Abstract
Objective
To identify features associated with multisystem involvement and therapeutic failure in patients with skin Langerhans cell histiocytosis (LCH).
Study design
We reviewed medical records of 71 consecutive LCH patients with skin involvement evaluated at Texas Children’s Hospital and analyzed clinical features, laboratory results, and presence of circulating cells with the BRAF-V600E mutation, with respect to initial staging and clinical outcomes.
Results
Skin disease in patients older than 18 months at diagnosis was associated with presence of multisystem disease (OR 9.65, 95% CI 1.17–79.4). Forty percent of patients referred for presumed skin-limited LCH had underlying multisystem involvement, half of these with risk-organ involvement. Patients with skin-limited LCH had 3-year progression-free survival (PFS) of 89% after initial therapy, and none developed multisystem disease. Patients with skin/multisystem involvement had 3 year PFS of 44% with vinblastine/prednisone therapy, and risk-organ involvement did not correlate with failure to achieve non-active disease. Circulating cells with BRAF-V600E were detected at higher frequency in multisystem patients (8/11 skin/multisystem, 1/13 skin-limited, P=0.002).
Conclusions
Skin-limited LCH requires infrequent therapeutic intervention and has lower risk of progression relative to skin plus multisystem LCH. The less aggressive clinical course and lack of circulating cells with BRAF-V600E mutation in skin-limited LCH suggest a different mechanism of disease origin compared with multisystem or risk-organ disease.
Keywords: rash, myeloid, BRAF-V600E
Langerhans cell histiocytosis (LCH) is an inflammatory myeloid neoplasia1 characterized by the accumulation of clonal CD207+ myeloid dendritic cells amidst an inflammatory background of macrophages, T-lymphocytes, and eosinophils.2–4 LCH presents most commonly in infants and children. The sites affected by LCH are highly variable, ranging from single-system disease in skin or bone to multisystem disease, potentially with risk-organ (liver, spleen, and bone marrow) involvement that predicts increased mortality.5 LCH patients frequently present with cutaneous signs that can sometimes resemble eczema or seborrheic dermatitis, with the scalp being the most common site of involvement.6 LCH lesions involving the skin may represent skin-limited disease that can resolve spontaneously or with minimal chemotherapy. Alternatively, skin lesions may represent the most clinically evident manifestation of potentially life-threatening multisystem disease. Previous studies have reported that 53% of patients with multisystem LCH present with skin lesions.7, 8 However, the true incidence of skin-limited LCH may be underestimated because patients with spontaneously-resolving or mild disease may go undiagnosed or may not be referred to specialists.9 The potential of skin-limited LCH to “metastasize” to other organs is not clear, with studies reporting widely variable occurrence of progression of isolated skin LCH to multisystem disease (0–60%).7, 9–16
Risk-organ involvement and inadequate response to initial therapy are the major clinical features currently associated with adverse outcomes in patients with skin LCH.5, 17, 18 Other predictors of disease extent, risk of progression or recurrence, or survival remain to be elucidated. The somatic BRAF-V600E mutation has been identified in pathologic dendritic cells of LCH lesions in approximately 60% of patients.19 Although BRAF status of the lesion does not predict risk-organ involvement, detection of BRAF-V600E in circulating blood cells has been associated with increased risk of disease recurrence independent of risk-organ involvement.1 Among patients with BRAF-V600E mutation identified in lesional biopsies, the presence of circulating cells with BRAF-V600E mutation was highly sensitive and specific for multi-system high risk LCH.1 In this study we characterize the significance of clinical variables, including age and presence of circulating cells with BRAF-V600E, with respect to extent of disease at diagnosis and clinical outcomes in patients with LCH skin lesions.
Methods
Medical records of 71 consecutive patients who presented with any LCH skin lesions, either as skin-limited disease or multisystem disease, at the Texas Children’s Cancer and Hematology Centers (TXCH) from March 2005 through October 2011 were reviewed. Patients who presented to TXCH either with de novo disease or by referral after diagnosis were included in this study. The age, date of diagnosis, date of symptom onset, location of LCH involvement, type of therapy, response to therapy, and time to recurrence or disease progression were recorded. Review of patient records was performed according to protocols approved by the Institutional Review Board of Baylor College of Medicine (BCM IRB).
Incidence and location of progression and recurrence were characterized as counts and proportions using categorical variables and age of symptoms onset, age of diagnosis, and time until disease progression were characterized with means and ranges. Disease state and response criteria were defined according to the Histiocyte Society Evaluation and Treatment Guidelines for LCH.20 Disease state was classified as non-active disease (NAD) or active disease (AD). Response in patients with AD was noted as AD/better (improving disease), AD/intermediate (stable disease, or regression with new sites of disease), or AD/worse. Progression was defined as development of AD/worse. Progression-free survival (PFS) was calculated using the Kaplan-Meier methods and compared by log-rank (Mantel-Cox) test, and data were censored at time of last follow-up. Clinical variables between groups of patients were compared using the two-tailed Mann-Whitney test for non-parametric data. Contingency analyses were performed using Fisher exact test. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to determine the association between clinical features of skin LCH and multisystem disease. Mantel-Haenszel method was used to determine hazard ratio of disease progression between skin-limited and skin plus multisystem disease. Data analyses were performed using Prism v. 5.0 (GraphPad, La Jolla, California).
A highly sensitive qPCR strategy was used to detect cells with the BRAF-V600E allele in LCH biopsy specimens and peripheral blood mononuclear cells (PMBCs) as described previously.1 Experiments were performed and clinical data were collected according to protocols approved by the BCM IRB.
Results
Seventy-one patients presented with LCH skin lesions between March 2005 and August 2012 (Table). Twenty-one patients were determined to have skin-limited disease, and 43 patients had multisystem involvement. Seven patients, referred late in their treatment course with multisystem disease, could not be categorized due to incomplete information at diagnosis, and they were not included in subsequent analyses.
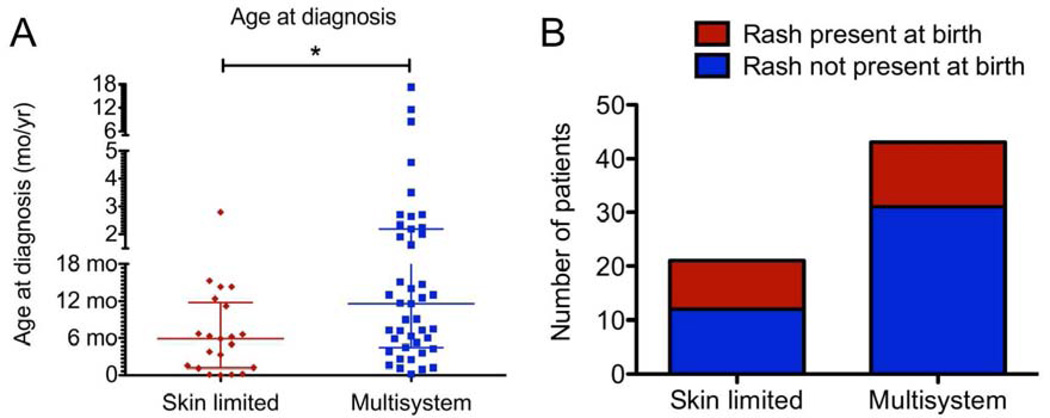
The majority of patients with skin-limited and skin plus multisystem disease were under 1 year of age at diagnosis (Table). Older age correlated with presence of multisystem disease (P=0.01; Figure 1, A). Skin disease diagnosed in patients older than 18 months of age was associated with the presence of multisystem disease (OR 9.65, 95% CI 1.17–79.4). However, LCH skin lesions at a younger age was not correlated with absence of multisystem disease, and LCH rash developing in the first week of life was not significantly associated with absence of multisystem disease (OR 0.51, 95% CI 0.17–1.5; Figure 1, B). Time interval of greater than 100 days between symptom onset and diagnosis correlated with presence of multisystem disease (OR 3.82, 95% CI 1.24–11.8).
Table I.
Patient characteristics
| Cohort | Number of Patients |
Gender | Median Age at Symptom Onset (Months) |
Median Age at Diagnosis (Months) |
|---|---|---|---|---|
| Skin-Only LCH | 21 | 14 Male 7 Female |
1.2 (Range: Birth – 30.5) |
5.9 (Range: Birth – 33.5) |
|
Multisystem LCH (Extent known at presentation) |
43 | 20 Male 23 Female |
1.8 (Range: 0.1 – 206) |
11.6 (Range: 0.2 –207) |
|
Multisystem LCH (Extent not known at presentation) |
7 | 4 Male 3 Female |
Birth (Range: Birth – 2.8) |
4.7 (Range: Birth – 27.1) |
| Total | 71 | 38 Male 33 Female |
1.6 (Range: Birth -206) |
7.2 (Range: Birth – 207) |
Figure 1.
A) Distribution of patient age at diagnosis. *: P<0.05 B) Onset of symptoms at birth was not significantly associated with absence of multisystem disease.
Patients with LCH skin lesions were evaluated for multisystem disease by radiographic skeletal survey and skull series, complete blood counts, hepatic function studies, and chest x-ray. Additional tests (ie, brain magnetic resonance imaging, chest or abdominal computed tomography) were obtained based on findings of the prior studies or the clinical presentation of the patients.21 Fourteen of 35 patients (40%) referred by primary care providers for presumed skin-limited LCH, ranging in age at symptom presentation from birth to 30 months of age, were subsequently found to have multisystem involvement. The most common additional site of involvement was bone, in 10/14 patients (71%). Risk-organ disease (liver, spleen, or bone marrow) occurred in 7/14 patients (50%). Lung disease (no longer considered a risk-organ site)22, 23 was also present in 3/14 patients at time of post-diagnostic evaluation.
Skin-Limited LCH
Skin-limited LCH in this cohort often resolved spontaneously, did not progress to involve other organs, and in most cases responded to primary therapy. Of the 20 skin-limited LCH patients with follow-up data, seventeen initially underwent a period of observation, and three were treated at diagnosis. Twelve patients (60%) had spontaneous resolution without any intervention or subsequent recurrence. Six patients (30%), five of whom were initially observed, experienced disease progression or recurrence. In this series there were no cases of patients with skin-limited LCH defined by comprehensive staging at diagnosis with evidence of subsequent progression to other organ systems.
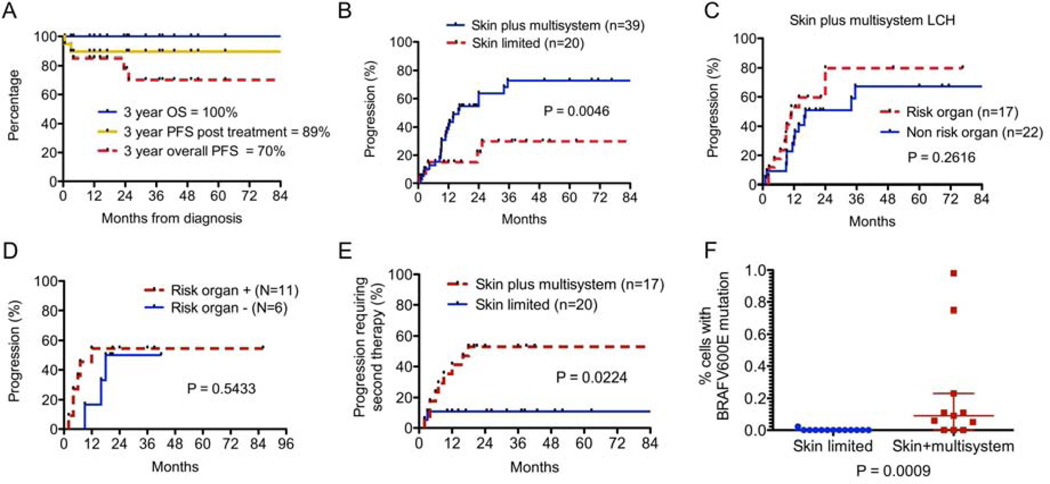
Only two patients with skin-limited LCH experienced disease progression after first therapy. Of the eight patients who were eventually treated, five patients achieved non-active disease (NAD) after initial therapy, two patients developed active disease (AD)/worse, and one patient was lost to follow-up before therapy response could be assessed. Both patients with AD/worse post treatment received oral methotrexate as initial therapy. Of the twenty skin-limited LCH patients, eighteen (90%) had NAD at time of last follow-up. Three year overall survival (OS) of all patients with skin-limited LCH was 100%. Progression was noted in six patients (3-year PFS 70%). If progression only after therapy initiation is considered, however, the 3-year PFS was 89% (Figure 2, A).
Figure 2.
A) OS and PFS of patients with skin-limited LCH. B) Progression in skin-limited LCH versus skin plus multisystem disease. C–D) Progression for risk-organ involvement in the entire series (C) and in patients receiving uniform initial therapy (D). E) Progression requiring need for second therapy in skin plus multisystem disease versus skin-limited disease. F) BRAF-V600E mutation was detected in circulating peripheral blood cells in 8/11 patients with active skin plus multisystem LCH, but only 1/13 patients with active skin-limited LCH, and only in a low percentage of cells (P = 0.0009).
Multisystem LCH Including Skin Lesions
Patients with skin plus multisystem LCH were treated with systemic chemotherapy. Thirty-nine patients had evaluable response to therapy, three were lost to follow-up, and one with risk-organ involvement died prior to initiation of therapy. Three year OS of patients with skin plus multisystem disease who received treatment was 100%. However, patients with skin plus multisystem disease (3-year progression 77%) were significantly more likely to progress than those with skin-limited disease (3-year progression 30%) (P=0.005; Figure 2, B). Forty-four percent of patients with skin plus multisystem disease had risk-organ involvement at diagnosis. There was no significant difference in progression between skin plus multisystem patients with risk-organ involvement (n = 17; 3-year progression 80%) versus non-risk involvement (n = 22; 3 year progression 68%) (P=0.26; Figure 2, C). Median time to progression was 11 months in risk-organ disease and 16.3 months without risk-organ disease.
To evaluate the effect of standard vinblastine/prednisone-based therapy in the management of skin plus multisystem LCH, and to minimize referral bias, we separately evaluated the 17 patients treated with this regimen as primary therapy within our institution and identified no significant difference in progression between patients with or without risk-organ disease (Figure 2, D). The majority of these patients with skin plus multisystem disease (56%) experienced disease progression during or after initial therapy, whereas patients with skin-limited disease rarely experienced post-treatment disease progression (11%) (Hazard ratio 4.00, 95% CI 1.22–13.18, P=0.02; Figure 2, E). Of the six patients with risk-organ involvement who experienced disease progression, five had persistence or progression of disease in risk organs, and none achieved NAD before disease progression. Of the three patients without risk-organ involvement that recurred, all achieved NAD before recurrence, and only one had disease recurrence in an organ besides skin.
Patients with skin plus multisystem disease usually responded to salvage therapy but often required multiple different regimens for a durable complete response. Of the 39 patients with evaluable therapy response, 29 (74%) had NAD at time of last follow-up after a median of 3 distinct therapy regimens. Among patients with AD at last evaluation, five patients were AD/better and five were AD/worse after a median of 2.5 regimens. Risk-organ involvement did not predict eventual failure to develop NAD (P=1.00). No deaths occurred after treatment initiation in this series.
Circulating Cells with BRAF-V600E Allele in Patients with Skin LCH
Pre-therapy peripheral blood samples were analyzed for circulating cells with the BRAF-V600E mutation. Of 13 patients with active skin-limited disease, only 1 patient (8%) had detectable circulating cells with BRAF-V600E mutation. These results were compared with previously-analyzed peripheral blood from eleven patients with skin plus multisystem LCH.1 BRAF-V600E was identified in the circulating cells of 8/11 (72%) patients, all of whom had BRAF-V600E. identified in the diagnostic biopsy. Six of the eight with circulating BRAF-V600E cells had risk-organs involved. Circulating cells with BRAF-V600E were detected not only at higher frequency in skin/multisystem patients but also in a higher percentage of cells: 0.05–0.98% in the multisystem patients and 0.02% in the one skin-limited patient with detectable mutation (Figure 2, F; P<0.001). In the one patient with skin-limited LCH and circulating BRAF-V600E cells at diagnosis, BRAF-V600E cells were not detected after effective treatment with oral methotrexate. Although diagnostic skin biopsy tissue was not available to evaluate the frequency of BRAF-V600E in skin-limited patients, we hypothesize that it occurs with similar frequency to LCH in general (50–65%) because all studies to date find common frequency across clinical risk groups.1, 19, 24, 25 We therefore hypothesize that these results reflect a lack of circulating LCH lesion precursors in patients with skin-limited disease.
Discussion
LCH occurs in 2–10 per million children per year and 1–2 per million adults, with extreme clinical heterogeneity.26–28 Involvement of risk organs (liver, spleen, and bone marrow) and failure to respond to the first 12 weeks of therapy predict increased mortality risk.5, 8 Accurate staging is essential to determine optimal therapy, as observation or curettage may be appropriate for single-system disease, whereas systemic chemotherapy is required for multisystem LCH. Survival of LCH patients is greater than 90% in non-risk organ disease, and the recent LCH-III trial has shown decreased disease reactivation rates in both risk and non-risk organ disease with prolongation of therapy from six months to one year.5, 29, 30 However, all LCH patients, especially those with prolonged uncontrolled disease, may develop long-term morbidity including chronic pain, problems with growth, endocrinopathies, and neurocognitive deficits.31 Timely recognition, staging, and therapy of LCH are therefore essential for optimal outcomes.
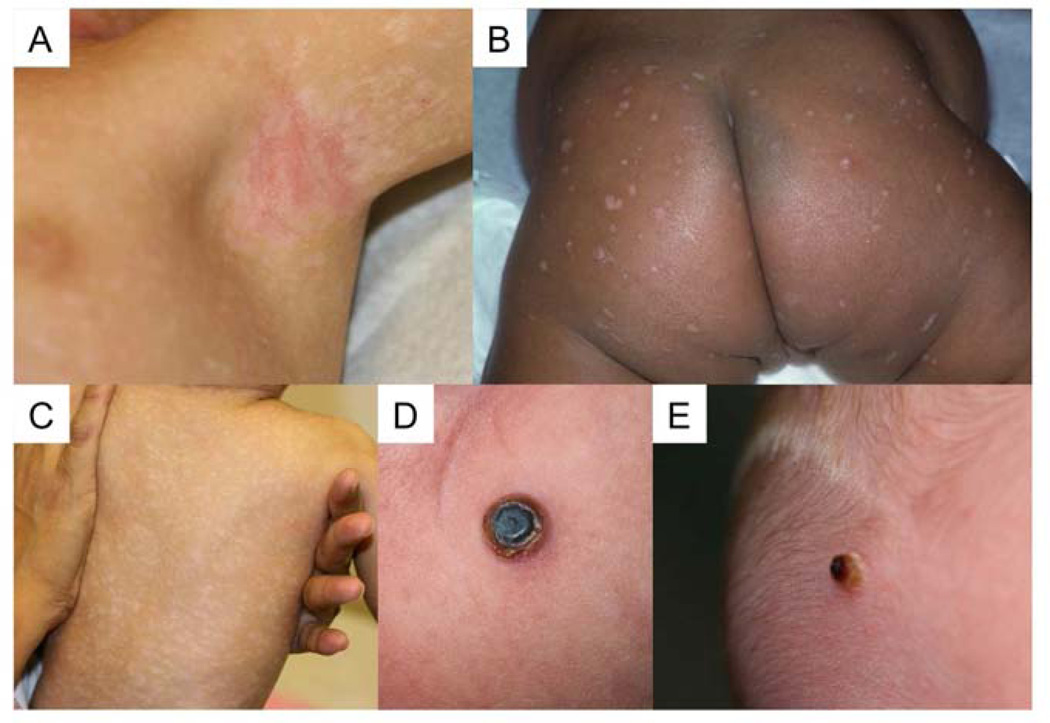
Skin lesions are a classic feature of LCH. Because the appearance of LCH skin lesions is variable and easily confused with more common childhood rashes such as eczema or seborrheic dermatitis (Figure 3), consideration of LCH and diagnostic biopsy are often delayed. In this series the median time from onset of symptoms to diagnostic biopsy was greater than 3 months. Although some patients have selfresolving skin lesions,7, 9 our experience has been that many patients who are diagnosed with LCH by skin biopsy are frequently assumed to have skin-limited disease without further evaluation.
Figure 3.
Skin LCH has variable appearance, including A) eczematous dermatitis, B) hypopigmented, eroded papules, C) hypopigmented macules, or D) and E) crusted papulonodules. Presentation does not reflect presence or absence of multisystem disease; despite similar appearance, the patient in D) had a single lesion, but the patient in E) had risk-organ involvement.
In order to identify clinical characteristics of skin LCH that might distinguish children with skin-limited from multisystem disease, we reviewed 71 patients treated at Texas Children’s Hospital over 5 years. In this series age of LCH diagnosis greater than 18 months was strongly associated with risk of multisystem disease. Similar to the DAL-HX 83/90 studies, in which the majority of patients with skin-only disease were also observed without therapy,7 patients with single-system skin disease at diagnosis did not typically progress to other organs besides skin. Overall survival in this series (OS=98%, with the only death occurring prior to therapy) was similar to previously published reports. Despite high OS and ability to treat recurrent or refractory disease with alternate chemotherapy agents, the majority of patients with skin plus multisystem LCH treated with standard of care (vinblastine/prednisone) did not have an initial durable response to therapy (PFS=44%), and disease persisted or progressed in risk organs in those patients with risk-organ involvement at diagnosis. Circulating cells with the BRAF-V600E mutation were frequently observed in patients with active multisystem LCH, but only in a single patient with skin-limited LCH.
Given the high frequency of multisystem involvement in patients with skin LCH, early biopsy of suspicious lesions and comprehensive organ evaluation has long been indicated for all patients diagnosed with skin LCH.9, 21 These current findings illustrate the high frequency of multisystem disease found at time of referral and continued delays between symptom onset and diagnosis. In this study 40% of patients referred for apparently skin-limited LCH also had multisystem disease when they were fully evaluated. Half of these multisystem patients had risk-organ involvement. The relative incidence of skin plus multisystem LCH relative to skin-limited LCH (2:1 in this series) is consistent with a previously published single-institution study,9 albeit with lower death rates in this series, likely due to improved salvage therapies in the last two decades.32–34 For reasons that remain unclear, time from symptom onset to diagnosis correlated with presence of multisystem involvement. It is possible that patients with skin-limited disease have more clinically impressive lesions. Data from this study do not suggest that time to diagnosis results in progression from skin-limited to multisystem disease, although we were unable to evaluate the possibility of progression from skin-limited to multisystem disease in infants under three months of age due to low numbers of patients referred in this age group. Thus, these data highlight the continued delays in identifying children with LCH, despite decades of evidence and recommendations supporting early biopsy of progressive or treatment refractory eczema or seborrheic dermatitis.9, 21 It is our view, consistent with others,9 that terms such as “Hashimoto-Pritzker disease” or “self-healing LCH” are diagnoses that can be made with certainty only in retrospect and therefore should not be used to describe patients with active disease. We urge providers to consider LCH in the differential diagnosis of such skin lesions to minimize morbidity of untreated lesions and potential for unrecognized high-risk disease.
Patients in this series with skin-limited LCH had lower frequency of recurrence/relapse compared with previous studies,9 and most skin-limited patients had self-resolving disease. No standard of care exists for the initial therapy of skin-limited LCH. Reported therapies include topical steroids, nitrogen mustard or imiquimod; surgical resection of isolated lesions; phototherapy or photochemotherapy; or systemic interferon a2b, methotrexate, 6-mercaptopurine, vincristine, vinblastine, steroids, thalidomide, cladribine, and/or cytarabine.35–46 No regimen, however, has been evaluated in a randomized-controlled trial, and most limited studies of these agents do not have significant long-term follow-up data. Many strategies were used to treat skin-limited LCH in this cohort, with variable efficacy; given small numbers of patients, we cannot make a recommendation about effective first-line treatment approach for patients with skin-limited disease requiring therapy. Because skin-limited disease in infants has a high rate of spontaneous regression, we recommend surveillance for patients with skin-limited disease except in cases of skin rashes that cause identifiable patient morbidity or discomfort.
No patient with skin-limited disease in this cohort developed disease in another organ site. Other series have shown rates of progression to other organ sites as high as 60%,7, 9–15 but it is unclear to what extent these patients underwent evaluation at diagnosis to ensure absence of multisystem disease. We have occasionally observed severe disease progression in patients with skin LCH who were evaluated outside of this cohort, including one patient who died from widespread risk-organ disease after several years of severe skin and mucosal disease.47 Although observation is a reasonable initial approach for skin-limited LCH after a comprehensive diagnostic evaluation, physicians should remain vigilant in follow-up of these patients, as the true risk of progression remains undefined.
The relative absence of circulating cells with BRAF-V600E mutations in patients with skin-limited LCH is consistent with previous findings showing circulating cells with BRAF-V600E mutation are generally found in patients with active multi-system risk-organ disease.1 This study was unable to define whether the BRAF-V600E mutation was absent in circulation due to its inherent absence in skin lesions or due to restricted migratory potential of precursor LCH cells in skin-limited disease. We would hypothesize the latter to be the case, considering that the mutation is present in equal frequency across clinical risk groups,1, 19 but further evaluation of skin lesions for the mutation is warranted. Although patients with multisystem disease without risk-organ involvement uncommonly have circulating BRAF-V600E cells, four of the five patients previously described had skin involvement, and this group had statistically increased relapse rates compared with other patients lacking risk-organ disease.1 We hypothesize that circulating LCH precursor cells in multisystem disease reflect a broader potential for migration of pathologic dendritic cells than in tissue-restricted low-risk LCH, in which circulating precursor cells are not identified. Previous results demonstrate that the state of differentiation of the cell that acquires the BRAF-V600E mutation defines disease-severity and tissue distribution of pathologic dendritic cells in LCH.1 We speculate that the relative absence of circulating cells with the BRAF-V600E mutation in patients with spontaneously resolving skin-limited LCH could be consistent with a transient skin-restricted precursor cell population specific to early hematopoiesis, analogous to transient myeloproliferative disorder of Down syndrome, which arises from abnormal hematopoietic cells from the fetal liver.48 The ability of circulating BRAF-V600E cells to predict skin-limited versus multisystem LCH will require validation in future prospective clinical trials.
LCH skin lesions are easily confused with other common pediatric dermatoses. Suspicion of LCH, biopsy, and comprehensive staging evaluation are essential first steps toward optimal clinical care for these patients, which may range from observation in mild skin-limited disease to cytotoxic chemotherapy in skin with associated multisystem disease. Prospective multicenter trials are needed to determine whether skin involvement is an independent predictor of poor therapeutic response in multisystem or risk-organ LCH, if alternative therapies to vinblastine/prednisone are superior for patients with multisystem LCH involving skin, and whether presence of circulating cells with the BRAF-V600E mutation risk-stratifies patients or serves as a marker of active disease.
Acknowledgments
The authors thank Munu Bilgi for data collection.
Supported by the HistioCure Foundation (Texas Children’s Cancer Center Histiocytosis Program), National Institutes of Health (R01 CA154489 [to C.A. and K.M], P50CA126752 [to C.A.], and NIH K12 CA090433 [to S.S.]), and Dan L. Duncan Cancer Center (P30CA125123).
Abbreviations
- AD
active disease
- CI
confidence interval
- LCH
Langerhans cell histiocytosis
- NAD
non-active disease
- OR
odds ratio
- OS
overall survival
- PFS
progression free survival
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of ineterset.
Portions of the study were presented as an abstract at the Histiocyte Society’s meeting, Washington, DC, October 19–21, 2013.
References
- 1.Berres ML, Lim KP, Peters T, Price J, Takizawa H, Salmon H, et al. BRAF-V600E expression in precursor versus differentiated dendritic cells defines clinically distinct LCH risk groups. J Exp Med. 2014;211:669–683. doi: 10.1084/jem.20130977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willman CL, Busque L, Griffith BB, Favara BE, McClain KL, Duncan MH, et al. Langerhans'-cell histiocytosis (histiocytosis X)--a clonal proliferative disease. N Engl J Med. 1994;331:154–160. doi: 10.1056/NEJM199407213310303. [DOI] [PubMed] [Google Scholar]
- 3.Yu RC, Chu C, Buluwela L, Chu AC. Clonal proliferation of Langerhans cells in Langerhans cell histiocytosis. Lancet. 1994;343:767–768. doi: 10.1016/s0140-6736(94)91842-2. [DOI] [PubMed] [Google Scholar]
- 4.Berres ML, Allen CE, Merad M. Pathological consequence of misguided dendritic cell differentiation in histiocytic diseases. Adv Immunol. 2013;120:127–161. doi: 10.1016/B978-0-12-417028-5.00005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gadner H, Minkov M, Grois N, Potschger U, Thiem E, Arico M, et al. Therapy prolongation improves outcome in multisystem Langerhans cell histiocytosis. Blood. 2013;121:5006–5014. doi: 10.1182/blood-2012-09-455774. [DOI] [PubMed] [Google Scholar]
- 6.Munn S, Chu AC. Langerhans cell histiocytosis of the skin. Hematol Oncol Clin North Am. 1998;12:269–286. doi: 10.1016/s0889-8588(05)70510-4. [DOI] [PubMed] [Google Scholar]
- 7.Titgemeyer C, Grois N, Minkov M, Flucher-Wolfram B, Gatterer-Menz I, Gadner H. Pattern and course of single-system disease in Langerhans cell histiocytosis data from the DAL-HX 83- and 90-study. Med Pediatr Oncol. 2001;37:108–114. doi: 10.1002/mpo.1178. [DOI] [PubMed] [Google Scholar]
- 8.Gadner H, Heitger A, Grois N, Gatterer-Menz I, Ladisch S. Treatment strategy for disseminated Langerhans cell histiocytosis. DAL HX-83 Study Group. Med Pediatr Oncol. 1994;23:72–80. doi: 10.1002/mpo.2950230203. [DOI] [PubMed] [Google Scholar]
- 9.Lau L, Krafchik B, Trebo MM, Weitzman S. Cutaneous Langerhans cell histiocytosis in children under one year. Pediatr Blood Cancer. 2006;46:66–71. doi: 10.1002/pbc.20479. [DOI] [PubMed] [Google Scholar]
- 10.Battistella M, Fraitag S, Teillac DH, Brousse N, de Prost Y, Bodemer C. Neonatal and early infantile cutaneous langerhans cell histiocytosis: comparison of self-regressive and non-selfregressive forms. Arch Dermatol. 2010;146:149–156. doi: 10.1001/archdermatol.2009.360. [DOI] [PubMed] [Google Scholar]
- 11.Minkov M, Prosch H, Steiner M, Grois N, Potschger U, Kaatsch P, et al. Langerhans cell histiocytosis in neonates. Pediatr Blood Cancer. 2005;45:802–807. doi: 10.1002/pbc.20362. [DOI] [PubMed] [Google Scholar]
- 12.Larralde M, Rositto A, Giardelli M, Gatti CF, Santos-Munoz A. Congenital self-healing Langerhans cell histiocytosis: the need for a long term follow up. Int J Dermatol. 2003;42:245–246. doi: 10.1046/j.1365-4362.2003.16142.x. [DOI] [PubMed] [Google Scholar]
- 13.Larralde M, Rositto A, Giardelli M, Gatti CF, Santos Munoz A. Congenital self-healing histiocytosis (Hashimoto-Pritzker) Int J Dermatol. 1999;38:693–696. doi: 10.1046/j.1365-4362.1999.00758.x. [DOI] [PubMed] [Google Scholar]
- 14.Willis B, Ablin A, Weinberg V, Zoger S, Wara WM, Matthay KK. Disease course and late sequelae of Langerhans' cell histiocytosis: 25-year experience at the University of California, San Francisco. J Clin Oncol. 1996;14:2073–2082. doi: 10.1200/JCO.1996.14.7.2073. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto K, Bale GF, Hawkins HK, Langston C, Pritzker MS. Congenital self-healing reticulohistiocytosis (Hashimoto-Pritzker type) Int J Dermatol. 1986;25:516–523. doi: 10.1111/j.1365-4362.1986.tb00867.x. [DOI] [PubMed] [Google Scholar]
- 16.Ng SS, Koh MJ, Tay YK. Cutaneous Langerhans cell histiocytosis: study of Asian children shows good overall prognosis. Acta Paediatr. 2013;102:e514–e518. doi: 10.1111/apa.12376. [DOI] [PubMed] [Google Scholar]
- 17.Gadner H, Grois N, Arico M, Broadbent V, Ceci A, Jakobson A, et al. A randomized trial of treatment for multisystem Langerhans' cell histiocytosis. J Pediatr. 2001;138:728–734. doi: 10.1067/mpd.2001.111331. [DOI] [PubMed] [Google Scholar]
- 18.Gadner H, Grois N, Potschger U, Minkov M, Arico M, Braier J, et al. Improved outcome in multisystem Langerhans cell histiocytosis is associated with therapy intensification. Blood. 2008;111:2556–2562. doi: 10.1182/blood-2007-08-106211. [DOI] [PubMed] [Google Scholar]
- 19.Badalian-Very G, Vergilio JA, Degar BA, MacConaill LE, Brandner B, Calicchio ML, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919–1923. doi: 10.1182/blood-2010-04-279083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minkov M, Grois N, McClain K, Nanduri V, Rodriguez-Galindo C, Simonitsch-Klupp I, et al. Histiocyte Society Evaluation and Treatment Guidelines. [Accessed 1 June 2013];2009 Apr; www.histiocytesociety.org/document.doc?id=290. Published 2009. Updated 2009. [Google Scholar]
- 21.Broadbent V, Gadner H, Komp DM, Ladisch S. Histiocytosis syndromes in children: II. Approach to the clinical and laboratory evaluation of children with Langerhans cell histiocytosis. Clinical Writing Group of the Histiocyte Society. Med Pediatr Oncol. 1989;17:492–495. doi: 10.1002/mpo.2950170527. [DOI] [PubMed] [Google Scholar]
- 22.Braier J, Latella A, Balancini B, Castanos C, Rosso D, Chantada G, et al. Outcome in children with pulmonary Langerhans cell Histiocytosis. Pediatr Blood Cancer. 2004;43:765–769. doi: 10.1002/pbc.20112. [DOI] [PubMed] [Google Scholar]
- 23.Ronceray L, Potschger U, Janka G, Gadner H, Minkov M. Pulmonary involvement in pediatric-onset multisystem Langerhans cell histiocytosis: effect on course and outcome. J Pediatr. 2012;161:129–133. e121–e123. doi: 10.1016/j.jpeds.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 24.Haroche J, Charlotte F, Arnaud L, von Deimling A, Helias-Rodzewicz Z, Hervier B, et al. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood. 2012;120:2700–2703. doi: 10.1182/blood-2012-05-430140. [DOI] [PubMed] [Google Scholar]
- 25.Satoh T, Smith A, Sarde A, Lu HC, Mian S, Trouillet C, et al. B-RAF mutant alleles associated with Langerhans cell histiocytosis, a granulomatous pediatric disease. PLoS One. 2012;7:e33891. doi: 10.1371/journal.pone.0033891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salotti JA, Nanduri V, Pearce MS, Parker L, Lynn R, Windebank KP. Incidence and clinical features of Langerhans cell histiocytosis in the UK and Ireland. Arch Dis Child. 2009;94:376–380. doi: 10.1136/adc.2008.144527. [DOI] [PubMed] [Google Scholar]
- 27.Minkov M. Multisystem Langerhans cell histiocytosis in children: current treatment and future directions. Paediatr Drugs. 2011;13:75–86. doi: 10.2165/11538540-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Allen CE, McClain KL. Langerhans cell histiocytosis: a review of past, current and future therapies. Drugs Today (Barc) 2007;43:627–643. doi: 10.1358/dot.2007.43.9.1088823. [DOI] [PubMed] [Google Scholar]
- 29.Morimoto A, Ikushima S, Kinugawa N, Ishii E, Kohdera U, Sako M, et al. Improved outcome in the treatment of pediatric multifocal Langerhans cell histiocytosis: Results from the Japan Langerhans Cell Histiocytosis Study Group-96 protocol study. Cancer. 2006;107:613–619. doi: 10.1002/cncr.21985. [DOI] [PubMed] [Google Scholar]
- 30.Ceci A, de Terlizzi M, Colella R, Loiacono G, Balducci D, Surico G, et al. Langerhans cell histiocytosis in childhood: results from the Italian Cooperative AIEOP-CNR-H.X '83 study. Med Pediatr Oncol. 1993;21:259–264. doi: 10.1002/mpo.2950210405. [DOI] [PubMed] [Google Scholar]
- 31.Haupt R, Nanduri V, Calevo MG, Bernstrand C, Braier JL, Broadbent V, et al. Permanent consequences in Langerhans cell histiocytosis patients: a pilot study from the Histiocyte Society-Late Effects Study Group. Pediatr Blood Cancer. 2004;42:438–444. doi: 10.1002/pbc.20021. [DOI] [PubMed] [Google Scholar]
- 32.Egeler RM, de Kraker J, Voute PA. Cytosine-arabinoside, vincristine, and prednisolone in the treatment of children with disseminated Langerhans cell histiocytosis with organ dysfunction: experience at a single institution. Med Pediatr Oncol. 1993;21:265–270. doi: 10.1002/mpo.2950210406. [DOI] [PubMed] [Google Scholar]
- 33.Weitzman S, Braier J, Donadieu J, Egeler RM, Grois N, Ladisch S, et al. 2'-Chlorodeoxyadenosine (2-CdA) as salvage therapy for Langerhans cell histiocytosis (LCH). results of the LCH-S-98 protocol of the Histiocyte Society. Pediatr Blood Cancer. 2009;53:1271–1276. doi: 10.1002/pbc.22229. [DOI] [PubMed] [Google Scholar]
- 34.Bernard F, Thomas C, Bertrand Y, Munzer M, Landman Parker J, Ouache M, et al. Multi-centre pilot study of 2-chlorodeoxyadenosine and cytosine arabinoside combined chemotherapy in refractory Langerhans cell histiocytosis with haematological dysfunction. Eur J Cancer. 2005;41:2682–2689. doi: 10.1016/j.ejca.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 35.Park L, Schiltz C, Korman N. Langerhans cell histiocytosis. J Cutan Med Surg. 2012;16:45–49. doi: 10.1177/120347541201600109. [DOI] [PubMed] [Google Scholar]
- 36.Neumann C, Kolde G, Bonsmann G. Histiocytosis X in an elderly patient. Ultrastructure and immunocytochemistry after PUVA photochemotherapy. Br J Dermatol. 1988;119:385–391. doi: 10.1111/j.1365-2133.1988.tb03233.x. [DOI] [PubMed] [Google Scholar]
- 37.Sakai H, Ibe M, Takahashi H, Matsuo S, Okamoto K, Makino I, et al. Satisfactory remission achieved by PUVA therapy in Langerhans cell hisiocytosis in an elderly patient. J Dermatol. 1996;23:42–46. doi: 10.1111/j.1346-8138.1996.tb03966.x. [DOI] [PubMed] [Google Scholar]
- 38.Kwon OS, Cho KH, Song KY. Primary cutaneous Langerhans cell histiocytosis treated with photochemotherapy. J Dermatol. 1997;24:54–56. doi: 10.1111/j.1346-8138.1997.tb02740.x. [DOI] [PubMed] [Google Scholar]
- 39.Hoeger PH, Nanduri VR, Harper JI, Atherton DA, Pritchard J. Long term follow up of topical mustine treatment for cutaneous langerhans cell histiocytosis. Arch Dis Child. 2000;82:483–487. doi: 10.1136/adc.82.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindahl LM, Fenger-Gron M, Iversen L. Topical nitrogen mustard therapy in patients with Langerhans cell histiocytosis. Br J Dermatol. 2012;166:642–645. doi: 10.1111/j.1365-2133.2011.10673.x. [DOI] [PubMed] [Google Scholar]
- 41.Failla V, Wauters O, Caucanas M, Nikkels-Tassoudji N, Nikkels AF. Photodynamic therapy for multi-resistant cutaneous Langerhans cell histiocytosis. Rare Tumors. 2010;2:e34. doi: 10.4081/rt.2010.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steen AE, Steen KH, Bauer R, Bieber T. Successful treatment of cutaneous Langerhans cell histiocytosis with low-dose methotrexate. Br J Dermatol. 2001;145:137–140. doi: 10.1046/j.1365-2133.2001.04298.x. [DOI] [PubMed] [Google Scholar]
- 43.McClain KL. Drug therapy for the treatment of Langerhans cell histiocytosis. Expert Opin Pharmacother. 2005;6:2435–2441. doi: 10.1517/14656566.6.14.2435. [DOI] [PubMed] [Google Scholar]
- 44.Do JE, Lee JY, Kim YC. Successful treatment of cutaneous Langerhans' cell histiocytosis with targeted narrowband ultraviolet B phototherapy in an infant. Clin Exp Dermatol. 2009;34:e280–e281. doi: 10.1111/j.1365-2230.2008.03198.x. [DOI] [PubMed] [Google Scholar]
- 45.Chang SE, Koh GJ, Choi JH, Lee KH, Sung KJ, Moon KC, et al. Widespread skin-limited adult Langerhans cell histiocytosis: long-term follow-up with good response to interferon alpha. Clin Exp Dermatol. 2002;27:135–137. doi: 10.1046/j.1365-2230.2002.00989.x. [DOI] [PubMed] [Google Scholar]
- 46.McClain KL, Kozinetz CA. A phase II trial using thalidomide for Langerhans cell histiocytosis. Pediatr Blood Cancer. 2007;48:44–49. doi: 10.1002/pbc.20578. [DOI] [PubMed] [Google Scholar]
- 47.Simko SJ, Tran HD, Jones J, Bilgi M, Beaupin LK, Coulter D, et al. Clofarabine salvage therapy in refractory multifocal histiocytic disorders, including Langerhans cell histiocytosis, juvenile xanthogranuloma and Rosai-Dorfman disease. Pediatr Blood Cancer. 2014;61:479–487. doi: 10.1002/pbc.24772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roy A, Cowan G, Mead AJ, Filippi S, Bohn G, Chaidos A, et al. Perturbation of fetal liver hematopoietic stem and progenitor cell development by trisomy 21. Proc Natl Acad Sci U S A. 2012;109:17579–17584. doi: 10.1073/pnas.1211405109. [DOI] [PMC free article] [PubMed] [Google Scholar]