Abstract
Background:
Robotic approaches have become increasingly used for colorectal surgery. The aim of this study is to examine the safety and efficacy of robotic colorectal procedures in an adult population.
Study Design:
A systematic review of articles in both PubMed and Embase comparing laparoscopic and robotic colorectal procedures was performed. Clinical trials and observational studies in an adult population were included. Approaches were evaluated in terms of operative time, length of stay, estimated blood loss, number of lymph nodes harvested, and perioperative complications. Mean net differences and odds ratios were calculated to examine treatment effect of each group.
Results:
Two hundred eighteen articles were identified, and 17 met the inclusion criteria, representing 4,342 patients: 920 robotic and 3,422 in the laparoscopic group. Operative time for the robotic approach was 38.849 minutes longer (95% confidence interval: 17.944 to 59.755). The robotic group had lower estimated blood loss (14.17 mL; 95% confidence interval: –27.63 to –1.60), and patients were 1.78 times more likely to be converted to an open procedure (95% confidence interval: 1.24 to 2.55). There was no difference between groups with respect to number of lymph nodes harvested, length of stay, readmission rate, or perioperative complication rate.
Conclusions:
The robotic approach to colorectal surgery is as safe and efficacious as conventional laparoscopic surgery. However, it is associated with longer operative time and an increased rate of conversion to laparotomy. Further prospective randomized controlled trials are warranted to examine the cost-effectiveness of robotic colorectal surgery before it can be adopted as the new standard of care.
Keywords: Colorectal surgery, Robotic, Laparoscopic
INTRODUCTION
Since the Clinical Outcomes of Surgical Therapy Study Group published its results in 2007 on the first multicenter, randomized controlled trial assessing the laparoscopic approach to colon resection for malignancy, many subsequent studies have reported similar findings. The laparoscopic approach was not only as safe and efficacious; it was superior in perioperative outcomes and postoperative patient recovery.1–5 Following these results, many institutions began using laparoscopy as the standard approach for treatment of colorectal diseases.
In the current era of minimally invasive surgery, however, use of the da Vinci robot (Intuitive Surgical, Sunnyvale, California) has become increasingly popular and an accepted modality in many fields, such as urology and gynecology.6,7 With regard to colorectal procedures, many studies have been published indicating that the robotic approach is equal in safety and efficacy to the conventional laparoscopic approach, broadening its application in this arena.8–24
Interest in expanding the robot's application to colorectal procedures has been driven in part by the theoretical technological advancements it has over conventional laparoscopy. With robotic surgery, the EndoWrist (Intuitive Surgical) function allows a greater range of articulation and tremor reduction.5,25 The operating field is represented in 3 dimensions, allowing significantly improved views and depth perception through a surgeon-operated camera.26 In contrast, the costs associated with the da Vinci robot are a significant prohibitive factor at many institutions to widespread use.13,18,20,27 Other drawbacks include the loss of haptic feedback and a steeper learning curve.20,28 Additionally, some studies have reported longer operating times with the robotic approach compared with traditional laparoscopy.8,12,14,16,18,22
Before accepting the robotic approach for widespread use in colorectal procedures, it is important to thoroughly evaluate it in comparison with the current standard of treatment, conventional laparoscopy. The aim of this study was to examine the safety and efficacy of robotic colorectal procedures in an adult population compared with laparoscopy using a systematic review and meta-analysis. The 2 operative approaches were evaluated in terms of operative time, estimated blood loss (EBL), conversion to open procedure, length of hospital stay (LOS), readmission rate, number of lymph nodes harvested, time to return of bowel function, time to initiation of soft diet, and perioperative complications.
MATERIALS AND METHODS
Search Methodology
Two independent reviewers conducted a systematic review of publications in PubMed and Embase comparing robotic and laparoscopic colorectal procedures. Databases were searched irrespective of publication date using the Medical Subject Headings “laparoscopic robotic colectomy,” “laparoscopic colon resection robot,” and “laparotomy colon robot.”
Publications were included in the study if they met the following criteria: (1) comparative studies examining laparoscopic versus robotic colorectal procedures, regardless of type (eg, right hemicolectomy, low anterior resection, sigmoid resection); (2) randomized controlled trials, controlled clinical trials, or observational studies, if they were comparative in nature; and (3) studies that reported various outcomes of interest, including but not limited to total operative time, LOS, conversions to open procedure, postoperative outcomes, and oncologic sufficiency. Non-English studies, noncomparative studies, preclinical studies (cadaveric experiences), studies on the pediatric population, nonclinical review articles, abstracts, case reports, editorials, expert opinions, commentary articles, and letters were excluded. The reference lists of articles were reviewed for additional relevant studies for inclusion in the pooled analysis. Results of the 2 reviews by each independent reviewer were compared for accuracy, with all disagreements resolved by consensus. The sole discrepancy between the 2 reviewers involved a specific institutional group experience reporting repeated data in different studies. Only the publication by the same author or institution with the largest sample size was included for the present analysis.
Statistical Analysis
The outcomes of interest for meta-analysis included total operative time, EBL, conversion to open procedure, LOS, readmission, number of nodes harvested, time to return of bowel function (flatus), time to initiation of soft diet, total number of postoperative complications, and number of readmissions. Conversion to open procedure was defined as a conversion to standard laparotomy through the extension or creation of a midline incision for purposes other than specimen removal. Data on conversions from a robotic procedure to standard laparoscopy were not analyzed in this study, because of insufficient reporting among the publications. Only 2 papers reported on robotic-to-laparoscopic conversions, yielding 4 total of such occurrences, compared with a raw total of 22 robotic-to-laparotomy conversions.10,16 A postoperative complication was defined as a complication arising within 30 days of the procedure, occurring as a direct result of the surgery. Because of the complexity and variation in the reporting of complications, statistical analysis was based on the absolute total number of complications reported and did not include analysis of individual types of complications.
Demographics and other relevant data were collected, including study design, number of patients who underwent robotic procedures, number of patients who underwent laparoscopic procedures, patient age, body mass index, numbers of men and women, history of abdominal surgery, and indication for surgery.
All data were extracted from the articles' text, tables, and figures and entered into a computerized spreadsheet for analysis. For continuous outcomes, mean net differences (baseline-to-treatment change in treatment group mirrors change in control group) were used as primary outcomes. For categorical outcomes, odds ratios were used to examine the treatment effect. DerSimonian and Laird random-effects models were used to pool mean net changes or odds ratios across the studies.29 The presence of heterogeneity was assessed with the Cochran Q test, and the extent of heterogeneity was quantified with the I2 index. To assess publication bias, funnel plots were constructed for each outcome. The Begg rank correlation test was used to examine the asymmetry of the funnel plot, and the Egger weighted linear regression test was used to examine the association between the mean effect estimate and its variance. No significant publication bias was detected for any study outcome using either statistical method. Additionally, sensitivity analyses were conducted by excluding each study in turn, to evaluate its relative influence on the pooled estimates. All analyses were conducted in Stata version 10 (StataCorp LP, College Station, Texas).
RESULTS
Literature Search Results
The literature search yielded 216 articles from 2 databases (PubMed and Embase) and 2 additional articles through a manual review of reference lists. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses were used as a model for mapping out the number of records identified and included and the reasons for exclusion (Figure 1).30 Of the 218 articles identified, 201 were excluded because of characterization as descriptive articles, pediatric studies, out-of-scope articles, case reports, and non-English-language articles. Seventeen full-text articles were then assessed for eligibility and included for final quantitative data analysis.
Figure 1.
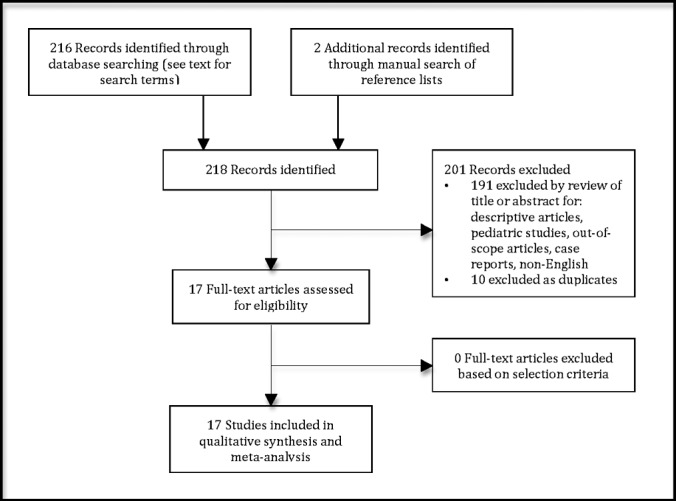
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart of literature search and study selection.
Description of Included Trials
In total, the analysis represents 4,342 patients across 17 comparative studies, 920 who underwent robotic colorectal procedures and 3,422 who underwent laparoscopic procedure (Table 1). Three articles further subdivided patients into 2 groups each, on the basis of the type of colorectal procedure.16,18,22 For example, Deutsch et al16 included 171 patients in total, 65 who underwent right colectomy (47 laparoscopic, 18 robotic) and 106 who underwent left colectomy (45 laparoscopic, 61 robotic). As a result, our study considered each subgroup divided by procedure to be separate for purposes of data analysis, yielding 20 individual study populations across 17 publications for each of the robotic and laparoscopic approaches. In terms of diagnoses, 12 studies examined robotic versus laparoscopic colorectal surgery for malignant conditions.8,9,11,12,14,15,17,19–21,23,24 One study exclusively examined patients with inflammatory bowel conditions.22 The remaining 4 studies included both benign and malignant colorectal diseases.10,13,16,18 With regard to procedure types, 2 study populations included only patients who underwent right hemicolectomy.16,18 Two studies examined patients who underwent low anterior resection.14,23 One group included sigmoid resections alone.18 Two study populations included patients who underwent total colectomy alone.22 The remaining 13 study groups included combinations of colorectal procedures.8–13,15,17,19–21,24
Table 1.
Studies Selected for Inclusion in Systematic Review
| Study | Year | Country | Study Design | Sample n | ROB n | LAP n | Indication |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Malignancy |
Diverticular Disease |
Benign |
Other |
|||||||||||
| ROB | LAP | ROB | LAP | ROB | LAP | ROB | LAP | |||||||
| Park et al8 | 2012 | Korea | Randomized controlled trial | 80 | 40 | 40 | 40 | 40 | 0 | 0 | 0 | 0 | 0 | 0 |
| Park et al9 | 2011 | Korea | Retrospective | 175 | 52 | 123 | 52 | 123 | 0 | 0 | 0 | 0 | 0 | 0 |
| D'Annibale et al10 | 2004 | Italy | Retrospective | 106 | 53 | 53 | 22 | 42 | 0 | 0 | 31 | 11 | 0 | 0 |
| Baek et al11 | 2013 | South Korea | Retrospective | 84 | 47 | 37 | 47 | 37 | 0 | 0 | 0 | 0 | 0 | 0 |
| Saklani et al12 | 2013 | South Korea | Retrospective | 138 | 74 | 64 | 74 | 64 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tyler et al13 | 2013 | USA | Retrospective | 2,583 | 160 | 2,423 | 58 | 1,032 | 58 | 843 | 44 | 548 | 0 | 0 |
| Lim et al14 | 2013 | South Korea | Retrospective | 180 | 34 | 146 | 34 | 146 | 0 | 0 | 0 | 0 | 0 | 0 |
| Shin15 | 2011 | Korea | Retrospective | 60 | 30 | 30 | 30 | 30 | 0 | 0 | 0 | 0 | 0 | 0 |
| Deutsch et al16 | 2012 | USA | Retrospective | 171 | 79 | 92 | 8 | 32 | 58 | 34 | 13 | 19 | 0 | 7 |
| Kwak et al17 | 2011 | Korea | Retrospective | 118 | 59 | 59 | 59 | 59 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rawlings et al18 | 2007 | USA | Retrospective | 57 | 30 | 27 | 5 | 8 | 8 | 12 | 16 | 6 | 1 | 1 |
| Helvind et al19 | 2012 | Denmark | Retrospective case control | 263 | 101 | 162 | 101 | 162 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bertani et al20 | 2011 | Italy | Retrospective | 64 | 34 | 30 | 34 | 30 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bianchi et al21 | 2010 | Italy | Retrospective | 50 | 25 | 25 | 25 | 25 | 0 | 0 | 0 | 0 | 0 | 0 |
| Miller22 | 2012 | USA | Retrospective | 34 | 17 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 17 | 17 |
| Baik23 | 2009 | Korea | Prospective comparative nonrandomized | 113 | 56 | 57 | 56 | 57 | 0 | 0 | 0 | 0 | 0 | 0 |
| Patriti24 | 2009 | Italy | Retrospective | 66 | 29 | 37 | 29 | 37 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: LAP, laparoscopic approach; ROB, robotic approach.
Operative Outcomes
Operative Time
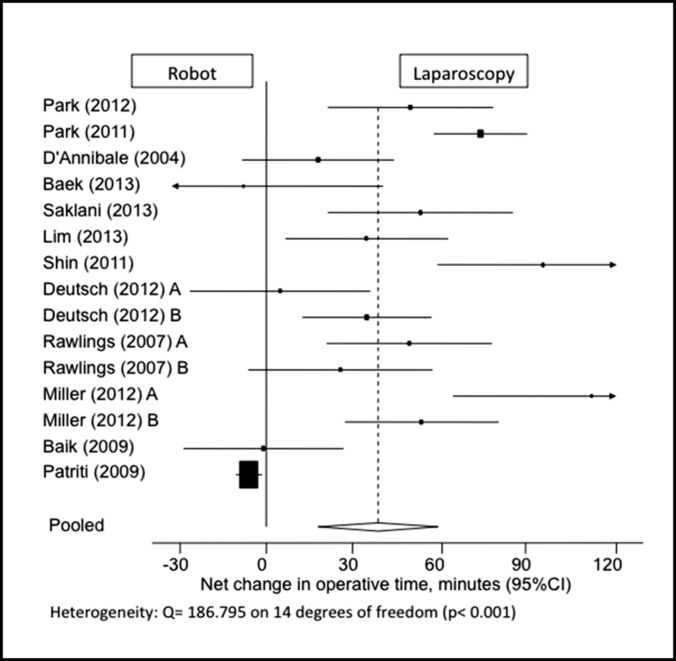
This parameter was adequately reported for 15 study populations, with a statistically significant pooled net mean difference of 38.849 minutes longer for robotic procedures compared with the laparoscopic approach (95% confidence interval [CI]: 17.944–59.755) (Figure 2). Although 3 studies reported that the robot had a trend toward slightly shorter operating times (less than a 10-minute difference), none of these findings were of statistical significance.11,23,24 One study did not report operative time.13 Four other studies reported operative time but did not include standard deviation values and were thus excluded from the data analysis.18,21–23
Figure 2.
Forest plot and pooled analysis of mean difference in operative time between robotic and laparoscopic approaches. The mean difference in operative time is reported for each study (black square) along with its 95% confidence interval (CI) (horizontal lines). The size of the square represents the weighted contribution of each study, and the diamond in the last line represents the pooled estimate and its 95% CI (width of diamond).
EBL
Thirteen study populations included data on EBL (Figure 3). The EBL was 14.17 mL less in the robotic group than in the laparoscopic group, a statistically significant value (95% CI: –27.63 to –1.60). Six studies did not report average EBL.9,13,17,19,21,23 One study included a mean value but did not report the standard deviation and was thus excluded.20
Figure 3.
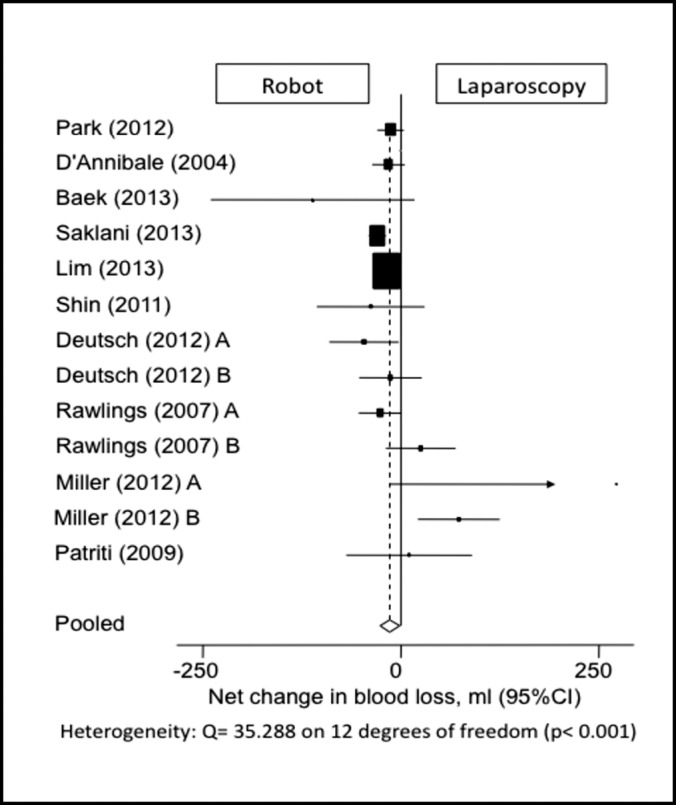
Forest plot and pooled analysis of mean difference in blood loss between robotic and laparoscopic approaches. The mean difference in blood loss is reported for each study (black square) along with its 95% confidence interval (CI) (horizontal lines). The size of the square represents the weighted contribution of each study, and the diamond in the last line represents the pooled estimate and its 95% CI (width of diamond).
Number of Harvested Lymph Nodes
Among the 9 publications that reported on the number of harvested lymph nodes, there was no significant difference between the robotic and laparoscopic approaches, with a pooled mean net change of –0.686 nodes (95% CI: –2.402 to 1.030) (Figure 4). Six study populations were excluded from data analysis because no standard deviations were reported with the values.16,17,19–23 It should be noted that in 1 laparoscopic group, the average number of harvested lymph nodes was <12, compared with 4 robotic groups in which this occurred.11,12,16,24
Figure 4.
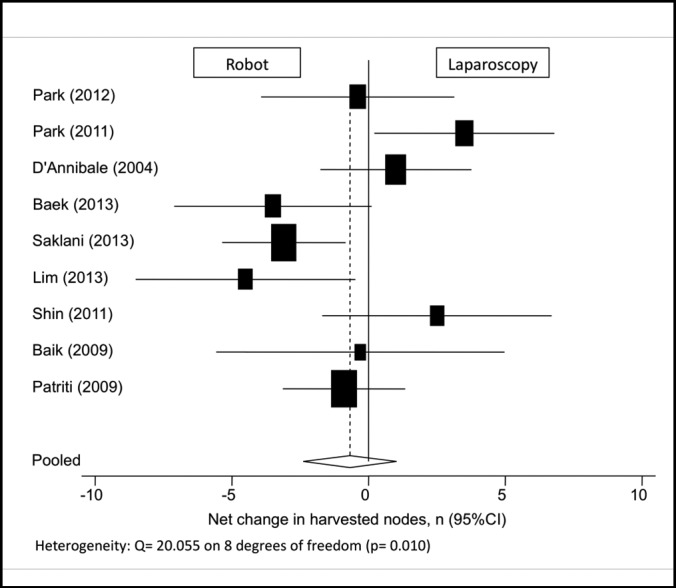
Forest plot and pooled analysis of mean difference in the number of harvested lymph nodes between robotic and laparoscopic approaches. The mean difference in number of harvested lymph nodes is reported for each study (black square) along with its 95% confidence interval (CI) (horizontal lines). The size of the square represents the weighted contribution of each study, and the diamond in the last line represents the pooled estimate and its 95% CI (width of diamond).
Days to Return of Bowel Function
Eleven study groups reported the number of days to flatus, yielding no difference between the robotic and laparoscopic approaches, with a pooled mean net change of 0.061 days (95% CI: –0.232 to 0.355 days) (Figure 5). One study was excluded because of missing data for standard deviation.21
Figure 5.
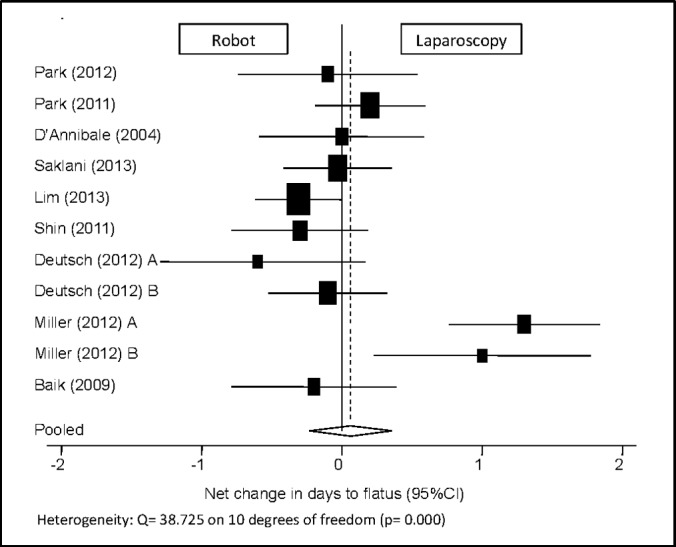
Forest plot and pooled analysis of mean difference in the number of days until return of bowel function (flatus) between robotic and laparoscopic approaches. The mean difference in the number of days until return of bowel function is reported for each study (black square) along with its 95% confidence interval (CI) (horizontal lines). The size of the square represents the weighted contribution of each study, and the diamond in the last line represents the pooled estimate and its 95% CI (width of diamond).
Days to Soft Diet
Among the 7 publications reporting the number of days to the initiation of a soft diet, there was no statistically significant difference between the 2 groups, with a pooled mean net change of –0.349 days (95% CI: –0.701 to 0.002) (Figure 6). One study was excluded because of a lack of reported standard deviation.21
Figure 6.
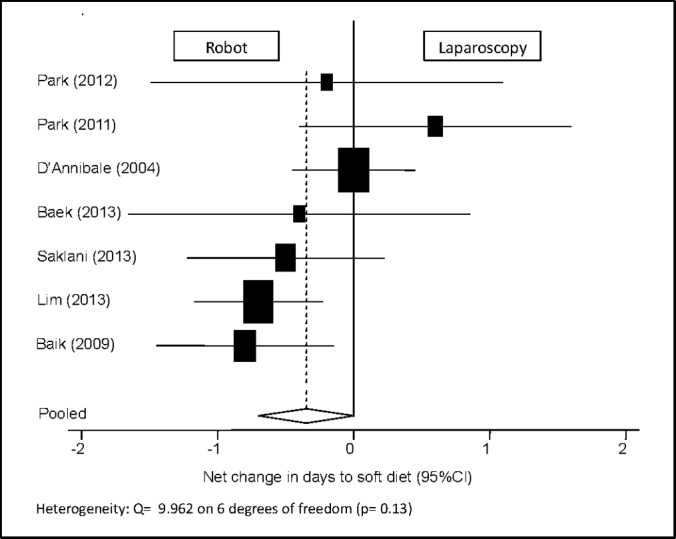
Forest plot and pooled analysis of mean difference in the number of days until initiation of soft diet between robotic and laparoscopic approaches. The mean difference in the number of days until initiation of soft diet is reported for each study (black square) along with its 95% confidence interval (CI) (horizontal lines). The size of the square represents the weighted contribution of each study, and the diamond in the last line represents the pooled estimate and its 95% CI (width of diamond).
LOS
This parameter was reported in 14 study populations. Analysis yielded no difference in LOS between the robotic and laparoscopic groups, with a pooled mean net change of 0.159 days (95% CI: 0.698 to 1.016) (Figure 7).
Figure 7.
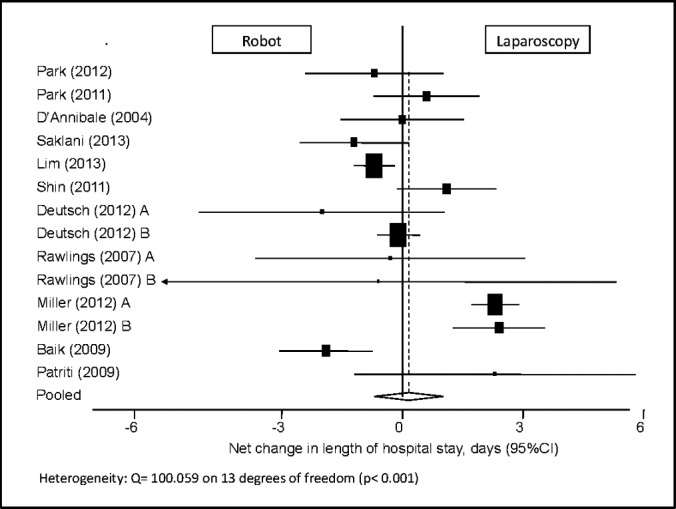
Forest plot and pooled analysis of mean difference in the length of hospital stay between robotic and laparoscopic approaches. The mean difference in the length of hospital stay is reported for each study (black square) along with its 95% confidence interval (CI) (horizontal lines). The size of the square represents the weighted contribution of each study, and the diamond in the last line represents the pooled estimate and its 95% CI (width of diamond).
Conversion to Open Procedure
All 20 study samples reported data on whether the procedure was converted to open. Five studies reported values without standard deviations and were thus excluded from analysis.11,13,19–21 Using calculated odds ratios, robotic colorectal procedures were found to be 1.78 times significantly more likely to be converted to an open procedure compared with the laparoscopic approach (95% CI: 1.24–2.55) (Figure 8).
Figure 8.
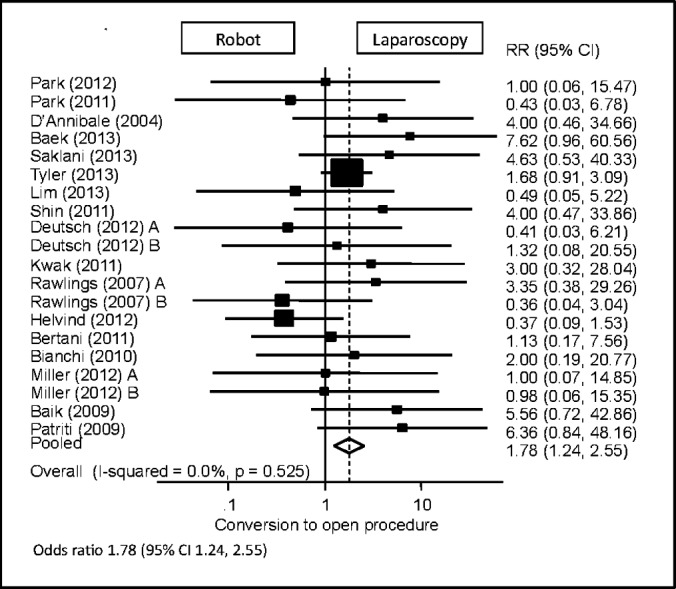
Forest plot and pooled analysis of odds ratio for conversion to laparotomy between robotic and laparoscopic approaches. The odds ratio for conversion to laparotomy is reported for each study (black square) along with its 95% confidence interval (CI) (horizontal lines). The size of the square represents the weighted contribution of each study, and the diamond in the last line represents the pooled estimate and its 95% CI (width of diamond).
Hospital Readmission
Three publications included data on hospital readmissions.11,12,14 There was a trend toward higher 30-day readmission rates for patients undergoing robotic procedures, but this was found not to be significantly different compared with laparoscopic procedures (95% CI: 0.20–1.79) (Figure 9).
Figure 9.
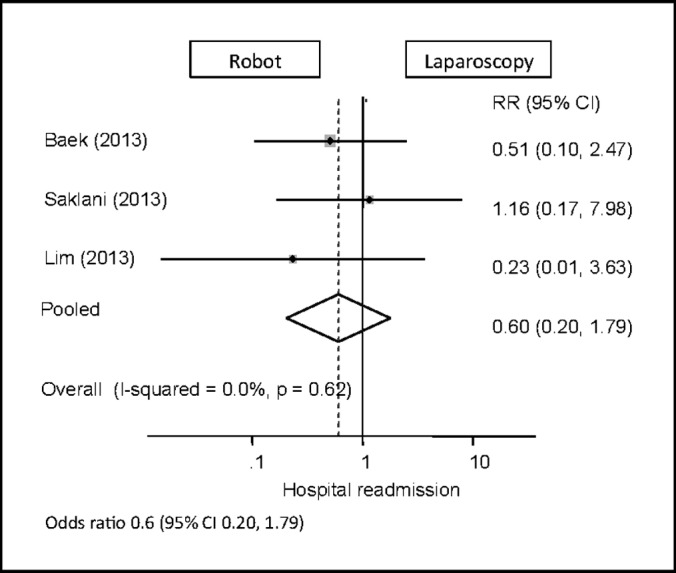
Forest plot and pooled analysis of odds ratio for hospital readmission between robotic and laparoscopic approaches. The odds ratio for hospital readmission is reported for each study (black square) along with its 95% confidence interval (CI) (horizontal lines). The size of the square represents the weighted contribution of each study, and the diamond in the last line represents the pooled estimate and its 95% CI (width of diamond).
Postoperative Complications
Seventeen study populations reported data on postoperative complications. There was no standard for reporting complications across the articles, with varying types and numbers of categories. Some studies included an “other” category but did not further specify the type of complication. Raw totals for complications included 188 in the laparoscopic group and 133 in the robotic group. Some of the most commonly reported complications included wound infection, anastomotic leak, intra-abdominal abscess, postoperative bleeding, and ileus. Of the 3,422 laparoscopic procedures and 920 robotic procedures, there were 18 and 9 reported wound infections (0.53% vs 0.98%), 48 and 36 reported anastomotic leaks (1.40% vs 3.91%), 17 and 11 intra-abdominal abscesses (0.50% vs 1.20%), 15 and 12 cases of postoperative bleeding (0.44% vs 1.30%), and 35 and 19 reported cases of ileus (1.02% vs 2.07%), respectively.
Statistical analysis in this study was based on the total number of complications. The robotic approach to colorectal procedures was found to have similar overall complication rates compared with the laparoscopic approach (95% CI: 0.90–1.34) (Figure 10).
Figure 10.
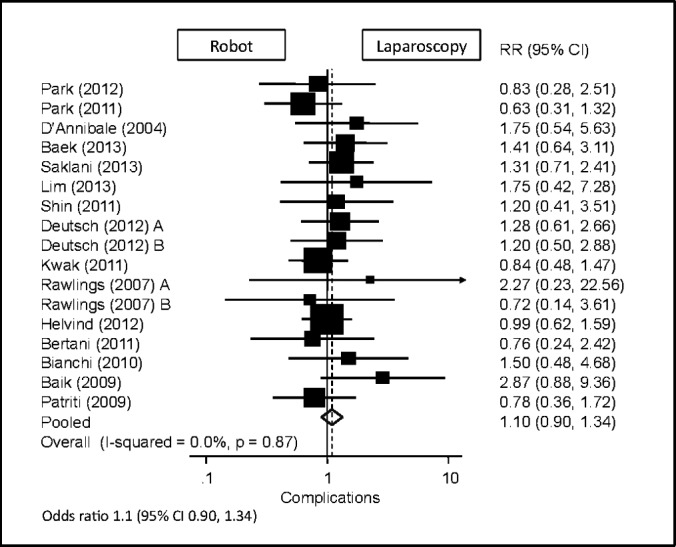
Forest plot and pooled analysis of odds ratio for total overall complications between robotic and laparoscopic approaches. The odds ratio for total overall complications is reported for each study (black square) along with its 95% confidence interval (CI) (horizontal lines). The size of the square represents the weighted contribution of each study, and the diamond in the last line represents the pooled estimate and its 95% CI (width of diamond).
DISCUSSION
This meta-analysis of 17 publications and >4,300 patients comparing robotic and laparoscopic colorectal surgery comprises the most comprehensive and current results available on the subject. The data suggest that the robotic approach is as safe and effective as the laparoscopic approach. However, the operative time in robotic procedures was approximately 40 minutes longer than in laparoscopic procedures. Of the studies included for analysis, 6 of 15 reported significant differences, with all 6 indicating that robotic procedures had longer operating times.8,12,14,16,18,22 The time spent docking the robot as well as the time necessary for maintenance of equipment and drapes specific to the robot have been shown to decrease with experience.9,19 However, despite the learning curve that exists with implementing new equipment and technology,20,28 most of these studies involved colon and rectal procedures performed by either a single surgeon or a small group of 2 to 4 surgeons, all of whom had prior training and experience with robotic surgery.8–11,15–18,23
Although cost analysis was not conducted in this study, previous studies have consistently demonstrated increased costs when performing robotic colon and rectal surgery, and that part of these costs is related predominantly to longer procedure times.27,28,31 When considering the future of colorectal surgery, the extent to which the da Vinci robot will be used over conventional laparoscopy will be partially dependent on the valuation of the time and cost associated with longer procedures.
EBL was 14 mL less in robotic procedures than in laparoscopic cases. Although statistically significant, there was no clinically relevant difference. Although 1 of the theoretical advantages of robotic over conventional laparoscopic surgery is that a more precise technique and tremor reduction would minimize tissue trauma and blood loss, this claim has not been consistently substantiated in the literature. Of the 13 studies included for analysis here, only 1 yielded a statistically significant difference in blood loss. Furthermore, it demonstrated greater blood loss in the robotic group compared with the laparoscopic group (123 vs 76 mL, P = .0358).16 Unlike the more pronounced reductions in blood loss with conventional laparoscopy over the open approach, this meta-analysis found a statistically, but not clinically, significant reduction with robotic over laparoscopic procedures.28,32
In addition to minimizing blood loss, the biggest theoretical advantage touted by proponents of the robotic approach is the decreased necessity for converting the procedure to laparotomy. This is especially true when operating in the narrow space of the pelvis, as seen in cases involving the rectum and some gynecologic and urologic procedures.6,7 However, this meta-analysis showed that the robotic approach was 1.78 times more likely to be converted to an open procedure than the laparoscopic approach. This important finding contradicts the popular belief that the robot's benefit of finer dissection over laparoscopy allows the decreased need to convert procedures to open. In fact, it negates one of the most significant theoretical advantages of robotic surgery over laparoscopy. In assessing the rate of conversion to laparotomy, a history of previous abdominal surgery in a patient can be a confounding factor. Studies with a higher proportion of patients who have had prior surgery may have a higher rate of conversions because of the presence of adhesions and scarring. Baik et al23 attempted to address this issue in their study. Their study showed that of the 6 laparoscopic procedures converted, no patient had a history of previous abdominal surgery. Additionally, no robotic cases were converted. In this particular study, it appeared that the type of approach (robotic vs laparoscopic) played a greater role in influencing conversion rates than did a history of abdominal surgery, indicating that the latter may not be a contraindication to robotic surgery. No other studies have addressed this particular issue at this time.
With respect to oncologic resection, there was no difference between the robotic and laparoscopic approaches in terms of the number of lymph nodes harvested in colorectal procedures performed for malignancy. Of note, among the 15 study populations included for this analysis, 1 of the laparoscopic groups yielded an average number of nodes <12 (when ≥12 is generally considered the standard for sufficient oncologic resection), compared with 2 of the robotic groups. Laparoscopy has been previously shown to yield sufficient nodal retrieval for an oncologic resection, and this meta-analysis showed similar outcomes for both laparoscopic and robotic approaches.4
The LOS likewise did not differ between the laparoscopic and robotic groups. As with operative time, the number of days a patient spends postoperatively in the hospital has significant implications consequential to the associated costs. There was no difference between the 2 groups in terms of number of days to flatus, number of days to initiation of soft diet, or readmission rates.
Intra-operative complications beyond the scope of conversions to laparotomy were sparsely reported. One study reported a nonsignificant rate of intra-operative complications between robotic and laparoscopic groups (P = .657) but did not define what these complications were.13 Across 5 of the included studies, there were 4 bowel injuries in the robotic group,10,18,20 2 bowel injuries in the laparoscopic group,20,23 1 case of ischemia in a laparoscopic patient,21 and 1 patient in the robotic group who fell off the operating table.18
The reporting of postoperative complications differed greatly between studies. Because of the complexity and variation in reporting, statistical analysis was based on the absolute total number of complications reported and yielded no significant difference between robotic and laparoscopic approaches. Four studies included in this meta-analysis quantitatively analyzed individual complications, and 3 of the studies found no significant difference between the robotic and laparoscopic groups.17,19,24 The study by Tyler et al13 found that the rates of postoperative ileus, anastomotic complications, and mortality were greater in the laparoscopic group and that wound infections, deep vein thromboses and pulmonary emboli, and fistulas were more common in the robotic group (P < .05). The lack of subanalysis of specific complications resulting from either surgical approach is a limitation of this study. Further analyses that break down specific complications that may be directly related to colon and rectal surgery, such as wound infections and anastomotic leak, may be useful and warranted in the future.
In addition to evaluating new technology for safety and efficacy in comparison to the current standard of treatment, the issue of cost is of great importance given the fluctuating state of the health care system. As mentioned previously, cost-effectiveness was not an outcome that was specifically addressed within this study, but a few reports warrant mentioning here as they pertain to discussing robotic and laparoscopic approaches for colon and rectal surgery. Tyler et al13 reported that the total cost of a hospital encounter for a robotic colectomy was $3,424 greater than laparoscopy when adjusted for variations in geographic location and hospital status (eg, private for-profit, public, teaching hospital) (P < .001). A study by Park et al27 in Korea showed that the total cost of a robotic colectomy was $1,916 more than that of a laparoscopic colectomy (P = .013). They also reported that the charges to patients were $3,604 more for the robotic approach (P = .018). International discrepancies in health care costs aside, studies in the United States and elsewhere have reported similar findings suggesting that robotic surgery costs thousands more per patient both to the hospital and to patients themselves.18,20 The significantly higher costs of robot-assisted surgery may not justify the few and arguably minor advantages over conventional laparoscopy. This must be further investigated with cost-effectiveness analysis to determine the long-term utility of robotic surgery in colorectal procedures after proficiency has been reached with the robotic approaches.
Previous studies have also compared published literature on robotic colorectal surgery. In a systematic review by Kim et al33 examining studies encompassing 2,644 patients who underwent open, laparoscopic, and robotic colorectal surgery between 2002 and 2013, the investigators found longer operative times, lower blood loss, shorter LOS, and higher cost associated with the robot. In stark contrast however, more than half of their included studies reported lower conversion rates with the robot compared with the laparoscopic approach. Unfortunately, no meta-analysis was performed. Another systematic review by Witkiewicz et al34 examining right robotic hemicolectomies exclusively also demonstrated longer operative times and higher cost with robotic procedures. They observed that right hemicolectomies could serve as a training platform in a surgeon's early experience with the da Vinci robot.
Although this meta-analysis is comprehensive and the most current evaluation of robotic and laparoscopic approaches to colon and rectal surgery to date, it should be interpreted in the context of some limitations. First, 4 of the included studies were composed only of American populations, compared with 7 with Asian populations. Studies with Asian populations reported lower patient body mass index than studies with American populations. This has a number of implications on the data, including effects on the operative time and perioperative complications. Second, the paucity of current randomized controlled trials further limits the results of our meta-analysis. Furthermore, not all of the studies reported on all outcomes examined within this analysis, leading to variations in the included sample sizes and statistical power between outcomes. Each study has its own biases and limitations, with different inclusion and exclusion criteria, varying indications for surgery, and different types of included colorectal procedures.
CONCLUSIONS
In conclusion, this systematic literature review and meta-analysis suggests that the robotic approach is equally safe and efficacious in comparison with the conventional laparoscopic colorectal approach. However, the robotic approach tended to have longer operating times, less blood loss, and a higher rate of conversion to an open procedure compared with laparoscopy. On the basis of the findings of this meta-analysis, there does not appear to be any clear advantage of a robotic approach over a laparoscopic one for colorectal surgery. Future studies encompassing prospective randomized controlled trials and cost-effectiveness are warranted to determine whether a robotic approach will have any place in colorectal procedures.
Contributor Information
Becky B. Trinh, Department of Surgery, Tulane University School of Medicine, New Orleans, Louisiana, USA.
Nicole R. Jackson, Department of Surgery, Tulane University School of Medicine, New Orleans, Louisiana, USA.
Adam T. Hauch, Department of Surgery, Tulane University School of Medicine, New Orleans, Louisiana, USA.
Tian Hu, Department of Epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana, USA..
Emad Kandil, Department of Surgery, Tulane University School of Medicine, New Orleans, Louisiana, USA.
References:
- 1. Fleshman J, Sargent D, Green E, et al. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST study group trial. Ann Surg. 2007;246:655–664. [DOI] [PubMed] [Google Scholar]
- 2. Jayne DG, Guillou PJ, Thorpe H, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC trial group. J Clin Oncol. 2007;25:3061–3068. [DOI] [PubMed] [Google Scholar]
- 3. Law WL, Poon JT, Fan JM, Lo SH. Comparison of outcome of open and laparoscopic resection for stage II and stage III rectal cancer. Ann Surg Oncol. 2009;16:1488–1493. [DOI] [PubMed] [Google Scholar]
- 4. Bonjer HJ, Hop WC, Nelson H, et al. Laparoscopically assisted vs open colectomy for colon cancer: a meta-analysis. Arch Surg. 2007;142:298–303. [DOI] [PubMed] [Google Scholar]
- 5. Balch GC. Emerging role of laparoscopic and robotic surgery for rectal cancers. Ann Surg Oncol. 2009;16:1451–1453. [DOI] [PubMed] [Google Scholar]
- 6. McClain PD, Mufarrij PW, Hemal AK. Robot-assisted reconstructive surgery for ureteral malignancy: analysis of efficacy and oncologic outcomes. J Endourol. 2012;26(12):1614–1617. [DOI] [PubMed] [Google Scholar]
- 7. Lambaudie E, Houvenaeghel G, Walz J, et al. Robot-assisted laparoscopy in gynecologic oncology. Surg Endosc. 2008;22(12):2743–2747. [DOI] [PubMed] [Google Scholar]
- 8. Park SY, Choi G, Park JS, Kim HJ, Ryuk JP. Short-term clinical outcome of robot-assisted intersphincteric resection for low rectal cancer: a retrospective comparison with conventional laparoscopy. Surg Endosc. 2013;27:48–55. [DOI] [PubMed] [Google Scholar]
- 9. Park JS, Choi GS, Lim KH, Jang YS, Jun SH. s052: a comparison of robot-assisted, laparoscopic, and open surgery in the treatment of rectal cancer. Surg Endosc. 2011;25:240–248. [DOI] [PubMed] [Google Scholar]
- 10. D'Annibale A, Morpurgo E, Fiscon V, et al. Robotic and laparoscopic surgery for treatment of colorectal diseases. Dis Colon Rectum. 2004;47:2162–2168. [DOI] [PubMed] [Google Scholar]
- 11. Baek SJ, Al-Asari S, Jeong DH, et al. Robotic versus laparoscopic coloanal anastomosis with or without intersphincteric resection for rectal cancer. Surg Endosc. 2013;27:4157–4163. [DOI] [PubMed] [Google Scholar]
- 12. Saklani AP, Lim DR, Hur H, et al. Robotic versus laparoscopic surgery for mid-low rectal cancer after neoadjuvant chemoradiation therapy: comparison of oncologic outcomes. Int J Colorectal Dis. 2013;28(12):1689–1698. [DOI] [PubMed] [Google Scholar]
- 13. Tyler JA, Fox JP, Desai MM, Perry WB, Glasgow SC. Outcomes and costs associated with robotic colectomy in the minimally invasive era. Dis Colon Rectum. 2013;56:458–466. [DOI] [PubMed] [Google Scholar]
- 14. Lim DR, Min BS, Kim MS, et al. Robotic versus laparoscopic anterior resection of sigmoid colon cancer: comparative study of long-term oncologic outcomes. Surg Endosc. 2013;27:1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shin JY. Comparison of short-term surgical outcomes between a robotic colectomy and a laparoscopic colectomy during early experience. J Korean Soc Coloproctol. 2012;28(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deutsch GB, Sathyanarayana SA, Gunabushanam V, et al. Robotic vs. laparoscopic colorectal surgery: an institutional experience. Surg Endosc. 2012;26:956–963. [DOI] [PubMed] [Google Scholar]
- 17. Kwak JM, Kim SH, Kim J, Son DN, Baek SJ, Cho JS. Robotic vs laparoscopic resection of rectal cancer: short-term outcomes of a case-control study. Dis Colon Rectum. 2011;54:151–156. [DOI] [PubMed] [Google Scholar]
- 18. Rawlings AL, Woodland JH, Vegunta RK, Crawford DL. Robotic versus laparoscopic colectomy. Surg Endosc. 2007;21:1701–1708. [DOI] [PubMed] [Google Scholar]
- 19. Helvind NM, Eriksen JR, Mogensen A, et al. No differences in short-term morbidity and mortality after robot-assisted laparoscopic versus laparoscopic resection for colonic cancer: a case-control study of 263 patients. Surg Endosc. 2013;27:2575–2580. [DOI] [PubMed] [Google Scholar]
- 20. Bertani E, Chiappa A, Biffi R, et al. Assessing appropriateness for elective colorectal cancer surgery: clinical, oncological, and quality-of-life short-term outcomes employing different treatment approaches. Int J Colorectal Dis. 2011;26:1317–1327. [DOI] [PubMed] [Google Scholar]
- 21. Bianchi PP, Ceriani C, Locatelli A, et al. Robotic versus laparoscopic total mesorectal excision for rectal cancer: a comparative analysis of oncological safety and short-term outcomes. Surg Endosc. 2010;24:2888–2894. [DOI] [PubMed] [Google Scholar]
- 22. Miller AT, Berian JR, Rubin M, Hurst RD, Fichera A, Umanskiy K. Robotic-assisted proctectomy for inflammatory bowel disease: a case-matched comparison of laparoscopic and robotic technique. J Gastrointest Surg. 2012;16:587–594. [DOI] [PubMed] [Google Scholar]
- 23. Baik SH, Kwon HY, Kim JS, et al. Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol. 2009;16:1480–1487. [DOI] [PubMed] [Google Scholar]
- 24. Patriti A, Ceccarelli G, Bartoli A, Spaziani A, Biancafarina A, Casciola L. Short- and medium-term outcome of robot-assisted and traditional laparoscopic rectal resection. JSLS. 2009;13:176–183. [PMC free article] [PubMed] [Google Scholar]
- 25. Lanfranco AR, Castellanos AE, Desai JP, Meyers WC. Robotic surgery: a current perspective. Ann Surg. 2004;239:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rockall TA, Darzi A. Robot-assisted laparoscopic colorectal surgery. Surg Clin N Am. 2003;83:1463–1468. [DOI] [PubMed] [Google Scholar]
- 27. Park JS, Choi GS, Park SY, Kim HJ, Ryuk JP. Randomized clinical trial of robot-assisted versus standard laparoscopic right colectomy. Br J Surg. 2012;99:1219–1226. [DOI] [PubMed] [Google Scholar]
- 28. Fung AK, Aly EH. Robotic colonic surgery: is it advisable to commence a new learning curve? Dis Colon Rectum. 2003;56:786–796. [DOI] [PubMed] [Google Scholar]
- 29. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 30. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scarpinata R, Aly EH. Does robotic rectal cancer surgery offer improved early postoperative outcomes? Dis Colon Rectum. 2013;56(2):253–262. [DOI] [PubMed] [Google Scholar]
- 32. Lacy AM, García-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomized trial. Lancet. 2002;359:2224–2229. [DOI] [PubMed] [Google Scholar]
- 33. Kim CW, Kim CH, Baik SH. Outcomes of robotic-assisted colorectal surgery compared with laparoscopic and open surgery: a systematic review. J Gastrointest Surg. 2014;18:816–830. [DOI] [PubMed] [Google Scholar]
- 34. Witkiewicz W, Zawadzki M, Rzaca M, et al. Robot-assisted right colectomy: surgical technique and review of the literature. Videosurgery Miniinv. 2013;8(3):253–7. [DOI] [PMC free article] [PubMed] [Google Scholar]