Abstract
Potent gastric acid suppression using proton pump inhibitors (PPIs) is common in clinical practice yet may have important effects on human health that are mediated through changes in the gastrointestinal microbiome. Acting through pH-dependent or pH-independent mechanisms, PPIs have the potential to alter the normal microbiota throughout the human gastrointestinal lumen. In the esophagus, PPIs change the normal bacterial milieu to decrease distal esophageal exposure to inflammatory Gram-negative bacteria which may lower the risk of Barrett's esophagus. In the stomach, PPIs alter the abundance and location of gastric Helicobacter pylori and other bacteria, which has implications for peptic ulcer disease and gastric malignancy. In the small bowel, PPIs cause polymicrobial small bowel bacterial overgrowth and have been associated with the diagnosis of celiac disease. In the colon, PPIs associate with incident but not recurrent Clostridium difficile infection, putatively through alterations in commensal colonic anaerobes. Our understanding of the effect of gastric acid suppression on the human gastrointestinal microbiome is incomplete but is rapidly advancing.
Keywords: Proton pump inhibitors, Gastric acid suppression, Hypergastrinemia, Human microbiome, Barrett's esophagus, Helicobacter pylori, Small bowel bacterial overgrowth, Clostridium difficile infection
INTRODUCTION
For centuries, it has been known that dietary factors influence gastrointestinal bacteria; Dorlencourt hypothesized that pH differences between breast milk and cow’s milk explained the higher proportions of Lactobacillus observed in the stools of breastfed children.1 Today, the role of gastric acidity in the human gastrointestinal microbiome is intertwined with the development and increasing use of proton pump inhibitors (PPIs). Other medications can alter the pH of the human gastrointestinal lumen. However, PPIs are the most potent, the most common, and have received the most attention. This review focuses on PPIs and covers the physiology of gastric acid production and suppression, and the evidence and clinical consequences of acid-related changes in the normal microbiome.
PROTON PUMP INHIBITORS AND GASTROINTESTINAL ACIDITY
Normal gastrointestinal acidity
Acidity within the human gastrointestinal tract varies by anatomic location and is part of essential physiologic processes including digestion and nutrient absorption.2 In the stomach, lumenal pH can approach 1.0; gastric acid plays a role in breakdown of food particles, and the pH-dependent separation of intrinsic factor from R-protein.3 Outside of the stomach, lumenal pH is often discussed in the context of optimizing drug delivery. In general, pH tends to rise gradually from 6.5 in the small bowel to a high of 7.5, drop in the cecum (to as low as 5.5), and again rise gradually in the left colon to a high of 6.5 – 7.0.4 The invariant pattern of gastrointestinal pH seen between individuals suggests that pH plays crucial physiologic roles throughout the gastrointestinal tract. Local pH partially determines the absorption of biotin and folate in the small bowel,5,6 vitamin B12 in the distal ileum,7 and calcium and other electrolytes in the colon.8 Thus, in addition to the influence that pH exerts on the microbiome, gastrointestinal acidity is important and tightly regulated.
Physiology of gastric acid production
Food, stress, and other central and hormonal mechanisms stimulate gastric acid secretion acting via autonomic and paracrine signals. The primary signals are gastrin from pyloric and duodenal G-cells, acetylcholine from postganglionic neurons in the gastric submucosa, and histamine from enterochromaffin-like cells; the common target of these signals and the acid-producing cell of the stomach is the parietal cell.9 In response to stimuli, transmembrane H+/K+-ATPase pumps are translocated from tubulovesicles into parietal cell canaliculi, increasing their concentration on the cell surface by 10-fold. These powerful pumps then acidify the stomach by utilizing ATP for energy to drive protons or hydronium ions against enormous concentration gradients.10
Proton pump inhibitors
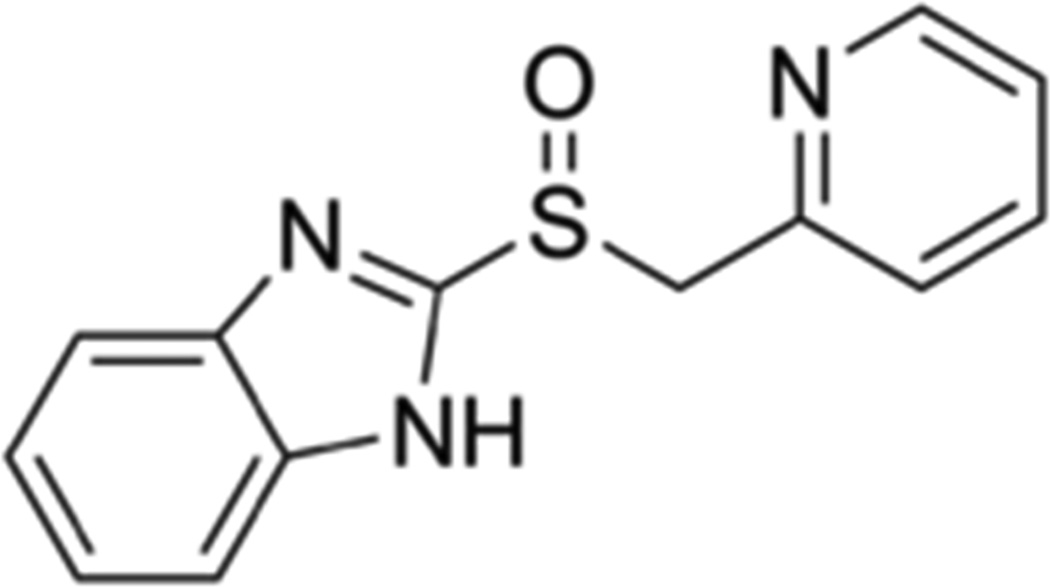
Proton pump inhibitors were independently synthesized by two companies from 2-pyridylthioacetamide by screening modified compounds (Figure 1); the first PPIs were omeprazole (1988) and lansoprazole (1991).11 There were initial safety concerns surrounding omeprazole, which was linked to increased risk for gastric carcinoids.12 Subsequent studies suggested that PPIs did not confer increased risk for malignancy and more PPIs were developed including enantiomers (esomeprazole and dexlansoprazole) of the original PPIs.13 There are currently 7 PPIs available in the United States by prescription, 2 PPIs (omeprazole and lansoprazole) that are available over-the-counter, and 3 PPIs (omeprazole, lansoprazole, and pantoprazole) which are available as generics. Because PPIs are metabolized through the hepatic cytochrome P450 system, drug levels can vary between formulations for individuals with certain pharmacogenetic characteristics.14 However, there is little evidence that the various PPI formulations differ significantly in clinical efficacy or in side effects.15
Figure 1.
Common structure of proton pump inhibitors (PPIs). All PPIs share a common backbone, with a pyridine linked to a benzimadazole.
All PPIs are pro-drugs that are concentrated in a pH-dependent manner in the canaliculi of parietal cells. PPIs are concentrated within acidic parietal cell canaliculi, protonated, and covalently bound to cysteine residues of parietal cell H+/K+-ATPase antiporter pumps.16 Because stimulation at the prospect of food causes H+/K+-ATPases to be translocated into parietal cell canaliculi, PPIs are most effective if taken before meals when the maximal number of H+/K+-ATPases are available as targets. Once bound by PPIs, parietal cell H+/K+-ATPases are irreversibly fixed into an inactive configuration, which lasts approximately 24 hours until more H+/K+-ATPases can be inserted from resting intracellular vesicles into the apical membrane of the parietal cell. The key to the tremendous efficacy of PPIs is that they inhabit the end pathway of gastric acid production and thus, unlike other acid suppressive medications, cannot be overwhelmed by normal physiologic compensatory mechanisms.
In the stomach, PPIs induce profound hypochlorhydria. Serum concentration peaks after 2–5 hours; after 3–4 hours, a single oral PPI dose will raise gastric pH in most patients from 2.0 to over 6.0, a 10,000-fold change.17 The pH-raising effect of PPIs persist in the proximal duodenum, but are attenuated by the distal duodenum. In a study of healthy volunteers who underwent continuous pH monitoring, median pH in the distal duodenum was 5.85 after 1 week of PPIs compared to 5.95 after 1 week of placebo.18 Using wireless capsule pH measurement, there is similar overall small bowel pH between users and non-users of high-dose PPIs.19 The best available evidence thus suggests that, by the proximal jejunum, the direct pH effect of PPIs has been fully attenuated and is no longer significant.
Proton pump inhibitors have established clinical efficacy for many health conditions including peptic ulcer disease, gastroesophageal reflux, eosinophilic esophagitis, and acid hypersecretory conditions (e.g., Zollinger-Ellison syndrome). Because they are effective and are believed to be benign, PPIs have gained widespread use. They are perennially among the top three drug classes by sales in the world; one PPI, esomeprazole, was the fourth most prescribed drug by sales in the United States in 2012 and the top drug by sales through the first six months of 2013.20 When used for appropriate indications, PPIs have great benefits. However, they are often prescribed in situations where they have no potential clinical benefit.21 Over half of all inpatients who receive PPIs do not have an appropriate indication for the drugs and, among these patients, over one third are discharged on PPIs.22 Among outpatients, 80% of PPI prescriptions are repeats and 40 to 50% are for non-specific abdominal pain.23
Non-pH dependent effects of PPIs
The influence of PPIs on the gastrointestinal microbiome is presumed to depend upon their capacity to raise gastric pH. However, PPIs also have the potential to influence the microbiome through pH-independent mechanisms. First, proton pump inhibitors induce hormonal changes including hypergastrinemia and hyperparathyroidism that have the potential to alter the gastrointestinal bacterial milieu.24 Second, PPIs can alter lumenal contents to interfere with nutrient absorption and change the amount or location of bacterial food substrates. Case reports and cross-sectional studies document increased hypomagnesemia among patients on longterm PPIs, suggesting the possibility that PPIs interfere with small bowel magnesium transport.25,26 Finally, PPIs have been shown to bind non-gastric H+/K+-ATPases, both on human cells and on commensal bacteria and fungi.27 The P-type family of ATPases, which includes H+/K+-ATPases, is present on fungi, Helicobacter pylori,28 and Streptococcus pneumoniae,29 but little is known about the effect of PPIs on specific bacteria aside from H. pylori.
EFFECTS OF PROTON PUMP INHIBITORS ON THE MICROBIOME
Esophagus
Proton pump inhibitors are first-line treatment for acid-related esophageal disorders including gastroesophageal reflux disease (GERD), erosive esophagitis, Barrett’s esophagus (BE), suspected eosinophilic esophagitis, and non-erosive reflux disease.30–32 Esophageal disorders are the most common reason for prescribing a PPI. Since the 1970s, there has been a 5 to 10-fold rise in BE and esophageal adenocarcinoma (EAC), with a parallel rise in GERD.33,34 In a large pharmacy database, over 60% of patients on long-term PPIs reported heartburn and 68% carried diagnoses of GERD, dyspepsia, or both.35 But diagnostic testing was rare; only 27% of these patients underwent upper endoscopy and only 3% had testing for Helicobacter pylori.
The esophageal microbiome is altered in esophagitis and BE compared to normal controls.36 A study of distal esophageal specimens from 34 subjects who had esophagitis, BE, or an endoscopically normal esophagus found that the microbiome could be separated into 2 types: a pattern dominated by Streptococcus that associated with a normal esophagus, and a pattern dominated by Gram-negative anaerobes or microaerophilic bacteria that associated with esophagitis or Barrett's. These Gram-negative bacteria may increase esophageal inflammation by activating Toll-like receptor 4 and the NF-kB pathway through surface lipopolysaccharides (LPS).37 Alternatively, these bacteria may increase distal esophageal acid exposure by lowering lower esophageal sphincter tone or by delaying gastric emptying.38,39
Proton pump inhibitors are believed to protect against progression of Barrett's esophagus to EAC by decreasing distal esophageal mucosal acid exposure. PPIs simultaneously alter the distal esophageal microbiome in ways that may affect inflammation and carcinogenesis. The mucosal-associated microbiota of the distal esophagus, which is altered in patients with esophagitis or BE,36,40 is further modified by PPIs. A study of 34 patients with Barrett's, esophagitis, or a normal distal esophagus used 16S rRNA gene sequencing to assess the microbiome from distal esophageal biopsies and gastric aspirates, comparing results before versus after PPIs.41 Before PPIs were administered, there were no major differences in distal esophageal mucosal bacteria comparing patients with esophagitis/BE to controls. After PPIs were administered, however, there were significant increases in distal esophageal Lachnospiraceae, Comamonadaceae, and unclassified Clostridial families. The family Methylobacteriaceae, which were increased in gastric aspirates among BE/esophagitis patients before PPIs, were dramatically depleted in these patients after PPI therapy. This bacterial family has also been associated with inflamed tissue in patients with inflammatory bowel disease and found in patients with irritable bowel syndrome, suggesting that these bacteria can only thrive on altered mucosa.42
Although Helicobacter is not a dominant organism in the esophagus, H. pylori exerts control over the distal esophageal microbiome. There is a strong inverse correlation between H. pylori infection (especially cagA positive H. pylori) and Barrett's esophagus or EAC.43,44 A recent study by Fischbach et al investigated the role of acid suppression in the H. pylori-Barrett's relationship.45 The authors found that the odds ratio for the association between H. Pylori and BE was 0.56 among those who used PPIs compared to 0.90 among those who did not, implying that PPIs augment the protective effects of H. pylori for BE. These results are surprising because PPIs have powerful anti-H. pylori activity and H. pylori appears to be protective for esophageal neoplasia. The most likely explanation is that the direct protective effects of PPIs in Barrett's (via decreased distal esophageal acid exposure) outweigh indirect and less potent anti-H. pylori effects. Future studies should further elaborate the influence of PPIs in the H. pylori-Barrett's relationship and determine the precise mechanisms by which PPIs alter the distal esophageal microbiome.
Stomach
Proton pump inhibitors are a mainstay of H. pylori eradication therapy and have direct bacteriostatic activity against H. pylori46 as well as indirect Helicobacter activity via increases in gastric pH. Because H. pylori and an acidic environment are necessary for the formation of most gastric and duodenal ulcers, PPIs effectively prevent peptic ulcer disease and dramatically speed the healing of ulcers that have already formed.47 PPIs are often used in non-ulcer dyspepsia and other functional gastric conditions, although their utility under these circumstances is less clear.
The acidity of the stomach distinguishes the gastric niche from the rest of the human gastrointestinal tract and determines the composition of the gastric flora. H. pylori is the dominant microorganism of the stomach, accounting for at least 70% of the gastric microbiome by 16S rRNA sequencing in positive individuals.48 Gastric acidity both allows Helicobacter pylori to thrive and is influenced by the presence of H. pylori. Acid suppression with PPIs decreases H. pylori abundance and, in antrum-predominant infection, shifts H. pylori’s location to the corpus; meanwhile, corpus-predominant H. pylori infection can cause atrophic gastritis and achlorhydria.49
PPIs cause gastric bacterial overgrowth, and PPI-induced gastric bacterial overgrowth is related to H. pylori infection. H. pylori-infected individuals have greater pH changes with PPIs than do uninfected individuals and are consequently more susceptible to overgrowth.50 When H. pylori is absent, dominant gastric bacteria include oral flora such as Streptococcus (primarily in the mitis group)51 and common commensals such as Lactobacillus and Clostridium spp. that are seen elsewhere in the gastrointestinal tract.52,53 When gastric pH is raised above 4.0 by PPIs, Lactobacillus spp., Streptococcus spp., and other gastric bacteria proliferate and can cause nausea, bloating, and altered concentrations of upper GI anaerobes, which in turn affects conjugation of bile acids and can lead to diarrhea.54,55
In susceptible individuals, chronic H. pylori infection leads to multifocal atrophic gastritis, gastric epithelial dysplasia, and gastric cancer.56 This stepwise inflammatory process, termed the Correa cascade, has been demonstrated in animal models and corroborated by human studies; in 1994, H. pylori was recognized as a class I (definite) carcinogen by the World Health Organization.57 Because of improved hygiene and increased use of antibiotics, H. pylori infection is declining in the developed world. However, in areas at high risk for gastric cancer, PPIs have been successfully used with antibiotics to eradicate H. pylori for the chemoprevention of gastric cancer. Two large, randomized and placebo-controlled trials have been conducted in areas in China with very high baseline rates of gastric cancer. The first study, conducted among 1,630 participants with H. pylori infection in the Fujian Province showed that antibiotics and PPIs decreased incident gastric cancer among those without precursor lesions after 7.5 years of follow-up.58 A second, larger trial in the Shandong province showed a significant reduction in incident gastric cancer among all participants, comparing PPIs and amoxicillin versus placebo after 15 years of follow-up.59 Because of this and similar data, short courses of PPIs are recommended as part of a chemopreventive strategy in high-risk individuals with H. pylori infection in guidelines from the United States, Europe, and Asia.47,60,61
In H. pylori negative individuals, the effect of chronic PPI use on gastric dysplasia is less clear, and recent data suggest that H. pylori is not the only gastric microorganism that contributes to dysplasia and gastric cancer. Ironically, H. pylori does not thrive in the relatively high pH environment associated with gastric cancer. In patients with gastric cancer, H. pylori decreases in abundance and there is a shift towards Streptococci genera that are not often found in normal individuals.62 Recent data from mouse models have contributed to our understanding of the role of the non-H. pylori gastric microbiome in the pathogenesis of gastric cancer, although the high gastric pH of mice (baseline 3.0 to 4.0) may limit the ability to generalize murine microbiome findings to humans.63 A well-established mouse model of gastric cancer is the transgenic INS-GAS mouse, which overexpresses gastrin and almost invariably develops gastric cancer.64 When raised in a germfree environment, H. pylori-monoinfected INS-GAS mice had delayed progression of gastric dysplasia compared to mice with a complex gastric microbiome.65 Introduction of complex microbiota or of defined species (altered Schaedler’s flora) into the stomachs of INS-GAS mice was sufficient to accelerate dysplasia.66 This interesting finding raises the possibility that PPIs, if continued after H. pylori eradication, could promote gastric cancer pathogenesis by causing non-H. pylori gastric dysbiosis that perpetuates the Correa cascade.
The preponderance of data does not support the idea that PPIs accelerate cancer in the stomach in humans through the microbiome or other mechanisms. Early animal studies of omeprazole showed increased rates of enterochromaffin (ECL) cell carcinoids, but subsequent lifelong studies of rats failed to confirm this finding.12 Further animal studies did not indicate risk,13 and longterm prospective cohort data in humans have not shown an association between PPIs and gastric carcinoids or gastric adenocarcinoma.67
Small Bowel
The profound effect of PPIs on pH is limited to the stomach and proximal duodenum, with little-to-no effect on the pH of the majority of the small bowel.18 Nevertheless, gastric acid suppression by PPIs exerts a downstream effect on small intestinal bacterial composition. The increase in the quantity and diversity of the gastric microbiome in PPI users is paralleled by an increase in the quantity of bacteria in the proximal small bowel. A study of 450 consecutive patients undergoing glucose hydrogen breath test for suspected small intestinal bacterial overgrowth (SIBO) found that 50% of PPI users tested positively, compared to 6% of non-users.68 Using duodenal aspirates and a diagnostic criteria of 103 colonic-type organisms per cc of fluid, a study of over 300 patients found that that 36% of PPI users had SIBO compared to 22% of non-users.69 The most common organisms in SIBO patients were Escherichia coli (37%), Enterococcus spp. (32%), and Klebsiella pneumoniae (24%). Finally, a recent meta-analysis found a nearly three-fold increase in the risk of SIBO among adult users of PPIs compared to non-users (OR 2.28, 95% CI 1.24–4.21).70 The association was particularly strong when the endpoint of SIBO was classified solely on the gold standard of duodenal or jejunal aspirates (OR 7.59). While individuals with SIBO are often asymptomatic, clinical sequelae can include gas and bloating sensation due to increased intralumenal carbohydrate fermentation, iron and vitamin B12 deficiency due to competitive microbial uptake, and fat malabsorption as a consequence of bacterial deconjugation of bile acids.71,72
PPIs are often prescribed to provide gastric protection in patients who are co-ingesting nonsteroidal anti-inflammatory drugs (NSAIDs), but the combined use of these agents may exert a paradoxical cytotoxic effect on the small bowel. In a study of rats administered omeprazole or lansoprazole for 9 days plus celecoxib or naproxen for the final 4 days, PPI use was associated with reductions in jejunal Actinobacteria and Bifidobacteria, and exacerbated intestinal damage.73 This injury appeared to be mediated by dysbiosis, because injury was ameliorated when PPI-treated rats were repleted with a Bifidobacteria-enriched microbiota. Also, when germ-free mice were given jejunal bacteria from PPI-treated rats, the germ-free mice had more severe NSAID-related injuries than did germ-free mice given bacteria from control rats. While this effect has not been demonstrated in humans, these results suggest that PPIs, when co-administered with NSAIDs, may potentiate cytoxocity in the small bowel via a microbiome-mediated effect.
The rise of PPI use in recent decades has coincided temporally with an increased incidence of celiac disease, an immune-mediated enteropathy characterized by intraepithelial lymphocytosis and villous atrophy in response to the ingestion of gluten. Children with celiac disease appear to have distinct duodenal microbial characteristics including reduced Lactobacillus and Bifidobacterium and increased Bacteroides and E. coli.74 A population-based case-control study found that a prescription of a PPI was far more likely in patients prior to being diagnosed with celiac disease compared to age and sex-matched controls (OR 4.79; 95% CI 4.17–5.51). Given the possibility that PPIs may have been prescribed in response to symptoms of undiagnosed celiac disease, a sensitivity analysis excluding all PPI prescriptions in the one year immediately preceding this diagnosis found that the effect, though diminished, remained significant (OR 2.28; 95% CI 1.67–3.10).75 While these results do not prove causality, the potentially mediating effect of the microbiome on the PPI-celiac disease relationship warrants further investigation.
A second link between PPI use and the increased risk of celiac disease may relate to the effect of PPIs on H. pylori. In a large cross-sectional study of simultaneously submitted gastric and duodenal biopsy specimens to a national commercial pathology laboratory, there was a strong inverse association between H. pylori and celiac disease; this remained significant after adjusting for age, gender, and socioeconomic status (OR 0.48; 95% CI 0.40–0.58).76 This apparently protective effect of H. pylori may be due to the local recruitment of regulatory T lymphocytes, damping the immune response to potentially antigenic dietary exposures.77 Because PPIs exert a bacteriostatic effect on H. pylori, the potentially protective effect of this bacteria on celiac disease risk may be diminished by PPIs; in support of this hypothesis, the rise in diagnosis of celiac disease correlates with decreased rates of Helicobacter infection in western societies.
Colon
The large intestine contains most of the human gastrointestinal microbiome in part because the colonic pH of 5.5 to 7.0 is permissive for the growth of many microbial species.78 Elegant interspecies experiments show that, when a mammalian microbiome is transplanted into a germ-free zebrafish, the microbial structure quickly changes to resemble a conventional zebrafish microbiome.79 This suggests that very basic host characteristics – pH, temperature, and motility – are important determinants of overall microbiome structure. PPIs do not directly alter the pH of the colon, yet they may have clinically important effects on the distal gut, and interest has focused around the relationship between PPIs and Clostridium difficile infection (CDI).
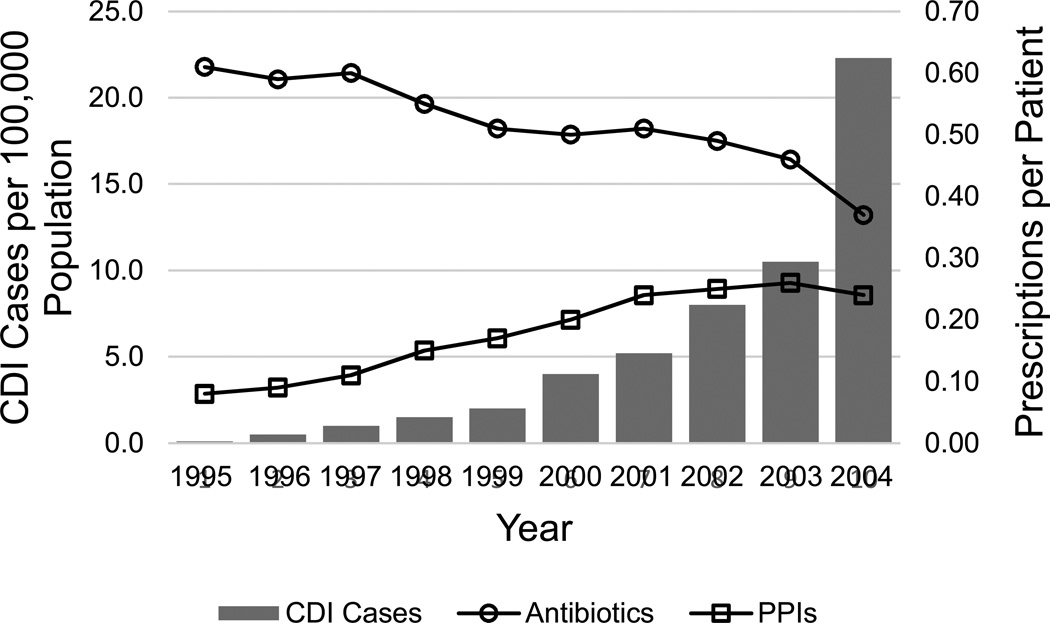
C. difficile infection is a highly morbid form of infectious colitis that has been associated with exposure to PPIs in over thirty observational studies.80,81 During a period of declining use of antibiotics, the rise in CDI correlates with increased use of proton pump inhibitors (Figure 2).82 An association between PPIs and CDI has been found among outpatients,83 inpatients,84 and patients in intensive care units.85 Multiple meta-analyses and population-based data support these findings.80,81 A program of active surveillance undertaken by the Centers for Disease Control that covers over 11 million people found that PPI exposure was 5% higher among those with CDI who did not report exposure to antibiotics, compared to those with CDI who did report exposure to antibiotics.86
Figure 2.
Corresponding rises in the incidence of C. difficile infection (CDI) and rate of proton pump inhibitor (PPI) use, during a time of decreasing antibiotic use. Adapted from: Dial S, Delaney JA, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. Dec 21 2005;294(23):2989–2995.
The mechanism linking PPIs and CDI is uncertain, but is believed to be via the microbiome. C. difficile spores are acid resistant, and acid suppression has little impact on their survival.87,88 Antibiotic use causes CDI by depleting commensal bacteria that normally block C. difficile proliferation and reducing the diversity of the colonic microbiome.89,90 Proton pump inhibitors cause small bowel bacterial overgrowth with predominantly colonic species; it follows that, with overgrowth in the proximal gut, an altered bacterial load is delivered to the colon that may predispose to CDI. Distal gut bacteria interact with colonic epithelial cells and patients using PPIs have been shown to have increased colonic intraepithelial leukocytes91 and fecal calprotectin levels,92 suggesting colonic mucosal inflammation. Proton pump inhibitors may also directly bind colonic epithelial H+/K+-ATPases, or act on the colonic mucosa through NF-kB or other systemic immune pathways.93 Further evidence supporting the hypothesis that the microbiome mediates the PPI-CDI relationship can be found in studies examining PPIs as a risk factor for recurrent CDI. Unlike studies of incident CDI, the relationship between PPIs and recurrent CDI is not clear.94,95 It is biologically plausible that PPIs cause incident CDI by altering the colonic microbiome and that this effect is blunted after the microbiome has already been perturbed by CDI.
Few studies have investigated the changes within the fecal microbiome that precede CDI. In a prospective cohort study of 599 patients during a C. difficile outbreak, decreased Bacteroidetes at the time of admission was associated with subsequent development of CDI.96 When 16S rRNA sequencing was used to compare the fecal microbiome from 25 patients who developed CDI to 25 randomly selected controls who did not, the patients who developed CDI had significant depletions in Clostridiales Incertae Sedis XI, a family that belongs to the same order as C. difficile.97 In humans, the combination of antibiotics and PPIs produced a pattern of reduced fecal bacterial diversity and reduced Bacteroidetes abundance although the effect of PPIs alone in humans is unknown.98
Under controlled conditions, PPIs have effects on the fecal microbiome of animals. In dogs, administration of high-dose PPIs increased Lactobacillus and, in male dogs, reduced commensal fecal bacterial types including Bacteroidetes.99 Another study used quantitative real-time PCR to assess the effect of achlorhydria on the fecal microbiome in Wistar rats treated with high-dose PPIs and humans with chronic atrophic gastritis.100 They observed significant increases in the levels of Lactobacillus in acid-suppressed rats and achlorhydric humans compared to controls, without comparable increases in Bacteroidetes. These fecal microbiome changes resemble some of the alterations seen after administration of antibiotics, raising the possibility that PPIs may act like antibiotics to decrease microbiome diversity or otherwise alter normal microbiome structure and lower normal colonization resistance to C. difficile.90
SUMMARY
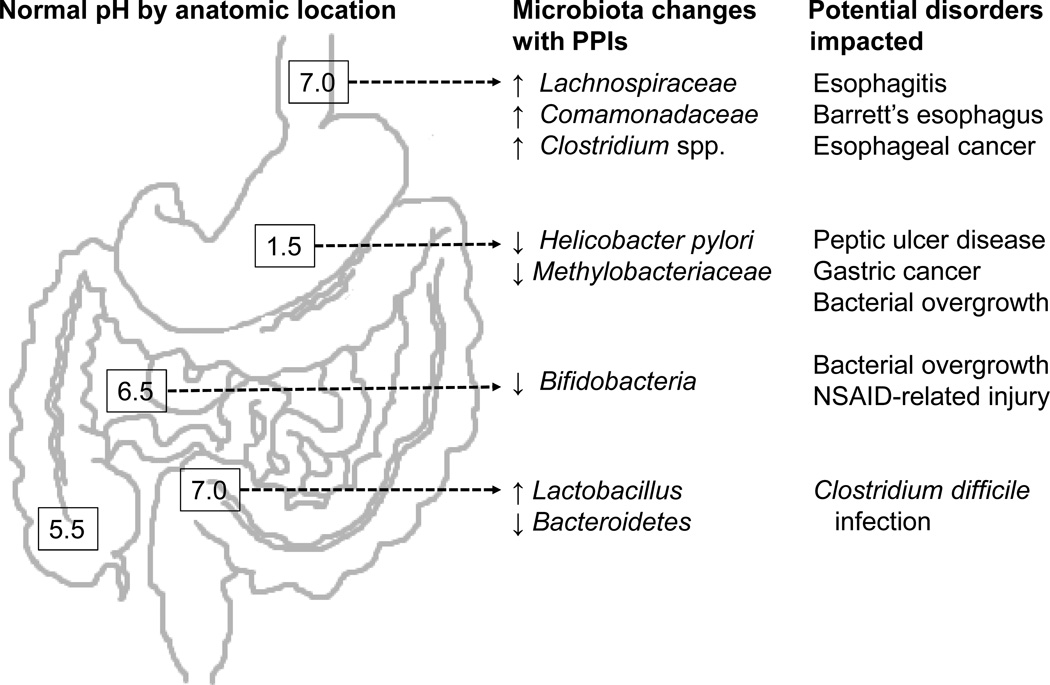
Proton pump inhibitors irreversibly bind and inactivate gastric H+/K+-ATPases to induce profound gastric achlorhydria. PPIs are highly effective treatment for acid-related disorders but are widely overused. PPIs alter the microbiome throughout the human gastrointestinal tract with important potential consequences for human health (Figure 3). The ability of PPIs to heal erosive esophagitis and slow progression of Barrett's esophagus may be partly mediated by PPI-related decreases in Gram negative bacteria. In the stomach, PPIs have a chemopreventive effect when used for eradication of Helicobacter pylori, yet contribute to gastric carcinogenesis in animals by causing dysbiosis if given after H. pylori is eradicated. In the small bowel, PPIs may cause diarrhea through bacterial overgrowth and may be a risk factor for celiac disease. Finally, epidemiological studies show that PPIs are associated with Clostridium difficile infection, although the mechanism linking PPIs and incident C. difficile remains unclear. Further research is needed to determine the effect of PPIs on the gastrointestinal microbiome and on human health.
Figure 3.
Bacteria that may be affected by proton pump inhibitors (PPIs) are shown by anatomical area; small arrows indicate directionality of changes with PPIs.
Key points.
Proton pump inhibitors (PPIs) have the potential to affect human health via interactions with the gastrointestinal microbiome.
PPIs reduce esophageal Gram negative bacteria and may decrease risk for distal esophageal neoplasia.
Given for Helicobacter pylori eradication, PPIs can prevent gastric cancer yet may cause gastric dysbiosis after H. pylori has been eradicated.
PPIs may cause small intestinal bacterial overgrowth and are associated with the diagnosis of celiac disease.
PPIs are associated with Clostridium difficile infection (CDI), although the mechanism linking PPIs and CDI is uncertain.
Acknowledgments
Funding: Daniel E. Freedberg was supported in part by NIH training grants (T32 DK083256 and UL1 RR024156); Benjamin Lebwohl was supported in part by The National Center for Advancing Translational Sciences, NIH (UL1 TR000040) and the American Gastroenterological Association Research Scholar Award; Julian A. Abrams was supported in part by Columbia University’s Irving Scholar Award and an NIH grant (U54 CA 163004).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: The authors have nothing to disclose.
References
- 1.Dorlencourt H, Lavaudon R. Les pH des selles des nourrissons sains et malades. Nourrisson. 1931;19:147–153. [Google Scholar]
- 2.Evans DF, Pye G, Bramley R, Clark AG, Dyson TJ, Hardcastle JD. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988 Aug;29(8):1035–1041. doi: 10.1136/gut.29.8.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berk L, Castle WB, et al. Observations on the etiologic relationship of achylia gastrica to pernicious anemia; activity of vitamin B12 as food, extrinsic factor. The New England journal of medicine. 1948 Dec 9;239(24):911–913. doi: 10.1056/NEJM194812092392402. [DOI] [PubMed] [Google Scholar]
- 4.Fallingborg J. Intraluminal pH of the human gastrointestinal tract. Danish medical bulletin. 1999 Jun;46(3):183–196. [PubMed] [Google Scholar]
- 5.Said HM, Redha R, Nylander W. Biotin transport in the human intestine: site of maximum transport and effect of pH. Gastroenterology. 1988 Nov;95(5):1312–1317. doi: 10.1016/0016-5085(88)90366-6. [DOI] [PubMed] [Google Scholar]
- 6.Strum WB. Enzymatic reduction and methylation of folate following pH-dependent, carrier-mediated transport in rat jejunum. Biochimica et biophysica acta. 1979 Jun 13;554(1):249–257. doi: 10.1016/0005-2736(79)90022-1. [DOI] [PubMed] [Google Scholar]
- 7.Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013 Dec 11;310(22):2435–2442. doi: 10.1001/jama.2013.280490. [DOI] [PubMed] [Google Scholar]
- 8.Carmel R, Rosenberg AH, Lau KS, Streiff RR, Herbert V. Vitamin B12 uptake by human small bowel homogenate and its enhancement by intrinsic factor. Gastroenterology. 1969 Mar;56(3):548–555. [PubMed] [Google Scholar]
- 9.Feldman M, Friedman LS, Sleisenger MH. Sleisenger & Fordtran's gastrointestinal and liver disease : pathophysiology, diagnosis, management. 7th ed. Philadelphia: Saunders; 2002. [Google Scholar]
- 10.Munson K, Lambrecht N, Shin JM, Sachs G. Analysis of the membrane domain of the gastric H(+)/K(+)-ATPase. The Journal of experimental biology. 2000 Jan;203(Pt 1):161–170. doi: 10.1242/jeb.203.1.161. [DOI] [PubMed] [Google Scholar]
- 11.Chiba T, Malfertheiner P, Satoh H. Proton pump inhibitors : a balanced view [Google Scholar]
- 12.Larsson H, Carlsson E, Mattsson H, et al. Plasma gastrin and gastric enterochromaffinlike cell activation and proliferation. Studies with omeprazole and ranitidine in intact and antrectomized rats. Gastroenterology. 1986 Feb;90(2):391–399. doi: 10.1016/0016-5085(86)90938-8. [DOI] [PubMed] [Google Scholar]
- 13.Olbe L, Carlsson E, Lindberg P. A proton-pump inhibitor expedition: the case histories of omeprazole and esomeprazole. Nat Rev Drug Discov. 2003 Feb;2(2):132–139. doi: 10.1038/nrd1010. [DOI] [PubMed] [Google Scholar]
- 14.Sim SC, Risinger C, Dahl ML, et al. A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther. 2006 Jan;79(1):103–113. doi: 10.1016/j.clpt.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Kahrilas PJ, Shaheen NJ, Vaezi MF, et al. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008 Oct;135(4):1383–1391. doi: 10.1053/j.gastro.2008.08.045. 1391 e1381–1385. [DOI] [PubMed] [Google Scholar]
- 16.Shin JM, Munson K, Vagin O, Sachs G. The gastric HK-ATPase: structure, function, and inhibition. Pflugers Arch. 2009 Jan;457(3):609–622. doi: 10.1007/s00424-008-0495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laine L, Shah A, Bemanian S. Intragastric pH with oral vs intravenous bolus plus infusion proton-pump inhibitor therapy in patients with bleeding ulcers. Gastroenterology. 2008 Jun;134(7):1836–1841. doi: 10.1053/j.gastro.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Gan KH, Geus WP, Lamers CB, Heijerman HG. Effect of omeprazole 40 mg once daily on intraduodenal and intragastric pH in H. pylori-negative healthy subjects. Digestive diseases and sciences. 1997 Nov;42(11):2304–2309. doi: 10.1023/a:1018827003641. [DOI] [PubMed] [Google Scholar]
- 19.Michalek W, Semler JR, Kuo B. Impact of acid suppression on upper gastrointestinal pH and motility. Dig Dis Sci. 2011 Jun;56(6):1735–1742. doi: 10.1007/s10620-010-1479-8. [DOI] [PubMed] [Google Scholar]
- 20. [Accessed Accessed March 23, 2014];IMS Health, Top-Line Market Data. http://www.imshealth.com/portal/site/ims/menuitem.5ad1c081663fdf9b41d84b903208c22a/?vgnextoid=fbc65890d33ee210VgnVCM10000071812ca2RCRD.
- 21.Heidelbaugh JJ, Goldberg KL, Inadomi JM. Overutilization of proton pump inhibitors: a review of cost-effectiveness and risk [corrected] The American journal of gastroenterology. 2009 Mar;104(Suppl 2):S27–S32. doi: 10.1038/ajg.2009.49. [DOI] [PubMed] [Google Scholar]
- 22.Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Alimentary pharmacology & therapeutics. 2005 May 15;21(10):1203–1209. doi: 10.1111/j.1365-2036.2005.02454.x. [DOI] [PubMed] [Google Scholar]
- 23.Bashford JN, Norwood J, Chapman SR. Why are patients prescribed proton pump inhibitors? Retrospective analysis of link between morbidity and prescribing in the General Practice Research Database. Bmj. 1998 Aug 15;317(7156):452–456. doi: 10.1136/bmj.317.7156.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang YX, Metz DC. Safety of proton pump inhibitor exposure. Gastroenterology. 2010 Oct;139(4):1115–1127. doi: 10.1053/j.gastro.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 25.Epstein M, McGrath S, Law F. Proton-pump inhibitors and hypomagnesemic hypoparathyroidism. N Engl J Med. 2006 Oct 26;355(17):1834–1836. doi: 10.1056/NEJMc066308. [DOI] [PubMed] [Google Scholar]
- 26.Markovits N, Loebstein R, Halkin H, et al. The Association of Proton Pump Inhibitors and Hypomagnesemia in the Community Setting. J Clin Pharmacol. 2014 Apr 28; doi: 10.1002/jcph.316. [DOI] [PubMed] [Google Scholar]
- 27.Vesper BJ, Jawdi A, Altman KW, Haines GK, 3rd, Tao L, Radosevich JA. The effect of proton pump inhibitors on the human microbiota. Current drug metabolism. 2009 Jan;10(1):84–89. doi: 10.2174/138920009787048392. [DOI] [PubMed] [Google Scholar]
- 28.Melchers K, Herrmann L, Mauch F, et al. Properties and function of the P type ion pumps cloned from Helicobacter pylori. Acta physiologica Scandinavica. Supplementum. 1998 Aug;643:123–135. [PubMed] [Google Scholar]
- 29.Hoskins J, Alborn WE, Jr, Arnold J, et al. Genome of the bacterium Streptococcus pneumoniae strain R6. Journal of bacteriology. 2001 Oct;183(19):5709–5717. doi: 10.1128/JB.183.19.5709-5717.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. The American journal of gastroenterology. 2013 Mar;108(3):308–328. doi: 10.1038/ajg.2012.444. quiz 329. [DOI] [PubMed] [Google Scholar]
- 31.Kahrilas PJ, Shaheen NJ, Vaezi MF, Clinical P, Quality Management C American Gastroenterological Association I. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterologyt. 2008 Oct;135(4):1392–1413. doi: 10.1053/j.gastro.2008.08.044. 1413 e1391–1395. [DOI] [PubMed] [Google Scholar]
- 32.Dellon ES, Gonsalves N, Hirano I, et al. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE) The American journal of gastroenterology. 2013 May;108(5):679–692. doi: 10.1038/ajg.2013.71. quiz 693. [DOI] [PubMed] [Google Scholar]
- 33.El-Serag HB. Time trends of gastroesophageal reflux disease: a systematic review. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2007 Jan;5(1):17–26. doi: 10.1016/j.cgh.2006.09.016. * [DOI] [PubMed] [Google Scholar]
- 34.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. Journal of the National Cancer Institute. 2005 Jan 19;97(2):142–146. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 35.Jacobson BC, Ferris TG, Shea TL, Mahlis EM, Lee TH, Wang TC. Who is using chronic acid suppression therapy and why? The American journal of gastroenterology. 2003 Jan;98(1):51–58. doi: 10.1111/j.1572-0241.2003.07186.x. [DOI] [PubMed] [Google Scholar]
- 36.Yang L, Lu X, Nossa CW, Francois F, Peek RM, Pei Z. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology. 2009 Aug;137(2):588–597. doi: 10.1053/j.gastro.2009.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang L, Francois F, Pei Z. Molecular pathways: pathogenesis and clinical implications of microbiome alteration in esophagitis and Barrett esophagus. Clin Cancer Res. 2012 Apr 15;18(8):2138–2144. doi: 10.1158/1078-0432.CCR-11-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan YP, Chakder S, Gao F, Rattan S. Inducible and neuronal nitric oxide synthase involvement in lipopolysaccharide-induced sphincteric dysfunction. Am J Physiol Gastrointest Liver Physiol. 2001 Jan;280(1):G32–G42. doi: 10.1152/ajpgi.2001.280.1.G32. [DOI] [PubMed] [Google Scholar]
- 39.Calatayud S, Garcia-Zaragoza E, Hernandez C, et al. Downregulation of nNOS and synthesis of PGs associated with endotoxin-induced delay in gastric emptying. Am J Physiol Gastrointest Liver Physiol. 2002 Dec;283(6):G1360–G1367. doi: 10.1152/ajpgi.00168.2002. [DOI] [PubMed] [Google Scholar]
- 40.Liu N, Ando T, Ishiguro K, et al. Characterization of bacterial biota in the distal esophagus of Japanese patients with reflux esophagitis and Barrett's esophagus. BMC infectious diseases. 2013;13:130. doi: 10.1186/1471-2334-13-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amir I, Konikoff FM, Oppenheim M, Gophna U, Half EE. Gastric microbiota is altered in oesophagitis and Barrett's oesophagus and further modified by proton pump inhibitors. Environmental microbiology. 2013 Sep 20; doi: 10.1111/1462-2920.12285. [DOI] [PubMed] [Google Scholar]
- 42.Rajilic-Stojanovic M, Biagi E, Heilig HG, et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011 Nov;141(5):1792–1801. doi: 10.1053/j.gastro.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 43.Wang C, Yuan Y, Hunt RH. Helicobacter pylori infection and Barrett's esophagus: a systematic review and meta-analysis. The American journal of gastroenterology. 2009 Feb;104(2):492–500. doi: 10.1038/ajg.2008.37. quiz 491, 501.* [DOI] [PubMed] [Google Scholar]
- 44.Rokkas T, Pistiolas D, Sechopoulos P, Robotis I, Margantinis G. Relationship between Helicobacter pylori infection and esophageal neoplasia: a meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2007 Dec;5(12):1413–1417. doi: 10.1016/j.cgh.2007.08.010. 1417 e1411–1412.* [DOI] [PubMed] [Google Scholar]
- 45.Fischbach LA, Graham DY, Kramer JR, et al. Association between Helicobacter pylori and Barrett's Esophagus: A Case-Control Study. Am J Gastroenterol. 2014 Mar;109(3):357–368. doi: 10.1038/ajg.2013.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iwahi T, Satoh H, Nakao M, et al. Lansoprazole, a novel benzimidazole proton pump inhibitor, and its related compounds have selective activity against Helicobacter pylori. Antimicrob Agents Chemother. 1991 Mar;35(3):490–496. doi: 10.1128/aac.35.3.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chey WD, Wong BC Practice Parameters Committee of the American College of G. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. The American journal of gastroenterology. 2007 Aug;102(8):1808–1825. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 48.Andersson AF, Lindberg M, Jakobsson H, Backhed F, Nyren P, Engstrand L. Comparative analysis of human gut microbiota by barcoded pyrosequencing. PloS one. 2008;3(7):2836. doi: 10.1371/journal.pone.0002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanduleanu S, Jonkers D, De Bruine A, Hameeteman W, Stockbrugger RW. Non-Helicobacter pylori bacterial flora during acid-suppressive therapy: differential findings in gastric juice and gastric mucosa. Alimentary pharmacology & therapeutics. 2001 Mar;15(3):379–388. doi: 10.1046/j.1365-2036.2001.00888.x. [DOI] [PubMed] [Google Scholar]
- 50.Labenz J, Tillenburg B, Peitz U, et al. Helicobacter pylori augments the pH-increasing effect of omeprazole in patients with duodenal ulcer. Gastroenterology. 1996 Mar;110(3):725–732. doi: 10.1053/gast.1996.v110.pm8608881. [DOI] [PubMed] [Google Scholar]
- 51.Engstrand L, Lindberg M. Helicobacter pylori and the gastric microbiota. Best practice & research. Clinical gastroenterology. 2013 Feb;27(1):39–45. doi: 10.1016/j.bpg.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 52.Zilberstein B, Quintanilha AG, Santos MA, et al. Digestive tract microbiota in healthy volunteers. Clinics. 2007 Feb;62(1):47–54. doi: 10.1590/s1807-59322007000100008. [DOI] [PubMed] [Google Scholar]
- 53.Li XX, Wong GL, To KF, et al. Bacterial microbiota profiling in gastritis without Helicobacter pylori infection or non-steroidal anti-inflammatory drug use. PloS one. 2009;4(11):e7985. doi: 10.1371/journal.pone.0007985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology. 2009 May;136(6):1863–1873. doi: 10.1053/j.gastro.2009.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pereira SP, Gainsborough N, Dowling RH. Drug-induced hypochlorhydria causes high duodenal bacterial counts in the elderly. Alimentary pharmacology & therapeutics. 1998 Jan;12(1):99–104. doi: 10.1046/j.1365-2036.1998.00275.x. [DOI] [PubMed] [Google Scholar]
- 56.Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005 May;128(6):1567–1578. doi: 10.1053/j.gastro.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 57.IARC working group on the evaluation of carcinogenic risks to humans: some industrial chemicals. Lyon, 15–22 February 1994. IARC monographs on the evaluation of carcinogenic risks to humans / World Health Organization, International Agency for Research on Cancer. 1994;60:1–560. [Google Scholar]
- 58.Wong BC, Lam SK, Wong WM, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004 Jan 14;291(2):187–194. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 59.Ma JL, Zhang L, Brown LM, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. Journal of the National Cancer Institute. 2012 Mar 21;104(6):488–492. doi: 10.1093/jnci/djs003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012 May;61(5):646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 61.Liu WZ, Xie Y, Cheng H, et al. Fourth Chinese National Consensus Report on the management of Helicobacter pylori infection. J Dig Dis. 2013 May;14(5):211–221. doi: 10.1111/1751-2980.12034. [DOI] [PubMed] [Google Scholar]
- 62.Dicksved J, Lindberg M, Rosenquist M, Enroth H, Jansson JK, Engstrand L. Molecular characterization of the stomach microbiota in patients with gastric cancer and in controls. Journal of medical microbiology. 2009 Apr;58(Pt 4):509–516. doi: 10.1099/jmm.0.007302-0. [DOI] [PubMed] [Google Scholar]
- 63.Tan MP, Kaparakis M, Galic M, et al. Chronic Helicobacter pylori infection does not significantly alter the microbiota of the murine stomach. Applied and environmental microbiology. 2007 Feb;73(3):1010–1013. doi: 10.1128/AEM.01675-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang TC, Dangler CA, Chen D, et al. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000 Jan;118(1):36–47. doi: 10.1016/s0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- 65.Lofgren JL, Whary MT, Ge Z, et al. Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology. 2011 Jan;140(1):210–220. doi: 10.1053/j.gastro.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lertpiriyapong K, Whary MT, Muthupalani S, et al. Gastric colonisation with a restricted commensal microbiota replicates the promotion of neoplastic lesions by diverse intestinal microbiota in the Helicobacter pylori INS-GAS mouse model of gastric carcinogenesis. Gut. 2014 Jan;63(1):54–63. doi: 10.1136/gutjnl-2013-305178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klinkenberg-Knol EC, Nelis F, Dent J, et al. Long-term omeprazole treatment in resistant gastroesophageal reflux disease: efficacy, safety, and influence on gastric mucosa. Gastroenterology. 2000 Apr;118(4):661–669. doi: 10.1016/s0016-5085(00)70135-1. [DOI] [PubMed] [Google Scholar]
- 68.Lombardo L, Foti M, Ruggia O, Chiecchio A. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2010 Jun;8(6):504–508. doi: 10.1016/j.cgh.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 69.Pyleris E, Giamarellos-Bourboulis EJ, Tzivras D, Koussoulas V, Barbatzas C, Pimentel M. The prevalence of overgrowth by aerobic bacteria in the small intestine by small bowel culture: relationship with irritable bowel syndrome. Digestive diseases and sciences. 2012 May;57(5):1321–1329. doi: 10.1007/s10620-012-2033-7. [DOI] [PubMed] [Google Scholar]
- 70.Lo WK, Chan WW. Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: a meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013 May;11(5):483–490. doi: 10.1016/j.cgh.2012.12.011. * [DOI] [PubMed] [Google Scholar]
- 71.Bures J, Cyrany J, Kohoutova D, et al. Small intestinal bacterial overgrowth syndrome. World journal of gastroenterology : WJG. 2010 Jun 28;16(24):2978–2990. doi: 10.3748/wjg.v16.i24.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Williams C, McColl KE. Review article: proton pump inhibitors and bacterial overgrowth. Alimentary pharmacology & therapeutics. 2006 Jan 1;23(1):3–10. doi: 10.1111/j.1365-2036.2006.02707.x. [DOI] [PubMed] [Google Scholar]
- 73.Wallace JL, Syer S, Denou E, et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 2011 Oct;141(4):1314–1322. doi: 10.1053/j.gastro.2011.06.075. 1322 e1311–1315. [DOI] [PubMed] [Google Scholar]
- 74.Nadal I, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Imbalance in the composition of the duodenal microbiota of children with coeliac disease. Journal of medical microbiology. 2007 Dec;56(Pt 12):1669–1674. doi: 10.1099/jmm.0.47410-0. [DOI] [PubMed] [Google Scholar]
- 75.Lebwohl B, Spechler SJ, Wang TC, Green PH, Ludvigsson JF. Use of proton pump inhibitors and subsequent risk of celiac disease. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2014 Jan;46(1):36–40. doi: 10.1016/j.dld.2013.08.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lebwohl B, Blaser MJ, Ludvigsson JF, et al. Decreased risk of celiac disease in patients with Helicobacter pylori colonization. American journal of epidemiology. 2013 Dec 15;178(12):1721–1730. doi: 10.1093/aje/kwt234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robinson K, Kenefeck R, Pidgeon EL, et al. Helicobacter pylori-induced peptic ulcer disease is associated with inadequate regulatory T cell responses. Gut. 2008 Oct;57(10):1375–1385. doi: 10.1136/gut.2007.137539. [DOI] [PubMed] [Google Scholar]
- 78.Walter J, Ley R. The human gut microbiome: ecology and recent evolutionary changes. Annual review of microbiology. 2011;65:411–429. doi: 10.1146/annurev-micro-090110-102830. [DOI] [PubMed] [Google Scholar]
- 79.Rawls JF, Mahowald MA, Ley RE, Gordon JI. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell. 2006 Oct 20;127(2):423–433. doi: 10.1016/j.cell.2006.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012 Jul;107(7):1011–1019. doi: 10.1038/ajg.2012.108. * [DOI] [PubMed] [Google Scholar]
- 81.Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012 Jul;107(7):1001–1010. doi: 10.1038/ajg.2012.179. * [DOI] [PubMed] [Google Scholar]
- 82.Dial S, Delaney JA, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005 Dec 21;294(23):2989–2995. doi: 10.1001/jama.294.23.2989. [DOI] [PubMed] [Google Scholar]
- 83.Dial S, Delaney JA, Schneider V, Suissa S. Proton pump inhibitor use and risk of community-acquired Clostridium difficile-associated disease defined by prescription for oral vancomycin therapy. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2006 Sep 26;175(7):745–748. doi: 10.1503/cmaj.060284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dial S, Alrasadi K, Manoukian C, Huang A, Menzies D. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2004 Jul 6;171(1):33–38. doi: 10.1503/cmaj.1040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Buendgens L, Bruensing J, Matthes M, et al. Administration of proton pump inhibitors in critically ill medical patients is associated with increased risk of developing Clostridium difficile-associated diarrhea. Journal of critical care. 2014 Mar 7; doi: 10.1016/j.jcrc.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 86.Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA internal medicine. 2013 Jul 22;173(14):1359–1367. doi: 10.1001/jamainternmed.2013.7056. [DOI] [PubMed] [Google Scholar]
- 87.Rao A, Jump RL, Pultz NJ, Pultz MJ, Donskey CJ. In vitro killing of nosocomial pathogens by acid and acidified nitrite. Antimicrob Agents Chemother. 2006 Nov;50(11):3901–3904. doi: 10.1128/AAC.01506-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wilson KH, Sheagren JN, Freter R. Population dynamics of ingested Clostridium difficile in the gastrointestinal tract of the Syrian hamster. J Infect Dis. 1985 Feb;151(2):355–361. doi: 10.1093/infdis/151.2.355. [DOI] [PubMed] [Google Scholar]
- 89.Kelly CP, LaMont JT. Clostridium difficile--more difficult than ever. N Engl J Med. 2008 Oct 30;359(18):1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 90.De La Cochetiere MF, Durand T, Lepage P, Bourreille A, Galmiche JP, Dore J. Resilience of the dominant human fecal microbiota upon short-course antibiotic challenge. Journal of clinical microbiology. 2005 Nov;43(11):5588–5592. doi: 10.1128/JCM.43.11.5588-5592.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu YH, Han DS, Choi EY, et al. Is use of PPIs related to increased intraepithelial lymphocytes in the colon? Dig Dis Sci. 2012 Oct;57(10):2669–2674. doi: 10.1007/s10620-012-2315-0. [DOI] [PubMed] [Google Scholar]
- 92.Poullis A, Foster R, Mendall MA, Shreeve D, Wiener K. Proton pump inhibitors are associated with elevation of faecal calprotectin and may affect specificity. Eur J Gastroenterol Hepatol. 2003 May;15(5):573–574. doi: 10.1097/00042737-200305000-00021. author reply 74. [DOI] [PubMed] [Google Scholar]
- 93.Rechkemmer G, Frizzell RA, Halm DR. Active potassium transport across guinea-pig distal colon: action of secretagogues. J Physiol. 1996 Jun 1;493(Pt 2):485–502. doi: 10.1113/jphysiol.1996.sp021398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Freedberg DE, Salmasian H, Friedman C, Abrams JA. Proton pump inhibitors and risk for recurrent Clostridium difficile infection among inpatients. The American journal of gastroenterology. 2013 Nov;108(11):1794–1801. doi: 10.1038/ajg.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Linsky A, Gupta K, Lawler EV, Fonda JR, Hermos JA. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Archives of internal medicine. 2010;170(9):772–778. doi: 10.1001/archinternmed.2010.73. [DOI] [PubMed] [Google Scholar]
- 96.Manges AR, Labbe A, Loo VG, et al. Comparative metagenomic study of alterations to the intestinal microbiota and risk of nosocomial Clostridum difficile-associated disease. J Infect Dis. 2010 Dec 15;202(12):1877–1884. doi: 10.1086/657319. [DOI] [PubMed] [Google Scholar]
- 97.Vincent C, Stephens DA, Loo VG, et al. Reductions in intestinal Clostridiales precede the development of nosocomial Clostridium difficile infection. Microbiome. 2013;1(1):18. doi: 10.1186/2049-2618-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jakobsson HE, Jernberg C, Andersson AF, Sjolund-Karlsson M, Jansson JK, Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One. 2010;5(3):e9836. doi: 10.1371/journal.pone.0009836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Garcia-Mazcorro JF, Suchodolski JS, Jones KR, et al. Effect of the proton pump inhibitor omeprazole on the gastrointestinal bacterial microbiota of healthy dogs. FEMS Microbiol Ecol. 2012 Jun;80(3):624–636. doi: 10.1111/j.1574-6941.2012.01331.x. [DOI] [PubMed] [Google Scholar]
- 100.Kanno T, Matsuki T, Oka M, et al. Gastric acid reduction leads to an alteration in lower intestinal microflora. Biochemical and biophysical research communications. 2009 Apr 17;381(4):666–670. doi: 10.1016/j.bbrc.2009.02.109. [DOI] [PubMed] [Google Scholar]