Abstract
Background
Weight loss and malnutrition are poorly tolerated by geriatric patients, and pancreaticoduodenectomy (PD) can result in chronic malabsorption and weight loss. We sought to determine how preoperative severe nutritional risk (SNR), as defined by the American College of Surgeons National Surgical Quality Improvement Program / American Geriatric Society Best Practice Guidelines, impacts long-term survival following PD for benign disease among geriatric and non-geriatric patients.
Methods
All patients undergoing PD for non-malignant conditions at a single center between 1995 and 2013 were followed for survival, excluding patients who died within 90 days of surgery. Survival of geriatric (age ≥65 years) and non-geriatric (age < 65 years) patients with and without SNR was compared using Kaplan Meier methods. Cox-regression was performed.
Results
320 patients underwent PD for benign disease. Over the course of the study, the proportion of geriatric patients undergoing PD for benign conditions increased from 25% to 46%. In addition to being older, geriatric patients undergoing PD for benign disease were significantly more likely to have coronary artery disease (CAD) and hypertension. Geriatric patients with preoperative SNR had significantly decreased long-term survival following PD for benign disease (p<0.001), with roughly 1 in 3 patients dead at 5 years compared to 1 in 14 patients without SNR. Survival was not significantly different among non-geriatric patients with and without SNR. In geriatric patients, age, CAD, and SNR were significantly associated with decreased survival on both univariate and multivariate analysis.
Conclusion
SNR can be a useful predictor of long-term survival in geriatric patients undergoing PD, and could improve patient risk stratification preoperatively. Non-operative management should be strongly considered in geriatric patients with SNR, when malignancy is not suspected.
Introduction
Pancreaticoduodenectomy (PD) is usually performed for underlying periampullary malignancy; however, PD is increasingly being performed for nonmalignant conditions. In modern series, roughly 25-30% of PDs are performed for benign disease, such as pancreatitis, intraductal papillary mucinous neoplasia (IPMN), and adenoma.1 While many studies have examined the long-term survival of patients undergoing PD for cancer, few studies have focused on the long-term outcomes of patients with nonmalignant disease. PD frequently results in chronic malabsorption and weight loss,2 but little is known about the impact of the operation itself on survival.
The proportion of the US population age 65 and older is growing rapidly. From 2010 to 2050, this segment of the US population is expected to more than double in size.3 Coupled with this increase, there has been a significant rise in the detection of benign, potentially malignant, pancreatic conditions, such as intraductal papillary mucinous neoplasms (IPMN), which has led to an increase in the number of elderly patients who undergo PD.4 Unfortunately, little is known about the long-term outcome of elderly patients following PD for benign conditions, as most studies have focused exclusively on perioperative outcomes in patients with both benign and malignant disease.5
Nutritional status in elderly patients is a critical, often underappreciated, element that strongly influences quality of life, functional status, and survival.6-10 Weight loss and malnutrition are poorly tolerated by geriatric patients as they have little nutritional reserve, and are unable to compensate for decreased caloric intake or absorption in the way that younger adults can.6, 9, 11 The American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) / American Geriatric Society (AGS) Best Practices Guidelines: Optimal Preoperative Assessment of the Geriatric Surgical Patient is a resource designed to aid surgeons in the preoperative evaluation of geriatric patients.12 These guidelines recommend that surgeons screen patients pre-operatively for severe nutritional risk (SNR), which incorporates serum albumin, body mass index (BMI), and weight loss, as such patients are at increased risk of perioperative morbidity and mortality.13, 14 While impaired nutritional status has been demonstrated to correlate with poor perioperative outcomes, it is possible that preoperative nutritional risk adversely affects long-term outcomes as well, especially after PD where patients can suffer from chronic malabsorption and weight loss.2 As the perioperative mortality for PD has decreased substantially over the last 30 years,15 enhancing our understanding of the long-term effect of PD on survival becomes imperative, especially in elderly patients with benign conditions, where the underlying disease process is unlikely to be their cause of death.16
In this study, we examine how the proportion of geriatric patients undergoing PD for benign disease has risen from 1995 to 2013 at our institution. Additionally, we explore the impact of preoperative SNR on long-term survival after PD for benign disease. We hypothesize that elderly patients with preoperative SNR have decreased long-term survival following PD for benign conditions.
Methods
Study Population
All patients who underwent pancreaticoduodenectomy (PD) for benign disease at Barnes-Jewish Hospital from February 1995 until December of 2013 were identified from an institutional database, and included in the study. Since 2007, the database has been maintained prospectively. Prior to 2007, the database was created from medical records. Our institutional review board approves this database and resulting studies. To clarify, all patients with invasive cancer of any kind on final pathology, including all neuroendocrine tumors, IPMN with invasive carcinoma, adenomas with invasive carcinoma, lymphomas, and gastrointestinal stromal tumors, were excluded from the study. In addition, we excluded patients who died within 90 days of surgery and those with missing survival data from the final analyses.
Variables
Patients were categorized as geriatric (age 65 years or older) or non-geriatric (age less than 65 years). The threshold of age 65 years was based on that used in the ACS NSQIP / AGS Best Practice Guidelines.12 All patients were screened for weight loss at their initial office visit and the amount lost as well as the time period were recorded in the medical record. Severe nutritional risk (SNR) was defined using criteria set forth by the ACS NSQIP / AGS Best Practice Guidelines.12 Specifically, patients were considered to have SNR if they had any of the following features: a BMI less than 18.5 kg/m2, an albumin less than 3.0 g/dl, or an average weight loss of 10% or more of total body weight in the preceding 6 months. In the event that one of the three variables that define SNR was missing from the database, the other two variables were considered; no patients were missing 2 or 3 of these variables simultaneously. Patient age, BMI, smoking status, serum albumin, and comorbidities were determined at the time of PD. Patients with acute or chronic pancreatitis were categorized as having “pancreatitis” due to the frequent presence of both findings on final pathology. Patients without the diagnosis of “IPMN”, “adenoma”, or “pancreatitis” were classified as “other,” which consisted of less common indications for PD such as trauma, benign strictures, and ulcers. Survival status and date of death were determined from medical records or using the Social Security Death Index.
Statistical Analysis
The primary exposure was severe nutritional risk, and the primary outcome was death. Chi-square and student's t tests were used to compare categorical and continuous data, respectively. Kaplan Meier methods were used to estimate survival among patients with and without severe nutritional risk. Univariate Cox proportional hazard models were used to identify factors associated with overall survival among geriatric patients. Multivariate Cox regression with backward selection was performed, which included all variables with a P value <0.3 on univariate analysis in the final model. All P values were two-sided, and a P value < 0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.3 (SAS Inc. Cary, NC).
RESULTS
Baseline Patient Characteristics
A total of 1526 patients underwent PD between 1995 and 2013. Of these, 320 patients (21%) underwent PD for nonmalignant conditions. The median length of follow up was 7.4 years (interquartile range: 3.6 – 11.0 years). One-hundred and forty-nine (47%) were men, and 284 (89%) were white. The mean age was 59 years (SD: +/− 13.7), and 120 (38%) were age 65 or older (geriatric). The mean BMI was 26.2 kg/m2 (SD: +/−6.4), and 70 (22%) were obese. The most common comorbidities were hypertension (49%), diabetes (19%), and coronary artery disease (16%). Patients' characteristics are summarized in Table 1.
Table 1.
Baseline Characteristics of Patients Undergoing Pancreaticoduodenectomy for Benign Disease
| No. (Column %) |
||||
|---|---|---|---|---|
| Variables | All Patients (n=320) | Non-Geriatric Patients* (n=191) | Geriatric Patients* (n=114) | P Value** |
| Age, mean years, (SD) | 59.0 (13.7) | 50.8 (9.7) | 72.7 (5.0) | <0.001 |
| Sex | ||||
| Male | 149 (46.6%) | 89 (46.6%) | 55 (48.3%) | 0.780 |
| Female | 169 (52.8%) | 102 (53.4%) | 59 (51.8%) | |
| Missing | 2 (0.6%) | NA | NA | |
| Race | ||||
| White | 284 (88.8%) | 168 (88.0%) | 104 (91.2%) | 0.736 |
| Black | 29 (9.1%) | 19 (10.0%) | 9 (7.9%) | |
| Other | 4 (1.3%) | 3 (1.6%) | 1 (0.9%) | |
| Missing | 3 (0.9%) | 1 (0.5%) | NA | |
| Comorbidities | ||||
| CAD | 52 (16.3%) | 22 (11.5%) | 27 (23.7%) | 0.005 |
| Diabetes | 61 (19.1%) | 36 (18.9%) | 23 (20.2%) | 0.777 |
| Hypertension | 158 (49.4%) | 71 (37.2%) | 79 (69.3%) | <0.001 |
| COPD | 30 (9.4%) | 12 (10.5%) | 0.529 | |
| Obesity | 70 (21.9%) | 43 (22.5%) | 24 (21.1%) | 0.766 |
| Stroke | 6 (1.9%) | 1 (0.5%) | 3 (2.6%) | 0.117 |
| Smoking Status | ||||
| Never | 114 (35.6%) | 66 (34.6%) | 41 (36.0%) | <0.001 |
| Current | 115 (35.9%) | 90 (47.1%) | 21 (18.4%) | |
| Former | 91 (28.4%) | 35 (18.3%) | 52 (45.6%) | |
| BMI, mean kg/m2 (SD) + | 26.2 (6.4) | 26.3 (6.8) | 26.1 (5.7) | 0.777 |
| Albumin, mean g/dl (SD) ++ | 4.1 (0.6) | 4.0 (0.6) | 4.2 (0.5%) | 0.078 |
| Diagnosis | ||||
| IPMN | 88 (27.5%) | 24 (12.6%) | 62 (54.4%) | <0.001 |
| Adenoma | 81 (25.3%) | 51 (26.7%) | 26 (22.8%) | |
| Pancreatitis | 91 (28.4%) | 74 (38.7%) | 12 (10.5%) | |
| Other | 60 (18.8%) | 42 (22.0%) | 14 (12.3%) | |
| Severe Nutritional Risk | 88 (27.5%) | 59 (30.9%) | 26 (22.8%) | 0.1277 |
Excludes 9 patients who died within 90 days of operation and 6 patients with missing survival data.
Compares geriatric to non-geriatric patients.
6 missing values
16 missing values
Benign Indications for Pancreaticoduodenectomy and Trends Over Time
The most common benign indications for PD were pancreatitis (n=91, 28%), IPMN (n=88, 28%), adenomas (n=81, 25%), and other diagnoses (n=60, 19%). We divided the study into 3 nearly equal time periods in order to examine how the indications for PD in the setting of benign disease evolved over time. Over the course of the study, the reasons for PD changed, with pancreatitis being the most common indication at the beginning and IPMN becoming the most common reason by the end (Figure 1A). We categorized patients as geriatric (age ≥ 65 years) or non-geriatric (age<65) in order to better understand how the indications for surgery and postoperative outcomes differed between these populations. At the beginning of the study, geriatric patients accounted for 25.6% of all patients undergoing PD for benign disease, whereas by the end of the study, this population made up 43.9% of patients (Figure 1B). Geriatric patients most commonly underwent PD for IPMN, while the most common indication in non-geriatric patients was pancreatitis (Figure 1C).
Figure 1.
Indications for pancreaticoduodenectomy by age, and changes over time. (A) Benign indications for pancreaticoduodenectomy over time. (B) Pancreaticoduodenectomy for benign disease by age over time. (C) Benign indications for pancreaticoduodenectomy by age group.
The Impact of Severe Nutritional Risk on Long-Term Survival
We examined the effect of SNR on long-term survival following PD for benign disease. For this analysis, we focused specifically on long-term survival rather than peri-operative outcomes following PD; thus, we excluded 9 patients who died within 90 days of surgery and 6 patients with missing survival data, leaving 305 patients for the analysis. Overall, 85 patients (28%) undergoing PD for benign disease had SNR. When comparing geriatric to non-geriatric patients undergoing PD, geriatric patients were more likely to have coronary artery disease and hypertension, and were more likely to be former smokers and have IPMN (Table 1). Non-geriatric patients were more likely to be current smokers, and were more likely to undergo PD for pancreatitis and other diagnoses. Notably, geriatric patients undergoing PD were not more likely to have SNR; however, we hypothesized that the effect of SNR on long-term survival might differ between geriatric and non-geriatric patients, as geriatric patients have less nutritional reserve.11
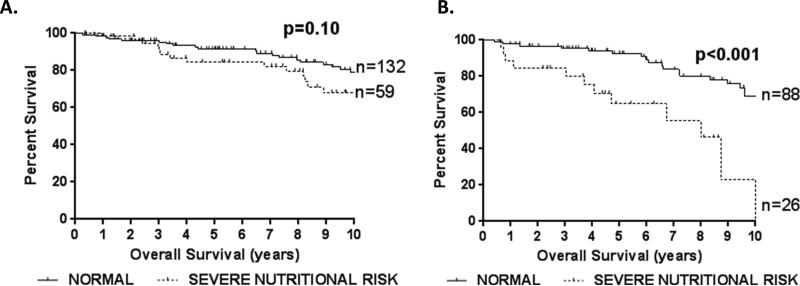
When we examined the effect of SNR on survival, SNR did not significantly affect overall survival in non-geriatric patients, though there was a non-significant trend towards decreased survival (p=0.10) (Figure 2A). However, geriatric patients with SNR had significantly decreased overall survival following PD compared to geriatric patients without SNR (5- year survival: 64.8% vs 92.5%, p<0.001) (Figure 2B). Indeed, roughly 1 in every 3 geriatric patients with preoperative SNR was dead at 5 years following PD for benign disease compared to 1 in every 14 patients without SNR. The 7- and 10- year survival following PD for geriatric patients with and without SNR was 55.6% vs 83.8% and 23.2% vs 68.9%, respectively.
Figure 2.
The impact of severe nutritional risk on survival following pancreaticoduodenectomy for benign disease among non-geriatric (A) and geriatric (B) patients. P-values are by log-rank test.
When we compared geriatric patients with and without SNR, there was a non-significant trend for patients with SNR to have lower albumin levels and BMI, but there were no significant differences in the patient characteristics examined (Table 2). Among geriatric patients undergoing PD, age (HR=1.12 per year increase, p<0.001), coronary artery disease (HR=2.32, p=0.032), and SNR (HR=3.91, p<0.001) were significantly associated with decreased survival by univariate analysis (Table 3). Upon multivariate analysis, age (HR=1.10 per year increase, p=0.032), coronary artery disease (HR=2.48, p=0.024), and SNR (HR=2.74, p=0.012) were independently associated with decreased survival following PD for benign disease (Table 3). When we repeated the multivariate analysis in patients age 60 and older as well as patients age 70 and older, SNR remained an independent predictor of long-term survival after risk adjustment (age≥60: OR=2.74, p=0.012; age≥70: OR=2.59, p=0.021). However, SNR was not significantly associated with overall survival among non-geriatric patients by univariate or multivariate analysis. This suggests that the effect of SNR on long-term survival differs between older and younger patients.
Table 2.
Characteristics of Geriatric Patients with and without Severe Nutritional Risk (SNR)
| No. (Column %) | |||
|---|---|---|---|
| Variables | No SNR (n=88) | SNR (n=26) | P Value |
| Age, mean (SD), y | 72.3 (4.5) | 74.2 (6.4) | 0.168 |
| Sex | |||
| Male | 41 (46.6%) | 14 (53.9%) | 0.515 |
| Female | 47 (53.4%) | 12 (46.2%) | |
| Race | |||
| White | 79 (89.8%) | 25 (96.2%) | 0.582 |
| Black | 8 (9.1%) | 1 (3.9%) | |
| Other | 1 (1.1%) | 0 (0%) | |
| Comorbidities | |||
| CAD | 18 (20.5%) | 9 (34.6%) | 0.136 |
| Diabetes | 19 (21.6%) | 4 (15.4%) | 0.488 |
| Hypertension | 61 (69.3%) | 18 (69.2%) | 0.993 |
| COPD | 8 (9.1%) | 4 (15.4%) | 0.358 |
| Obesity | 21 (23.9%) | 3 (11.5%) | 0.176 |
| Stroke | 2 (2.3%) | 1 (3.9%) | 0.660 |
| Smoking Status | |||
| Never | 35 (39.8%) | 6 (23.1%) | 0.114 |
| Current | 13 (14.8%) | 8 (30.8%) | |
| Former | 40 (45.5%) | 12 (46.2%) | |
| BMI, mean kg/m2 (SD)* | 26.6 (5.8) | 24.5 (4.7) | 0.105 |
| Albumin, mean g/dl (SD)** | 4.2 (0.41) | 4.0 (0.686) | 0.103 |
| Diagnosis | |||
| IPMN | 48 (54.5%) | 14 (53.9%) | 0.9220 |
| Adenoma | 21 (23.9%) | 5 (19.2%) | |
| Pancreatitis | 9 (10.2%) | 3 (11.5%) | |
| Other | 10 (11.4%) | 4 (15.4%) | |
1 missing value
5 missing values
Table 3.
Univariate and Multivariate Predictors of Survival Among Geriatric Patients Undergoing Pancreaticoduodenectomy for Benign Disease
| Univariate |
Multivariate* |
|||||
|---|---|---|---|---|---|---|
| Variables | Hazard Ratio | 95% CI | P Value | Hazard Ratio | 95% CI | P Value |
| Age, per year | 1.12 | 1.05 - 1.20 | <0.001 | 1.10 | 1.03 - 1.18 | 0.005 |
| Sex | ||||||
| Male | [reference] | |||||
| Female | 1.06 | 0.53 - 1.12 | 0.864 | |||
| Race** | ||||||
| White | [reference] | |||||
| Black | 0.7 | 0.21 - 2.34 | 0.564 | |||
| Comorbidities | ||||||
| CAD | 2.32 | 1.08 - 4.99 | 0.031 | 2.48 | 1.13 - 5.45 | 0.024 |
| Diabetes | 0.76 | 0.325 - 1.75 | 0.512 | |||
| Hypertension | 1.94 | 0.84 - 4.48 | 0.121 | |||
| COPD | 1.98 | 0.58 - 6.72 | 0.272 | |||
| Obesity | 1.41 | 0.64 - 3.15 | 0.397 | |||
| Stroke | 2.72 | 0.64 - 11.51 | 0.174 | |||
| Smoking Status | ||||||
| Never | [reference] | |||||
| Current | 1.26 | 0.49 - 3.25 | 0.639 | |||
| Former | 1.17 | 0.54 - 2.56 | 0.153 | |||
| Albumin | ||||||
| ≥ 3.5 g/dl | [reference] | |||||
| < 3.5 g/dl | 2.25 | 0.67 - 7.56 | 0.188 | |||
| Diagnosis | ||||||
| Adenoma | [reference] | |||||
| IPMN | 1.29 | 0.58 - 2.86 | 0.5320 | |||
| Pancreatitis | 0.75 | 0.23 - 2.43 | 0.6340 | |||
| Other | 0.33 | 0.07 - 1.50 | 0.1500 | |||
| Severe Nutritional Risk | 3.91 | 1.86 - 8.21 | <0.001 | 2.74 | 1.25 - 6.02 | 0.012 |
Used Cox regression with backward selection, including all variables with a p<0.3 on univariate analysis.
The category “other race” was excluded as only one geriatric patient fell into this category
Discussion
Weight loss and malnutrition portend an extremely poor prognosis in geriatric patients,6-10 and PD frequently induces a state of chronic malabsorption and weight loss.2 We found that elderly patients’ preoperative nutritional status predicts long-term survival following PD for benign disease. We excluded all patients with malignant disease in order to study the true impact of PD in the setting of SNR, thus eliminating the effect of cancer on long-term survival. While it is striking how SNR adversely impacted long-term survival in geriatric patients following PD for benign disease, it is perhaps equally as impressive how well patients without SNR did following PD, as these patients had a mean age of 73 years at surgery and had a 7-year survival of 84%. We recognize that selection bias likely contributes to the excellent outcomes in these patients; however, surgeons rely on appropriate patient selection to enhance outcomes.
In our study, we demonstrated that the proportion of geriatric patients undergoing PD for benign disease at our institution increased by 59% over the 19 year study. At the same time, the proportion of patients undergoing PD for IPMN increased nearly 3-fold. While geriatric patients were most likely to be diagnosed with IPMN, the disproportionate increase in IPMN relative to the increase in geriatric patients likely reflects a true rise in the incidence and/or detection of IPMN, rather than being solely attributable to an increase in the aging patient population.4 When examining the survival curves of geriatric patients with and without SNR (Figure 2B), it appears that there is a sharp decrease in survival which occurs towards the end of the first year after surgery, followed by a more gradual decline in survival over time. This initial decline could reflect patients unable to recover from the operation, who eventually succumb to the sequelae of complication or failure to thrive. While many authors have focused on 30-day or 90-day mortality as an endpoint for PD, it is clear from our data that patients with SNR who survive more than 90 days postoperatively are still at serious risk of poor outcomes.
From a risk-benefit standpoint, it is of utmost importance to optimize patient selection when considering PD in geriatric patients with benign conditions, as most of these patients will not die from their benign disease process. Cauley et al. demonstrated that among 48 patients with high-risk IPMN initially managed non-operatively, only 2 patients died from pancreatic cancer during a mean follow up of 42 months.16 Therefore, non-operative management should be strongly considered in geriatric patients with SNR and benign disease.
Preoperative nutritional status is an important prognostic factor for perioperative outcomes. Serum albumin has long been used as a surrogate for nutritional status. Low serum albumin has been associated with increased peri-operative morbidity and mortality.17 Because of this, serum albumin has been used to risk stratify patients undergoing major abdominal surgery, such as PD.1 Venkat et al. devised a risk score which included serum albumin to predict 30- and 90-day mortality following PD.18 However, using serum albumin as a sole determinant of nutritional status has limitations, as albumin is a negative acute phase reactant and can be abnormal in other conditions besides malnutrition, such as chronic illness.19 Clavien's group introduced the concept of a nutritional risk score in patients undergoing gastrointestinal surgery, and later demonstrated that this simple test outperformed other more complex methods of assessing nutritional status, such as bioimpedence analysis.13, 14 Recognizing the importance of incorporating validated measures of patients' preoperative nutritional status into risk stratification, the ACS NSQIP / AGS Best Practice Guidelines adopted and modified this measure, and recommends screening all geriatric patients preoperatively for SNR.12 We demonstrate that the risk of death associated with SNR extends far beyond the initial perioperative period, as we excluded patients who died within 90 days of surgery. In agreement with ACS NSQIP/AGS Best Practice Guidelines, our data supports the recommendation that a formal nutritional evaluation should be a routine part of the preoperative work up for geriatric patients being considered for PD. This assessment is especially important in order to have an accurate discussion regarding the risks and benefits of surgery with patients who have benign disease prior to the decision to operate.
As with any observational study, we recognize that there are limitations to our work. Unfortunately, we were unable to obtain the exact causes of death in these patients due to limitations of our database. Chronic malnutrition can lead to death from numerous causes, such as infection or end organ damage, and attributing a patient's death to poor nutritional status is difficult. We also recognize the obvious selection bias involved in choosing geriatric patients for PD. It is not always clear which patients have benign diagnoses preoperatively. As a result, patients with non-malignant disease may undergo PD due to concern for underlying malignancy, but are ultimately found to have benign disease after resection. Alternatively, some patients with benign disease processes require PD for life threatening complications, such as bleeding or recurrent pancreatitis; however, we were not able to account for this in our database. Additionally, formal measures of performance status were not included in our database which could impact long term survival in these patients as well. We were also unable to examine whether efforts were made to improve nutritional and/or functional status pre- or postoperatively in patients with SNR. Further study is needed to determine whether optimizing preoperative nutrition and function improves long-term outcomes. At the very least, our study suggests that close long-term follow up is essential in elderly patients with baseline SNR undergoing PD. Perhaps, better incorporation of geriatric specialists in the care of these patients could help improve outcomes.
In conclusion, the number of geriatric patients undergoing PD for benign conditions is increasing. This study demonstrates that utilizing the ACS NSQIP / AGS Best Practice Guidelines’ criteria for preoperative SNR can be a useful method to better stratify geriatric patients who are at high risk of poor long-term survival following PD. As the geriatric population continues to grow and perioperative patient care continues to improve, the importance of appropriate patient selection to maximize long-term outcomes in those with benign disease will become increasingly important.
Acknowledgements
The authors would like to thank Allison Jaeger and Lori Worley for managing the database used in this study.
Grant Support: This study was supported by the NCI grant T32 CA 009621.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
References
- 1.Greenblatt DY, Kelly KJ, Rajamanickam V, et al. Preoperative factors predict perioperative morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol. 2011;18(8):2126–35. doi: 10.1245/s10434-011-1594-6. [DOI] [PubMed] [Google Scholar]
- 2.Huang JJ, Yeo CJ, Sohn TA, et al. Quality of life and outcomes after pancreaticoduodenectomy. Ann Surg. 2000;231(6):890–8. doi: 10.1097/00000658-200006000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United States Census Bureau The Next Four Decades - The Older Population in the United States: 2010 to 2050. 2010 May; [Google Scholar]
- 4.Schmidt CM, Lillemoe KD. IPMN-controversies in an “epidemic”. J Surg Oncol. 2006;94(2):91–3. doi: 10.1002/jso.20571. [DOI] [PubMed] [Google Scholar]
- 5.Faraj W, Alameddine R, Mukherji D, et al. Postoperative outcomes following pancreaticoduodenectomy: how should age affect clinical practice? World J Surg Oncol. 2013;11:131. doi: 10.1186/1477-7819-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morley JE. Undernutrition in older adults. Fam Pract. 2012;29(Suppl 1):i89–i93. doi: 10.1093/fampra/cmr054. [DOI] [PubMed] [Google Scholar]
- 7.Saka B, Kaya O, Ozturk GB, et al. Malnutrition in the elderly and its relationship with other geriatric syndromes. Clin Nutr. 2010;29(6):745–8. doi: 10.1016/j.clnu.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Soderstrom L, Rosenblad A, Adolfsson ET, et al. Nutritional status predicts preterm death in older people: A prospective cohort study. Clin Nutr. 2014;33(2):354–9. doi: 10.1016/j.clnu.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Miller SL, Wolfe RR. The danger of weight loss in the elderly. J Nutr Health Aging. 2008;12(7):487–91. doi: 10.1007/BF02982710. [DOI] [PubMed] [Google Scholar]
- 10.Winter JE, Macinnis RJ, Wattanapenpaiboon N, Nowson CA. BMI and all-cause mortality in older adults: a meta-analysis. Am J Clin Nutr. 2014;99(4):875–90. doi: 10.3945/ajcn.113.068122. [DOI] [PubMed] [Google Scholar]
- 11.Bozzetti F. Nutritional issues in the care of the elderly patient. Crit Rev Oncol Hematol. 2003;48(2):113–21. doi: 10.1016/j.critrevonc.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Chow WB, Cho CY, Rosenthal RA, Esnaola NF. ACS NSQIP/AGS Best Practice Guidelines: Optimal Preoperative Assessment of the Geriatric Surgical Patient. 2012 doi: 10.1016/j.jamcollsurg.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Schiesser M, Kirchhoff P, Muller MK, et al. The correlation of nutrition risk index, nutrition risk score, and bioimpedance analysis with postoperative complications in patients undergoing gastrointestinal surgery. Surgery. 2009;145(5):519–26. doi: 10.1016/j.surg.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Schiesser M, Muller S, Kirchhoff P, et al. Assessment of a novel screening score for nutritional risk in predicting complications in gastro-intestinal surgery. Clin Nutr. 2008;27(4):565–70. doi: 10.1016/j.clnu.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Winter JM, Brennan MF, Tang LH, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol. 2012;19(1):169–75. doi: 10.1245/s10434-011-1900-3. [DOI] [PubMed] [Google Scholar]
- 16.Cauley CE, Waters JA, Dumas RP, et al. Outcomes of primary surveillance for intraductal papillary mucinous neoplasm. J Gastrointest Surg. 2012;16(2):258–67. doi: 10.1007/s11605-011-1757-6. discussion 266. [DOI] [PubMed] [Google Scholar]
- 17.Gibbs J, Cull W, Henderson W, et al. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg. 1999;134(1):36–42. doi: 10.1001/archsurg.134.1.36. [DOI] [PubMed] [Google Scholar]
- 18.Venkat R, Puhan MA, Schulick RD, et al. Predicting the risk of perioperative mortality in patients undergoing pancreaticoduodenectomy: a novel scoring system. Arch Surg. 2011;146(11):1277–84. doi: 10.1001/archsurg.2011.294. [DOI] [PubMed] [Google Scholar]
- 19.Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17(6):432–7. doi: 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed] [Google Scholar]