Abstract
Introduction
There is epidemiological evidence that metal contaminants may play a role in the development of atherosclerosis and its complications. Moreover, a recent clinical trial of a metal chelator had a surprisingly positive result in reducing cardiovascular events in a secondary prevention population, strengthening the link between metal exposure and cardiovascular disease (CVD). This is, therefore, an opportune moment to review evidence that exposure to metal pollutants, such as arsenic, lead, cadmium, and mercury, are significant risk factors for CVD.
Methods
We reviewed the English-speaking medical literature to assess and present the epidemiological evidence that 4 metals having no role in the human body (xenobiotic), mercury, lead, cadmium, and arsenic, have epidemiologic and mechanistic links to atherosclerosis and CVD. Moreover, we briefly review how the results of the Trial to Assess Chelation Therapy strengthen the link between atherosclerosis and xenobiotic metal contamination in humans.
Conclusions
There is strong evidence that xenobiotic metal contamination is linked to atherosclerotic disease and is a modifiable risk factor.
Keywords: metal, myocardial infarction, atherosclerosis, oxidative stress, chelation
1. Introduction
The result of a recent clinical trial of a metal chelator showing reduced cardiovascular events in a secondary prevention population highlights the potential connection between metal pollutants and cardiovascular disease (CVD). This is, therefore, an opportune moment to review the causal link between metal exposure and CVD. The most commonly used terms for metal pollutants, “heavy metals” or “toxic heavy metals”, refer to specific density, atomic weight, atomic number, or other chemical properties. We have chosen to use the term “xenobiotic”, to denote a foreign chemical substance found within an organism; – thus, xenobiotic metal.
2. Definitions
Xenobiotic metals have no biological role at any dose. These include lead, arsenic, mercury, cadmium, and many others. We will focus on these four toxic, xenobiotic, metals that are ranked among the top 10 on the current Agency for Toxic Substances and Disease Registry Priority List of Hazardous Substances1. Arsenic, lead, and mercury are ranked as the top 3 hazardous substances.
3. Lead
Distribution
Lead is the most common toxic element. Volcanic activity and geochemical weathering are the greatest natural sources. Lead-based paints, gasoline additives, food-can soldering, battery making, and soldered joints of drinking water pipe systems represent anthropogenic sources of lead in the environment2,3. Recommendations to limit lead paints since 1978 have led to substantial reductions in childhood lead toxicity4. Many children, however, continue to live in houses with either non-intact lead-based paint or high levels of lead in dust. Exposure to lead also occurs through airborne emissions and occupational exposures, water, foods, or occasionally through the use of alternative health-care products, such as herbal remedies5 (Table 1). Tetraethyl lead as a gasoline additive for land-based vehicles has now been largely banned worldwide. However it is still present in aviation fuel for piston engine aircraft. Particles of lead suspended in the atmosphere, along with fuel-based and other sources of lead3,6, can represent a source of continued exposure.
Table 1.
Metal exposure, half-life, ingestion, excretion, ways to measure
| Metal | Exposure | Half-life | Ingestion | Excretion | Measure |
|---|---|---|---|---|---|
| Lead | food, water, air, gasoline additives, food-can soldering, lead-based paints, ceramic glazes, drinking water pipe systems, folk remedies |
In the blood 36 days In bones 20–30 years |
Inhalation with 30–40% absorbed Ingestion with 5% absorbed in adults and up to 50% in children |
Urine Sweat Hair Nails |
Blood level X- ray fluorescence of bone Urine level |
| Cadmium | contaminated food (leafy vegetables, grains, organ meats, crustaceans), drinking water, inhalation of polluted air, occupational exposure in industries, tobacco smoke. |
In liver 4–19 years In kidneys 6–38 years |
Inhalation with 40–50% absorbed Ingestion with 3- 7% absorbed |
No efficient excretory mechanism, small amounts excreted via urine |
Blood level Urine level Biopsy of the liver, kidneys Hair |
| Mercury | contaminated fish, meat and organ tissue of marine mammals or feral wildlife, dental amalgams, skin- lightening creams, antiseptic facial products, mercury- containing laxatives or diuretics, teething powders, latex paint, thimerosal- containing vaccines |
Elemental: In the blood 1–3 days In the whole body1- 3 weeks Inorganic: In the blood 1–3 weeks Organic: In the blood and the whole body 50 days |
Elemental: Inhalation with 80% absorbed Ingestion with 0.01% absorbed Inorganic: Inhalation or inhalation with 10% absorbed Skin with 2–3% absorbed Organic: Inhalation or inhalation with 95–100% absorbed |
Metabolized in the liver and excreted through the bile duct 10% excreted via urine |
Blood level Urine level Toenail level |
| Arsenic | contaminated fish, tobacco smoke, arsenic treated wood, ingestion of high-arsenic drinking water |
Inorganic: In the blood 4–6 hours Methylated: In the blood 20–30 hours |
Inhalation with 40–60% absorbed Ingestion with 95% absorbed |
Urine Nails Hair | Blood level Urine level Hair and nail levels |
Absorption, body distribution and excretion
Approximately 30–40% of inhaled and 5–10% of ingested lead is absorbed into the bloodstream. GI absorption, however, can reach as high as 30–50% in children3 (Table 1). Once absorbed, 99% of lead binds to red blood cells and 1% remains in serum3. The half-life of lead in the bloodstream is relatively short (36 days); while in bones it is 20–30 years3. Absorbed lead is excreted from the body via urine, sweat, hair and nails2,3.
Exposure evaluation
Blood lead assesses acute exposure to lead3. Blood lead levels, however, represent only recent short-term exposures, and account for about 1–5% of total body lead burden; the rest is stored in bone and other tissues3. Bone acts as an endogenous source of lead by continuous release of the metal to the plasma, long after exposure has ended, when the rate of bone turnover increases. Indeed, noninvasive X-ray fluorescence of bone is the most accurate technique to assess body lead burden7,8. Urine lead may be used to assess lead exposure and for monitoring of therapy for lead toxicity.
Blood lead levels have exhibited a steady decline over the last decades, concurrent with the mandated discontinuation of leaded gasoline for ground vehicles. The mean blood lead level in the US population 30 years ago was 12.8 µg/dL, which decreased to 1.45 µg/dL more recently, with 99% of US adults having blood lead levels below 10 µg/dL9. Epidemiological data strongly suggest that there may be no safe threshold level for lead, however10.
Cardiovascular effects
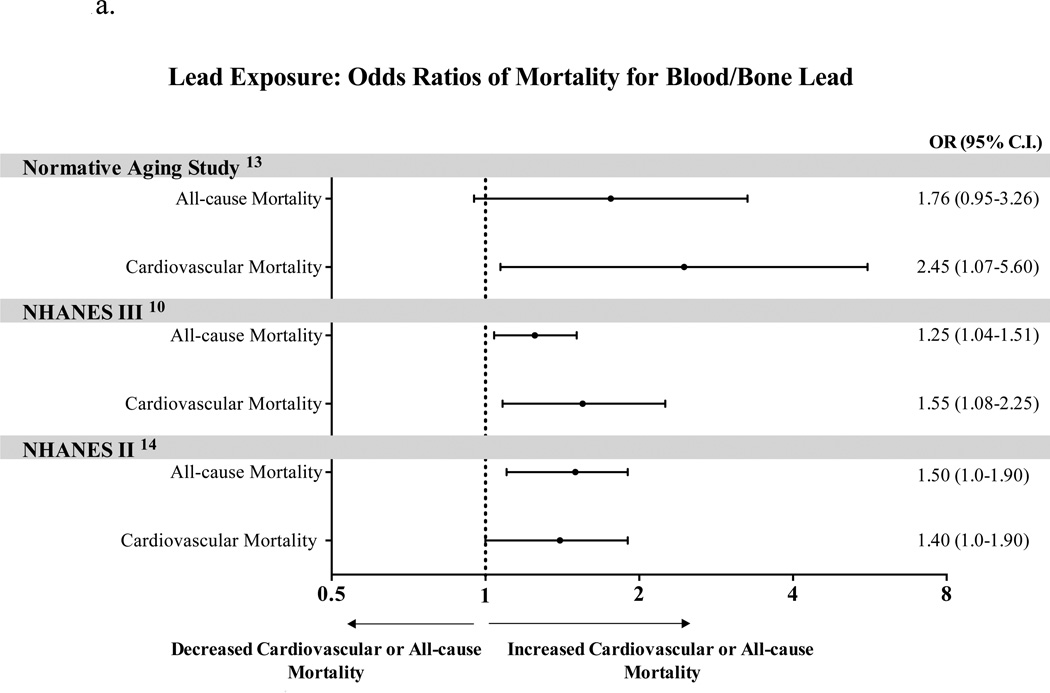
Increased cardiovascular mortality has been attributed to both elevated blood and bone lead levels with stronger association shown for bone levels. 11,12 (Figure 1a). Weisskopf et al. analyzed the association between tertiles of patella and tibia lead and mortality in 868 male participants of the Veterans’ Affairs Normative Aging Study13. After multivariable adjustments, when compared to the first tertile, participants in the third tertile were more likely to die from all causes (HR 2.52, 95% CI, 1.17–5.41, p=0.02), from cardiovascular causes (HR 5.63, 95% CI, 1.73–18.3, p=0.003), and nearly 10 times more likely to die from ischemic heart disease (HR 8.37, 95% CI, 1.29–54.4, p=0.01). In the second National Health and Nutrition Examination Survey (NHANES II) survey14, Lustberg et al reported that subjects with blood lead levels of 20–29 µg/dL had increased all-cause mortality (RR 1.46, 95% CI, 1.14–1.86) and circulatory mortality (RR 1.39, 95% CI, 1.01–1.91) compared with those having blood lead levels below10 µg/dL.
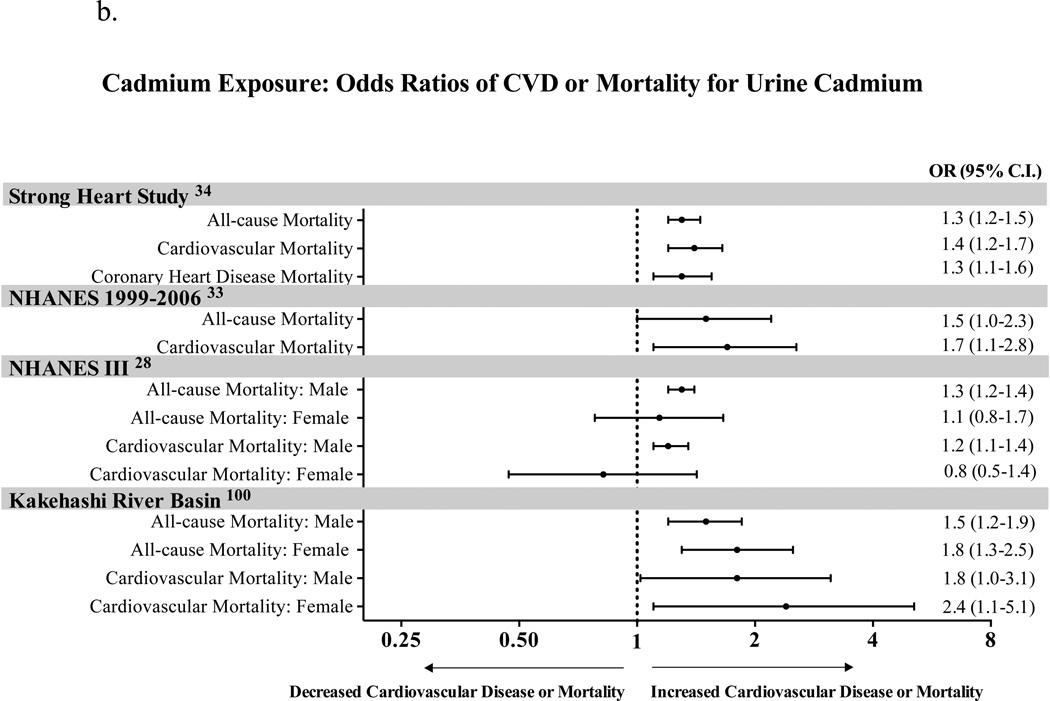
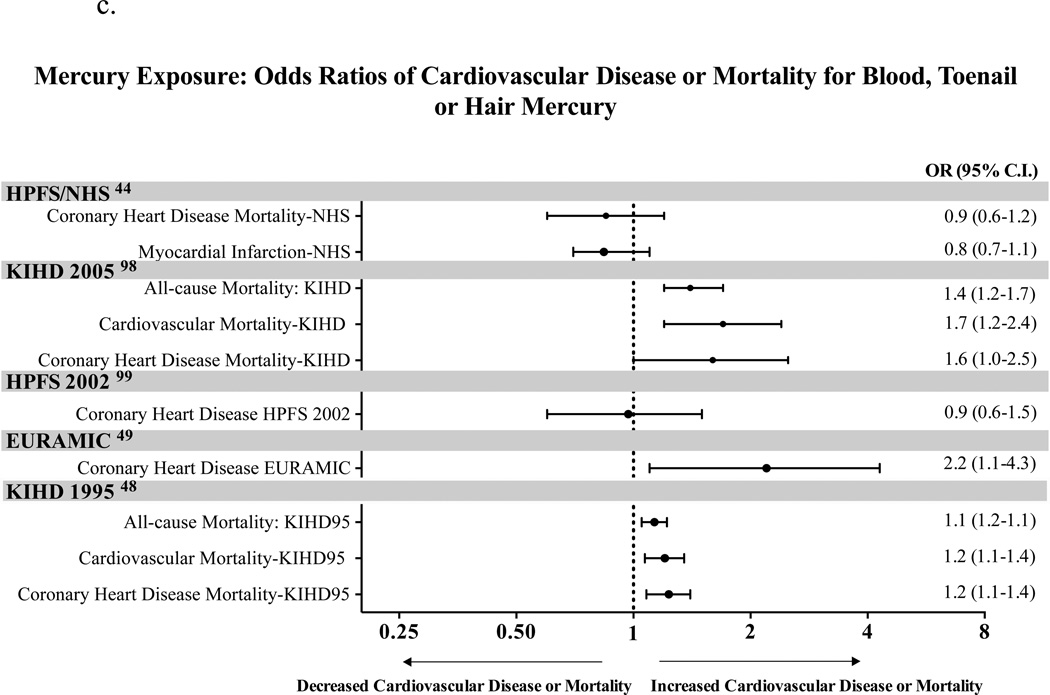
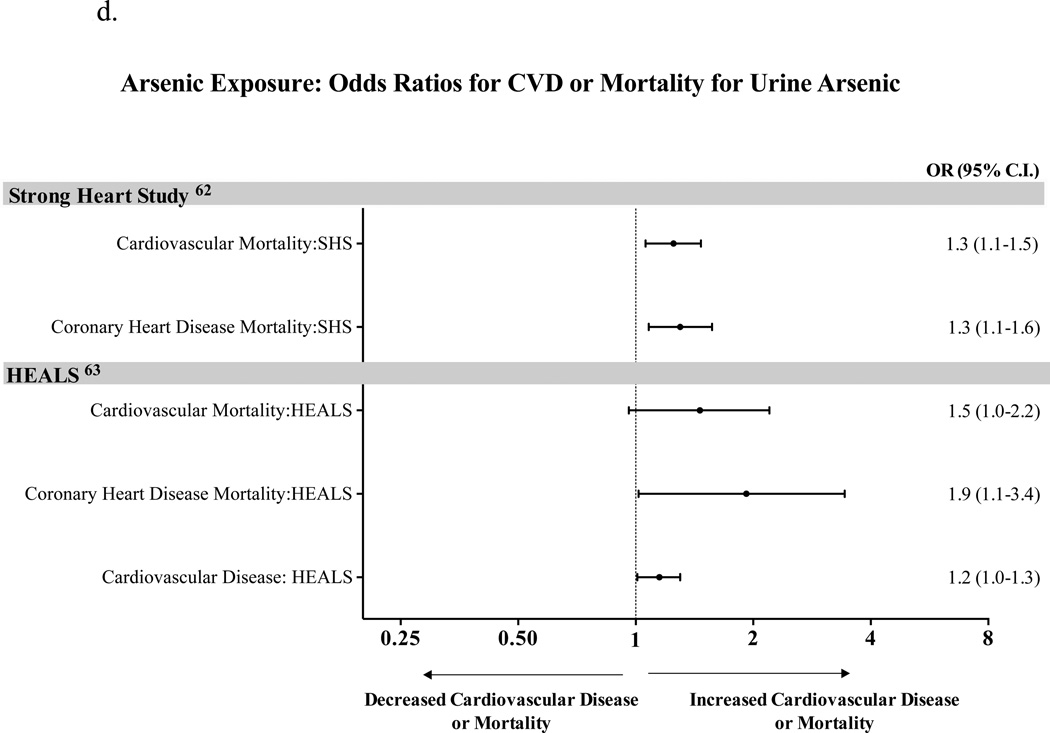
Figure 1.
a. Lead exposure: Odds Ratios for Mortality for Blood/Bone Lead
b. Cadmium Exposure: Odds Ratios for CVD or Mortality for Urine Cadmium
c. Mercury Exposure: Odds Ratios for CVD or Death
d. Arsenic Exposure: Odds Ratios for CVD and Death for Urine Arsenic
Menke et al. studied 13,946 adult participants of the NHANES III survey with blood lead levels <0.48 µmol/L (10 µg/dL) followed up for up to 12 years10. After multivariate adjustment, the risk of cardiovascular events was significantly greater in participants with the highest tertile of lead exposure (≥0.17 µmol/L or 3.62 µg/dL), compared with those in the lowest tertile (<0.09 µmol/L or 1.94 µg/dL). All-cause mortality was higher by 25% (HR 1.25, 95% CI, 1.04–1.51) in the third tertile vs. the first tertile, while cardiovascular mortality was higher (HR 1.55, 95% CI, 1.08–2.24), mortality from myocardial infarction was higher (HR 1.89, 95% CI, 1.04–3.43), and mortality from stroke was higher by more than 2-fold (HR 2.51, 95% CI, 1.20–5.26)10. The NHANES survey (1999–2000) showed that blood lead was associated with an increased prevalence of PAD, even at levels below current safety standards11.
The association of lead exposure with hypertension is one of the best-established cardiovascular effects of this metal15,16. Meta-analyses of 61 original studies, including approximately 60,000 participants17 showed that a doubling of blood lead was associated with an increase in SBP of 1.0–1.25 mmHg and DBP of 0.6 mmHg15. While modest in magnitude, these increases in SBP and DBP may have significant clinical relevance in large populations.
Lead exposure has also been linked to dyslipidemia and atherosclerosis14,18,19. Experimental and human autopsy studies showed an association between lead exposure and aortic atherosclerotic plaque burden14,19,20,21. There are some interesting findings that support the association of lead with atherosclerosis. For example, the cardioprotective antioxidant activity of HDL is partially mediated by paraoxonase activity, an enzyme that is closely bound to the HDL particle and involved in inhibition of LDL oxidation. Lead, as well as other metals, can inactivate paraoxonase and, therefore, promote LDL oxidation and atherosclerosis development22,23, 24.
4. Cadmium
Distribution
Cadmium is considered one of the most toxic environmental substances due to its ubiquity, toxicity, and long half-life. Exposure to cadmium occurs through inhalation (particularly in active cigarette smokers), water consumption, industrial exposure, and contaminated food (Table 1). Tobacco plants are highly efficient in absorbing cadmium from soil, and accumulate it in the leaf25. Therefore, any exposure to tobacco smoke leads to high exposure to cadmium26, and smokers have cadmium levels that are at least twice as high as those of nonsmokers25,27. High levels of cadmium can be found in vegetables, fruits, and grains, with the highest levels in greens and potatoes. Shellfish and organ meats contain elevated cadmium concentrations as well, and agricultural fertilizer has also been reported to contain cadmium25,28,29.
Absorption, body distribution and excretion
Approximately 40–50% of inhaled and 3–7% of ingested cadmium is absorbed. Similar to lead, GI absorption of cadmium is greater in the young25,30 (Table 1). Intestinal cadmium absorption occurs through a transporter shared with iron and when accompanied by iron deficiency, GI cadmium absorption may increase31. Once absorbed, cadmium is protein-bound via erythrocytes or albumin and undergoes hepatic conjugation to metallothionein, a cysteine-rich protein25. This cadmium-metallothionein-cadmium complex then accumulates in the kidneys and may cause renal impairment25. Cadmium is also stored in bones, pancreas, adrenals, testes, and placenta25.
Cadmium has no efficient excretory mechanism
It is excreted in the urine, but it remains bound to metallothionein, which is almost completely reabsorbed in the renal tubules25. Cadmium half-life in the liver is between 4 and 19 years, and in the kidneys is between 6 and 38 years25,27.
Exposure evaluation
Cadmium levels can be measured in blood, urine, liver, kidney, hair and other tissues25. Blood cadmium level is indicative of recent exposure25. The geometric mean level in occupationally non-exposed adults in the United States is 0.315 µg/L. In heavy smokers this level may be as high as 1.58 µg/L (Table 1).
Urine cadmium reflects mainly total body burden, although urine levels change with recent exposure as well. In the U.S. general population, the geometric mean urinary cadmium level in adults is 0.232 µg/L (or 0.247 µg/g creatinine)32 (Table 1).
Cardiovascular effects
Cadmium is associated with cardiovascular and all-cause mortality (Figure 1b). Menke et al.28 reported that every 2-fold increase in creatinine adjusted urinary cadmium levels in men is associated with an increase in risk of all-cause (HR 1.28, 95% CI, 1.15–1.23) and cardiovascular mortality (HR 1.21, 95% CI, 1.07–1.36). The risk of CAD-associated mortality was also increased (HR 1.36, 95% CI, 1.11–1.66). While these associations were not observed in women, other studies showed cardiovascular mortality to be associated with urinary cadmium levels in both genders33,34. In a review published recently by Tellez-Plaza et al35, based on 12 studies the pooled RRs for cardiovascular disease, CAD, stroke, and PAD were 1.36 (95% CI, 1.11–1.66), 1.30 (95% CI, 1.12–1.52), 1.18 (95% CI, 0.86–1.59), and 1.49 (95% CI, 1.15–1.92), respectively35. The pooled RRs for CAD in men, women and never smokers were 1.29 (1.12, 1.48), 1.20 (0.92, 1.56) and 1.27 (0.97, 1.67), respectively35. These are modest in magnitude, but quite consistent.
Cadmium has also been associated with PAD in both men and women11,35,36,37. Blood and urine cadmium levels were 16% (95% CI, 4.7–28.7) and 36% (95% CI, 1–83) higher11,37, respectively, in patients with PAD. After adjustment for age, sex, race, smoking status, and urinary creatinine, the odds ratio for PAD comparing the highest versus lowest quartile of urine cadmium distribution was 3.05 (95% CI, 0.97–9.58)37.
The largest epidemiologic examination of the association between cadmium exposure and BP change was based on the 1999–2004 NHANES survey38. Among 15,332 participants over 20 years of age, Tellez-Plaza et al. reported an association of blood, but not urine, cadmium levels with a modest elevation of blood pressure. The geometric mean of blood cadmium was 3.77 nmol/L (0.42 µg/L). Participants in the 90th percentile of blood cadmium distribution had 1.36 mmHg (95% CI, 0.24–3.24) higher SBP and 1.68 mmHg (95% CI, 0.57–2.78) higher DBP levels when compared to participants in the 10th percentile of blood cadmium distribution.
5. Mercury
Distribution
Mercury has been ranked as the third most toxic environmental hazard after arsenic and lead1. Common sources of mercury exposure include proximity to mercury mining sites, recycling facilities, medical or municipal incinerators, coal-fired power plants, or mercury-containing latex paint39. Dietary sources include fresh water fish or seafood40 with high mercury content, high fructose corn syrup, rice, and other dietary products. Additionally, dental amalgam is a historic source of mercury exposure38. There have also been reports of mercury contamination in beauty products, laxatives, and infant products39, 41. Another potential source of mercury is thimerosal-containing vaccines (Table 1). Thimerosal, a controversial ethylmercury compound that has been used as a preservative in vaccines, has been completely removed from pediatric vaccines, and mostly removed from adult products.
Absorption, body distribution and excretion
Approximately 80% of inhaled and 0.01% of ingested elemental mercury is absorbed38. For inorganic mercury, absorption of inhaled versus ingested mercury is equal (10%), while 2–3% of inorganic mercury is absorbed through the skin38. Organic mercury (most commonly found in fish), if ingested or inhaled, is almost completely (95–100%) absorbed and is the most toxic form of mercury that is distributed to all organs and tissues including brain and placenta38 (Table 1). Elimination of organic mercury from the body occurs through either demethylation to inorganic mercury or degradation to L-cystein complex in bile. About 10% of organic mercury is excreted through the urine. Selenium, vitamin C, and vitamin E can decrease toxic effects of mercury by multiple mechanisms42,43,44.
Exposure evaluation
Blood, urine and toenail levels of mercury have been used to estimate mercury exposure38 (Table 1). Blood mercury levels peak sharply during exposure and then decrease rapidly45. The mean total mercury levels in whole blood and urine of the general population are approximately 1–8 µg/L and 4–5 µg/L, respectively46. Mercury levels as high as 200 µg/L have been reported in individuals with high fish intake46, which is striking in the context of United States’ occupational exposures being limited to less than 15 µg/L38,47. Urine mercury may be used for assessment of inorganic mercury exposure, as organic mercury represents only a small fraction of urinary mercury. Urine mercury levels may vary greatly during the day and from day to day in the same individual, as well as show inter-individual variability, even in a setting of constant exposure38. Current Occupational Safety and Health Administration (OSHA) recommendations require urinary mercury levels not to exceed 35 µg mercury per g of creatinine47.
Cardiovascular effects
When evaluating the association of mercury levels and cardiovascular disease, it is important to note that this relationship may be confounded by fish consumption, which raises mercury levels, but lowers cardiovascular risk (Figure 1c).
In 1995, Salonen et al. reported an association between high levels of mercury exposure via freshwater fish consumption and risk of acute myocardial infarction (AMI), all-cause, and cardiovascular mortality48. Men in the highest tertile of hair mercury content when compared to the lowest tertile had relative risk of fatal or non-fatal AMI of 1.69 (95% CI, 1.03–2.76, p=0.038), RR of cardiovascular disease of 2.9 (95% CI, 1.2–6.6, p=0.014) and relative risk of death from any cause of 2.3 (95% CI, 1.4–3.6, p<0.001). The relative risk of coronary death in this study was not associated with hair mercury content. In a case-control study, Guallar et al. showed an association between higher levels of toenail mercury and risk of non-fatal AMI49. More recently, Mozaffarian et al. found no association between toenail mercury and CAD, stroke, or total cardiovascular disease in participants with either normal or low levels of selenium, which may protect against mercury toxicity44.
Data regarding a relationship between mercury exposure and blood pressure changes are inconsistent50,51,52,53. Studies of chronic occupational mercury exposure in miners revealed a 46% increase in incidence of hypertension when compared to age-matched controls54. Correlations have been shown between hair or blood mercury and elevated BP50,51.
6. Arsenic
Distribution
Arsenic is highly toxic to human health1. Inorganic and most toxic forms of arsenic (arsenate and arsenite) are found in soils, crops and water, particularly in groundwater from deep wells, often used as drinking water. These compounds are also found in environmental tobacco smoke and arsenic-treated wood, used in the majority of outdoor wooden structures in the US55. High levels of arsenic are present in agricultural fertilizer that is used for soil treatment; therefore, vegetables and fruits, if grown in this soil, contain high levels of arsenic55 (Table 1). Arsenic has also been used as an additive to poultry feed to inhibit parasites. Arsenic is emitted by coal-burning power plants. As for organic forms of arsenic, large amounts of arsenobetaine or arsenocholine are found in contaminated fish; however, these forms are considered to be essentially nontoxic55,56,57.
Absorption, body distribution and excretion
The primary routes of arsenic absorption are gastrointestinal and respiratory55 (Table 1). Approximately 40–60% of inhaled and 95% of ingested arsenic is absorbed55. Arsenic metabolism includes two main reactions: conversion of arsenate to arsenite by oxidation/reduction reactions forming glutathione-arsenic complexes, and methylation that occurs mainly in the liver producing water soluble monomethylarsinic acid and dimethylarsinic acid that are eliminated through the urine. Arsenic metabolism is an area of active investigation, as differences in methylation of arsenic have been associated with differences in health outcomes, including cardiovascular disease55,58,59.
Exposure evaluation
Since arsenic is cleared from the blood within a few hours of exposure, measurement of blood arsenic can only be used to assess a very recent exposure55 (Table 1). Typical values in non-exposed individuals should be less than 1 µg/L60.
Urine is considered to be the most reliable body sample to detect arsenic exposure. The American Conference of Governmental Industrial Hygienists considers urine arsenic below 35 µg/L to be acceptable for non-toxic exposed individuals. Yet other reports61 suggest there may be no safe threshold of arsenic exposure. Arsenic can be detected in urine of people with no known exposure55. This could be due to high consumption of certain seafood that contain non-toxic organic form of arsenic, arsenobetaine55. Therefore, the measurement of speciated urinary arsenic, rather than total urinary arsenic, is preferred for assessments of cardiovascular toxicity. Finally, arsenic tends to accumulate in nails and hair, wherein acceptable levels of arsenic are less than 1 ppm60.
Cardiovascular effects
There is only limited evidence on the relationship between arsenic and cardiovascular morbidity and mortality (Figure 1d). The only prospective cohort study, published recently by Moon et al, reported that long term exposure to low to moderate arsenic levels is associated with cardiovascular disease incidence and cardiovascular mortality62. Participants of this study had a median urinary arsenic level of 9.7 µg/g creatinine, with a range between 1 and 183.4 µg/g creatinine, and interquartile range (IQR) between 5.8 and 15.7 µg/g creatinine. The hazard ratios for cardiovascular disease mortality, CAD mortality, and stroke mortality per IQR were 1.65 (95% CI, 1.20–2.27; p<0.001 for trend), 1.71 (95% CI, 1.19–2.44; p<0.001 for trend), and 3.03 (95% CI, 1.08–8.50; p<0.001 for trend), respectively. The association of arsenic with CVD mortality was stronger in participants with diabetes. Evidence is also accumulating on the association between higher levels of arsenic exposure and CV morbidity and mortality in Bangladesh63.
High levels of well-water arsenic exposure are recognized as being causative in the development of peripheral arterial disease37,64,65, such as blackfoot disease. This is a severe form of PAD endemic to Taiwan characterized by thromboangiitis obliterans, severe arteriosclerosis and high levels of vessel wall arsenic37,64,65. However, the generalizability of these findings is limited, due to the nature of the exposure (deep well water) and the extremely high estimated levels of arsenic exposure.
Finally, though the literature is limited, there is evidence to suggest that a positive relationship between arsenic exposure and hypertension66.
7. Hypothetical mechanisms of metal toxicity
There are general mechanisms that apply to all toxic metals, and specific mechanisms that are idiosyncratic to the individual metal in question. These mechanisms center on oxidative stress. While the science underlying these mechanisms is accurately quoted, attribution of benefit to metal chelation because of these mechanisms has to be considered speculative. Moreover, the oxidative-stress = oxidative-damage hypothesis has been challenged as well.
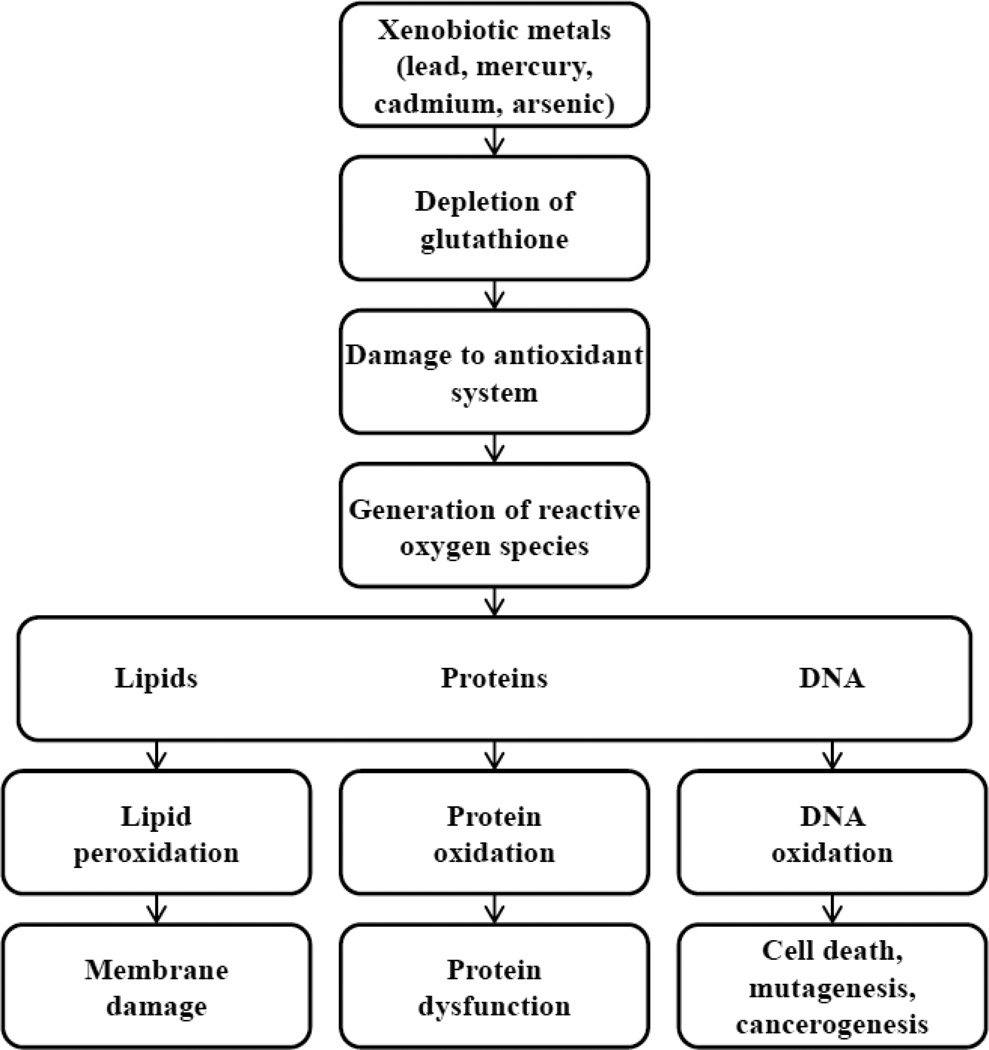
Oxidative stress results from an imbalance between the production and detoxification of reactive oxygen species (ROS). The toxicity of ROS is based on their ability to oxidize intra- and extracellular structures such as proteins, lipids, and nucleic acids (Figure 2). Several enzyme systems are known to protect the body against ROS. These enzymes include superoxide dismutase, catalase, glutathione peroxidase, paraoxonase, thioredoxin, heme oxygenase, and others. Glutathione peroxidase is of particular interest.
Figure 2.
Toxicity of ROS (please see attached file)
Many metals have electron-sharing properties and, therefore, are capable of forming covalent bonds with sulfhydryl groups of proteins (e.g., glutathione, cystein, homocysteine, metallothionein, albumin). By binding to glutathione, these metals deplete its levels, and, therefore, increase the intracellular concentration of ROS. The consequences include promotion of lipid peroxidation, cell membrane damage, DNA damage, oxidation of aminoacids in proteins and, therefore, changes in their conformation and function, and inactivation of enzymes. According to current concepts of atherogenesis, oxidative modification of LDL, a free radical-driven lipid peroxidation process, is an early event in atherosclerosis development68.
Many metals have been shown to increase lipid peroxidation69,70. In addition, metal-related ROS-mediated changes include microtubule destruction, mitochondrial damage by disruption of the membrane potential, inhibition of ATP production, followed by dysfunction of ion transporters such as Ca-ATPase and Na-K-ATPase causing changes in calcium homeostasis71.
By binding to sulfhydryl groups of proteins not involved in the detoxification of ROS, metals may cause other biological impairments. Lead causes endothelial dysfunction by binding and inhibiting endothelial nitric oxide synthase (eNOS) and decreasing nitric oxide (NO) production72,73. Mercury has also been reported to impair nitric oxide metabolism by binding to SH groups of NF-kB and changing its effects on gene expression, and, thus, resulting in decreased expression of inducible NOS (iNOS)74. Cadmium has been shown to inhibit endothelial and calcium-calmodulin constitutive NOS as well75. Arsenic exposure was linked to impairment of NO production and increased generation of ROS, perhaps by uncoupling of eNOS production76.
There are additional, idiosyncratic mechanisms of toxicity. Thus, lead, competing with zinc, binds to sulfhydryl groups of delta-aminolevulinic acid dehydratase (ALAD, the enzyme involved in heme metabolism), preventing binding of ALAD to aminolevulinic acid (ALA)77, generating ROS78. Lead has also been shown to promote endothelial release of endothelin, to elevate serum levels of norepinephrine, angiotensin-converting enzyme (ACE) and thromboxane, and to decrease production of prostacyclin79,80. All these changes may mediate vascular constriction. In addition, lead, being one of the calcium-like elements, competes with calcium for transport by channels and pumps in endoplasmic reticulum. Lead may also substitute for calcium in calcium-dependent processes, and can interact with calmodulin.
Arsenic inhibits pyruvate and alpha-ketoglutarate dehydrogenases, important enzymes of gluconeogenesis and glycolysis81. It can also replace phosphate in glycolysis, generating arseno-3-phosphoglycerate instead of 1,3-bisphosphoglycerate, which leads to uncoupling of oxidative phosphorylation81. Moreover, arsenic has been linked to increased intravascular inflammation by up-regulating interleukin 6, tumor necrosis factor alpha, and monocyte chemotactic protein, vascular cell adhesion molecule and intercellular adhesion molecule82. Furthermore, arsenic inhibits expression of peroxisome proliferator-activated receptor gamma causing hyperglycemia and dyslipidemia83.
Macrophages and endothelial cells take up cadmium by endocytosis causing foam cell production followed by foam cell necrosis and endothelial cell disruption followed by endothelial cell necrosis. Once the endothelial layer is disrupted, cadmium reaches smooth muscle cells and accumulates there, activating smooth muscle cell proliferation and apoptosis. Cadmium may also substitute for iron and copper in proteins that contain these biologically necessary metals. As a result, iron and copper, after being released from its usual binding proteins, may produce ROS, as both elements can be more easily involved in reduction-oxidation reactions84. Cadmium is also associated with perturbations in inflammation and coagulation, including elevated blood C-reactive protein and fibrinogen in a general US population, even after adjustment for other cardiovascular disease risk factors such as smoking82,85,86. Moreover, cadmium exposure has been associated with elevations of mediators or markers of systemic inflammation, including IL-6, TNF-alpha, VCAM-187.
8. Oxidative stress, metals, and diabetes
Patients with diabetes are thought to be especially susceptible to oxidative stress. The formation of advanced glycation end-products, advanced lipoxygenation products, and protein oxidation products require non-enzymatically, metal catalyzed oxygen chemistry. These oxidized and crosslinked complexes are long-lived, and form the basis of diabetes complications, activating the receptor for advanced glycation end products88 and multiple other downstream inflammatory cascades89,90,91. And while the transition elements most closely associated with these reactions are iron and copper, xenobiotic transition elements, such as cadmium and others (cobalt and tungsten)92 might be also involved.
9. Potential Interventions
Chelation therapy with ethylenediamine tetraacetic acid (EDTA, edetate disodium) has been used to treat atherosclerotic disease since 195693, without a solid scientific base. In 2002, the Cochrane Collaborative94 reported that there was insufficient evidence for or against chelation therapy to make a recommendation. Yet patients continued to seek, and practitioners to use, EDTA to prevent or treat atherosclerotic disease of the coronaries, carotids, and peripheral arteries. In 2002, the Trial to Assess Chelation Therapy (TACT), a 2 × 2 factorial trial testing EDTA infusions versus placebo and oral high-dose multivitamins and minerals versus oral placebo was designed95 and funded. TACT enrolled 1708 patients96 who had sustained a prior myocardial infarction, were at least 50 years old, and had a creatinine of 2.0 mg/dL or less. TACT administered 55,222 infusions of EDTA-based chelation or placebo. EDTA chelation significantly reduced a combined cardiovascular endpoint, with a 5-year number needed to treat of 18. In 633 patients who had diabetes97, a diagnosis associated with a strong pro-oxidant state, the reduction in events was greater, with a 41% reduction in events and a number needed to treat of 6.5 patients over 5 years (unadjusted p=0.0002). Thus, although mechanisms were not elucidated nor care recommendations yet changed based on these extraordinary results, TACT provides a strong inferential basis for the conclusion that metal burden may be an under-recognized and modifiable risk factor for atherosclerotic disease.
10. Conclusions
Prudent public health measures should be taken to fully assess, then minimize, the public’s exposure to xenobiotic metals. In addition, given the present state of the science, it appears reasonable to consider the results of the recently published chelation trial (TACT) as biologically plausible and, in the selected patients, actionable.
Acknowledgments
The National Heart, Lung, and Blood Institute (NHLBI), grant # U01 HL92607 and the National Center for Complementary and Alternative Medicine (NCCAM), grant # U01AT001156 provided funding and oversight to support the TACT study and creation of the paper.
The authors are solely responsible for the design and conduct of the TACT study, all study analyses, the drafting and editing of the paper and its final contents.
Dr. Lamas was supported in part by the TACT grants from the National Center for Complementary and Alternative Medicine (U01AT001156) and the National Heart, Lung and Blood Institute (U01HL092607).
Dr. Thurston was supported by the NYU-NIEHS Center of Excellence Grant ES00260.
Dr. Hochman was supported in part by the NYU CTSA grant (UL1TR000038) from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agency for Toxic Substances and Disease Registry (ATSDR) The priority list of hazardous substances. Atlanta (GA): U.S. Department of Health and Human Services, Public Health Service; 2011. [Google Scholar]
- 2.Abadin H, Ashizawa A, Stevens YW, Llados F, Diamond G, Sage G, et al. Toxicological Profile for Lead. Atlanta (GA): 2007. Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles. [PubMed] [Google Scholar]
- 3.Skerfving S, Bergdahl IA. Lead. In: Nordberg GF, Fowler BA, Nordberg M, Friberg LT, editors. Third Edition. Elsevier: Handbook on the Toxicology of Metals; 2007. pp. 599–643. [Google Scholar]
- 4.Chisolm JJ, Jr., Harrison HE. The exposure of children to lead. Pediatrics. 1956;18(6):943–958. [PubMed] [Google Scholar]
- 5.Papanikolaou NC, Hatzidaki EG, Belivanis S, Tzanakakis GN, Tsatsakis AM. Lead toxicity update. A brief review. Medical science monitor : international medical journal of experimental and clinical research. 2005;11(10):RA329–RA336. [PubMed] [Google Scholar]
- 6.Kessler R. Sunset for leaded aviation gasoline? Environmental health perspectives. 2013;121(2):a54–a57. doi: 10.1289/ehp.121-a54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahlgren L, Liden K, Mattsson S, Tejning S. X-ray fluorescence analysis of lead in human skeleton in vivo. Scandinavian journal of work, environment & health. 1976;2(2):82–86. doi: 10.5271/sjweh.2815. [DOI] [PubMed] [Google Scholar]
- 8.Rosen JF, Markowitz ME, Bijur PE, Jenks ST, Wielopolski L, Kalef-Ezra JA, et al. L-line x-ray fluorescence of cortical bone lead compared with the CaNa2EDTA test in lead-toxic children: public health implications. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(2):685–689. doi: 10.1073/pnas.86.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosnett MJ, Wedeen RP, Rothenberg SJ, Hipkins KL, Materna BL, Schwartz BS, et al. Recommendations for medical management of adult lead exposure. Environmental health perspectives. 2007;115(3):463–471. doi: 10.1289/ehp.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menke A, Muntner P, Batuman V, Silbergeld EK, Guallar E. Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults. Circulation. 2006;114(13):1388–1394. doi: 10.1161/CIRCULATIONAHA.106.628321. [DOI] [PubMed] [Google Scholar]
- 11.Navas-Acien A, Selvin E, Sharrett AR, Calderon-Aranda E, Silbergeld E, Guallar E. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation. 2004;109(25):3196–201. doi: 10.1161/01.CIR.0000130848.18636.B2. [DOI] [PubMed] [Google Scholar]
- 12.Nash D, Magder L, Lustberg M, Sherwin RW, Rubin RJ, Kaufmann RB, et al. Blood lead, blood pressure, and hypertension in perimenopausal and postmenopausal women. JAMA : the journal of the American Medical Association. 2003;289(12):1523–1532. doi: 10.1001/jama.289.12.1523. [DOI] [PubMed] [Google Scholar]
- 13.Weisskopf MG, Jain N, Nie H, Sparrow D, Vokonas P, Schwartz J, et al. A prospective study of bone lead concentration and death from all causes, cardiovascular diseases, and cancer in the Department of Veterans Affairs Normative Aging Study. Circulation. 2009;120(12):1056–1064. doi: 10.1161/CIRCULATIONAHA.108.827121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lustberg M, Silbergeld E. Blood lead levels and mortality. Archives of internal medicine. 2002;162(21):2443–2449. doi: 10.1001/archinte.162.21.2443. [DOI] [PubMed] [Google Scholar]
- 15.Pirkle JL, Schwartz J, Landis JR, Harlan WR. The relationship between blood lead levels and blood pressure and its cardiovascular risk implications. American journal of epidemiology. 1985;121(2):246–258. doi: 10.1093/oxfordjournals.aje.a113995. [DOI] [PubMed] [Google Scholar]
- 16.Harlan WR, Landis JR, Schmouder RL, Goldstein NG, Harlan LCBlood lead, blood pressure. Relationship in the adolescent and adult US population. JAMA : the journal of the American Medical Association. 1985;253(4):530–534. doi: 10.1001/jama.253.4.530. [DOI] [PubMed] [Google Scholar]
- 17.Nawrot TS, Thijs L, Den Hond EM, Roels HA, Staessen JA. An epidemiological re-appraisal of the association between blood pressure and blood lead: a meta-analysis. Journal of human hypertension. 2002;16(2):123–131. doi: 10.1038/sj.jhh.1001300. [DOI] [PubMed] [Google Scholar]
- 18.Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free radical biology & medicine. 1995;18(2):321–36. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- 19.Revis NW, Zinsmeister AR, Bull R. Atherosclerosis and hypertension induction by lead and cadmium ions: an effect prevented by calcium ion. Proceedings of the National Academy of Sciences of the United States of America. 1981;78(10):6494–6498. doi: 10.1073/pnas.78.10.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aalbers TG, Houtman JP. Relationships between trace elements and atherosclerosis. The Science of the total environment. 1985;43(3):255–283. doi: 10.1016/0048-9697(85)90133-0. [DOI] [PubMed] [Google Scholar]
- 21.Voors AW, Shuman MS, Johnson WD. Additive statistical effects of cadmium and lead on heart-related disease in a North Carolina autopsy series. Archives of environmental health. 1982;37(2):98–102. doi: 10.1080/00039896.1982.10667544. [DOI] [PubMed] [Google Scholar]
- 22.Li WF, Pan MH, Chung MC, Ho CK, Chuang HY. Lead exposure is associated with decreased serum paraoxonase 1 (PON1) activity and genotypes. Environmental health perspectives. 2006;114(8):1233–1236. doi: 10.1289/ehp.9163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters JL, Kubzansky LD, Ikeda A, Fang SC, Sparrow D, Weisskopf MG, et al. Lead concentrations in relation to multiple biomarkers of cardiovascular disease: the Normative Aging Study. Environmental health perspectives. 2012;120(3):361–366. doi: 10.1289/ehp.1103467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skoczynska A, Smolik R, Jelen M. Lipid abnormalities in rats given small doses of lead. Archives of toxicology. 1993;67(3):200–204. doi: 10.1007/BF01973308. [DOI] [PubMed] [Google Scholar]
- 25.Nordberg GF, Nogawa K, Nordberg M, Friberg LT. In: Cadmium. Third Edition. Nordberg GF, Fowler BA, Nordberg M, Friberg LT, editors. Elsevier: Handbook on the Toxicology of Metals; 2007. pp. 445–486. [Google Scholar]
- 26.Wagner GJ, Yeargan R. Variation in cadmium accumulation potential and tissue distribution of cadmium in tobacco. Plant physiology. 1986;82(1):274–279. doi: 10.1104/pp.82.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure--a review of the literature and a risk estimate. Scandinavian journal of work, environment & health. 1998;(24 Suppl 1):1–51. [PubMed] [Google Scholar]
- 28.Menke A, Muntner P, Silbergeld EK, Platz EA, Guallar E. Cadmium levels in urine and mortality among U.S adults. Environmental health perspectives. 2009;117(2):190–196. doi: 10.1289/ehp.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nawrot TS, Staessen JA, Roels HA, Munters E, Cuypers A, Richart T, et al. Cadmium exposure in the population: from health risks to strategies of prevention. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2010;23(5):769–782. doi: 10.1007/s10534-010-9343-z. [DOI] [PubMed] [Google Scholar]
- 30.Horiguchi H, Oguma E, Sasaki S, Miyamoto K, Ikeda Y, Machida M, et al. Comprehensive study of the effects of age, iron deficiency, diabetes mellitus, and cadmium burden on dietary cadmium absorption in cadmium-exposed female Japanese farmers. Toxicology and applied pharmacology. 2004;196(1):114–123. doi: 10.1016/j.taap.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 31.Berglund M, Akesson A, Nermell B, Vahter M. Intestinal absorption of dietary cadmium in women depends on body iron stores and fiber intake. Environmental health perspectives. 1994;102(12):1058–1066. doi: 10.1289/ehp.941021058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Report on Human Exposure to Environmental Chemicals. CDC. 2009 [Google Scholar]
- 33.Tellez-Plaza M, Navas-Acien A, Menke A, Crainiceanu CM, Pastor-Barriuso R, Guallar E. Cadmium exposure and all-cause and cardiovascular mortality in the U.S. general population. Environmental health perspectives. 2012;120(7):1017–1022. doi: 10.1289/ehp.1104352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tellez-Plaza M, Guallar E, Howard BV, Umans JG, Francesconi KA, Goessler W, et al. Cadmium exposure and incident cardiovascular disease. Epidemiology. 2013;24(3):421–429. doi: 10.1097/EDE.0b013e31828b0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tellez-Plaza M, Jones MR, Dominguez-Lucas A, Guallar E, Navas-Acien A. Cadmium exposure and clinical cardiovascular disease: a systematic review. Current atherosclerosis reports. 2013;15(10):356. doi: 10.1007/s11883-013-0356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, Sharrett AR, Guallar E. Cadmium and peripheral arterial disease: gender differences in the 1999–2004 US National Health and Nutrition Examination Survey. American journal of epidemiology. 2010;172(6):671–681. doi: 10.1093/aje/kwq172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Navas-Acien A, Silbergeld EK, Sharrett R, Calderon-Aranda E, Selvin E, Guallar E. Metals in urine and peripheral arterial disease. Environmental health perspectives. 2005;113(2):164–169. doi: 10.1289/ehp.7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, Guallar E. Cadmium exposure and hypertension in the 1999–2004 National Health and Nutrition Examination Survey (NHANES) Environmental health perspectives. 2008;116(1):51–56. doi: 10.1289/ehp.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berlin M, Zalups RK, Fowler BA. In: Mercury. Third Edition. Nordberg GF, Fowler BA, Nordberg M, Friberg LT, editors. Elsevier: Handbook on the Toxicology of Metals; 2007. pp. 675–729. [Google Scholar]
- 40.Mercury in canned tuna still a concern: new tests reinforce a need for some people to limit consumption. Consumer reports. 2011;76(1):20–21. [PubMed] [Google Scholar]
- 41.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles. Toxicological profile for mercury. Atlanta (GA): U.S. Department of Health and Human Services, Public Health Service; 1999. [Google Scholar]
- 42.Ganther HE. Modification of methylmercury toxicity and metabolism by selenium and vitamin E: possible mechanisms. Environmental health perspectives. 1978;25:71–76. doi: 10.1289/ehp.782571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mozaffarian D. Fish, mercury, selenium and cardiovascular risk: current evidence and unanswered questions. International journal of environmental research and public health. 2009;6(6):1894–1916. doi: 10.3390/ijerph6061894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mozaffarian D, Shi P, Morris JS, Spiegelman D, Grandjean P, Siscovick DS, et al. Mercury exposure and risk of cardiovascular disease in two U.S cohorts. The New England journal of medicine. 2011;364(12):1116–1125. doi: 10.1056/NEJMoa1006876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.WHO (World Health Organization) Concise International Chemical Assessment Document 50. WHO; Geneva, Switzerland: 2003. Elemental mercury and inorganic mercury compounds: human health aspects. [Google Scholar]
- 46.WHO (World Health Organization) Environmental Health Criteria 101, Methylmercury. Geneva, Switzerland: WHO; 1990. [Google Scholar]
- 47.Occupational Safety and Health Standards. Mercury: US Department of Labor, Occupational Safety and Health Administration (OSHA); 2007. [Google Scholar]
- 48.Salonen JT, Seppanen K, Nyyssonen K, Korpela H, Kauhanen J, Kantola M, et al. Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation. 1995;91(3):645–655. doi: 10.1161/01.cir.91.3.645. [DOI] [PubMed] [Google Scholar]
- 49.Guallar E, Sanz-Gallardo MI, van’t Veer P, Bode P, Aro A, Gomez-Aracena J, et al. Mercury, fish oils, and the risk of myocardial infarction. The New England journal of medicine. 2002;347(22):1747–1754. doi: 10.1056/NEJMoa020157. [DOI] [PubMed] [Google Scholar]
- 50.Salonen JT, Seppanen K, Lakka TA, Salonen R, Kaplan GA. Mercury accumulation and accelerated progression of carotid atherosclerosis: a population-based prospective 4-year follow-up study in men in eastern Finland. Atherosclerosis. 2000;148(2):265–273. doi: 10.1016/s0021-9150(99)00272-5. [DOI] [PubMed] [Google Scholar]
- 51.Valera B, Dewailly E, Poirier P. Environmental mercury exposure and blood pressure among Nunavik Inuit adults. Hypertension. 2009;54(5):981–986. doi: 10.1161/HYPERTENSIONAHA.109.135046. [DOI] [PubMed] [Google Scholar]
- 52.Vupputuri S, Batuman V, Muntner P, Bazzano LA, Lefante JJ, Whelton PK, et al. Effect of blood pressure on early decline in kidney function among hypertensive men. Hypertension. 2003;42(6):1144–1149. doi: 10.1161/01.HYP.0000101695.56635.31. [DOI] [PubMed] [Google Scholar]
- 53.Pedersen EB, Jorgensen ME, Pedersen MB, Siggaard C, Sorensen TB, Mulvad G, et al. Relationship between mercury in blood and 24-h ambulatory blood pressure in Greenlanders and Danes. American journal of hypertension. 2005;18(5 Pt 1):612–618. doi: 10.1016/j.amjhyper.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 54.Kobal AB, Horvat M, Prezelj M, Briski AS, Krsnik M, Dizdarevic T, et al. The impact of long-term past exposure to elemental mercury on antioxidative capacity and lipid peroxidation in mercury miners. Journal of trace elements in medicine and biology : organ of the Society for Minerals and Trace Elements. 2004;17(4):261–274. doi: 10.1016/S0946-672X(04)80028-2. [DOI] [PubMed] [Google Scholar]
- 55.Fowler BA, Selene CH, Chou J, Jones RJ, Chen CJ. In: Arsenic. Handbook on the Toxicology of Metals. Third Edition. Nordberg GF, Fowler BA, Nordberg M, Friberg LT, editors. Elsevier: 2007. pp. 367–406. [Google Scholar]
- 56.WHO (World Health Organization) Environmental Health Criteria 18, Arsenic. Geneva, Switzerland: WHO; 1981. [Google Scholar]
- 57.Smith AH, Lopipero PA, Bates MN, Steinmaus CMPublic health. Arsenic epidemiology and drinking water standards. Science. 2002;296(5576):2145–2146. doi: 10.1126/science.1072896. [DOI] [PubMed] [Google Scholar]
- 58.Tellez-Plaza M, Gribble MO, Voruganti VS, Francesconi KA, Goessler W, Umans JG, et al. Heritability and preliminary genome-wide linkage analysis of arsenic metabolites in urine. Environmental health perspectives. 2013;121(3):345–351. doi: 10.1289/ehp.1205305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y, Wu F, Liu M, Parvez F, Slavkovich V, Eunus M, et al. A prospective study of arsenic exposure, arsenic methylation capacity, and risk of cardiovascular disease in Bangladesh. Environmental health perspectives. 2013;121(7):832–838. doi: 10.1289/ehp.1205797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles. Toxicological profile for arsenic. Atlanta (GA): U.S. Department of Health and Human Services, Public Health Service; 2007. [Google Scholar]
- 61.American Conference of Government Industrial Hygienists (ACGIH) Documentation of biological exposure indices. Cincinnati (OH: ACGIH Worldwide; 2001. [Google Scholar]
- 62.Moon KA, Guallar E, Umans JG, Devereux RB, Best LG, Francesconi KA, et al. Association Between Exposure to Low to Moderate Arsenic Levels and Incident Cardiovascular Disease: A Prospective Cohort Study. Annals of internal medicine. 2013 doi: 10.7326/0003-4819-159-10-201311190-00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen Y, Graziano JH, Parvez F, Liu M, Slavkovich V, Kalra T, Argos M, Islam T, Ahmed A, Rakibuz-Zaman M, Hasan R, Sarwar G, Levy D, van Geen A, Ahsan H. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: prospective cohort study. British Med J. 2011 May;5:342, d2431. doi: 10.1136/bmj.d2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Engel RR, Smith AH. Arsenic in drinking water and mortality from vascular disease: an ecologic analysis in 30 counties in the United States. Archives of environmental health. 1994;49(5):418–427. doi: 10.1080/00039896.1994.9954996. [DOI] [PubMed] [Google Scholar]
- 65.Tseng CH, Chong CK, Chen CJ, Tai TY. Dose-response relationship between peripheral vascular disease and ingested inorganic arsenic among residents in blackfoot disease endemic villages in Taiwan. Atherosclerosis. 1996;120(1–2):125–33. doi: 10.1016/0021-9150(95)05693-9. [DOI] [PubMed] [Google Scholar]
- 66.Abhyankar LN, Jones MR, Guallar E, Navas-Acien A. Arsenic exposure and hypertension: a systematic review. Environmental health perspectives. 2012;120(4):494–500. doi: 10.1289/ehp.1103988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gilbert HF. Thiol/disulfide exchange equilibria and disulfide bond stability. Methods in enzymology. 1995;251:8–28. doi: 10.1016/0076-6879(95)51107-5. [DOI] [PubMed] [Google Scholar]
- 68.Leonarduzzi G, Gamba P, Gargiulo S, Biasi F, Poli G. Inflammation-related gene expression by lipid oxidation-derived products in the progression of atherosclerosis. Free radical biology & medicine. 2012;52(1):19–34. doi: 10.1016/j.freeradbiomed.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 69.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Current medicinal chemistry. 2005;12(10):1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 70.Yiin SJ, Lin TH. Lead-catalyzed peroxidation of essential unsaturated fatty acid. Biological trace element research. 1995;50(2):167–172. doi: 10.1007/BF02789419. [DOI] [PubMed] [Google Scholar]
- 71.Yucebilgic G, Bilgin R, Tamer L, Tukel S. Effects of lead on Na(+)-K(+) ATPase and Ca(+2) ATPase activities and lipid peroxidation in blood of workers. International journal of toxicology. 2003;22(2):95–97. doi: 10.1080/10915810305096. [DOI] [PubMed] [Google Scholar]
- 72.Vaziri ND, Ding Y, Ni Z. Nitric oxide synthase expression in the course of lead-induced hypertension. Hypertension. 1999;34(4 Pt 1):558–562. doi: 10.1161/01.hyp.34.4.558. [DOI] [PubMed] [Google Scholar]
- 73.Vaziri ND, Ding Y. Effect of lead on nitric oxide synthase expression in coronary endothelial cells: role of superoxide. Hypertension. 2001;37(2):223–226. doi: 10.1161/01.hyp.37.2.223. [DOI] [PubMed] [Google Scholar]
- 74.Eckhardt W, Bellmann K, Kolb H. Regulation of inducible nitric oxide synthase expression in beta cells by environmental factors: heavy metals. The Biochemical journal. 1999;338(Pt 3):695–700. [PMC free article] [PubMed] [Google Scholar]
- 75.Demontis MP, Varoni MV, Volpe AR, Emanueli C, Madeddu P. Role of nitric oxide synthase inhibition in the acute hypertensive response to intracerebroventricular cadmium. British journal of pharmacology. 1998;123(1):129–135. doi: 10.1038/sj.bjp.0701573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bhatnagar A. Environmental cardiology: studying mechanistic links between pollution and heart disease. Circulation research. 2006;99(7):692–705. doi: 10.1161/01.RES.0000243586.99701.cf. [DOI] [PubMed] [Google Scholar]
- 77.Zhao Y, Wang L, Shen HB, Wang ZX, Wei QY, Chen F. Association between delta-aminolevulinic acid dehydratase (ALAD) polymorphism and blood lead levels: a meta-regression analysis. Journal of toxicology and environmental health Part A. 2007;70(23):1986–1994. doi: 10.1080/15287390701550946. [DOI] [PubMed] [Google Scholar]
- 78.Flora SJ, Flora G, Saxena G, Mishra M. Arsenic and lead induced free radical generation and their reversibility following chelation. Cellular and molecular biology. 2007;53(1):26–47. [PubMed] [Google Scholar]
- 79.Carmignani M, Boscolo P, Poma A, Volpe AR. Kininergic system and arterial hypertension following chronic exposure to inorganic lead. Immunopharmacology. 1999;44(1–2):105–110. doi: 10.1016/s0162-3109(99)00115-0. [DOI] [PubMed] [Google Scholar]
- 80.Vander AJ. Chronic effects of lead on the renin-angiotensin system. Environmental health perspectives. 1988;78:77–83. doi: 10.1289/ehp.887877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bergquist ER, Fischer RJ, Sugden KD, Martin BD. Inhibition by methylated organo-arsenicals of the respiratory 2-oxo-acid dehydrogenases. Journal of organometallic chemistry. 2009;694(6):973–980. doi: 10.1016/j.jorganchem.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu F, Jasmine F, Kibriya MG, Liu M, Wojcik O, Parvez F, et al. Association between arsenic exposure from drinking water and plasma levels of cardiovascular markers. American journal of epidemiology. 2012;175(12):1252–1261. doi: 10.1093/aje/kwr464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tseng CH. The potential biological mechanisms of arsenic-induced diabetes mellitus. Toxicology and applied pharmacology. 2004;197(2):67–83. doi: 10.1016/j.taap.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 84.Messner B, Knoflach M, Seubert A, Ritsch A, Pfaller K, Henderson B, et al. Cadmium is a novel and independent risk factor for early atherosclerosis mechanisms and in vivo relevance. Arteriosclerosis, thrombosis, and vascular biology. 2009;29(9):1392–1398. doi: 10.1161/ATVBAHA.109.190082. [DOI] [PubMed] [Google Scholar]
- 85.Lin YS, Rathod D, Ho WC, Caffrey JJ. Cadmium exposure is associated with elevated blood C-reactive protein and fibrinogen in the U. S. population: the third national health and nutrition examination survey (NHANES III, 1988–1994) Annals of epidemiology. 2009;19(8):592–596. doi: 10.1016/j.annepidem.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 86.Jiang SJ, Lin TM, Wu HL, Han HS, Shi GY. Decrease of fibrinolytic activity in human endothelial cells by arsenite. Thrombosis research. 2002;105(1):55–62. doi: 10.1016/s0049-3848(01)00397-8. [DOI] [PubMed] [Google Scholar]
- 87.Knoflach M, Messner B, Shen YH, Frotschnig S, Liu G, Pfaller K, et al. Non-toxic cadmium concentrations induce vascular inflammation and promote atherosclerosis. Circulation journal : official journal of the Japanese Circulation Society. 2011;75(10):2491–2495. doi: 10.1253/circj.cj-11-0196. [DOI] [PubMed] [Google Scholar]
- 88.Manigrasso MB, Juranek J, Ramasamy R, Schmidt AM. Unlocking the biology of RAGE in diabetic microvascular complications. Trends in endocrinology and metabolism: TEM. 2013 doi: 10.1016/j.tem.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nagai R, Murray DB, Metz TO, Baynes JW. Chelation: a fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes. 2012;61(3):549–559. doi: 10.2337/db11-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40(4):405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 91.Monnier VM. Transition metals redox: reviving an old plot for diabetic vascular disease. The Journal of clinical investigation. 2001;107(7):799–801. doi: 10.1172/JCI12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Agarwal S, Zaman T, Tuzcu EM, Kapadia SR. Heavy metals and cardiovascular disease: results from the National Health and Nutrition Examination Survey (NHANES) 1999–2006. Angiology. 2011;62(5):422–429. doi: 10.1177/0003319710395562. [DOI] [PubMed] [Google Scholar]
- 93.Clarke CN, Clarke NE, Mosher RE. Treatment of angina pectoris with disodium ethylene diamine tetraacetic acid. The American journal of the medical sciences. 1956;232(6):654–666. doi: 10.1097/00000441-195612000-00006. [DOI] [PubMed] [Google Scholar]
- 94.Villarruz MV, Dans A, Tan F. Chelation therapy for atherosclerotic cardiovascular disease. The Cochrane database of systematic reviews. 2002;(4):CD002785. doi: 10.1002/14651858.CD002785. [DOI] [PubMed] [Google Scholar]
- 95.Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, Nahin RL, et al. Design of the Trial to Assess Chelation Therapy (TACT) American heart journal. 2012;163(1):7–12. doi: 10.1016/j.ahj.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, Nahin RL, et al. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA : the journal of the American Medical Association. 2013;309(12):1241–1250. doi: 10.1001/jama.2013.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Escolar E, Lamas GA, Mark DB, Boineau R, Goertz C, Rosenberg Y, et al. The Effect of an EDTA-based Chelation Regimen on Patients With Diabetes Mellitus and Prior Myocardial Infarction in the Trial to Assess Chelation Therapy (TACT) Circulation Cardiovascular quality and outcomes. 2013 doi: 10.1161/CIRCOUTCOMES.113.000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Salonen JT, Seppänen K, Nyyssönen K, Korpela H, Kauhanen J, Kantola M, Tuomilehto J, Esterbauer H, Tatzber F, Salonen R. Mercury, Fish Oils, and Risk of Acute Coronary Events and Cardiovascular Disease, Coronary Heart Disease, and All-Cause Mortality in Men in Eastern Finland. Arteriosclerosis, Thrombosis, and Vascular Biology [Internet] 2004;91:645–655. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=7828289&retmode=ref&cmd=prlinks. [Google Scholar]
- 99.Yoshizawa K, Rimm EB, Morris JS, Spate VL, Hsieh C-C, Spiegelman D, Stampfer MJ, Willett WC. Mercury and the risk of coronary heart disease in men. N Engl J Med. 2002;347:1755–1760. doi: 10.1056/NEJMoa021437. [DOI] [PubMed] [Google Scholar]
- 100.Nakagawa H, Nishijo M, Morikawa Y, Miura K, Tawara K, Kuriwaki J-I, Kido T, Ikawa A, Kobayashi E, Nogawa K. Urinary cadmium and mortality among inhabitants of a cadmium-polluted area in Japan. Environ Res. 2006;100:323–329. doi: 10.1016/j.envres.2005.08.014. [DOI] [PubMed] [Google Scholar]