Abstract
Hispanic Americans (HA) are a significant and increasing segment of the population who must be considered in future health planning. HA, compared to European Americans (EA), have a lower prevalence of coronary artery disease, but higher burden of cardiovascular disease risk factors. It remains unclear if this observation termed the ‘Hispanic Paradox’ also exists for vascular beds outside the heart. We present a review of the literature which suggests that this paradox may also exist for arteries in the extremities and neck.
Keywords: Carotid artery atherosclerosis, Coronary artery disease, Hispanic ethnicity, Hispanic Paradox, Peripheral artery disease, Subclavian artery stenosis
Introduction
Peripheral artery disease (PAD) is typically characterized by atherosclerotic blood flow obstruction to the upper and lower extremity.1 The definition of PAD may be broadened to include any arterial bed exclusive of the heart (i.e. carotid and subclavian arteries, aorta and visceral arterial beds). For the purposes of this review, PAD refers to atherosclerotic obstruction of blood flow to leg arteries, occurring along the arterial tree from the aortic bifurcation to the tibial arteries. Atherosclerotic obstruction in other non-coronary arterial beds will be identified and discussed separately.
A diagnosis of PAD conveys a significant risk for cardiovascular disease (CVD) morbidity and mortality.2–4 Also, PAD is a preventable cause of walking difficulties,5 disability, 6 and leg amputations.7 In 2010, 202 million individuals around the world were estimated to be living with PAD, a 25% increase in the global burden of PAD from the preceding decade.8 In the United States (U.S), 5% of individuals age 60 to 70 years, 10% age 70 to 80 years, and 20% age > 80 years are estimated to have PAD.9 Given our aging population, PAD represents an important public health issue, which must be considered in future public health planning.
Our knowledge of PAD in Hispanics Americans (HA) and other minority groups is limited, with the exception of African Americans (AA). In the 2000 census, HA represented 16% (50.5 million) of U.S. residents while accounting for more than half the growth of the total U.S. population in the preceding decade.10 It is estimated that by the year 2060, 129 million HA (31% of the total population) will reside in the U.S.11 Future public health planning must also account for this demographic change.
PAD has been characterized as a coronary heart disease (CHD) equivalent in terms of future CVD risk. Both PAD and CHD are manifestations of CVD, and share similar atherosclerotic pathology, as well as common risk factors.12 Its been previously reported that HA have a higher prevalence of CVD risk factors compared to European Americans (EA), but lower rates of CHD and CVD death.13,14 This observation termed the “Hispanic Paradox,” was recently confirmed in a meta-analysis of 18 studies (meeting stringent criteria) conducted between 1950 to 2009.14 The purpose of this review article is to investigate whether the ‘Hispanic Paradox’ exists for PAD. We will also investigate this paradox in other non-coronary vascular beds.
Diagnosis of PAD
Most individuals with PAD are asymptomatic, with less than 10% presenting with classic symptoms of intermittent claudication (IC; pain experienced in the calf, hip, and/or buttock on walking that subsides with rest).15 In a diverse population of participants with known PAD, the prevalence of IC was low among HA (6%), EA (11%), and AA (7%). Critical limb ischemia (CLI; pain experience in the calf, hip and/or buttock at rest, or non-healing ulcers and/or gangrene in the foot) is less common than IC in the general population, and its prevalence in HA with PAD is unknown.15 For these reasons, symptoms of IC or CLI underestimate the full PAD burden.
Standard diagnosis for PAD is with the ankle brachial index (ABI), a non-invasive physiologic test, commonly performed using a simple blood pressure cuff and a continuous Doppler ultrasound device. The ABI for each leg is calculated separately by dividing the higher of the systolic blood pressure (SBP) of either ipsilateral superficial ankle artery (posterior tibial or dorsalis pedis) by the higher of the right or left brachial artery SBP.16 The higher of the two brachial pressures should be used because of the association between PAD and subclavian artery stenosis.17 An ABI ≤ 0.90 in either leg is diagnostic for PAD, and has a 79% sensitivity and 96% specificity for ≥ 50% stenosis of the arterial lumen in the affected leg.18 While a low ABI (≤ 0.90) has strong prognostic value, borderline ABI (0.90–1.00, 1.30–1.40) and high ABI (≥ 1.40) values are also significantly associated with increased risk of CVD morbidity and mortality.19 Digital subtraction angiography (DSA), the gold standard diagnosis for PAD, is expensive, invasive, utilizes potentially nephrotoxic contrast media, and is thus reserved for the management of severe disease.20 For this reason, most epidemiologic studies of PAD have used the ABI.
In recent years the singular threshold for and use of the ABI for PAD diagnosis has been questioned.21 In a group of participants without PAD or major risk factors for PAD, and after extensive covariate adjustments, the ABI varied across gender and ethnic groups.22 This suggests that optimum ABI thresholds for PAD may differ among genders and different ethnicities. Also, the ABI has reduced sensitivity for PAD in diabetic patients who often develop stiffness in their tibial arteries from medial artery calcification (MAC).23 MAC leads to less compressible tibial arteries, which artificially elevate ankle SBP and the calculated ABI, and may obscure underlying atherosclerotic obstruction. Diabetes, a significant risk factor for PAD, is more prevalent in HA and other minority popopulations.24
Diabetic patients suspected with stiff vessels (ABI ≥ 1.3) should be further evaluated for PAD in a vascular lab with the toe brachial index (TBI, ratio of SBP in the toe to the arm).25 A TBI ≤ 0.7 is suggestive of PAD, and can identify disease in individuals with stiff vessels because toe arteries are less affected by calcification.26 Also, while the prognostic value of the ABI for CVD mortality is affected by type 2 diabetes mellitus (T2DM) status (low and high ABI convey increased risk CVD mortality), the association between the TBI and CVD mortality is linear (only low values convey increased risk).27 The usefulness of the TBI and other vascular lab assessments for PAD has not been extensively studied in HA.
Prevalence of PAD
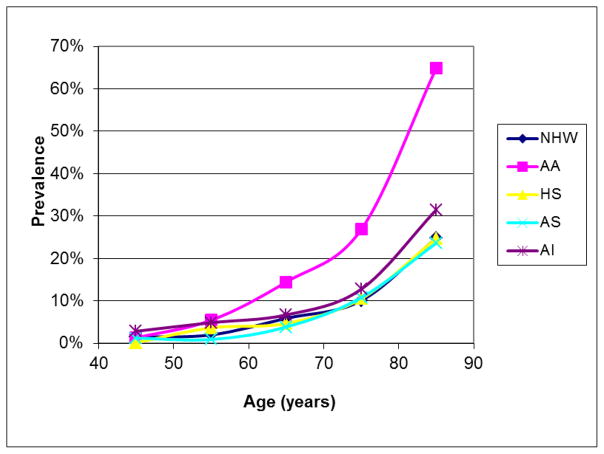
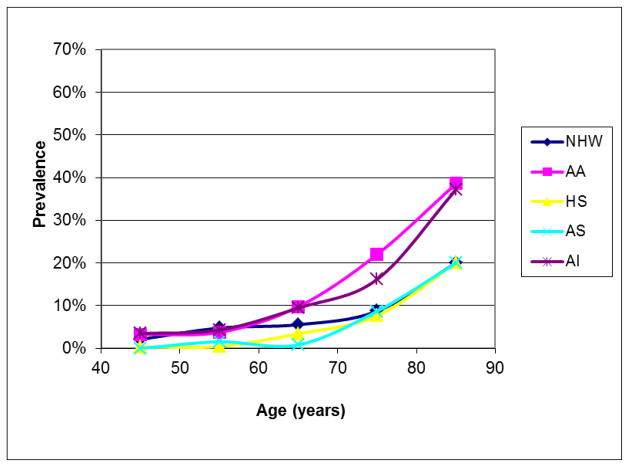
Our best estimates of the ethnic distribution of PAD in the U.S. are from the year 2000.9 At that time, 6.5 million (5.8% of the population) individuals aged ≥ 40 years were estimated to be living with PAD, of which 3.8% were HA, 78% EA, 15.9% AA, 1.5% Asian Americans (AS), and 0.7% American Indian (AI).9 For both men and women, and across most age groups AA had the highest rates of PAD, AI had intermediate rates, while HA and EA had the lowest rates (Figure 1 & 2). In this report, three studies provided much of the prevalence data for PAD in HA; the National Health and Nutrition Examination Survey28 (NHANES, a national sample of non institutionalized individuals), the San Diego Population Study29 (SDPS, mostly employees, retires and their spouses recruited from an academic institution), and the Multi-ethnic Study of Atherosclerosis30 (MESA, community living individuals free from clinically manifest CVD at recruitment). All three studies were heavily skewed towards individuals age ≥ 40 years, oversampled HA and other minority groups, and Mexican Americans comprised a large portion of the HA population.
Figure 1.
Ethnic-specific prevalence of peripheral arterial disease in men. NHW = non-Hispanic white, AA = African American, HS = Hispanic Americans, AS = Asian- Americans, AI = American Indian
Figure 2.
Ethnic-specific prevalence of peripheral arterial disease in women. NHW = non-Hispanic white, AA = African American, HS = Hispanic Americans, AS = Asian Americans, AI = American Indian
Table 1 shows the prevalence of PAD in the three multi ethnic cohorts which included HA and other minority populations; NHANES, SDPS, and MESA. For all three studies, AA had the highest rates of PAD, followed by EA, while rates in HA and AS were the lowest. In MESA, using an ABI ≤ 1.0 as a threshold, PAD prevalence was determined in Mexican-, Puerto Rican-, Dominican-, and other HA (included Cuban Americans). Puerto Ricans had the highest prevalence of PAD (13%), followed by Mexicans (10.7%), Dominicans (9.4), and other HA (6.0%).31
Table 1.
Prevalence of PAD in Multi-ethnic Cohorts
| Ethnicities | NHANES N=2157 |
SDPS N=2343 |
MESA N=6653 |
|---|---|---|---|
| European Americans | |||
| % of population | 85.9 | 59.8 | 38 |
| % with PAD | 4.5 | 4.9 | 3.6 |
| Hispanic Americans | |||
| % of population | 4.5 | 14.6 | 22 |
| % with PAD | 3 | 1.8 | 2.4 |
| African Americans | |||
| % of population | 9.6 | 13.7 | 28 |
| % with PAD | 7.9 | 7.8 | 7.2 |
| Asian Americans | |||
| % of population | N/A | 11.9 | 12 |
| % with PAD | N/A | 1.4 | 2 |
PAD = peripheral artery disease, NHANES = National Health and Nutrition Examination Survey, SDPS = San Diego Population Study, MESA = Multi-ethnic Study of Atherosclerosis
Risk Factors for PAD
T2DM and smoking are the major risk factors for PAD, with other CVD risk factors such as hypertension (HTN) and dyslipidemia (DYS) also showing a strong association.15 Higher levels of the novel CVD risk factors C-reactive protein (CRP) and fibrinogen are associated with PAD.30 In MESA, after adjustments for age and gender, T2DM rates were significantly higher in HA (19.1) compared to EA (6.7%), while cigarette smoking rates did not differ significantly (12% vs. 10.8% respectively). Also in MESA, after similar adjustments, HA had significantly higher rates of HTN and DYS, along with higher levels of CRP and fibrinogen compared to EA.30 In the SDPS, T2DM rates were higher in HA (6.5%) compared to EA (3.8%), though not significant, while mean pack years of smoking were significantly lower for HA (7.1% vs 10.2 respectively).29 HA had poorer lipid profiles, but similar rates of HTN and HTN medication use compared to EA in the SDPS.29
National data have consistently identified HA as a population subgroup significantly and adversely affected by biological and environmental CVD risk factors, which also increase risk for PAD.24 HA also experience higher rates of inactivity, poor diet, lower socioeconomic status, and limited access to healthcare compared to EA. A recent report of adults age ≥ 18 showed a higher proportion of HA with multiple CVD risk factors than EA (39.6 vs 35.5 respectively).32 Reduction of these disparities are important goals in the Healthy People 2020 Initiatve.33 Despite having poorer CVD risk factor profiles than CA, national data have also reported that HA have lower rates of CHD.14,24
Hispanic Paradox in PAD
Evidence suggests that the Hispanic Paradox may exist in PAD. In both the SDPS and MESA where ethnic comparisons were investigated, HA had greater burden PAD risk factors than EA.29,30 Particularly in MESA, T2DM rates for HA were as high as those for AA, a subgroup of the population who consistently have the highest rates of PAD. Adverse social determinants of health such as low education and income were also greater in HA compared to EA for both the SDPS and MESA.29,30 Despite having a poorer PAD risk factor profile than EA, HA had a lower prevalence of PAD in both studies.
Ethnic susceptibility to PAD was reported in NHANES, SDPS, and MESA. Hispanic compared to European ethnicity was protective against odds of PAD in minimally adjusted models in all three studies, though odd ratios were not significant. In MESA, Hispanic ethnicity was significantly protective for PAD in models fully adjusted for PAD risk factors, and adverse social determinants of health (OR = 0.49, 95% CI: 0.23 to 0.76).30 In summary, HA compared to EA have a lower prevalence of and apparently lower susceptibility to PAD, despite having a higher burden of PAD risk factors.
Carotid Artery Atherosclerosis
Carotid artery atherosclerosis refers to narrowing of the carotid arteries as a result of luminal stenosis or atherosclerotic plaque, is considered a strong predictor of future ischemic stroke, and has been associated with increased CVD risk.34 Carotid artery plaque and intima-media thickness (cIMT), in particular, are markers of subclinical atherosclerosis. Carotid plaque is a recognized phenotype of atherosclerosis with a potentially high likelihood of rapid progression, thrombotic complications, and rupture.34 Carotid IMT, however, signifies HTN hypertrophy of the arterial media, and has been proposed to be more influenced by genetics than is plaque development.34 These two subclinical markers identify two disease processes that contribute to carotid artery narrowing, although the exact mechanisms remain unclear.34
Most of the research regarding risk factors and outcomes associated with carotid artery atherosclerosis has been among EA, but several research groups throughout the U.S. have investigated these relationships in HA. This review includes various subgroups within the HA ethnicity, such as Mexican and Dominican Republic. Further, it includes several measures and definitions of carotid atherosclerosis, such as carotid plaque and IMT, and the extent of stenosis and arterial stiffness, in the investigation of risk factors and outcomes associated with carotid artery atherosclerosis in the Hispanic population.
Traditional CVD risk factors
The Northern Manhattan Stroke Study (NOMASS) and Northern Manhattan Study (NOMAS) are prospective studies of participants recruited from northern Manhattan, and were designed to determine and compare incidence rates of stroke and vascular outcomes, investigate risk factors for first stroke, and identify predictors of stroke severity outcomes after the first stroke.35 This northern Manhattan community of approximately 260,000 residents is composed of 63% HA (from Dominican Republic), 20% AA, and 15% EA.35 In NOMASS, maximal internal carotid artery plaque thickness (MICPT) was less in HS (1.2 ± 1.5mm) compared to AA and EA (1.7 ± 1.3mm), even after adjustment for socio-demographic variables and atherosclerotic risk factors. MICPT was positively associated with low-density lipoprotein cholesterol (LDL-C), however, where a 60 mg/dL increase in LDL-C would lead to a 1.26 fold increase in MICPT in a HA, but no MICPT increase EA.35 In a cohort of overweight Latino children 8–13 years old in southern California (The Study of Latino Adolescents at Risk for Diabetes – SOLAR), a similar association was observed, where the odds of cIMT progression (cIMT ≥ 0.01mm over 2 years) increased 3% for each 1 mg/dL increase of baseline LDL-C, independent of glucose effectiveness (a compilation of measures of insulin resistance) and other covariates.36
In addition to LDL-C, cigarette smoking is a traditional CVD risk factor. When cigarette smoking was evaluated as a risk factor by Mast and colleagues in the NOMASS cohort (n=431) combined with the Berlin Cerebral Ischemia Data Bank (n=483), current smoking was significantly associated with carotid artery stenosis >60% among EA smokers, but not in HA.37 And, the attributable risk of regular smoking on carotid stenosis for stroke patients in the combined cohort was 50% for EA, 15% for AA, and 13% for HA. 37 Therefore, cigarette smoking in HS does not account for prevalent carotid stenosis as strongly as it does for EA. These studies were consistent in demonstrating that HA had less carotid plaque and intima-media thickness, but displayed stronger associations with LDL-C compared to AA and EA. Of additional interest is that the strong association between LDL-C and cIMT was detected in two distinct Hispanic populations of two age groups at opposite ends of the age spectrum, which suggests an early and persistent association between this modifiable risk factor and carotid artery atherosclerosis in HA.
Ethnicity as a risk factor
In multivariate analyses of 3,291,382 individuals who self-referred for vascular screening tests across the U.S., and adjusting for age and atherosclerotic risk factors, the risk of carotid artery stenosis [defined as stenosis ≥ 50% in either internal carotid artery] was 30% lower in AA, AS, and HA, compared with EA.38 Among college-age students at the University of Southern California, ethnicity was strongly associated with cIMT, independent of underlying CVD risk factors.39 In particular, AA had 17.3 mm μg greater cIMT (95% CI: −0.3, 34.8) compared to EA, whereas AS and HA had 14.3 (95% CI: −24.3, −4.4) and 15.4 (95% CI: −26.2, −4.7) μm smaller cIMT, respectively.39 These findings are consistent with results for cIMT in older adults and for ethnic differences in carotid plaques.39 Markert et. al. were specifically interested in ethnic differences in carotid stiffness and arterial diameter, indicative of compensatory dilation of larger vessels, in the NOMAS cohort of 2189 individuals.40 This outcome measure indicates compensatory dilation of the carotid artery in response to intima-media thickening, and is an early predictor of CVD and stroke.40 They found that Hispanic ethnicity was significantly associated with diastolic intraluminal common carotid artery diameter (DDIAM) compared to European ethnicity in fully adjusted analyses.40 DDIAM was greater with age among HA (β=0.02, p<0.0001), but not among AA or EA. Stiffness was also greater with age among HA (β=0.01, p<0.0001) and AA (β=0.01, p=0.006) but not among EA.40 As previous studies have shown that HA have a reduced rate of mortality from ischemic stroke compared to EA (RR=0.51, 95% CI: 0.50–0.52), these authors interpreted their findings that increased DDIAM with older age in HS may be protective of stroke, regardless of increased stiffness.40 In summary, when specifically examined as a risk factor for carotid atherosclerosis, Hispanic ethnicity was inversely associated with cIMT; and positively associated with DDIAM.
Heritability within Hispanic families
The observation of differential associations of ethnicity with carotid atherosclerosis has lead to the investigation of heritability within Hispanic families. Among the San Antonio Family Heart Study (SAFHS) of 620 individuals from 24 families, Kao observed that heritability of subclinical atherosclerosis as assessed by cIMT is low (~16%), but is increased in families with a larger burden of T2DM.41 The increased cIMT in these families persists after adjustment for body mass index (BMI), systolic and diastolic blood pressure (BP), and serum lipid levels, suggesting potential shared genetic risk factors for carotid atherosclerosis and T2DM in Hispanic families.41 Within hypertensive Hispanic families, it is known that HTN and cIMT aggregate.42 Therefore, Chen and colleagues investigated whether there is a genetic basis for the associations between cIMT, BP, and renal function in 149 HA families in the Los Angeles Molecular Genetics of Hypertension Specialized Center of Research (SCOR).43 These authors found that HA recruited through a HTNparent demonstrated that cIMT (0.37), BP (0.24), urine microalbumin (0.11), and measures of renal function (blood urea nitrogen (−0.02), serum creatinine (−0.06), creatinine clearance (0.04)) are heritable (p<0.05), and suggests that phenotypic correlations between cIMT and measures of renal function cannot be explained only by direct effects of BP on the kidney.43 Through these studies, it appears as though heritability for cIMT in isolation is low. Carotid IMT in conjunction with other comorbidities, such as T2DM and HTN, however, demonstrates stronger heritability among HA families.
Vascular events and atherosclerosis in other vascular beds
Associations of carotid artery atherosclerosis with vascular events and peripheral arterial stiffness have been evaluated in a multiethnic cohort and a Latino cohort with prevalent HTN. Rundek and colleagues found that HA with MCPT ≥ 1.9 mm had a 3- to 4-fold increased risk of CVD events compared to a 1- to 2-fold increased risk among EA and AA in NOMAS.34 In general, EA and AA had a higher amount of carotid atherosclerosis than HA, but if carotid plaque was present, it had a significant impact on the CVD risk only among HA.34 In a population of Latino patients with HTN, Krantz and colleagues determined that pulse wave velocity, a measure of arterial stiffness, was associated with cIMT, independent of CVD risk factors (p<0.0001).44 Taken together, these findings indicate that while carotid atherosclerosis is less prevalent in HA populations, it is strongly associated with atherosclerosis in other vascular beds and subsequent vascular outcomes.
Hispanic Paradox in Carotid Artery Atherosclerosis
With regard to the ‘Hispanic Paradox,’ in carotid artery atherosclerosis, individuals of HA ethnicity demonstrated less carotid stenosis and IMT compared to AA and EA. They also had a larger diastolic carotid luminal diameter with age, suggesting that their lower stroke mortality in older age groups may potentially be due to an increased diameter. Prevalent carotid disease was associated with other markers of atherosclerosis, which is consistent with other studies that have found atherosclerosis in multiple vascular beds. Further, the heritability of carotid atherosclerosis in families indicated shared genetic components of carotid plaque, T2DM, and BP, independent of environmental risk factors. The effect of higher LDL-C on carotid atherosclerosis was robust in various populations and age groups, and indicates this disease process among HA begins early in life. Therefore, consistent with the ‘Hispanic Paradox,’ HA have less prevalent carotid artery atherosclerosis, yet have stronger associations with LDL-C, a strong risk factor for CVD. They also have heritable and statistical indicators directly implying differential associations of carotid disease with risk factors and outcomes based solely on Hispanic ethnicity
Subclavian Stenosis
Upper extremity atherosclerotic obstruction is largely due to subclavian artery stenosis.17 The standard screening procedure for determining obstruction of upper extremity vasculature is bilateral brachial artery systolic BP, and is indicated by a difference ≥ 15 mmHg between each extremity.17,45 Subclavian stenosis (SS) defined by this ≥ 15 mmHg difference has been linked to coronary artery calcification,46 cIMT,46 PAD,17 and total mortality,45 independent of traditional CVD risk factors.
Few studies have evaluated ethnic differences in SS, but several have presented ethnic specific prevalences, and one has assessed the association between ethnicity and SS. Shadman and colleagues determined the prevalence of SS in 4 cohorts, 2 clinical and 2 population, totaling 4223 subjects in all.17 Among the population cohorts, the overall prevalence of SS was 1.9% (1.4, 2.4). The prevalence of SS in HA was 1.7% (0.6, 3.8) compared to 2.3% in EA, and 0.6% (0.1, 2.2) in AA. Among the clinic cohorts, the overall prevalence of SS was 7.1% (5.7, 8.7). The prevalence of SS in HA was 10.5% (2.9, 24.8), compared to 6.0% (4.6, 7.6) in EA, and 14.0% (8.8, 20.8) in AA. Thus, the prevalence of SS in HA in the population cohorts is lower than the overall prevalence, but is greater than the overall SS prevalence in the clinic cohorts. In multivariable analyses evaluating risk factors for SS and CVD (e.g. PAD, stroke, CHD) with SS, HA ethnicity was not significantly associated with SS in either analyses, with odds ratios of 1.14 (p=0.688) and 1.35 (p=0.330), respectively, suggesting that HA ethnicity alone is not a significant risk factor for SS.
Aboyans and colleagues sought to determine associations between SS and markers of atherosclerosis in multiple ethnic groups in the MESA.46 While the prevalence of SS in HA and AS were too small (1.9% and 1.0%, respectively) to test for associations of SS with markers of subclinical atherosclerosis, these authors demonstrated that women had significantly higher prevalence of SS in all ethnic groups.46 Consistent with Shadman et. al.’s findings, the prevalence of SS in HA (Men:1.5%, Women:2.2%) was less than EA (Men:4.6%, Women:5.6%) and AA (Men:6.3%, Women:8.3%), and greater than AS (Men:0.8%, Women:1.2%).
Conclusion
We present a review of the literature which suggests the ‘Hispanic Paradox’ in CVD may not be limited to the coronary arteries. HA, compared to EA, have a lower prevalence of atherosclerotic obstruction in vascular beds located in the lower extremities and neck, despite also having an increased burden of CVD risk factors. However, there were no consistent differences in the risk of SS. The mechanism underlying HA reduced susceptibility to the development of atherosclerosis is unclear. Possible explanations for the ‘Hispanic Paradox’ include levels of acculturation and genetic differences, including gene-environment interactions. More studies are needed to further investigate the epidemiology of atherosclerosis in HA.
Acknowledgments
Support in part by Research Supplements to Promote Diversity in Health-Related Research (N01 HC95160 Administrative Supplement NIH-NHLBI) and UCSD Integrated Cardiovascular Epidemiology Fellowship (T32 HL079891 Training Grant NIH-NHLBI)
Abbreviations
- AA
African American
- ABI
Ankle-brachial index
- AI
American Indian
- AS
Asian American
- BMI
Body mass index
- BP
Blood pressure
- CHD
Coronary heart disease
- CLI
Critical limb ischemia
- CVD
Cardiovascular disease
- cIMT
Carotid artery plaque and intima-media thickness
- DDIAM
Diastolic intraluminal common carotid artery diameter
- DSA
Digital subtraction angiography
- DYS
Dyslipidemia
- EA
European American
- HA
Hispanic American
- HTN
Hypertension
- IC
Intermittent claudication
- LDL-C
Low density lipoprotein cholesterol
- MAC
Medial artery calcification
- MICPT
Maximal internal carotid artery plaque thickness
- PAD
Peripheral artery disease
- SBP
Systolic blood pressure
- T2DM
Diabetes mellitus
- SS
Subclavian stenosis
- TBI
Toe Brachial Index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Creager MA, White CJ, Hiatt WR, et al. Atherosclerotic Peripheral Vascular Disease Symposium II Executive Summary. Circulation. 2008;118(25):2811–25. doi: 10.1161/CIRCULATIONAHA.108.191170. [DOI] [PubMed] [Google Scholar]
- 2.Criqui MH, Langer RD, Fronek A, Feigelson HS. Coronary disease and stroke in patients with large-vessel peripheral arterial disease. Drugs. 1991;42 (Suppl 5):16–21. doi: 10.2165/00003495-199100425-00005. [DOI] [PubMed] [Google Scholar]
- 3.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326(6):381–6. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 4.Leng GC, Lee AJ, Fowkes FG, et al. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1996;25(6):1172–81. doi: 10.1093/ije/25.6.1172. [DOI] [PubMed] [Google Scholar]
- 5.McDermott MM, Greenland P, Liu K, et al. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136(12):873–83. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 6.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pohjolainen T, Alaranta H. Lower limb amputations in southern Finland 1984–1985. Prosthet Orthot Int. 1988;12(1):9–18. doi: 10.3109/03093648809079386. [DOI] [PubMed] [Google Scholar]
- 8.Fowkes FGR, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013 doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 9.Allison MA, Ho E, Denenberg JO, et al. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32(4):328–33. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Ennis SR, Rios-Vargas M, Albert NG. The Hispanic Population: 2010 - c2010br-04.pdf [Internet] [cited 2014 Feb 12]; Available from: http://www.census.gov/prod/cen2010/briefs/c2010br-04.pdf.
- 11.U. S. Census Bureau DIS. National Population Projections [Internet] 2012 [cited 2014 Feb 12]; Available from: http://www.census.gov/population/projections/data/national/2012.html.
- 12.Criqui MH, Denenberg JO. The generalized nature of atherosclerosis: how peripheral arterial disease may predict adverse events from coronary artery disease. Vasc Med Lond Engl. 1998;3(3):241–5. doi: 10.1177/1358836X9800300311. [DOI] [PubMed] [Google Scholar]
- 13.Willey JZ, Rodriguez CJ, Moon YP, et al. Coronary Death and Myocardial Infarction among Hispanics in the Northern Manhattan Study: Exploring the Hispanic Paradox. Ann Epidemiol. 2012;22(5):303–9. doi: 10.1016/j.annepidem.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mery Cortes-Bergoderi KG. Cardiovascular mortality in Hispanics compared to non-Hispanic whites: A systematic review and meta-analysis of the Hispanic paradox. Eur J Intern Med. 2013 doi: 10.1016/j.ejim.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg. 2007;33 (Suppl 1):S1–75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 16.Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126(24):2890–909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 17.Shadman R, Criqui MH, Bundens WP, et al. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44(3):618–23. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 18.Lijmer JG, Hunink MG, van den Dungen JJ, Loonstra J, Smit AJ. ROC analysis of noninvasive tests for peripheral arterial disease. Ultrasound Med Biol. 1996;22(4):391–8. doi: 10.1016/0301-5629(96)00036-1. [DOI] [PubMed] [Google Scholar]
- 19.Ankle Brachial Index Collaboration. Fowkes FGR, Murray GD, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA J Am Med Assoc. 2008;300(2):197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins R, Cranny G, Burch J, et al. A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease. Health Technol Assess Winch Engl. 2007;11(20):iii–iv. xi–xiii, 1–184. doi: 10.3310/hta11200. [DOI] [PubMed] [Google Scholar]
- 21.Rosero EB, Kane K, Clagett GP, Timaran CH. A systematic review of the limitations and approaches to improve detection and management of peripheral arterial disease in Hispanics. J Vasc Surg. 2010;51(4, Supplement):S27–S35. doi: 10.1016/j.jvs.2009.08.085. [DOI] [PubMed] [Google Scholar]
- 22.Aboyans V, Criqui MH, McClelland RL, et al. Intrinsic contribution of gender and ethnicity to normal ankle-brachial index values: the Multi-Ethnic Study of Atherosclerosis (MESA) J Vasc Surg. 2007;45(2):319–27. doi: 10.1016/j.jvs.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 23.Dachun Xu, Jue Li, Liling Zou, et al. Sensitivity and specificity of the ankle--brachial index to diagnose peripheral artery disease: a structured review. Vasc Med Lond Engl. 2010;15(5):361–9. doi: 10.1177/1358863X10378376. [DOI] [PubMed] [Google Scholar]
- 24.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111(10):1233–41. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26(12):3333–41. doi: 10.2337/diacare.26.12.3333. [DOI] [PubMed] [Google Scholar]
- 26.Høyer C, Sandermann J, Petersen LJ. The toe-brachial index in the diagnosis of peripheral arterial disease. J Vasc Surg. 2013;58(1):231–8. doi: 10.1016/j.jvs.2013.03.044. [DOI] [PubMed] [Google Scholar]
- 27.Hyun S, Forbang NI, Allison MA, Denenberg JO, Criqui MH, Ix JH. Ankle-brachial index, toe-brachial index, and cardiovascular mortality in persons with and without diabetes mellitus. J Vasc Surg. doi: 10.1016/j.jvs.2014.02.008. [Internet] [cited 2014 Jul 18]; Available from: http://www.sciencedirect.com/science/article/pii/S0741521414002365. [DOI] [PMC free article] [PubMed]
- 28.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110(6):738–43. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 29.Criqui MH, Vargas V, Denenberg JO, et al. Ethnicity and peripheral arterial disease: the San Diego Population Study. Circulation. 2005;112(17):2703–7. doi: 10.1161/CIRCULATIONAHA.105.546507. [DOI] [PubMed] [Google Scholar]
- 30.Allison MA, Criqui MH, McClelland RL, et al. The effect of novel cardiovascular risk factors on the ethnic-specific odds for peripheral arterial disease in the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2006;48(6):1190–7. doi: 10.1016/j.jacc.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 31.Allison MA, Budoff MJ, Wong ND, Blumenthal RS, Schreiner PJ, Criqui MH. Prevalence of and risk factors for subclinical cardiovascular disease in selected US Hispanic ethnic groups: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2008;167(8):962–9. doi: 10.1093/aje/kwm402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention (CDC) Racial/ethnic and socioeconomic disparities in multiple risk factors for heart disease and stroke--United States, 2003. MMWR Morb Mortal Wkly Rep. 2005;54(5):113–7. [PubMed] [Google Scholar]
- 33.Healthy People 2020 - Improving the Health of Americans [Internet] [cited 2014 Feb 21]; Available from: http://www.healthypeople.gov/2020/default.aspx.
- 34.Rundek T, Arif H, Boden-Albala B, Elkind MS, Paik MC, Sacco RL. Carotid plaque, a subclinical precursor of vascular events: the Northern Manhattan Study. Neurology. 2008;70(14):1200–7. doi: 10.1212/01.wnl.0000303969.63165.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sacco RL, Roberts JK, Boden-Albala B, et al. Race-ethnicity and determinants of carotid atherosclerosis in a multiethnic population. The Northern Manhattan Stroke Study. Stroke J Cereb Circ. 1997;28(5):929–35. doi: 10.1161/01.str.28.5.929. [DOI] [PubMed] [Google Scholar]
- 36.Toledo-Corral CM, Davis JN, Alderete TL, et al. Subclinical atherosclerosis in Latino youth: progression of carotid intima-media thickness and its relationship to cardiometabolic risk factors. J Pediatr. 2011;158(6):935–40. doi: 10.1016/j.jpeds.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mast H, Thompson JL, Lin IF, et al. Cigarette smoking as a determinant of high-grade carotid artery stenosis in Hispanic, black, and white patients with stroke or transient ischemic attack. Stroke J Cereb Circ. 1998;29(5):908–12. doi: 10.1161/01.str.29.5.908. [DOI] [PubMed] [Google Scholar]
- 38.Rockman CB, Hoang H, Guo Y, et al. The prevalence of carotid artery stenosis varies significantly by race. J Vasc Surg. 2013;57(2):327–37. doi: 10.1016/j.jvs.2012.08.118. [DOI] [PubMed] [Google Scholar]
- 39.Breton CV, Wang X, Mack WJ, et al. Carotid artery intima-media thickness in college students: race/ethnicity matters. Atherosclerosis. 2011;217(2):441–6. doi: 10.1016/j.atherosclerosis.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markert MS, Della-Morte D, Cabral D, et al. Ethnic differences in carotid artery diameter and stiffness: the Northern Manhattan Study. Atherosclerosis. 2011;219(2):827–32. doi: 10.1016/j.atherosclerosis.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kao WHL, Hsueh W-C, Rainwater DL, et al. Family history of type 2 diabetes is associated with increased carotid artery intimal-medial thickness in Mexican Americans. Diabetes Care. 2005;28(8):1882–9. doi: 10.2337/diacare.28.8.1882. [DOI] [PubMed] [Google Scholar]
- 42.Arnett DK, Baird AE, Barkley RA, et al. Relevance of genetics and genomics for prevention and treatment of cardiovascular disease: a scientific statement from the American Heart Association Council on Epidemiology and Prevention, the Stroke Council, and the Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2007;115(22):2878–901. doi: 10.1161/CIRCULATIONAHA.107.183679. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y-C, Guo X, Raffel LJ, et al. Carotid intima-media thickness (cIMT) cosegregates with blood pressure and renal function in hypertensive Hispanic families. Atherosclerosis. 2008;198(1):160–5. doi: 10.1016/j.atherosclerosis.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 44.Krantz MJ, Long CS, Hosokawa P, et al. Pulse wave velocity and carotid atherosclerosis in white and Latino patients with hypertension. BMC Cardiovasc Disord. 2011;11:15. doi: 10.1186/1471-2261-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aboyans V, Criqui MH, McDermott MM, et al. The vital prognosis of subclavian stenosis. J Am Coll Cardiol. 2007;49(14):1540–5. doi: 10.1016/j.jacc.2006.09.055. [DOI] [PubMed] [Google Scholar]
- 46.Aboyans V, Kamineni A, Allison MA, et al. The Epidemiology of Subclavian Stenosis and its Association with Markers of Subclinical Atherosclerosis: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2010;211(1):266–70. doi: 10.1016/j.atherosclerosis.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]