Abstract
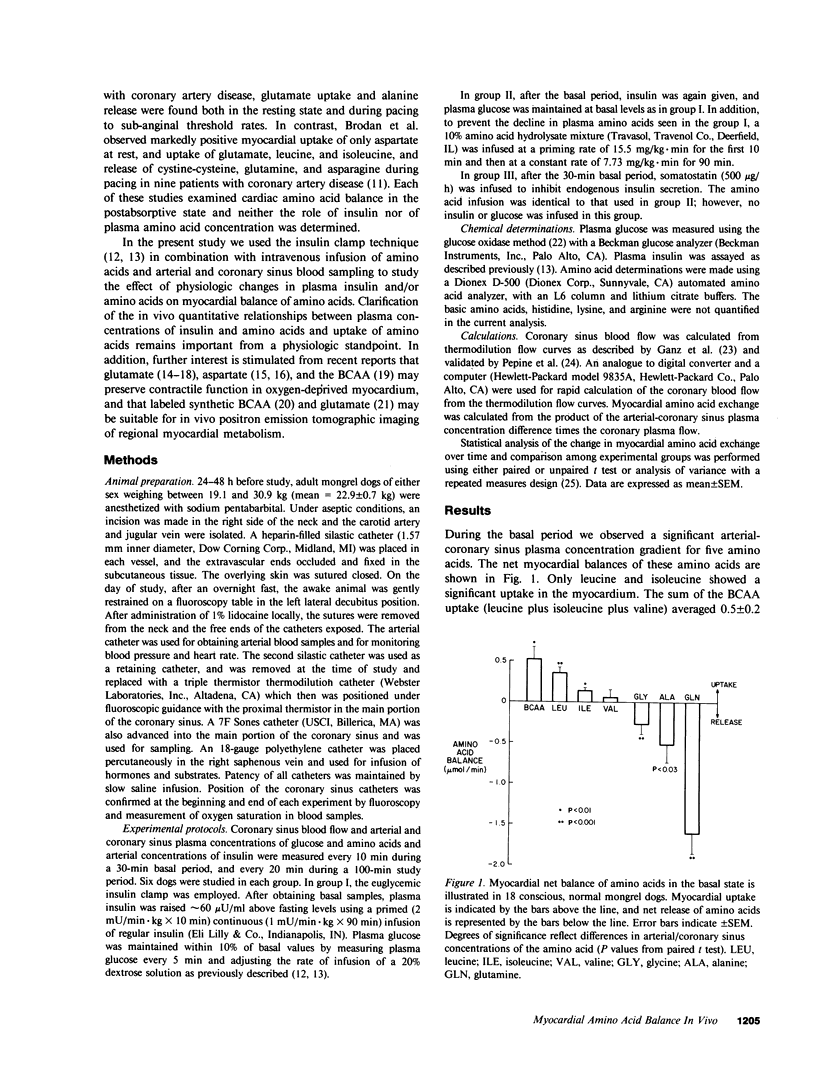
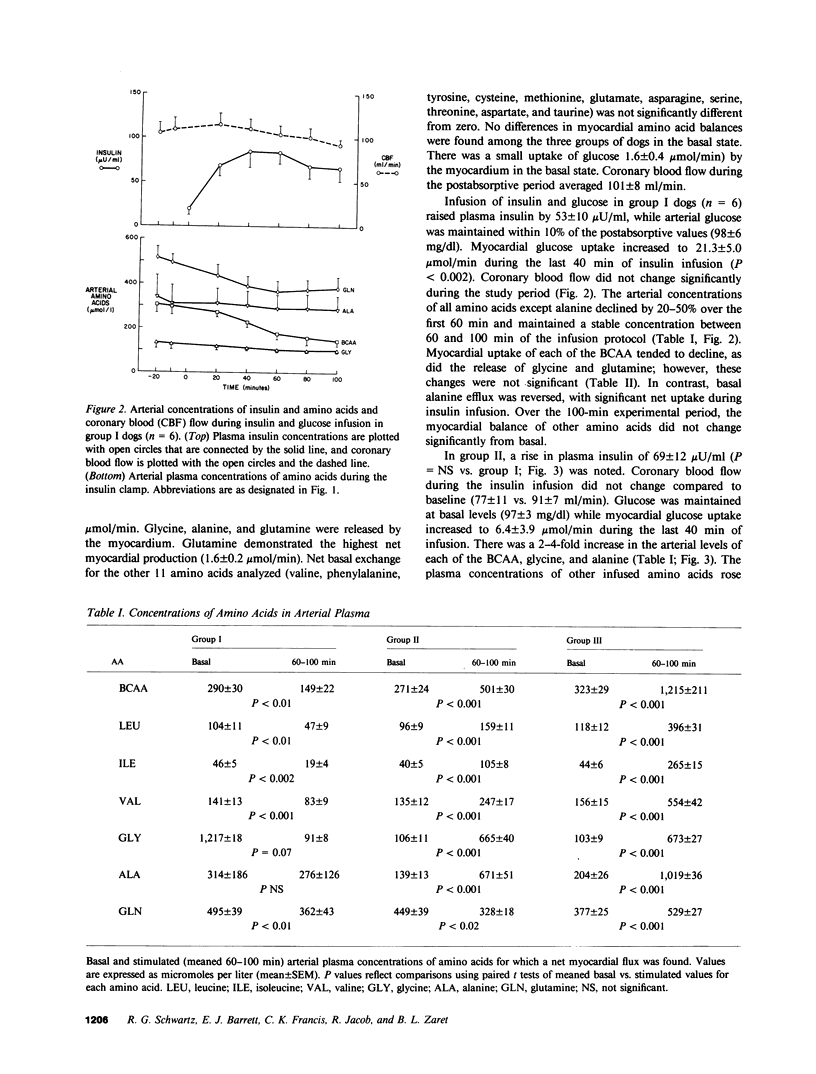
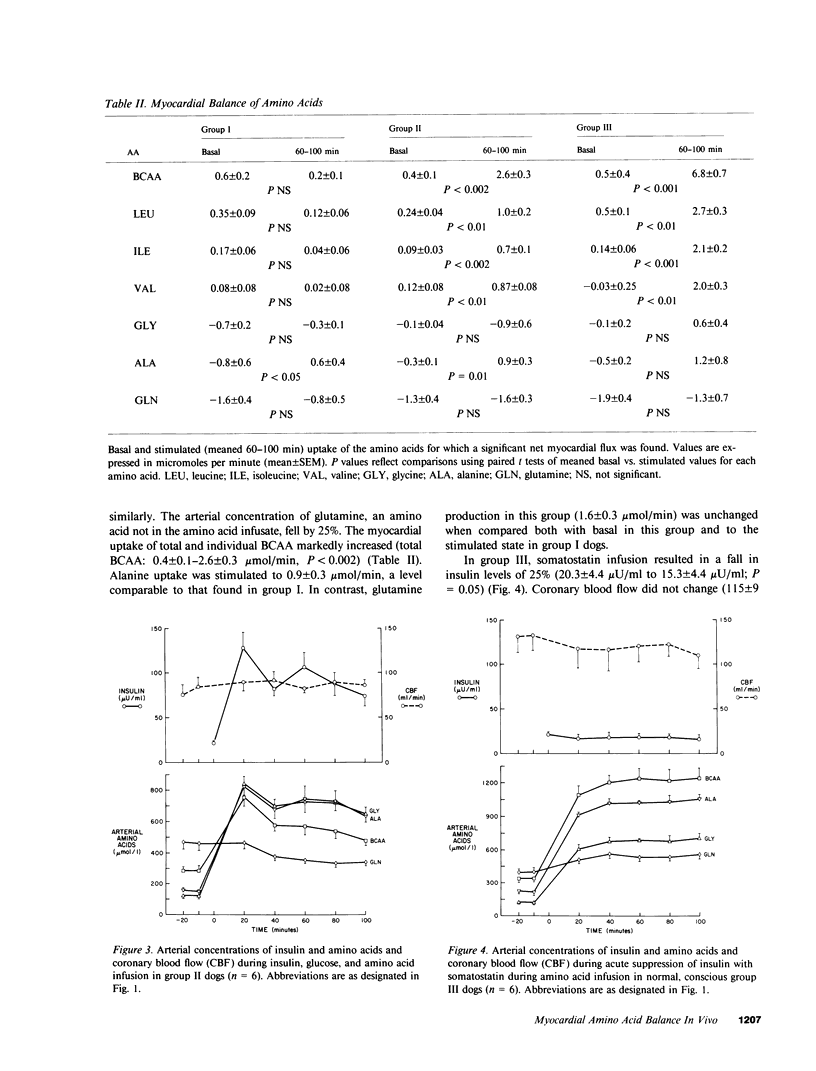
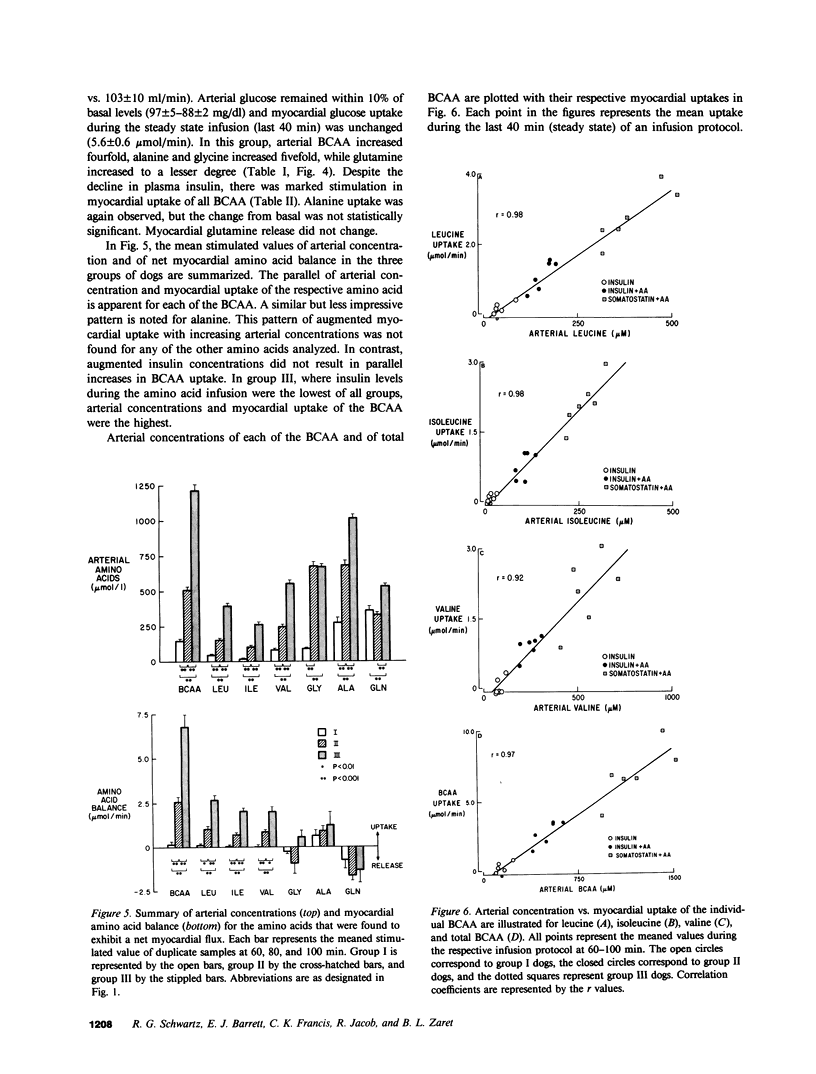
The effects in vivo of physiologic increases in insulin and amino acids on myocardial amino acid balance were evaluated in conscious dogs. Arterial and coronary sinus concentrations of amino acids and coronary blood flow were measured during a 30-min basal and a 100-min experimental period employing three protocols: euglycemic insulin clamp (plasma insulin equaled 70 +/- 11 microU/ml, n = 6); euglycemic insulin clamp during amino acid infusion (plasma insulin equaled 89 +/- 12 microU/ml, n = 6); and suppression of insulin with somatostatin during amino acid infusion (plasma insulin equaled 15 +/- 4 microU/ml, n = 6). Basally, only leucine and isoleucine were removed significantly by myocardium (net branched chain amino acid [BCAA] uptake equaled 0.5 +/- 0.2 mumol/min), while glycine, alanine, and glutamine were released. Glutamine demonstrated the highest net myocardial production (1.6 +/- 0.2 mumol/min). No net exchange was seen for valine, phenylalanine, tyrosine, cysteine, methionine, glutamate, asparagine, serine, threonine, taurine, and aspartate. In group I, hyperinsulinemia caused a decline of all plasma amino acids except alanine; alanine balance switched from release to an uptake of 0.6 +/- 0.4 mumol/min (P less than 0.05), while the myocardial balance of other amino acids was unchanged. In group II, amino acid concentrations rose, and were accompanied by a marked rise in myocardial BCAA uptake (0.4 +/- 0.1-2.6 +/- 0.3 mumol/min, P less than 0.001). Uptake of alanine was again stimulated (0.9 +/- 0.3 mumol/min, P less than 0.01), while glutamine production was unchanged (1.3 +/- 0.4 vs. 1.6 +/- 0.3 mumol/min). In group III, there was a 4-5-fold increase in the plasma concentration of the infused amino acids, accompanied by marked stimulation in uptake of only BCAA (6.8 +/- 0.7 mumol/min). Myocardial glutamine production was unchanged (1.9 +/- 0.4-1.3 +/- 0.7 mumol/min). Within the three experimental groups there were highly significant linear correlations between myocardial uptake and arterial concentration of leucine, isoleucine, valine, and total BCAA (r = 0.98, 0.98, 0.92, and 0.97, respectively); P less than 0.001 for each). In vivo, BCAA are the principal amino acids taken up by the myocardium basally and during amino acid infusion. Plasma BCAA concentration and not insulin determines the rate of myocardial BCAA uptake. Insulin stimulates myocardial alanine uptake. Neither insulin nor amino acid infusion alters myocardial glutamine release.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abumrad N. N., Rabin D., Wise K. L., Lacy W. W. The disposal of an intravenously administered amino acid load across the human forearm. Metabolism. 1982 May;31(5):463–470. doi: 10.1016/0026-0495(82)90235-9. [DOI] [PubMed] [Google Scholar]
- BING R. J. CARDIAC METABOLISM. Physiol Rev. 1965 Apr;45:171–213. doi: 10.1152/physrev.1965.45.2.171. [DOI] [PubMed] [Google Scholar]
- Barrett E. J., Schwartz R. G., Francis C. K., Zaret B. L. Regulation by insulin of myocardial glucose and fatty acid metabolism in the conscious dog. J Clin Invest. 1984 Sep;74(3):1073–1079. doi: 10.1172/JCI111474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrio J. R., Baumgartner F. J., Henze E., Stauber M. S., Egbert J. E., MacDonald N. S., Schelbert H. R., Phelps M. E., Liu F. T. Synthesis and myocardial kinetics of N-13 and C-11 labeled branched-chain L-amino acids. J Nucl Med. 1983 Oct;24(10):937–944. [PubMed] [Google Scholar]
- Bergmann S. R., Lerch R. A., Fox K. A., Ludbrook P. A., Welch M. J., Ter-Pogossian M. M., Sobel B. E. Temporal dependence of beneficial effects of coronary thrombolysis characterized by positron tomography. Am J Med. 1982 Oct;73(4):573–581. doi: 10.1016/0002-9343(82)90338-2. [DOI] [PubMed] [Google Scholar]
- Bittl J. A., Shine K. I. Protection of ischemic rabbit myocardium by glutamic acid. Am J Physiol. 1983 Sep;245(3):H406–H412. doi: 10.1152/ajpheart.1983.245.3.H406. [DOI] [PubMed] [Google Scholar]
- Brodan V., Fabián J., Andel M., Pechar J. Myocardial amino acid metabolism in patients with chronic ischemic heart disease. Basic Res Cardiol. 1978 Mar-Apr;73(2):160–170. doi: 10.1007/BF01906751. [DOI] [PubMed] [Google Scholar]
- Buse M. G., Biggers J. F., Friderici K. H., Buse J. F. Oxidation of branched chain amino acids by isolated hearts and diaphragms of the rat. The effect of fatty acids, glucose, and pyruvate respiration. J Biol Chem. 1972 Dec 25;247(24):8085–8096. [PubMed] [Google Scholar]
- CARLSTEN A., HALLGREN B., JAGENBURG R., SVANBORG A., WERKO L. Myocardial metabolism of glucose, lactic acid, amino acids and fatty acids in healthy human individuals at rest and at different work loads. Scand J Clin Lab Invest. 1961;13:418–428. [PubMed] [Google Scholar]
- Chua B. H., Siehl D. L., Morgan H. E. A role for leucine in regulation of protein turnover in working rat hearts. Am J Physiol. 1980 Dec;239(6):E510–E514. doi: 10.1152/ajpendo.1980.239.6.E510. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Ganz W., Tamura K., Marcus H. S., Donoso R., Yoshida S., Swan H. J. Measurement of coronary sinus blood flow by continuous thermodilution in man. Circulation. 1971 Aug;44(2):181–195. doi: 10.1161/01.cir.44.2.181. [DOI] [PubMed] [Google Scholar]
- Gazzola G. C., Franchi R., Saibene V., Ronchi P., Guidotti G. G. Regulation of amino acid transport in chick embryo heart cells. I. Adaptive system of mediation for neutral amino acids. Biochim Biophys Acta. 1972 May 9;266(2):407–421. doi: 10.1016/0005-2736(72)90097-1. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Chang T. W. Regulation and significance of amino acid metabolism in skeletal muscle. Fed Proc. 1978 Jul;37(9):2301–2307. [PubMed] [Google Scholar]
- Guidotti G. G., Borghetti A. F., Gazzola G. C. The regulation of amino acid transport in animal cells. Biochim Biophys Acta. 1978 Dec 15;515(4):329–366. doi: 10.1016/0304-4157(78)90009-6. [DOI] [PubMed] [Google Scholar]
- Ichihara K., Neely J. R., Siehl D. L., Morgan H. E. Utilization of leucine by working rat heart. Am J Physiol. 1980 Dec;239(6):E430–E436. doi: 10.1152/ajpendo.1980.239.6.E430. [DOI] [PubMed] [Google Scholar]
- Jacob R., Barrett E. Chromatographic analysis of glutamine in plasma. J Chromatogr. 1982 Apr 16;229(1):188–192. doi: 10.1016/s0378-4347(00)86050-8. [DOI] [PubMed] [Google Scholar]
- Krebs H. A. Some aspects of the regulation of fuel supply in omnivorous animals. Adv Enzyme Regul. 1972;10:397–420. doi: 10.1016/0065-2571(72)90025-8. [DOI] [PubMed] [Google Scholar]
- Krivokapich J., Barrio J. R., Phelps M. E., Watanabe C. R., Keen R. E., Padgett H. C., Douglas A., Shine K. I. Kinetic characterization of 13NH3 and [13N]glutamine metabolism in rabbit heart. Am J Physiol. 1984 Feb;246(2 Pt 2):H267–H273. doi: 10.1152/ajpheart.1984.246.2.H267. [DOI] [PubMed] [Google Scholar]
- Lazar H. L., Buckberg G. D., Manganaro A. J., Becker H., Maloney J. V., Jr Reversal of ischemic damage with amino acid substrate enhancement during reperfusion. Surgery. 1980 Nov;88(5):702–709. [PubMed] [Google Scholar]
- Lowenstein J. M., Goodman M. N. The purine nucleotide cycle in skeletal muscle. Fed Proc. 1978 Jul;37(9):2308–2312. [PubMed] [Google Scholar]
- Marshall R. C., Tillisch J. H., Phelps M. E., Huang S. C., Carson R., Henze E., Schelbert H. R. Identification and differentiation of resting myocardial ischemia and infarction in man with positron computed tomography, 18F-labeled fluorodeoxyglucose and N-13 ammonia. Circulation. 1983 Apr;67(4):766–778. doi: 10.1161/01.cir.67.4.766. [DOI] [PubMed] [Google Scholar]
- Morgan H. E., Earl D. C., Broadus A., Wolpert E. B., Giger K. E., Jefferson L. S. Regulation of protein synthesis in heart muscle. I. Effect of amino acid levels on protein synthesis. J Biol Chem. 1971 Apr 10;246(7):2152–2162. [PubMed] [Google Scholar]
- Morgan H. E., Jefferson L. S., Wolpert E. B., Rannels D. E. Regulation of protein synthesis in heart muscle. II. Effect of amino acid levels and insulin on ribosomal aggregation. J Biol Chem. 1971 Apr 10;246(7):2163–2170. [PubMed] [Google Scholar]
- Mudge G. H., Jr, Mills R. M., Jr, Taegtmeyer H., Gorlin R., Lesch M. Alterations of myocardial amino acid metabolism in chronic ischemic heart disease. J Clin Invest. 1976 Nov;58(5):1185–1192. doi: 10.1172/JCI108571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain V. M., Garlick P. J. Effect of streptozotocin diabetes and insulin treatment on the rate of protein synthesis in tissues of the rat in vivo. J Biol Chem. 1974 Jul 25;249(14):4510–4514. [PubMed] [Google Scholar]
- Pepine C. J., Mehta J., Webster W. W., Jr, Nichols W. W. In vivo validation of a thermodilution method to determine regional left ventricular blood flow in patients with coronary disease. Circulation. 1978 Nov;58(5):795–802. doi: 10.1161/01.cir.58.5.795. [DOI] [PubMed] [Google Scholar]
- Pisarenko O. I., Solomatina E. S., Studneva I. M., Ivanov V. E., Kapelko V. I., Smirnov V. N. Effect of glutamic and aspartic acids on adenine nucleotides, nitrogenous compounds and contractile function during underperfusion of isolated rat heart. J Mol Cell Cardiol. 1983 Jan;15(1):53–60. doi: 10.1016/0022-2828(83)90307-3. [DOI] [PubMed] [Google Scholar]
- Rau E. E., Shine K. I., Gervais A., Douglas A. M., Amos E. C., 3rd Enhanced mechanical recovery of anoxic and ischemic myocardium by amino acid perfusion. Am J Physiol. 1979 Jun;236(6):H873–H879. doi: 10.1152/ajpheart.1979.236.6.H873. [DOI] [PubMed] [Google Scholar]
- Scharff R., Wool I. G. Accumulation of amino acids in muscle of perfused rat heart. Effect of insulin. Biochem J. 1965 Oct;97(1):257–271. doi: 10.1042/bj0970257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taegtmeyer H., Ferguson A. G., Lesch M. Protein degradation and amino acid metabolism in autolyzing rabbit myocardium. Exp Mol Pathol. 1977 Feb;26(1):52–62. doi: 10.1016/0014-4800(77)90065-x. [DOI] [PubMed] [Google Scholar]
- Taegtmeyer H., Peterson M. B., Ragavan V. V., Ferguson A. G., Lesch M. De novo alanine synthesis in isolated oxygen-deprived rabbit myocardium. J Biol Chem. 1977 Jul 25;252(14):5010–5018. [PubMed] [Google Scholar]
- Wahren J., Felig P., Hagenfeldt L. Effect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes mellitus. J Clin Invest. 1976 Apr;57(4):987–999. doi: 10.1172/JCI108375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Champlain J., Farley L., Cousineau D., van Ameringen M. R. Circulating catecholamine levels in human and experimental hypertension. Circ Res. 1976 Feb;38(2):109–114. doi: 10.1161/01.res.38.2.109. [DOI] [PubMed] [Google Scholar]



