Abstract
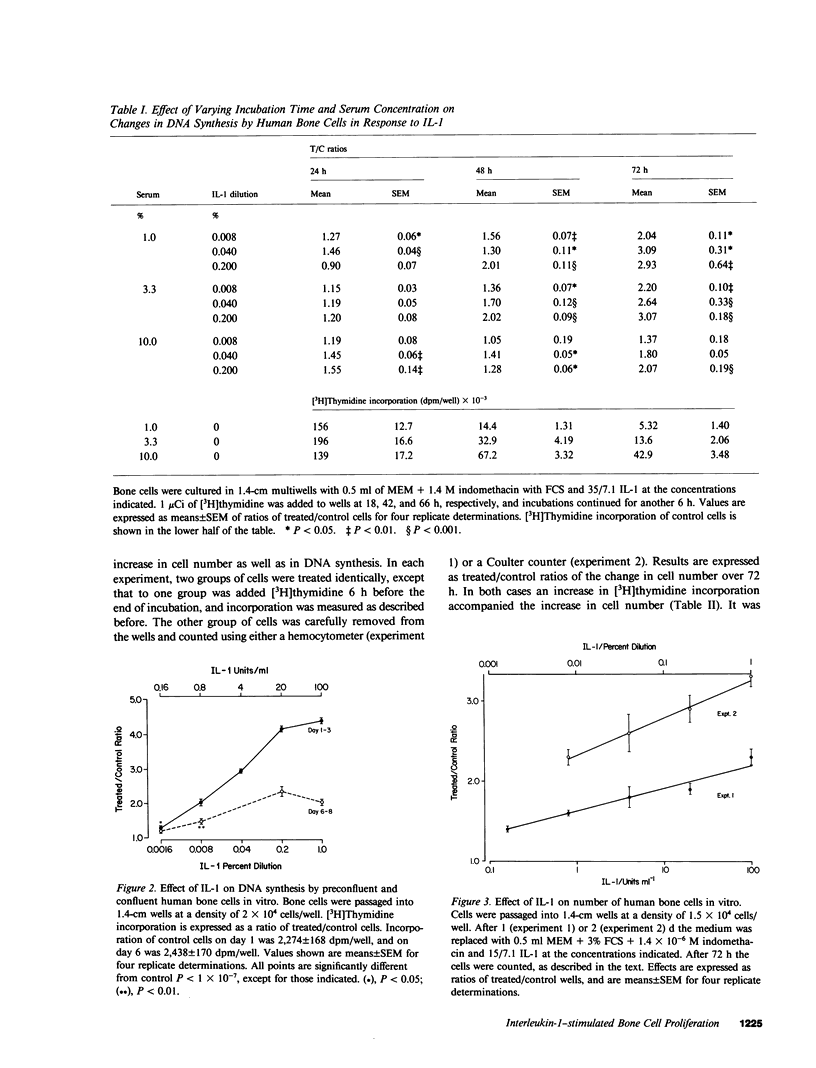
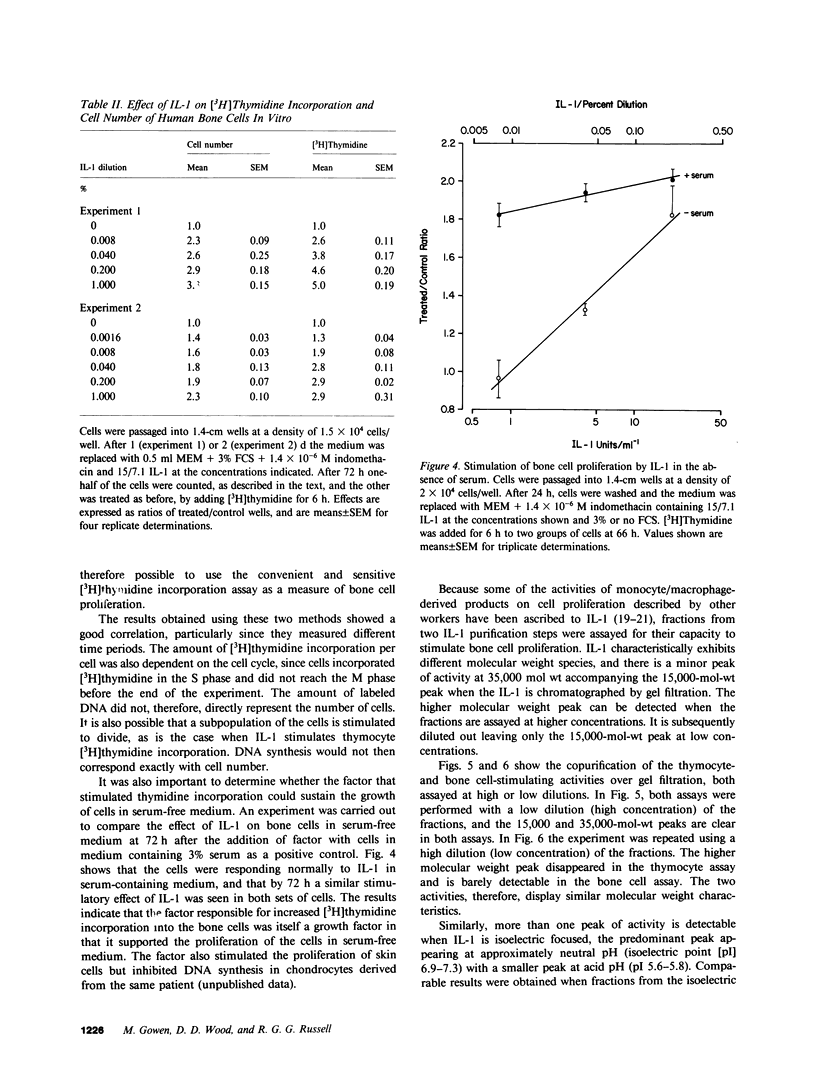
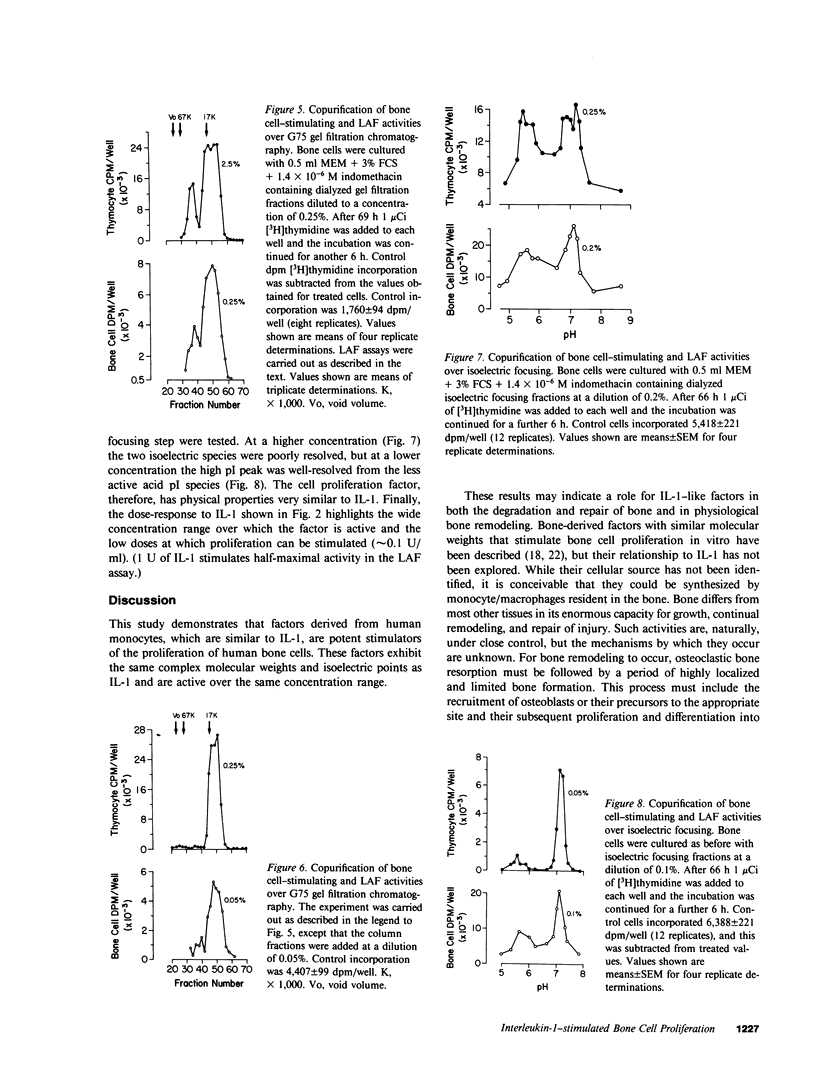
The process of induction of bone formation, which follows bone resorption during normal and pathological bone turnover, is well documented. However, the mechanisms responsible for this process are unclear. Mononuclear phagocytes present at the sites of bone remodeling could play a role in this "coupling" of bone formation to bone resorption. This study was designed to investigate such a possibility. By measuring both the increase in [3H]thymidine incorporation and in cell number, we found that human monocytes in culture released factors capable of stimulating the proliferation of osteoblast-like cells derived from human bone. Rapidly dividing cells exhibited a greater response to interleukin 1 (IL-1) than confluent cells. The factors are similar to IL-1 in that they exhibited the same molecular weight and isoelectric point, were present in fractions that contained IL-1 activity after gel filtration chromatography and isoelectric focusing, and showed similar dose-response characteristics. Proliferation was more marked when prostaglandin production by the cells, which was also stimulated by these factors, was inhibited by indomethacin. A factor produced by monocytes that affects osteoblast activity may be important in the coupling of osteoclast and osteoblast actions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beresford J. N., Gallagher J. A., Poser J. W., Russell R. G. Production of osteocalcin by human bone cells in vitro. Effects of 1,25(OH)2D3, 24,25(OH)2D3, parathyroid hormone, and glucocorticoids. Metab Bone Dis Relat Res. 1984;5(5):229–234. doi: 10.1016/0221-8747(84)90064-x. [DOI] [PubMed] [Google Scholar]
- Canalis E., Peck W. A., Raisz L. G. Stimulation of DNA and collagen synthesis by autologous growth factor in cultured fetal rat calvaria. Science. 1980 Nov 28;210(4473):1021–1023. doi: 10.1126/science.7434011. [DOI] [PubMed] [Google Scholar]
- DeLustro F., Sherer G. K., LeRoy E. C. Human monocyte stimulation of fibroblast growth by a soluble mediator(s). J Reticuloendothel Soc. 1980 Dec;28(6):519–532. [PubMed] [Google Scholar]
- Fleischmajer R., Perlish J. S., Reeves J. R. Cellular infiltrates in scleroderma skin. Arthritis Rheum. 1977 May;20(4):975–984. doi: 10.1002/art.1780200410. [DOI] [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., McGuire M. K., Russell R. G. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983 Nov 24;306(5941):378–380. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., Meats J. E., Russell R. G. Stimulation by human interleukin 1 of cartilage breakdown and production of collagenase and proteoglycanase by human chondrocytes but not by human osteoblasts in vitro. Biochim Biophys Acta. 1984 Feb 14;797(2):186–193. doi: 10.1016/0304-4165(84)90121-1. [DOI] [PubMed] [Google Scholar]
- Greenburg G. B., Hunt T. K. The proliferative response in vitro of vascular endothelial and smooth muscle cells exposed to wound fluids and macrophages. J Cell Physiol. 1978 Dec;97(3 Pt 1):353–360. doi: 10.1002/jcp.1040970310. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976 Sep;84(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- MacDonald B. R., Gallagher J. A., Ahnfelt-Ronne I., Beresford J. N., Gowen M., Russell R. G. Effects of bovine parathyroid hormone and 1,25-dihydroxyvitamin D3 on the production of prostaglandins by cells derived from human bone. FEBS Lett. 1984 Apr 9;169(1):49–52. doi: 10.1016/0014-5793(84)80287-2. [DOI] [PubMed] [Google Scholar]
- Martin B. M., Gimbrone M. A., Jr, Majeau G. R., Unanue E. R., Cotran R. S. Stimulation of human monocyte/macrophage-derived growth factor (MDGF) production by plasma fibronectin. Am J Pathol. 1983 Jun;111(3):367–373. [PMC free article] [PubMed] [Google Scholar]
- Martin B. M., Gimbrone M. A., Jr, Unanue E. R., Cotran R. S. Stimulation of nonlymphoid mesenchymal cell proliferation by a macrophage-derived growth factor. J Immunol. 1981 Apr;126(4):1510–1515. [PubMed] [Google Scholar]
- Schmidt J. A., Mizel S. B., Cohen D., Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J Immunol. 1982 May;128(5):2177–2182. [PubMed] [Google Scholar]
- Schmidt J. A., Oliver C. N., Lepe-Zuniga J. L., Green I., Gery I. Silica-stimulated monocytes release fibroblast proliferation factors identical to interleukin 1. A potential role for interleukin 1 in the pathogenesis of silicosis. J Clin Invest. 1984 May;73(5):1462–1472. doi: 10.1172/JCI111350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu N., Yoshikawa H., Takaoka K., Ono K. Extracts of cortical bone from adult rats stimulate DNA synthesis in osteoprogenitor cells from fetal rats. Clin Orthop Relat Res. 1983 Sep;(178):252–257. [PubMed] [Google Scholar]
- Urist M. R., Huo Y. K., Brownell A. G., Hohl W. M., Buyske J., Lietze A., Tempst P., Hunkapiller M., DeLange R. J. Purification of bovine bone morphogenetic protein by hydroxyapatite chromatography. Proc Natl Acad Sci U S A. 1984 Jan;81(2):371–375. doi: 10.1073/pnas.81.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Gillis S., Marbrook J., Mochizuki D., Smith K. A. Biochemical and biological characterization of lymphocyte regulatory molecules. I. Purification of a class of murine lymphokines. J Exp Med. 1979 Oct 1;150(4):849–861. doi: 10.1084/jem.150.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. D. Mechanism of action of human B cell-activating factor. I. Comparison of the plaque-stimulating activity with thymocyte-stimulating activity. J Immunol. 1979 Nov;123(5):2400–2407. [PubMed] [Google Scholar]
- Wood D. D. Purification and properties of human B cell-activating factor. J Immunol. 1979 Nov;123(5):2395–2399. [PubMed] [Google Scholar]
- Wyler D. J., Wahl S. M., Wahl L. M. Hepatic fibrosis in schistosomiasis: egg granulomas secrete fibroblast stimulating factor in vitro. Science. 1978 Oct 27;202(4366):438–440. doi: 10.1126/science.705337. [DOI] [PubMed] [Google Scholar]



