: 10.1073/pnas.0708100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Grunewald KK. Beneficial effects of exercise on growth of rats during intermittent fasting. J Nutr. 1987;117:390–395. doi: 10.1093/jn/117.2.390. [DOI] [PubMed] [Google Scholar]
- Samorajski T, Delaney C, Durham L, Ordy JM, Johnson JA, Dunlap WP. Effect of exercise on longevity, body weight, locomotor performance, and passive-avoidance memory of C57BL/6J mice. Neurobiol Aging. 1985;6:17–24. doi: 10.1016/0197-4580(85)90066-1. [DOI] [PubMed] [Google Scholar]
- Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED, Yamada KA, Imai SI. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18:416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpello JHB, Howlett HCS. Metformin therapy and clinical uses. Diab Vasc Dis Res. 2008;5:157–167. doi: 10.3132/dvdr.2008.027. [DOI] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Sharma PK, Agrawal V, Roy N. Mitochondria-mediated hormetic response in life span extension of calorie-restricted Saccharomyces cerevisiae. Age (Dordr) 2011;33:143–154. doi: 10.1007/s11357-010-9169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soda K, Dobashi Y, Kano Y, Tsujinaka S, Konishi F. Polyamine-rich food decreases age-associated pathology and mortality in aged mice. Exp Gerontol. 2009;44:727–732. doi: 10.1016/j.exger.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Solomon TP, Haus JM, Kelly KR, Cook MD, Filion J, Rocco M, Kashyap SR, Watanabe RM, Barkoukis H, Kirwan JP. A low-glycemic index diet combined with exercise reduces insulin resistance, postprandial hyperinsulinemia, and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. Am J Clin Nutr. 2010;92:1359–1368. doi: 10.3945/ajcn.2010.29771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernarakis N, Pasparaki A, Tasdemir E, Maiuri MC, Kroemer G. The effects of p53 on whole organism longevity are mediated by autophagy. Autophagy. 2008;4:870–873. doi: 10.4161/auto.6730. [DOI] [PubMed] [Google Scholar]
- Teng NIMF, Shahar S, Manaf ZA, Das SK, Taha CSC, Ngah WZW. Efficacy of fasting calorie restriction on quality of life among aging men. Physiol Behav. 2011;104:1059–1064. doi: 10.1016/j.physbeh.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Thorpe GW, Fong CS, Alic N, Higgins VJ, Dawes IW. Cells have distinct mechanisms to maintain protection against different reactive oxygen species: oxidative-stress-response genes. Proc Natl Acad Sci USA. 2004;101:6564–6569. doi: 10.1073/pnas.0305888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers S, Auwerx J, Schrauwen P. The journey of resveratrol from yeast to human. Aging (Albany NY) 2012;4:146–158. doi: 10.18632/aging.100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varady KA, Bhutani S, Church EC, Klempel MC. Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr. 2009;90:1138–1143. doi: 10.3945/ajcn.2009.28380. [DOI] [PubMed] [Google Scholar]
- Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Müller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- Walford RL, Mock D, Verdery R, MacCallum T. Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J Gerontol A Biol Sci Med Sci. 2002;57:B211–224. doi: 10.1093/gerona/57.6.b211. [DOI] [PubMed] [Google Scholar]
- Wang FM, Galson DL, Roodman GD, Ouyang H. Resveratrol triggers the pro-apoptotic endoplasmic reticulum stress response and represses pro-survival XBP1 signaling in human multiple myeloma cells. Exp Hematol. 2011;39:999–1006. doi: 10.1016/j.exphem.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang IF, Guo BS, Liu YC, Wu CC, Yang CH, Tsai KJ, Shen CKJ. Autophagy activators rescue and alleviate pathogenesis of a mouse model with proteinopathies of the TAR DNA-binding protein 43. Proc Natl Acad Sci USA. 2012;109:15024–15029. doi: 10.1073/pnas.1206362109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welborn TA, Dhaliwal SS, Bennett SA. Waist-hip ratio is the dominant risk factor predicting cardiovascular death in Australia. Med J Aust. 2003;179:580–585. doi: 10.5694/j.1326-5377.2003.tb05704.x. [DOI] [PubMed] [Google Scholar]
- Willcox BJ, Willcox DC. Caloric restriction, caloric restriction mimetics, and healthy aging in Okinawa: controversies and clinical implications. Curr Opin Clin Nutr Metab Care. 2014;17:51–58. doi: 10.1097/MCO.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82:222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- Witte AV, Fobker M, Gellner R, Knecht S, Flöel A. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci USA. 2009;106:1255–1260. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology. 2011;76:1568–1574. doi: 10.1212/WNL.0b013e3182190d09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xie Y, Berglund ED, Coate KC, He TT, Katafuchi T, Xiao G, Potthoff MJ, Wei W, Wan Y, et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife. 2012;1:e00065. doi: 10.7554/eLife.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, Benzer S, Kapahi P. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139:149–160. doi: 10.1016/j.cell.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]