Abstract
Background
Physical urticaria is a subtype of chronic urticaria induced by a physical stimulus.
Objective
To evaluate the consistency between a history of physical urticaria and results of challenge testing.
Methods
Seventy-six subjects, ages 3–77, were referred with the diagnoses of a physical urticaria and were evaluated using challenge testing directed toward the presenting diagnosis, yet included other stimuli based on history. The majority of subjects were tested to 3 or more stimuli, thus 294 provocation tests were performed. Fifty-seven subjects were surveyed for the status of their physical urticaria at least one year after initial evaluation.
Results
Of the 76 subjects with a positive history of a physical urticaria, 38 %(N=29) were challenge negative to the presenting diagnosis. Eight patients within the challenge negative group reacted positively to additional testing, thus 28 % (N=21) remained negative to all challenge testing, allowing discontinuation of medications and avoidance behavior. A negative challenge result was less likely in subjects presenting with cold induced urticaria (25 %), delayed pressure urticaria (25 %) and dermatographism (29 %), yet more common in cholinergic (65 %) and solar urticaria (67 %). A one-year follow-up survey of 57 subjects was consistent with initial results. Nineteen of this sub-group were rechallenged for the presenting diagnosis and the outcome was unchanged in 17 patients and in two patients the urticaria had resolved.
Conclusions
The diagnosis by history of a physical urticaria should be verified by testing whenever possible; and particularly if the condition is judged as severe and thus requires both significant life-style changes and pharmacologic intervention.
Keywords: Urticaria, physical urticaria, challenge testing, cold, cholinergic, dermatographism, delayed pressure
Introduction
Physical urticaria is a unique form of hives that is induced by specific physical stimuli. It is estimated that 0.5 % of the population has physically induced urticaria/angioedema and this population comprises 20–30 % of all cases of chronic urticaria (CU). (1, 2) The lifetime prevalence of a physical urticaria is estimated to be 4–6 %. (1) Disease resolution is quite variable depending on the subtype of physical urticaria, age of onset, and severity but has been estimated at 13–16 % after one year, 50 % after 5 years. (3, 4) The pathogenesis of physical urticaria is associated with the release of mediators from cutaneous mast cells, but the mechanisms underlying this mast cell activation remain unclear. (5, 6) The various forms of physical urticaria, their relevant stimuli and prevalence include: dermatographism (overall prevalence 2–5 %, 10 % of CU), cold urticaria (2 % of CU), delayed pressure urticaria/angioedema (1–2 % of CU), cholinergic urticaria (11 % of young adults, 2–5% of CU), exercise-induced urticaria, local heat urticaria (0.2 % of CU), vibratory urticaria (0.1 % of CU), solar urticaria (0.4–0.5 % of CU) and aquagenic urticaria (0.3 % of CU).(1, 3, 7–12) The diagnosis is based on a history of episodic physically induced urticaria ideally confirmed by the reproduction of this response following office-based provocation testing(13, 14). However, challenge testing requires proper equipment, training, and clinical support. Because of these requirements, caregivers may rely on the patient history for diagnosis and selection of intervention strategies. We thus ask the question as to how often the history would not be reproduced if challenge testing was performed.
In our study, we thus performed at total of 294 challenge tests prospectively on a cohort of 76 subjects that were diagnosed by a referring physician with a physical urticaria. Most patients were on medications and had altered their life style to avoid reactions. Challenge testing was directed by the presenting diagnosis and the clinical history. As will be shown, our study reveals that a significant portion of our subjects referred with the diagnosis of a specific physical urticaria were negative to challenge and these findings remained consistent upon follow-up at least one year later.
Methods
Subjects
A cohort of 76 subjects, ages 3–77 years, diagnosed with a physically induced urticaria by an internist, pediatrician, allergist or dermatologist, were referred to the NIH from 2009–2014 under protocol 09-I-0126 (Pathogenesis of Physical Induced Urticarial Syndromes) for further evaluation. At least one week prior to evaluation, all subjects refrained from taking anti-histamines and anti-leukotrienes or any agent that could affect the outcome of the challenge testing. Following informed consent, all subjects underwent a thorough history, which included a 36 question clinical survey of physical urticaria administered by clinical staff (supplemental material), and a physical examination. Subjects were acclimated to the ambient challenge room temperature for at least 2 hours before testing
Challenge testing
Challenge testing was directed toward the presenting diagnosis (e.g., cold urticaria), yet included other stimuli based on the history and survey results (Table I). For example, one subject was referred for the evaluation of cold urticaria while skiing. Upon questioning the subject reported the development of hives on non-cold exposed areas and after jogging in the cold. This subject was thus tested for cold induced urticaria and cholinergic/exercise induced urticaria. All subjects were tested for dermatographism and the majority of subjects were challenged to 3 or more other physical stimuli (median 4.0, IQR 1.0). Standard challenge testing was performed as described (Table I) (2, 9, 13) and included the following: Dermatographism: stroking of skin at various pressures (20–144 g/m2) using a dermographometer; (9) Cold induced urticaria: stimulation using a 50 ml glass beaker of ice water (0°–2°C) placed on the forearm for 1–10 minutes, cold hand water submersion (10° C) to 2 inches above the wrist for 5 minutes and in some cases total body cold exposure (4° C) for 10–20 minutes, evaporative cooling of water droplet with airflow at ~1 l/s ; Cholinergic urticaria/exercise induced: 15–25 minute treadmill exercise challenge until there was profuse sweating and continued exercise for >10 minutes, and/or 20–30 minutes hot water bath (40° C) until a (>1° C) rise in core body temperature was documented; Delayed pressure urticaria/angioedema: using a dermographometer set at 100 g/m2 imparting pressure for 5–180 seconds on the forearm and ~20 lbs. weight bearing on shoulder for 15–20 minutes; Solar Urticaria: direct exposure (1 × 3 cm area)using UVA, UVB and visible light for a range of time/joules depending on the Fitzpatrick phototyping scale to determine the minimual urticarial dose; Vibratory Urticaria/angioedema: vortex vibratory stimulation for 4 minutes at 2400 rpm. Local heat: 1–10 minute exposure to beaker of hot water (45–50°C); and Aquagenic urticaria: submersion of upper body limbs in water at room temperature and application of wet compresses for 20–30 minutes. Contact challenge stimuli was targeted in general to the volar surface of the arms and observed for the development of a wheal, flare and pruritus at 5, 10, 15 minutes post challenge and again at 30 minutes. In cases of cholinergic and aquagenic urticaria, the appearance of small macular or pinpoint lesions, were noted. For delayed and late phase reactions (pressure, vibration, solar), subjects were additionally observed at 4, 6, 8 and 24 hours post challenge. Figure 1 displays photographic examples of urticaria which developed 15–20 minutes (or 6 hours for delayed pressure) following challenge testing. All subjects (N=33) presenting with a history of cold urticaria underwent cold challenge testing to ice, cold hand immersion (if tolerated) and evaporative cooling and additional challenges based upon further questioning included 4 to generalized cold room, 8 to vibration, and 4 exercise. All subjects (N=20) with a history of cholinergic urticaria underwent exercise challenge plus other challenges (8-hot bath, 18-ice, 10-evaporative cooling, 11-vibration). For delayed pressure, all 8 subjects performed dermographometer pressure, weight bearing and vibration testing plus other testing (2-ice, 2-exercise, 1-solar). Other subjects were tested as follows: dermatographism, N=7 (7-dermographometer, 7-vibration, 4-ice, 1-pressure); solar, N=3 (3-solar, 1-vibration, 1-local heat); Vibratory, N=2 (2-vibration, 2-ice); aquagenic, N=1(1-compresses/immersion, bath); exercise induced, N=1 (exercise, vibration).
Table I.
Features and Testing of Physical Urticaria
| Distinguishing Features | Diagnostic testing* | |
|---|---|---|
| Dermatographism | linear, pruritic hives from shear force, most common physical urticaria | linear stroking at various pressures (20–144 g/m2) using dermatographometer, FricTest or ball point pen |
| Cold | pruritic wheal and flare from cold contact, up to 1/3 of cases of physical urticaria | ice water in 50 ml beaker placement for 1–10 min, cold hand immersion for 5 min, total body cold exposure, evaporative cooling |
| Cholinergic | pin-point diffuse papular lesions from increase in core body temperature | exercise challenge to induce sweat plus > 10 min, or passive warming using hot water bath to raise body temp > 1°C |
| Exercise induced | not induced by passive warming, larger lesions often associated with systemic symptoms | exercise challenge as above |
| Delayed Pressure | pruritus, swelling and pain 4–8 hrs. after exposure, may be associated with systemic symptoms of fatigue, arthralgia | 100 g/m2 of pressure for 5–180 sec duration on the forearm using a dermatographometer and 15 lbs. weight bearing on shoulder or lower leg for 15–20 min |
| Solar | immediate reaction to UV and visible light, resolves within 24 hrs., distinguish from polymorphous light eruption | UVA, UVB and visible light stimulation of variable intensity to establish minimal urticarial dose |
| Vibratory | erythema and swelling beyond provocation site | vortex vibratory stimulation for 4 min at 2500 rpm |
| Local Heat | rare, reaction limited to area of exposure | hot water (45–50°C) in glass beaker placement 1–10 min to establish threshold |
| Aquagenic | rare, distinguish from evaporative cooling and cold urticaria | submersion of hand/forearm at ~35° and application of wet compress for 20–30 min |
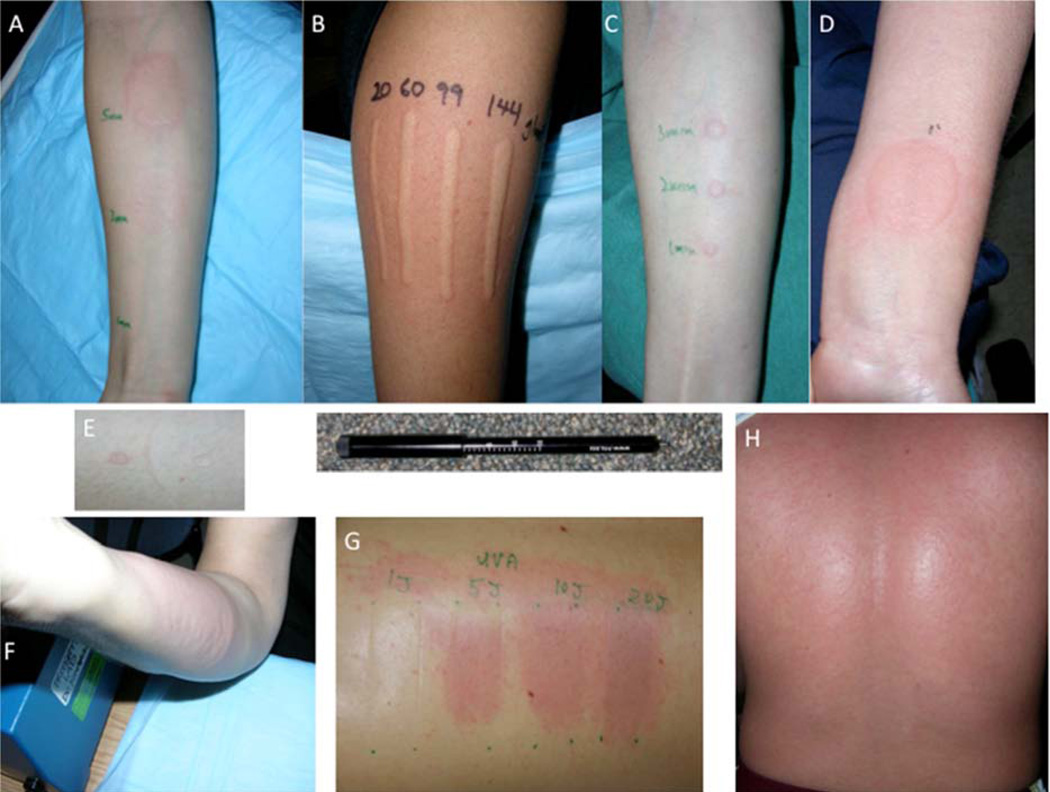
Figure 1. Physical Urticaria Images.
A) Cold-induced; 1, 3, and 5 min cold-contact. B) Dermatographism; linear scratch using dermographometer (below image) at 20, 60, 99 and 144 g/m2. C) Delayed-pressure; (at 6 hr.) using 100 g/m2 pressure for 1, 2, and 3 min. D) Local heat; 55° C contact for 5 sec. E) Evaporative cooling; on left, with right side airflow blocked. F) Vibratory; vortex stimulation for 4 min G) Solar; from UVA at 1, 5, 10, and 20 Joules. H) Cholinergic; following exercise.
Statistical Analysis
Calculation of median and interquartile range for tables and figures was performed using Excel and Prism.
Results
Overall demographics indicate the median (IQR) for the cohort was 32.0 (29.25) years, with a female predominance (67 %) and a median (IQR) duration of symptoms of 4 years 6.25). Approximately half overall were atopic, (49 %) consistent with a mildly elevated median (IQR) IgE of 53.6 IU/mL (147.8). Serum tryptase levels and inflammatory markers (CRP, ESR) were within normal range. The median (IQR) number of challenge test was 4.0(1.0) based on a total of 294 challenge tests performed. Table II also includes overall medication use and severity scaling (0–15) based on frequency and severity of symptoms. (15)
Table II.
Demographics and Results
| Overall | Challenge Positive History Positive |
Challenge Negative History Positive |
Other Positive |
All Positive | All Negative | |
|---|---|---|---|---|---|---|
| 76 | 47 (62%) | 29 (38%) | 8 (11%) | 55(72%) | 21 (28%) | |
| Age (median, IQR) (3–77) | 32.0(29.25) | 32.0(28.5) | 32.0 (29.5) | 17.0(34.5) | 32.0(30.0) | 32.0 (25.0) |
| Gender Female(%) | 51 (67%) | 30 (65%) | 21 (73%) | 6 (75%) | 36 (65%) | 15 (71%) |
| Duration of symptoms median yrs. (IQR) | 4.0(6.25) | 3.0(5.5) | 5.0(8.5) | 7.5(7.8) | 4.0(6.0) | 4.0(7.0) |
| Atopic (%) phadiatop, SPT | 36 (49%) | 24 (52%) | 12(43%) | 4 (50%) | 28(52%) | 8(40%) |
| IgE IU/mL (median, IQR) | 53.6(147.8) | 54.5 (148.7) | 41.3(117.9) | 40.8(232.8) | 53.6(150.9) | 45.3(130) |
| Tryptase ng/mL (median, IQR) | 4.0(2.2) | 3.8(2.2) | 4.2(2.5) | 4.2(1.6) | 3.8(2.2) | 4.7(2.5) |
| CRP mg/L (median, IQR) | 0.9(2.3) | 0.9(2.6) | 0.8(1.9) | 0.7(0.8) | 0.8(2.0) | 1.1(2.8) |
| ESR mm/hr (median, IQR) | 6.5(8.8) | 6.5(7.0) | 6.0(9.0) | 7.0(5.5) | 7.0(6.0) | 5.0(9.0) |
| Number of medications (median, IQR) | 3.0 (4.3) | 3.0 (4.5) | 3.0(4.8) | 2.0 (4.8) | 3.0(4.5) | 3 (5.0) |
| Anti histamines (median, IQR) | 1.0 (1.0) | 2.0 (1.0) | 1.0 (0.0) | 1.0 (0.3) | 1.0 (1.0) | 1.0 (1.0) |
| Epipen Rx (median, IQR) | 0.0 (1.0) | 0.0 (1.0) | 0.0 (0.0) | 0.0 (0.3) | 0.0 (1.0) | 0.0 (0.0) |
| Psychotropic meds (median, IQR) | 0.0 (1.0) | 0.0 (0.0) | 1.0 (2.0) | 0.0 (0.3) | 0.0 (0.0) | 1.0 (2.0) |
| Subjective Severity (mild/mod/sev)n=74 | 19/29/26 | 9/21/16 | 10/8/10 | 4/4/0 | 13/25/16 | 6/4/10 |
| Objective severity Scale 0–15(median, IQR) | 9.0(3.0) | 9.0 (2.5) | 8.0 (3.3) | 7.5 (3.3) | 9.0(2.0) | 9.0(3.5) |
| Number of Challenge tests (median, IQR) | 4.0 (1.0) | 4.0 (1.0) | 4.0 (2.0) | 4.5 (1.0) | 4.0(1.0) | 3.0(2.0) |
(IQR, interquartile range; SPT, skin prick testing)
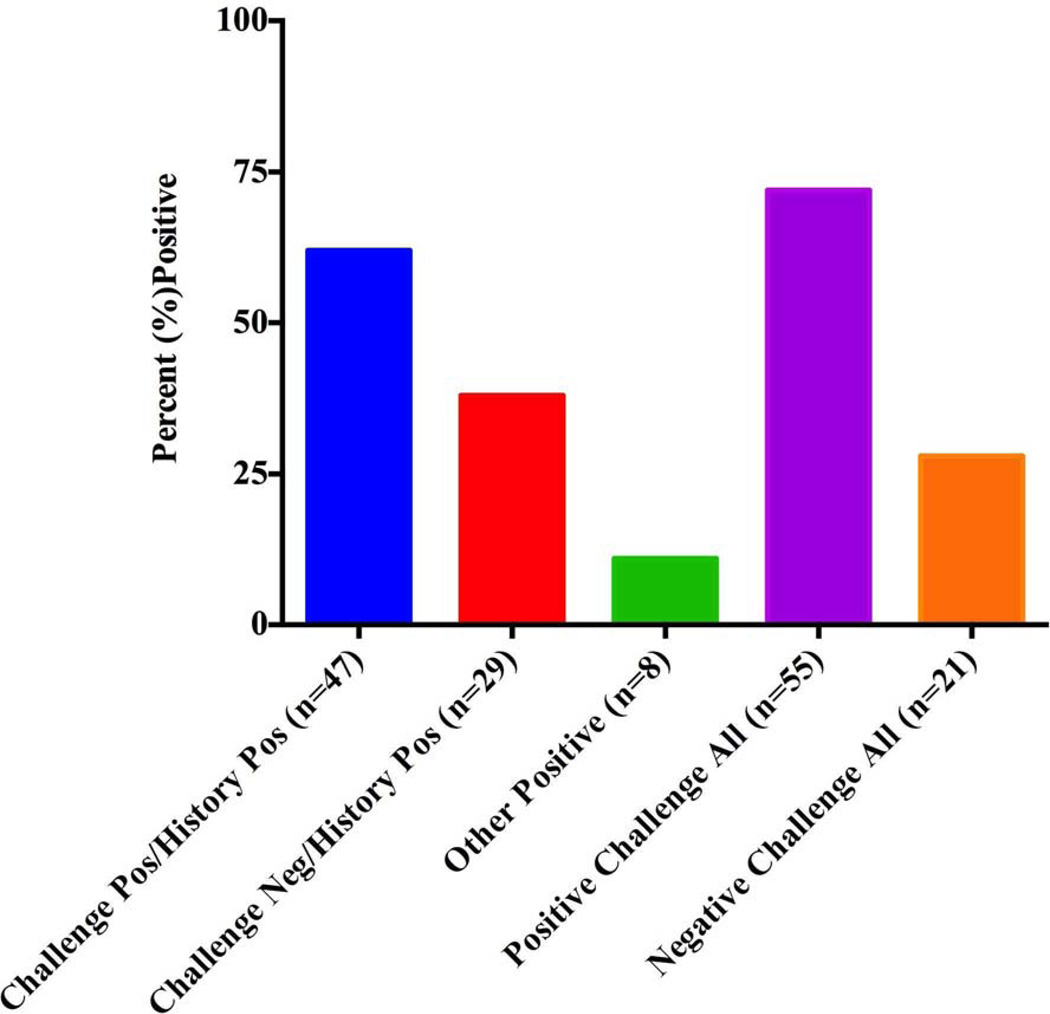
In response to challenge testing that was directed toward the presenting diagnosis, 62 % of 76 (n=47) patients developed urticaria (Challenge Positive/History Positive) and 38 % n=29) were challenge negative (Challenge Negative/History Positive), (see Table II). Based on further questioning and provocation, an additional eight patients) within the challenge negative group were diagnosed with another physical urticaria (Other Positive) so a final diagnosis of a physical urticaria was reached in 72 %(n=55) of subjects (All Positive) yet 28 % (n=21) remained negative to all challenge testing (All Negative) Figure 2).
Figure 2. Summary of Challenge Testing Results.
Of 76 subjects with a positive history of a physical urticaria, 47(62 %) were challenge positive and 29(38 %) were challenge negative to the presenting diagnosis. Eight within the challenge negative group were positive to another stimulus (Other Positive). Thus a physical urticaria was documented in 72 % (n=55) of subjects (All Positive) and 28 % (n=21) remained negative to all challenge testing (All Negative).
When comparing the groups as shown in Table II, the All (challenge) Positive group tended to be more atopic (52 % vs. 40 %), have a higher IgE level (median, 53.6 vs. 45.3 IU/mL), and exhibit a lower elevated CRP (median, 0.8 vs. 1.1 mg/but less psychotropic medication use (for mood and sleep disorders and ADHD; median 0.0 vs. 1.0) than in the All Negative group.
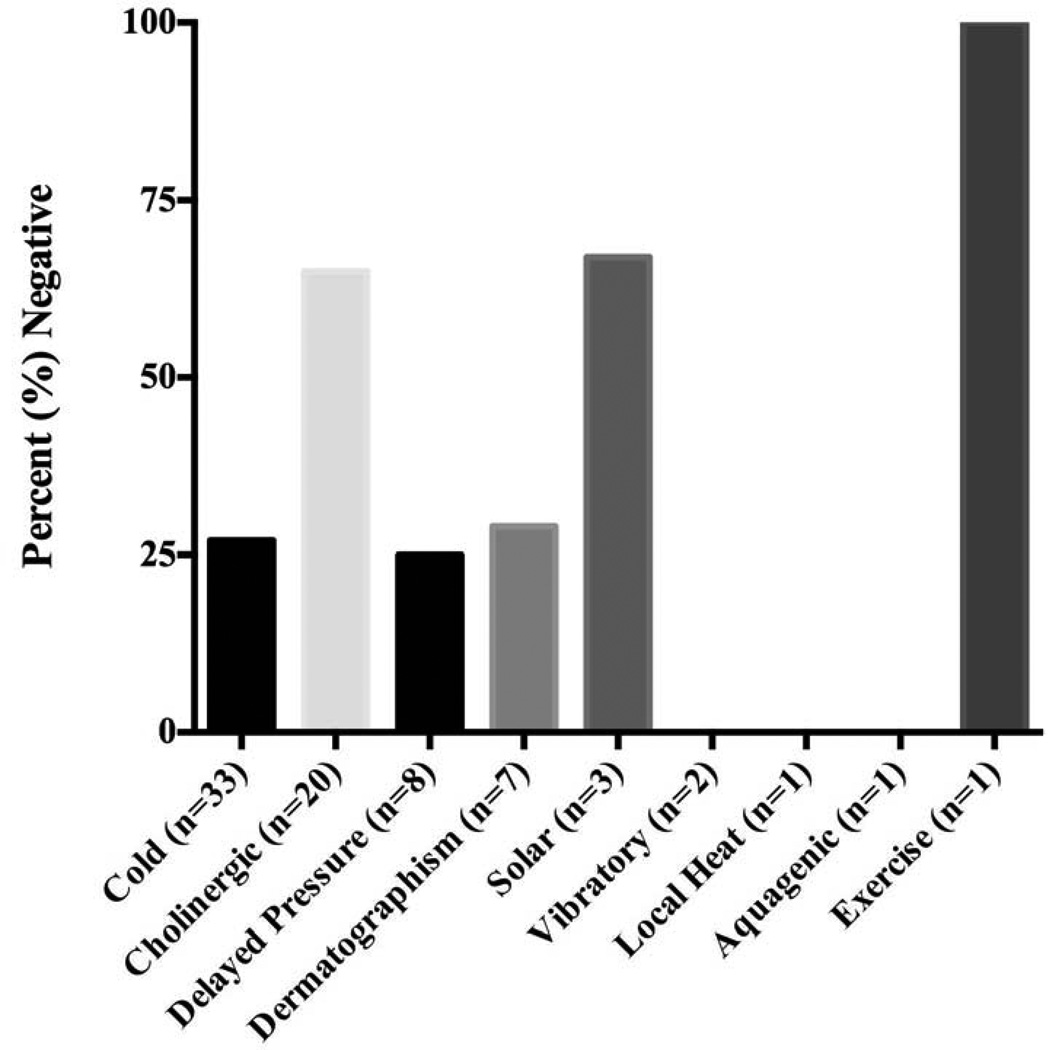
We also quantified the proportion of negative challenge results according to the various types of physical urticaria (Figure 3). The percentage of patients that had a negative response to challenge testing of their presenting diagnosis was relatively low for both cold and delayed pressure urticaria (25 %), yet high in cholinergic (65 %) and solar urticaria (67 %).
Figure 3. Percent Negative Challenge Testing by Subtype.
The proportion of negative challenge results based on the various types of physical urticaria indicates the percent negative was low (25 %) in cold-induced, and delayed pressure urticaria (25 %); yet high in cholinergic (65 %) and solar urticaria (67 %).
To further validate our provocation testing results and assess disease status, 57 of 76 patients were re-evaluated at least one year after their initial assessment (Table III). Our results indicate that all 19 patients that did not develop urticaria following challenge testing to their presenting diagnosis, maintained this negative diagnosis at least one year later. Amongst the 38 that were initially positive to challenge, there was resolution of disease in 4 while the remaining 34 subjects had unchanged manifestations. Based on subtype, there was resolution of disease in one patient in four urticarial subtypes: cold, cholinergic, delayed pressure and dermatographism. Other physical urticaria testing that was performed outside of presenting diagnosis remained unchanged (either positive or negative) at one-year follow-up. Thus follow-up results demonstrate an overall consistent pattern of disease at least one year after the initial evaluation, with disease resolution in 11% of patients.
Table III.
Follow-up Results
| Presenting Complaint |
N | Initial Visit Confirmed |
Follow-up at > 1year |
||||
|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Pos-->Neg | Neg-->Pos | ||
| Cold | 26 | 22 | 4 | 21 | 5 | 1 | 0 |
| Cholinergic | 13 | 4 | 9 | 3 | 10 | 1 | 0 |
| Delayed Pressure | 5 | 4 | 1 | 3 | 2 | 1 | 0 |
| Dermatographism | 6 | 4 | 2 | 3 | 3 | 1 | 0 |
| Vibratory | 2 | 2 | 0 | 2 | 0 | 0 | 0 |
| Delayed Cold | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
| Solar | 2 | 0 | 2 | 0 | 2 | 0 | 0 |
| Local Heat | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
| Exercise | 1 | 0 | 1 | 0 | 1 | 0 | 0 |
| Total | 57 | 38 | 19 | 34 | 23 | 4 | 0 |
Discussion
Physically induced urticaria is unique in that office-based challenge testing may be employed to determine the presence and extent of the reaction. This outcome not only substantiates the diagnosis, but also serves as a guide to both the patient and physician as to appropriate avoidance and treatment.
In our study, we performed a prospective survey of 76 subjects referred for a physical urticaria and determined that 38 % of the subjects were negative to the presenting diagnosis and 28 % were determined to be negative to all challenge testing performed. These findings have enabled individuals in the negative challenge group to decrease medication use and in some cases, with proper monitoring, to stop medicine and resume normal activities. In one example, a 36-year female presented with a 10 year history of severe and debilitating cholinergic urticaria. Her treatment regimen included over 20 medicines (antihistamines, leukotriene inhibitors, steroids, immunosuppressive agents, etc.) Following negative challenge testing for cholinergic urticaria, she resumed medicine-free normal life. The majority of our cohort (57 of 76) were reevaluated at least one year after the initial assessment and were found to have consistent manifestations, noting that of the 19 patients that were negative to provocation, all remained negative. These results support the value and reproducibility of challenge testing in patients with a history of a physical urticaria. Of the 38 patients that were initially positive to challenge, 4 patients (11 %) demonstrated resolution of symptoms. Our data is consistent with another report (n=73) that showed a resolution rate of 16 % at one-year follow-up.(4) While the majority of patients were diagnosed accurately and the condition may have resolved before challenge testing as reported in this paper, in a number of patients we did not verify the diagnosis at time of challenge, supporting the use of challenge testing to provide an objective diagnosis or to re-evaluate a previous diagnosis. Standard challenge testing also establishes individual thresholds for positivity, which can be used to determine disease severity and objectively evaluate response to therapy. One option for physicians who do not perform testing and where the diagnosis of a physical urticaria is associated with behavior intervention and pharmacologic therapy is to consider referral to a specialty clinic or tertiary care center for challenge testing.
Supplementary Material
Highlight Box.
Physical urticaria is a subtype of chronic urticaria that is induced by a physical stimulus.
In a study of patients referred for evaluation of physical urticaria, more than one-third of the subjects were consistently negative to the presumed physical stimulus reported by history to provoke urticaria.
Physicians who do not perform testing may wish to consider referral to a specialty clinic or tertiary care center for evaluation.
Acknowledgments
1This work was supported by the Division of Intramural Research, NIAID, NIH. 2Support by M.Y. for this project was funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported [in part] by the National Institute of Allergy and Infectious Diseases.
Abbreviations
- IQR
interquartile range
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors state no conflict of interest.
References
- 1.Dice JP. Physical urticaria. Immunology and allergy clinics of North America. 2004;24(2):225–246. doi: 10.1016/j.iac.2004.01.005. vi. [DOI] [PubMed] [Google Scholar]
- 2.Lang DM, Hsieh FH, Bernstein JA. Contemporary approaches to the diagnosis and management of physical urticaria. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2013;111(4):235–241. doi: 10.1016/j.anai.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 3.Silpa-archa N, Kulthanan K, Pinkaew S. Physical urticaria: prevalence, type and natural course in a tropical country. Journal of the European Academy of Dermatology and Venereology : JEADV. 2011;25(10):1194–1199. doi: 10.1111/j.1468-3083.2010.03951.x. [DOI] [PubMed] [Google Scholar]
- 4.Kozel MM, Mekkes JR, Bossuyt PM, Bos JD. Natural course of physical and chronic urticaria and angioedema in 220 patients. Journal of the American Academy of Dermatology. 2001;45(3):387–391. doi: 10.1067/mjd.2001.116217. [DOI] [PubMed] [Google Scholar]
- 5.Gorevic PD, Kaplan AP. The physical urticarias. Int J Dermatol. 1980;19(8):417–435. doi: 10.1111/j.1365-4362.1980.tb05893.x. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan AP. Urticaria: the relationship of duration of lesion to pathogenesis. Allergy Proc. 1990;11(1):15–18. doi: 10.2500/108854190778999546. [DOI] [PubMed] [Google Scholar]
- 7.Champion RH. Urticaria: then and now. The British journal of dermatology. 1988;119(4):427–436. doi: 10.1111/j.1365-2133.1988.tb03246.x. [DOI] [PubMed] [Google Scholar]
- 8.Humphreys F, Hunter JA. The characteristics of urticaria in 390 patients. The British journal of dermatology. 1998;138(4):635–638. doi: 10.1046/j.1365-2133.1998.02175.x. [DOI] [PubMed] [Google Scholar]
- 9.Abajian M, Schoepke N, Altrichter S, Zuberbier HC, Maurer M. Physical urticarias and cholinergic urticaria. Immunology and allergy clinics of North America. 2014;34(1):73–88. doi: 10.1016/j.iac.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Zuberbier T, Althaus C, Chantraine-Hess S, Czarnetzki BM. Prevalence of cholinergic urticaria in young adults. Journal of the American Academy of Dermatology. 1994;31(6):978–981. doi: 10.1016/s0190-9622(94)70267-5. [DOI] [PubMed] [Google Scholar]
- 11.Orfan NA, Kolski GB. Physical urticarias. Annals of allergy. 1993;71(3):205–212. quiz 12–5. [PubMed] [Google Scholar]
- 12.Kirby JD, Matthews CN, James J, Duncan EH, Warin RP. The incidence and other aspects of factitious wealing (dermographism) The British journal of dermatology. 1971;85(4):331–335. doi: 10.1111/j.1365-2133.1971.tb14027.x. [DOI] [PubMed] [Google Scholar]
- 13.Abajian M, Mlynek A, Maurer M. Physical urticaria. Curr Allergy Asthma Rep. 2012;12(4):281–287. doi: 10.1007/s11882-012-0269-0. [DOI] [PubMed] [Google Scholar]
- 14.Magerl M, Borzova E, Gimenez-Arnau A, Grattan CE, Lawlor F, Mathelier-Fusade P, et al. The definition and diagnostic testing of physical and cholinergic urticarias--EAACI/GA2LEN/EDF/UNEV consensus panel recommendations. Allergy. 2009;64(12):1715–1721. doi: 10.1111/j.1398-9995.2009.02177.x. [DOI] [PubMed] [Google Scholar]
- 15.Meyer J, Gorbach AM, Liu WM, Medic N, Young M, Nelson C, et al. Mast cell dependent vascular changes associated with an acute response to cold immersion in primary contact urticaria. PloS one. 2013;8(2):e56773. doi: 10.1371/journal.pone.0056773. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.