Abstract
Background
Neurocysticercosis is a leading cause of seizures and epilepsy in most of the world, and it occurs when Taenia solium larval cysts infect the central nervous system. T. solium tapeworm infection is endemic in much of Peru, but there are scarce data on the prevalence in many rural highland communities where it is likely to be hyper-endemic. Peace Corps Volunteers live and work in these communities; however, to our knowledge, they have not been used to facilitate public health research.
Materials and Methods
We utilized Peace Corps Volunteers to estimate the prevalence of T. solium tapeworm infection in seven rural communities in northern Peru. A convenience non-random sampling frame was used. Peace Corps Volunteers facilitated the collection of stool samples (N = 2,328), which were analyzed by sedimentation and microscopy. Niclosamide treatment and purgation preceded species identification, which was done by PCR-REA.
Results
Taenia sp. egg-positive stool samples were found in three of the seven communities we surveyed. The overall prevalence of Taenia sp. egg positivity was 2.1% (49/2,328) (95% CI = 1.6–2.8%) with prevalence up to 4.3% (42/977) (95% CI = 3.1–5.8%) by community. All 34 of the specimens tested by PCR-REA were T. solium. The overall prevalence of T. solium tapeworm infection was 1.5% (34/2,328) (95% CI = 1.0–2.0%). Prevalence up to 2.9% (28/977) (95% CI = 1.9–4.1%) by community was observed.
Conclusion/Significance
This study recorded high T. solium tapeworm prevalence, and identified hyper-endemic rural communities. It demonstrates that synergy between researchers and Peace Corps Volunteers can be an effective means to conducting large-scale, community-based studies in remote areas of Peru.
Introduction
Taenia solium (T. solium) neurocysticercosis (NCC) is the leading cause of adult-acquired epilepsy worldwide, and an increasingly important public health problem in developed countries with migrant populations. [1], [2] Cysticercosis has been shown to cluster around T. solium tapeworm carriers, [3] as T. solium tapeworm infection not only increases carriers’ risk for NCC, but also places other household members at substantially elevated risks. [4], [5] T. solium tapeworm infection may be underreported and difficult to detect in rural, Andean communities due to a lack of awareness and diagnostic facilities. [4].
Estimating the prevalence of T. solium infection in such communities is necessary to allow researchers and public health workers to address this health problem. Conducting epidemiologic research in remote communities can be costly, time-consuming, and difficult due to their geographic locations, linguistic and cultural barriers, as well as the need to establish a working relationship with community leaders and government. Therefore, our group utilized an existing network of Peace Corps Volunteers (PCVs) who were integrated into their communities to perform epidemiologic screening and surveillance. Our consortium aimed to perform a large-scale, cross-sectional epidemiologic study to examine the feasibility of bringing together PCVs and researchers to perform a T. solium tapeworm prevalence study in multiple rural regions of northern Peru. To our knowledge, collaboration between a group of university investigators and PCVs to perform local surveillance of T. solium tapeworm infection is unprecedented.
Materials and Methods
Study area and population
The study was conducted in seven communities in the northern departments of Piura and Cajamarca (Fig. 1) following PCV reports of free-roaming pigs and inadequate access to latrines. All communities are rural and most inhabitants do not have access to reliable potable water for drinking nor adequate sanitation systems. Study sites ranged in altitude between 236–2,667 meters above sea level. The climate is hot at lower elevations, but becomes increasingly temperate with altitude. May through December is typically dry, with heavy rainfall from January to April. Agriculture is the main economic activity in these regions where villagers frequently raise pigs for consumption and sale. Peace Corps Volunteers suggested communities for the study based on the guiding principles for selection: the observed presence of pigs (usually free-roaming) and an inadequate access to latrines. Persons were included in the study if they resided in these communities, and persons were excluded if they resided in a community where a mass-deworming program was in effect. The study was conducted in the following communities (population data obtained from the last available nation census, 2007): Joras (Ayabaca district, 38,730 inhabitants), Oxahuay (Sicchez district, 2,274 inhabitants), Pampa Elera Baja (Las Lomas district, 26,896 inhabitants), Baños del Inca (Baños del Inca district, 36,800 inhabitants), Chalamarca (Chalamarca district, 10,530 inhabitants), Iraca Grande (Chota district, 45,555 inhabitants), and Conchan (Conchan district, 6,449 inhabitants).
Figure 1. Map of Peru Showing Piura and Cajamarca.
Study design
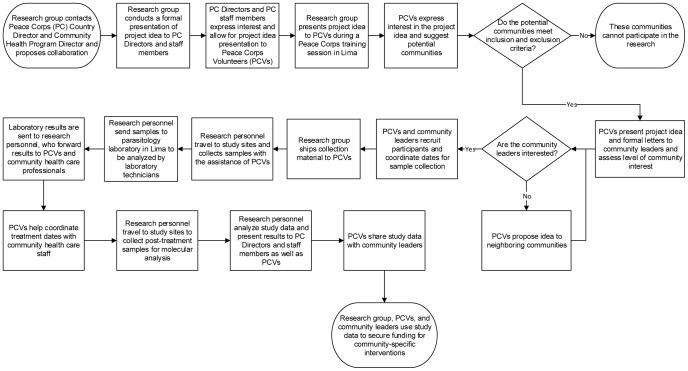
A community-based, cross-sectional prevalence study was performed using a convenience non-random sampling frame. The Cysticercosis Working Group (CWG) in Peru contacted the Country Director and Community Health Program Director at the Peace Corps office in Lima, Peru, and proposed collaboration. Researchers presented the study idea to Peace Corps staff and to 23 Health Program PCVs attending an in-service training session in Lima. Formal letters in Spanish explaining the research objective were sent to each PCV who suggested an eligible community. Peace Corps Volunteers presented these letters to their communities’ health posts, municipalities, schools, and community leaders. Peace Corps Volunteers worked in conjunction with leaders from these institutions to recruit participants. To maximize participation, all community residents were eligible to participate in this study. Figure 2 provides a process map detailing collaboration.
Figure 2. Process Map Demonstrating Key Steps in Collaboration.
Sample collection and analysis
Single stool specimens were collected from participants and analyzed for the presence of eggs, cysts, or parasite material using spontaneous sedimentation in tube technique (SSTT) and microscopy. [6] Each consenting resident was provided with a 500-ml plastic container with lid for collection of a whole, individual stool sample. Research personnel, with the assistance of PCVs, collected stool samples along with participant age and sex information. A 10cc fecal sample was placed in 40 cc of 5% of formol-Phosphate Buffered Saline, pH 7.2 (PBS) in a sealed propylene tube at room temperature. Fecal samples were transported at room temperature to the parasitology laboratory at Universidad Peruana Cayetano Heredia (UPCH) in Lima for analysis. Participants who were Taenia sp. egg-positive were invited to their community health clinic, where a local physician provided treatment. Treatment consisted of niclosamide and an electrolyte-polyethyleneglycol solution (EPS) purge (NuLYTELY, Asofarma, Argentina). [7] Adults were treated with 2 g of niclosamide and 2 L of EPS, which was given 2 h before and after treatment. Children>34 kg were given 1.5 g of niclosamide and 1 L of EPS. Children 11–34 kg were given 1 g of niclosamide and 25–40 mL/kg per h of EPS, divided into 8 doses. All participants were provided with detailed information on the risks associated with T. solium tapeworm infection and how to avoid repeat infection. Participants who did not expel the parasite were offered retreatment. All stools produced 24 hours post-treatment were examined for scoleces and proglottids, which were preserved in 95% alcohol for species identification by molecular analysis. Tapeworm material collected post-treatment was stored in 25% glycerol supplemented with penicillin (1000 Ul/ml) and gentamycin (100 Ug/ml) at 4°C until use. For Taenia species identification, DNA was isolated from proglottids expelled post-niclosamide treatment by phenol-chloroform, and a polymerase chain reaction (PCR) followed by restriction enzyme analysis (REA) was performed. [8] Briefly, PCR was performed in 25 µL master mix consisting in 1×PCR buffer (Gibco, Life Technologies) containing 2.5 mM MgCl2, 0.2 mM (each) dATP, dGTP, dCTP, and dTTP, 0.5 µM each primer, and 1 U of Taq polymerase. PCR was carried out in a thermocycler MJ Research MiniCycler PTC-150 with hot cover with the following temperature profile: 5 min at 94°C followed by 30 cycles consisting of 94°C for 1 min (denaturation), 56°C for 1 min (annealing), and 72°C for 2 min (elongation). Seventeen microliters of the PCR product was digested in 1 x enzyme buffer, 1 U of enzyme (AluI, DdeI, or MboI). The reaction mixture was incubated for 3 h at 37°C. Finally, the products were separated by electrophoresis on a 1.0% agarose gel containing 0.5 µg of ethidium bromide/ml to evaluate T. solium pattern.
Statistical Analysis
Prevalence (and 95% confidence intervals (CI)) of Taenia sp. and other intestinal parasitic infections was calculated as the number of stool samples that tested positive for eggs, or parasite material divided by the number of participants who contributed samples. Prevalence of T. solium tapeworm infection was calculated as the number of PCR-positive reaction divided by the number of participants who contributed samples. Pearson’s chi-square test was used to explore associations between age and sex and T. solium infection status, and a two proportions z-test was used to compare the proportion of parasitic infections between Cajamarca and Piura; p<0.05 was taken as statistically significant. All analyses were performed using Excel (Microsoft Office 2010) and STATA 10.1 (College Station, TX, USA).
Ethics Statement
We obtained Institutional Review Board (IRB) approval from Universidad Peruana Cayetano Heredia (Protocol IRB-61133) in Lima, Peru, as well as Asociación Benéfica Proyectos en Informática, Salud, Medicina y Agricultura (PRISMA), a non-governmental organization based in Lima, Peru. Appropriate informed consent procedures were followed. Adults (18 years of age and older) provided written informed consent. For subjects less than 18 years of age, written informed assent was obtained. Parents or guardians also provided written informed consent for minors.
Results
Study population
Eleven PCVs suggested a total of 10 potential communities for the study. One community was excluded because an existing cysticercosis elimination program was in effect, and community leaders from two communities declined to participate. A total of 2,328 participants from seven communities provided a stool sample. 2,169 (93.2%) participants provided information on sex and 2,136 (91.8%) provided information on age. 1,990 (85.5%) participants provided information on age and sex. 973 (44.9%) participants were male and 1,196 (55.1%) participants were female. The median age was 18 years IQR (9–40 years). Participants ranged in age from 1<year to 98 years. 1,485 (63.8%) participants were from Piura and 843 (36.2%) were from Cajamarca. The number of study participants in each community relative to the total inhabitants in the community is as follows: Joras (977/1,363), Oxahuay (88/640), Pampa Elera Baja (420/691), Baños del Inca (33/9,442), Chalamarca (550/776), Iraca Grande (96/957), and Conchan (164/419). Study population characteristics are presented in Table 1.
Table 1. Study Population Characteristics.
| Department | Piura | Cajamarca | ||||||
| Community | Joras | Oxahuay | Pampa EleraBaja | Baños delInca | Chalamarca | IracaGrande | Conchan | Total |
| No. of residents in communitya | 1,363 | 640 | 691 | 9,442 | 776 | 957 | 419 | 14,101 |
| Sample size (n) | 977 | 88 | 420 | 33 | 550 | 96 | 164 | N = 2,328 |
| Female, n (%) | 432 (52.3%) | 44 (50%) | 232 (55.4%) | 25 (75.8%) | 301 (55.4%) | 65 (67.7%) | 97 (59.2%) | 1,196 (55.1%) |
| Male, n (%) | 394 (47.7%) | 44 (50%) | 187 (44.6%) | 8 (24.2%) | 242 (44.6%) | 31 (32.3%) | 67 (40.9%) | 973 (44.9%) |
| No. who providedage, n (%) | 844 (86.4%) | 82 (93.2%) | 420 (100%) | 33 (100%) | 504 (91.6%) | 89 (92.7%) | 164 (100%) | 2,136 (91.8%) |
| No. who providedage, sex, n (%) | 699 (71.0%) | 82 (93.2%) | 419 (99.8%) | 33 (100%) | 504 (91.6%) | 89 (92.7%) | 164 (100%) | 1,990 (85.5%) |
| Median age (IQR) | 16 (9–45) | 15 (5–33) | 15 (7–35) | 14 (10–37) | 25 (10–41) | 22 (8–36) | 17 (8–39) | 18 (9–40) |
| Min, Max | <1, 90 | 1, 66 | <1, 85 | 6, 54 | <1, 98 | <1, 91 | 1, 87 | <1, 98 |
Data obtained from 2007 national census.
Prevalence of Taenia sp. egg positivity and T. solium tapeworm infection
In the total study population, the overall prevalence of Taenia sp. infection was 2.1% (49/2,328) (95% CI = 1.6–2.8%), and the overall prevalence of T. solium infection was 1.5% (34/2,328) (95% CI = 1.0–2.0%) (Table 2). Notably, no Taenia sp. egg-positive stool samples were found in Cajamarca. In Piura, the overall prevalence of Taenia sp. infection was 3.3% (49/1,458) (95% CI = 0.2–4.3%), reaching up to 4.3% (42/977) (95% CI = 3.1–5.8%) by community, and the overall prevalence of T. solium infection was 2.3% (34/1,485) (95% CI = 1.6–3.2%), reaching up to 2.9% (28/977) (95% CI = 1.9–4.1%) by community. Of the 49 Taenia sp. egg-positive cases, 39 were treated; 10 did not present for treatment for reasons unknown. From the treated group, 34 expelled tapeworm proglottids, which were all confirmed to be T. solium. Because we could not identify the species of Taenia in 15 egg-positive participants, T. solium tapeworm infection and Taenia sp. tapeworm infection prevalence are presented separately (Table 2). In Piura, T. solium was more prevalent in adults (18 years or older) (2.66%, 17/638) than children (1.84%, 13/708), although not statistically significant (χ 2 = 1.06, p = 0.30). T. solium tapeworm infection was also more prevalent in men (2.56%, 16/625) than women (2.54%,18/708), although this difference was not statistically significant (χ 2 = 0.0004, p = 0.98).
Table 2. Prevalence of Taenia solium and Taenia sp. Tapeworm Infection.
| Department | Piura | |||||||||
| Community | Joras | Oxahuay | Las Lomas | Total in Piura | Total in study population | |||||
| n = 977 | 95% CI | n = 88 | 95% CI | n = 420 | 95% CI | n = 1,485 | 95% CI | N = 2,328 | 95% CI | |
| Taenia solium | 2.9% | (1.9–4.1%) | 2.3% | (0.2–8.0%) | 1.0% | (0.3–2.4%) | 2.3% | (1.6–3.2%) | 1.5% | (1.0–2.0%) |
| Taenia sp. | 4.3% | (3.1–5.8%) | 3.4% | (0.7–9.6%) | 1.0% | (0.3–2.4%) | 3.3% | (0.2–4.3%) | 2.1% | (1.6–2.8%) |
Prevalence of other parasites
Other intestinal parasites found in stool samples were Ascaris lumbricoides (A. lumbricoides), hookworms, Enterobius vermicularis, Giardia lamblia (G. lamblia), Strongyloides stercoralis, and Trichuris trichiura (T. trichiura) (Tables 3 and 4). Prevalence of parasitic infection was found to be lower in Cajamarca than in Piura. This difference was statistically significant for A. lumbricoides (χ2 = 22.54, p<0.01), G. lamblia (χ2 = 23.63, p<0.01), and T. trichiura (χ2 = 5.84, p = 0.016). The most prevalent parasite in Cajamarca and Piura was G. lamblia.
Table 3. Prevalence of other Parasites by Community in Piura.
| Department | Piura | |||||||
| Community | Joras | Oxahuay | Las Lomas | Total | ||||
| n = 977 | 95% CI | n = 88 | 95% CI | n = 420 | 95% CI | n = 1,485 | 95% CI | |
| Ascaris lumbricoides | 6.7% | (5.1–8.3%) | 6.8% | (2.5–14.3%) | 0.7% | (0.1–2.1%) | 4.9% | (3.9–6.1%) |
| Hookworms | 0 | 0 | 0.7% | (0.1–2.1%) | 0.02% | (0.0–0.6%) | ||
| Enterobius vermicularis | 1.1% | (0.5–1.9%) | 0 | 0 | 0.7% | (0.3–1.2%) | ||
| Giardia lamblia | 8.1% | (6.3–9.7%) | 10.2% | (4.8–18.5%) | 16.7% | (10.6–13.2%) | 10.5% | (9.0–12.2%) |
| Strongyloides stercoralis | 0.5% | (0.1–1.2%) | 0 | 1.0% | (0.3–2.4%) | 0.6% | (0.2–1.1%) | |
| Trichuris trichiura | 2.3% | (1.4–3.4%) | 3.4% | (0.7–9.6%) | 0.2% | (0.0–1.3%) | 1.8% | (0.1–2.6%) |
Table 4. Prevalence of other Parasites by Community in Cajamarca.
| Department | Cajamarca | |||||||||
| Community | Baños del Inca | Chalamarca | Chota | Conchan | Total | |||||
| n = 33 | 95%CI | n = 550 | 95%CI | n = 96 | 95%CI | n = 164 | 95%CI | n = 843 | 95%CI | |
| Ascaris lumbricoides | 3.0% | (0.0–15.8%) | 2.2% | (1.1–3.8%) | 4.2% | (1.1–10.3%) | 1.8% | (0.3–5.3%) | 2.4% | (1.5–3.6%) |
| Hookworms | 0 | 0.2% | (0.0–5.7%) | 1.0% | (0.0–5.7%) | 0 | 0.2% | (0.0–0.9%) | ||
| Enterobius vermicularis | 3.0% | (0.0–15.8%) | 0.2% | (0.0–5.7%) | 0 | 0 | 0.2% | (0.0–0.9%) | ||
| Giardia lamblia | 3.0% | (0.0–15.8%) | 4.6% | (2.9–6.6%) | 2.1% | (0.2–7.3%) | 6.7% | (3.4–11.7%) | 4.7% | (3.3–6.3%) |
| Strongyloides stercoralis | 0 | 0 | 0 | 1.2% | (0.1–4.3%) | 0.2% | (0.0–0.9%) | |||
| Trichuris trichiura | 0 | 0.2% | (0.0–1.0%) | 2.1% | (0.2–7.3%) | 1.2% | (0.1–4.3%) | 0.6% | (0.2–1.4%) | |
Discussion
This study estimated the prevalence of T. solium tapeworm infection in seven rural communities where PCVs serve in northern Peru. The overall prevalence of Taenia sp. egg-positivity by sedimentation and microscopy was 2.1%, which is considered hyper-endemic. [4] Prevalence as high as 4.3% by community was observed. These findings are higher in comparison to the results of many community-based studies that also estimated Taenia sp. tapeworm prevalence by microscopy, including studies performed in endemic areas of Peru [3], [4], [9] and other countries in Latin America [10]–[15] Africa [16]–[18] and northern Vietnam. [19] The overall prevalence of T. solium tapeworm infection was 1.5% with prevalence up to 2.9% by community. Similarly, these findings are higher when compared to the results of previous studies which report T. solium tapeworm prevalence by microscopy and PCR;[18]–[20] however, they are lower when compared to the results of studies that used more sensitive diagnostic methods such as coproantigen ELISA and EITB assay.[4], [16]–[18] Despite being high, the prevalence of T. solium tapeworm infection detected by our survey is likely an underestimation, as microscopy is poorly sensitive, missing 60–70% of Taenia sp. cases. [2] Nevertheless, our findings identified hyper-endemic communities and support our study hypothesis that a consortium of PCVs and university investigators can effectively perform large-scale, community-based public health research in remote regions of Peru.
To our knowledge, ours is the first study utilizing PCVs to facilitate local surveillance for T. solium tapeworm infection. This expansive stool survey illustrates the feasibility of using PCVs to identify study sites, assist with access and entre into those sites, recruit participants, and facilitate specimen collection, as well as highlights the utility of this concept, providing a template for future research studies. As demonstrated, PCVs can be a practical intermediate between researchers and rural communities. 90% of all PCVs have at least a bachelor’s degree, live as their communities do, and work on their behalf. [21], [22] All PCVs receive a minimum of two months of cultural, linguistic, and project-specific training, and approximately 20% receive additional specific training in health issues. [22] There are isolated published instances of PCVs working with outside organizations to offer treatment to their communities, such as a community eye health program in Samoa and a hernia repair clinic in Latin America. [23], [24] Peace Corps Volunteers commonly work in training programs, health education, and health promotion initiatives – one notable example is the HIV/AIDS programs in Africa.[25]–[27] The medical literature contains studies showing that PCVs are indirectly involved in health related issues; however, no published examples of PCVs facilitating public health research amongst their communities exists.
The prevalence of parasitic infection was higher in Piura than Cajamarca, where high endemicity of intestinal parasites and the presence of Taenia sp. have been previously reported.[28]–[30] The fact that we did not find Taenia sp. egg-positive stool samples in Cajamarca, where pigs are also raised freely, was surprising; however, there are several possible explanations. We did not formally investigate pig husbandry, yet we observed a greater number of free-roaming pigs in the Piura sites compared to the Cajamarca sites. Similarly, we did not investigate access to sanitary facilities; however, the most recent national census (2007) indicates that the communities we surveyed in Cajamarca have better access to sanitary facilities, specifically latrines, than the communities we surveyed in Piura. The presence of free-roaming pigs and the absence of latrines increase the risk for porcine cysticercosis [31]–[34] which has been found to be hyper-endemic in Piura. Jayashi et al. reported seroprevalence up to 45.6% in Piura, Morropon district. [35] Proximity to a cysticercosis-infected pig increases the risk for human T. solium tapeworm infection. [9] Participants were not randomly selected, and it is possible that those who participated in Piura were more likely to have intestinal parasites, including Taenia sp., than those who participated in Cajamarca. We collected and analyzed a single stool sample from each participant. Parasites shed eggs intermittently, thus the stool could have been produced at a time when the tapeworm was not shedding eggs. In such a case, infection would have gone undetected. Projects designed to control parasitic infection in humans and animals (primarily cattle) have been implemented in several districts in Cajamarca, including communities in the district of Baños del Inca. [36] Such projects could also explain the observed differences; to our knowledge, however, no such programs were in effect in the communities we surveyed. It should be mentioned that several groups have actively attempted to control T. solium in endemic areas by applying mass human chemotherapy. [13], [37], [38] A combined mass human and porcine chemotherapy trial was performed by the CWG in Peru in the Quilcas district, department of Junín, [39] and a longitudinal cysticercosis elimination program in Tumbes, located along Peru’s northern coast, is currently in effect.[40]–[44] However, no such programs have been implemented by the CWG in Peru in the sites we investigated in this study.
This study had a few key strengths. We obtained a large sample size, as PCVs secured high participation rates. We also recorded high prevalence of T. solium tapeworm infection in communities not previously surveyed. This study also had a few limitations, the most notable of which is the absence of application of a sensitive coproantigen ELISA for human taeniasis, [45], [46] a test that can detect 2 to 3 times as many confirmed cases of taeniasis as microscopy. [4] To that point, Taenia sp. and T. solium tapeworm prevalence are likely to be underestimated. Similarly, we were unable to identify the species of Taenia in 15 egg-positive participants. All 34 participants who were treated and expelled Taenia proglottids were infected with T. solium, thus we expect the 15 participants for whom the species is unknown to have T. solium as well. Yet, we cannot rule out the possibility that these participants were infected with Taenia saginata (T. saginata), as this species of Taenia has been reported in northern Peru. It is important to note, however, that T. saginata is rare and has been found in only 10% of tapeworm carriers in the neighboring department of Tumbes (Garcia HH, unpublished data). [41].
Conclusion
This study demonstrates that synergy between researchers and the Peace Corps can be an effective means to conduct large-scale, community-based studies in remote areas. In our case, collaboration allowed for the discovery of hyper-endemic communities of T. solium tapeworm infection, and connected doctors, epidemiologists, and scientists to those communities through PCVs in a mutually beneficial manner. Peace Corps Peru and the CWG in Peru have begun discussions on subsequent projects, the development of appropriate, community-specific interventions, as well as the incorporation of basic epidemiologic principles and taeniasis/cysticercosis education into the PCV training continuum.
Acknowledgments
We are grateful to all the communities involved in this study and for support from Sanjay Mathur, Emilia Villanueva, Maria Elena Samplonius, Emily Rose Topalanchik, Andrea Doggett, and all PCVs involved in the project (Amanda McCullough, Jessica R. Gyourko, Hayden Mulligan, Eleanor Thompson, Michelle Polansky, Lauralee J. Woods, Katherine Murray, and Alison Clune). Additional support from CW, Catherine Anne Amutan, Cueva Marinas and Manuel Jesús from the Institucion de Investigacion y Capacitacion (IINCAP), Celia Luisa Espinoza, Fredy Ccopa Aguilar, and Angelica Ramirez Moreno is appreciated. The authors are grateful for the technical expertise of JB PHU and D. Sara. Dr. Jon S. Friedland is grateful for support from the Imperial College Biomedical Research Centre.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data have been deposited to Figshare (http://dx.doi.org/10.6084/m9.figshare.1213753).
Funding Statement
This study was funded by the D43 International Global Infectious Disease Research Training Grant 5D43TW006581-10. The authors also acknowledge the anonymous RG-ER fund for funding NW and TC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The anonymous RG-ER fund is an anonymous donor who is a person and not a fund. There is no expansion of the name possible. There is no conflict of interest with this fund. It is not an organization or a company.
References
- 1. Nash TE, Mahanty S, Garcia HH (2013) Neurocysticercosis-more than a neglected disease. PLoS neglected tropical diseases 7:e1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garcia HH, Gonzalez AE, Evans CA, Gilman RH (2003) Taenia solium cysticercosis. Lancet 362:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lescano AG, Garcia HH, Gilman RH, Gavidia CM, Tsang VC, et al. (2009) Taenia solium cysticercosis hotspots surrounding tapeworm carriers: clustering on human seroprevalence but not on seizures. PLoS neglected tropical diseases 3:e371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garcia HH, Gilman RH, Gonzalez AE, Verastegui M, Rodriguez S, et al. (2003) Hyperendemic human and porcine Taenia solium infection in Peru. The American journal of tropical medicine and hygiene 68:268–275. [PubMed] [Google Scholar]
- 5. Diaz Camacho S, Candil Ruiz A, Uribe Beltran M, Willms K (1990) Serology as an indicator of Taenia solium tapeworm infections in a rural community in Mexico. Transactions of the Royal Society of Tropical Medicine and Hygiene 84:563–566. [DOI] [PubMed] [Google Scholar]
- 6.Tello R (1988) Empleo de una nueva técnica parasitológica rápida de sedimentación espontánea en el diagnóstico de protozoarios y helmintos. V Jornadas Científicas Lima: UPCH.
- 7. Jeri C, Gilman RH, Lescano AG, Mayta H, Ramirez ME, et al. (2004) Species identification after treatment for human taeniasis. Lancet 363:949–950. [DOI] [PubMed] [Google Scholar]
- 8. Mayta H, Talley A, Gilman RH, Jimenez J, Verastegui M, et al. (2000) Differentiating Taenia solium and Taenia saginata infections by simple hematoxylin-eosin staining and PCR-restriction enzyme analysis. Journal of clinical microbiology 38:133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O’Neal SE, Moyano LM, Ayvar V, Gonzalvez G, Diaz A, et al. (2012) Geographic correlation between tapeworm carriers and heavily infected cysticercotic pigs. PLoS neglected tropical diseases 6:e1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sanchez AL, Gomez O, Allebeck P, Cosenza H, Ljungstrom L (1997) Epidemiological study of Taenia solium infections in a rural village in Honduras. Annals of tropical medicine and parasitology 91:163–171. [DOI] [PubMed] [Google Scholar]
- 11. Rodriguez-Hidalgo R, Benitez-Ortiz W, Praet N, Saa LR, Vercruysse J, et al. (2006) Taeniasis-cysticercosis in Southern Ecuador: assessment of infection status using multiple laboratory diagnostic tools. Memorias do Instituto Oswaldo Cruz 101:779–782. [DOI] [PubMed] [Google Scholar]
- 12. Sarti E, Schantz PM, Plancarte A, Wilson M, Gutierrez IO, et al. (1992) Prevalence and risk factors for Taenia solium taeniasis and cysticercosis in humans and pigs in a village in Morelos, Mexico. The American journal of tropical medicine and hygiene 46:677–685. [DOI] [PubMed] [Google Scholar]
- 13. Diaz Camacho SP, Candil Ruiz A, Suate Peraza V, Zazueta Ramos ML, Felix Medina M, et al. (1991) Epidemiologic study and control of Taenia solium infections with praziquantel in a rural village of Mexico. The American journal of tropical medicine and hygiene 45:522–531. [DOI] [PubMed] [Google Scholar]
- 14. Sarti E, Schantz PM, Plancarte A, Wilson M, Gutierrez OI, et al. (1994) Epidemiological investigation of Taenia solium taeniasis and cysticercosis in a rural village of Michoacan state, Mexico. Transactions of the Royal Society of Tropical Medicine and Hygiene 88:49–52. [DOI] [PubMed] [Google Scholar]
- 15. Rodriguez-Canul R, Fraser A, Allan JC, Dominguez-Alpizar JL, Argaez-Rodriguez F, et al. (1999) Epidemiological study of Taenia solium taeniasis/cysticercosis in a rural village in Yucatan state, Mexico. Annals of tropical medicine and parasitology 93:57–67. [DOI] [PubMed] [Google Scholar]
- 16. Mwanjali G, Kihamia C, Kakoko DV, Lekule F, Ngowi H, et al. (2013) Prevalence and risk factors associated with human Taenia solium infections in Mbozi District, Mbeya Region, Tanzania. PLoS neglected tropical diseases 7:e2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mwape KE, Phiri IK, Praet N, Speybroeck N, Muma JB, et al. (2013) The incidence of human cysticercosis in a rural community of Eastern Zambia. PLoS neglected tropical diseases 7:e2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mwape KE, Phiri IK, Praet N, Muma JB, Zulu G, et al. (2012) Taenia solium Infections in a rural area of Eastern Zambia-a community based study. PLoS neglected tropical diseases 6:e1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Somers R, Dorny P, Nguyen VK, Dang TC, Goddeeris B, et al. (2006) Taenia solium taeniasis and cysticercosis in three communities in north Vietnam. Tropical medicine & international health: TM & IH 11:65–72. [DOI] [PubMed] [Google Scholar]
- 20. Huisa BN, Menacho LA, Rodriguez S, Bustos JA, Gilman RH, et al. (2005) Taeniasis and cysticercosis in housemaids working in affluent neighborhoods in Lima, Peru. The American journal of tropical medicine and hygiene 73:496–500. [PubMed] [Google Scholar]
- 21.Peace Corps website. Available: http://files.peacecorps.gov/multimedia/pdf/about/pc_facts.pdf. Accessed 6 Jun 2014.
- 22.Peace Corps website. Available: http://files.peacecorps.gov/multimedia/pdf/about/pc_catalog.pdf. Accessed 24 Oct 2014.
- 23. Barnes S (2009) Using Peace Corps volunteers in community eye health. Community eye health/International Centre for Eye Health 22:S1. [PMC free article] [PubMed] [Google Scholar]
- 24. Turaga KK, Garg N, Coeling M, Smith K, Amirlak B, et al. (2006) Inguinal hernia repair in a developing country. Hernia: the journal of hernias and abdominal wall surgery 10:294–298. [DOI] [PubMed] [Google Scholar]
- 25. Nyberg BJ, Yates DD, Lovich R, Coulibaly-Traore D, Sherr L, et al. (2012) Saving lives for a lifetime: supporting orphans and vulnerable children impacted by HIV/AIDS. Journal of acquired immune deficiency syndromes (1999) 60 Suppl 3: S127–135. [DOI] [PubMed] [Google Scholar]
- 26.(2005) Volunteers. Peace Corps beefs up HIV/AIDS program assistance. AIDS policy & law 20:2. [PubMed] [Google Scholar]
- 27. Mullan F (2007) Responding to the global HIV/AIDS crisis: a Peace Corps for health. Jama 297:744–746. [DOI] [PubMed] [Google Scholar]
- 28. Roldan WH, Espinoza YA, Huapaya PE, Huiza AF, Sevilla CR, et al. (2009) Frequency of human toxocariasis in a rural population from Cajamarca, Peru determined by DOT-ELISA test. Revista do Instituto de Medicina Tropical de Sao Paulo 51:67–71. [DOI] [PubMed] [Google Scholar]
- 29. Gonzalez LC, Esteban JG, Bargues MD, Valero MA, Ortiz P, et al. (2011) Hyperendemic human fascioliasis in Andean valleys: an altitudinal transect analysis in children of Cajamarca province, Peru. Acta tropica 120:119–129. [DOI] [PubMed] [Google Scholar]
- 30. Esteban JG, Gonzalez C, Bargues MD, Angles R, Sanchez C, et al. (2002) High fascioliasis infection in children linked to a man-made irrigation zone in Peru. Tropical medicine & international health: TM & IH 7:339–348. [DOI] [PubMed] [Google Scholar]
- 31. Morales J, Martinez JJ, Rosetti M, Fleury A, Maza V, et al. (2008) Spatial distribution of Taenia solium porcine cysticercosis within a rural area of Mexico. PLoS neglected tropical diseases 2:e284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ngowi HA, Kassuku AA, Maeda GE, Boa ME, Carabin H, et al. (2004) Risk factors for the prevalence of porcine cysticercosis in Mbulu District, Tanzania. Veterinary parasitology 120:275–283. [DOI] [PubMed] [Google Scholar]
- 33. Sikasunge CS, Phiri IK, Phiri AM, Dorny P, Siziya S, et al. (2007) Risk factors associated with porcine cysticercosis in selected districts of Eastern and Southern provinces of Zambia. Veterinary parasitology 143:59–66. [DOI] [PubMed] [Google Scholar]
- 34. Krecek RC, Mohammed H, Michael LM, Schantz PM, Ntanjana L, et al. (2012) Risk factors of porcine cysticercosis in the Eastern Cape Province, South Africa. PloS one 7:e37718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jayashi CM, Arroyo G, Lightowlers MW, Garcia HH, Rodriguez S, et al. (2012) Seroprevalence and risk factors for Taenia solium cysticercosis in rural pigs of northern Peru. PLoS neglected tropical diseases 6:e1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rinaldi L, Gonzalez S, Guerrero J, Aguilera LC, Musella V, et al. (2012) A One-Health integrated approach to control fascioliasis in the Cajamarca valley of Peru. Geospatial health 6:S67–73. [DOI] [PubMed] [Google Scholar]
- 37. Cruz M, Davis A, Dixon H, Pawlowski ZS, Proano J (1989) Operational studies on the control of Taenia solium taeniasis/cysticercosis in Ecuador. Bulletin of the World Health Organization 67:401–407. [PMC free article] [PubMed] [Google Scholar]
- 38. Allan JC, Velasquez-Tohom M, Fletes C, Torres-Alvarez R, Lopez-Virula G, et al. (1997) Mass chemotherapy for intestinal Taenia solium infection: effect on prevalence in humans and pigs. Transactions of the Royal Society of Tropical Medicine and Hygiene 91:595–598. [DOI] [PubMed] [Google Scholar]
- 39. Garcia HH, Gonzalez AE, Gilman RH, Moulton LH, Verastegui M, et al. (2006) Combined human and porcine mass chemotherapy for the control of T. solium. The American journal of tropical medicine and hygiene 74:850–855. [PubMed] [Google Scholar]
- 40. Villaran MV, Montano SM, Gonzalvez G, Moyano LM, Chero JC, et al. (2009) Epilepsy and neurocysticercosis: an incidence study in a Peruvian rural population. Neuroepidemiology 33:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lescano AG, Garcia HH, Gilman RH, Guezala MC, Tsang VC, et al. (2007) Swine cysticercosis hotspots surrounding Taenia solium tapeworm carriers. The American journal of tropical medicine and hygiene 76:376–383. [PubMed] [Google Scholar]
- 42. Garcia HH, Gonzalez AE, Rodriguez S, Gonzalvez G, Llanos-Zavalaga F, et al. (2010) [Epidemiology and control of cysticercosis in Peru]. Revista peruana de medicina experimental y salud publica 27:592–597. [DOI] [PubMed] [Google Scholar]
- 43. Montano SM, Villaran MV, Ylquimiche L, Figueroa JJ, Rodriguez S, et al. (2005) Neurocysticercosis: association between seizures, serology, and brain CT in rural Peru. Neurology 65:229–233. [DOI] [PubMed] [Google Scholar]
- 44. Moyano LM, Saito M, Montano SM, Gonzalvez G, Olaya S, et al. (2014) Neurocysticercosis as a cause of epilepsy and seizures in two community-based studies in a cysticercosis-endemic region in Peru. PLoS neglected tropical diseases 8:e2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Allan JC, Velasquez-Tohom M, Torres-Alvarez R, Yurrita P, Garcia-Noval J (1996) Field trial of the coproantigen-based diagnosis of Taenia solium taeniasis by enzyme-linked immunosorbent assay. The American journal of tropical medicine and hygiene 54:352–356. [DOI] [PubMed] [Google Scholar]
- 46. Allan JC, Avila G, Garcia Noval J, Flisser A, Craig PS (1990) Immunodiagnosis of taeniasis by coproantigen detection. Parasitology 101 Pt 3:473–477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data have been deposited to Figshare (http://dx.doi.org/10.6084/m9.figshare.1213753).