Abstract
Background
In children with single right ventricular (RV) anomalies, changes in RV size and function may be influenced by shunt type chosen at time of the Norwood procedure.
Objectives
We sought to identify shunt-related differences in echocardiographic findings at 14 months and ≤6 months pre-Fontan in survivors of the Norwood procedure.
Methods
We compared 2-dimensional and Doppler echocardiographic indices of RV size and function, neo-aortic and tricuspid valve annulus dimensions and function, and aortic size and patency at 14.1 ± 1.2 and 33.6 ± 9.6 months () in subjects randomized to a Norwood procedure using either the modified Blalock-Taussig shunt (MBTS) or right ventricle-to-pulmonary artery shunt (RVPAS).
Results
Acceptable echocardiograms were available at both time points in 240 subjects (114 MBTS; 126 RVPAS). At 14 months, all indices were similar between shunt groups. From the 14-month to pre-Fontan echocardiogram, the MBTS group had stable indexed RV volumes and ejection fraction (EF), while the RVPAS group had increased RV end-systolic volume (p = 0.004) and decreased RVEF (p = 0.004). From 14 months to pre-Fontan, the treatment groups were similar with respect to decline in indexed neo-aortic valve area, >mild neo-aortic valve regurgitation (<5% at each time), indexed tricuspid valve area, and ≥moderate tricuspid valve regurgitation (<20% at each time).
Conclusions
Initial Norwood shunt type influences pre-Fontan RV remodeling during the 2nd and 3rd years of life in survivors with single RV anomalies, but with greater RVEF deterioration after RVPAS. Encouragingly, other indices of RV function remain stable prior to Fontan regardless of shunt type.
Keywords: echocardiography, hypoplastic left heart syndrome, Norwood
Introduction
The Pediatric Heart Network (1) Single Ventricle Reconstruction (SVR) trial compared outcomes in 549 infants undergoing a Norwood procedure for single right ventricle anomalies randomized to either a modified Blalock-Taussig shunt (MBTS) or right ventricle-to-pulmonary artery shunt (RVPAS) at 15 North American centers (2). The trial's primary finding was superior 1-year transplant-free survival in subjects who received a RVPAS compared with those who had an MBTS. (3) However, initial shunt type for the Norwood procedure did not impact echocardiographic indices measured after shunt removal (4). Specifically, at 14 months, right ventricular (RV) systolic, diastolic and global function, cardiac and vascular dimensions, neo-aortic and tricuspid annulus dimensions and valve function, and neo-aortic flow patterns all were similar for survivors with MBTS or RVPAS. Although significant interstage differences were noted in neo-aortic annular size and flow patterns between shunt types prior to stage II palliation, these were explained by the differing shunt physiologies.
Based on these results, we hypothesized that differences in echocardiographic findings between the shunt groups might emerge with longer-term follow-up. RV dysfunction related to the creation of a right ventriculotomy in the RVPAS group was of particular concern. The Pediatric Heart Network's SVR Extension Study was undertaken to provide longitudinal surveillance of the cohort, including echocardiography within 6 months of Fontan palliation. In this analysis, we compared 2-dimensional (2D) and Doppler indices of RV size and function, neo-aortic and tricuspid valve annulus dimensions and function, and aortic size and patency to evaluate these changes for the second and third years of life for our cohort, picking up at 14 months when the original analysis left off and then conducting echocardiographic analysis again prior to the Fontan procedure. By investigating changes over time, we sought to identify echocardiographic features at 14 months that predict clinical outcomes.
Methods
Subjects
Entry criteria for subjects in the SVR trial have been reported previously (2). In the current study, all SVR trial survivors who underwent a transthoracic echocardiogram within 6 months of planned Fontan palliation and had that study submitted to the SVR core echocardiography lab for interpretation were eligible for inclusion. We excluded subjects who underwent biventricular repair (n = 3), had their pre-Fontan echocardiogram outside the 6-month window prior to Fontan surgery (n = 2), or had no Fontan procedure scheduled at the time of this data collection (n = 16).
Study Design
The SVR study design also has previously been published (2). Briefly, SVR is a randomized clinical trial of infants with a diagnosis of single, morphologically right ventricle anomaly undergoing a Norwood procedure. The primary outcome has been published (3) (incidence of death or cardiac transplantation at 12 months after randomization), as have secondary outcomes, including hospital morbidity and rate of serious adverse events through 12 months, and risk factors for mortality and cardiac transplantation (5-7). The secondary echocardiographic markers of outcome for the SVR trial previously reported included 14-month indices of RV function, cardiac and vascular dimensions, valve annulus dimensions and function, and neo-aortic flow patterns. (4).
Echocardiographic Analysis
For the SVR Extension Study, an echocardiography core laboratory at the Medical College of Wisconsin reviewed 2D/Doppler echocardiograms performed at each clinical center within 6 months of a planned Fontan procedure. Core lab procedures for image analysis and data management have been previously described (4). Echocardiographic measures obtained from the imaging are summarized in Tables 1 and 2.
Table 1. Echocardiographic Measures of RV Size and Function.
| RV Size and Function |
|---|
| Systolic Size and Function |
| Indexed end-systolic volume |
| RV ejection fraction |
| RV % fractional area change |
| Tissue Doppler tricuspid peak annular systolic velocity |
| Tissue Doppler tricuspid annular isovolumic acceleration |
| Diastolic Size and Function |
| Indexed end-diastolic volume |
| Indexed end-diastolic area |
| Peak tricuspid E and A inflow velocity |
| Tricuspid E/A ratio |
| Tricuspid tissue Doppler E` and A` velocity |
| E/E` ratio |
| Presence of pulmonary vein reversal at atrial systole |
| Global Function |
| Myocardial performance index |
RV = right ventricle/ventricular
Table 2. Echocardiographic Measures of Valve and Aortic Size and Function.
| Valvular Size and Function |
|---|
| Tricuspid Valve |
| Indexed valve area |
| Severity of regurgitation |
| Neo-aortic Valve |
| Indexed valve area |
| Severity of regurgitation |
| Aortic Size and Patency |
| Ascending aortic diameter |
| Peak descending aortic velocity |
Statistical Analysis
Summary descriptive statistics of echocardiographic indices are presented by intended shunt type for the Norwood procedure. Distributions of echocardiographic indices were examined with histograms. Echocardiographic indices were compared between shunt groups at the pre-Fontan study. In addition, we performed paired comparisons of changes in these indices from 14 months of age (which marked the end of the SVR study visit from previously published data (4)) and the pre-Fontan study between shunt groups when a protocol echocardiogram was available at both time periods. Continuous measures were compared between assigned shunt types with either Student or Welch's t test or a Wilcoxon rank sum test as appropriate. Categorical measures were compared between shunt types with a Fisher exact test; ordinal measures were compared with a Fisher exact test and the Mantel-Haenszel test for trend. For paired analysis between values obtained at 14 months and the pre-Fontan study, the mean and standard deviation of the change over time in each of the echocardiographic measures were compared between shunt groups using a t test. Finally, echocardiographic indices obtained at 14 months were explored to assess whether they predicted transplant-free survival from the time of that study to Fontan surgery. The hazard ratios for death or heart transplant after the 14-month echocardiogram were assessed as a function of the echocardiographic measurement using Cox proportional hazards regression. The hazard ratio measures the association of the echocardiogram parameter with transplant-free survival, and the p value tests whether or not the hazard ratio is significantly different from 1 (no effect). Subjects who did not die or receive a heart transplant between the date of the 14-month echocardiogram and the date of Fontan surgery were censored at the time of their Fontan procedure. The p values were considered significant at <0.05.
Analyses were performed in SAS Version 9.3 (SAS Institute, Cary, NC). To adjust for the effect of somatic growth and age on the linear, area, and volumetric dimensions of cardiac structures and Doppler flow patterns, z-scores were used when normative data were available (8). The dimensions of the neo-aortic valve annulus were expressed as z-scores using normal values for the dimensions of the native aortic valve in a normal population. These z-scores are calculated with respect to body surface area (BSA), with the power of BSA determined by prior work examining linearity between echo measures and various functions of BSA (9).
All echocardiographic measures were performed by a single dedicated pediatric echocardiography technician and all measures were confirmed by a single experienced pediatric echocardiographer. To access reproducibility of echocardiogram measurements, approximately 5% of the echocardiograms at each time point were randomly selected to be read blindly a second time by the same echocardiographer. The intrarater intraclass correlations and percent agreement for continuous and dichotomous variables, respectively, were calculated. Agreement was good (>80%) for most variables; RV volume assessment had more variance, but this did not affect the consistency of RV ejection fraction (RVEF) estimates (86% agreement).
Results
Of the 262 SVR survivors who had echocardiographic studies submitted within 6 months of planned Fontan, 258 (98.5%; 125 MBTS and 133 RVPAS) were acceptable for core-lab analysis. The mean age at the time of the pre-Fontan echocardiogram was 33.6±9.6 months (median: 32.7 months; range: 13.6 to 65.9 months). Among the 258 subjects, 240 (93%; 114 MBTS and 126 RVPAS) also had undergone a 14-month protocol echocardiogram at a mean of 14.1 ± 1.2 months (median 14.1 months, range 11 to 20.9 months); of the remaining 18 subjects, 16 had no 14-month echocardiogram and 2 had undergone their Fontan at the time of or prior to the 14-month study. For the 240 subjects with paired echocardiograms, the mean duration between the 14-month echocardiogram and the pre-Fontan echocardiogram was 19.6 ± 9.5 months (range: 1.5 to 51.9 months).
Echocardiographic Assessment
Right Ventricular Systolic Size and Function
At the pre-Fontan echocardiogram, RVEF was significantly higher in the MBTS subjects compared with RVPAS (45 ± 6% vs. 42 ± 7%; p = 0.007). All other values at this time point were similar between shunt types including RV indexed systolic volume, indexed systolic area, percent fractional area change, peak tricuspid annular systolic velocity, and isovolumic acceleration (Table 3).
Table 3. Summary Statistics for Pre-Fontan Echocardiographic Measures by Initial Shunt Group.
| MBTS | RVPAS | ||||
|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | p Value | |
| RV systolic size and function | |||||
| End systolic volume, ml/BSA1.3 | 68 | 51.1 ± 15.7 | 79 | 54 ± 17.9 | 0.30 |
| End systolic area, cm2/BSA0.8 | 102 | 16.4 ± 4.2 | 112 | 17 ± 4.2 | 0.32 |
| Ejection fraction, % | 68 | 45 ± 6 | 79 | 42 ± 7 | 0.007 |
| Fractional area change, % | 102 | 31 ± 6 | 112 | 31 ± 6 | 0.36 |
| Peak systolic velocity, cm/sec | 75 | 5.68 ± 1.48 | 88 | 5.7 ± 1.6 | 0.93 |
| Isovolumic contraction acceleration, cm/sec2 | 43 | 33.1 ± 8.74 | 62 | 35.1 ± 11.9 | 0.34 |
| RV diastolic size and function | |||||
| End diastolic volume, ml/BSA1.3 | 68 | 92 ± 24.5 | 79 | 92.1 ± 26.3 | 0.98 |
| End diastolic area, cm2/BSA0.8 | 102 | 23.8 ± 5.1 | 112 | 24.4 ± 5.3 | 0.38 |
| Tricuspid valve peak early velocity, m/sec | 108 | 0.79 ± 0.23 | 109 | 0.7 6 ±0.20 | 0.27 |
| Tricuspid valve peak atrial velocity, m/sec | 82 | 0.70 ± 0.20 | 84 | 0.66 ± 0.21 | 0.30 |
| E/A ratio | 82 | 1.09 ± 0.32 | 84 | 1.16 ± 0.32 | 0.17 |
| Peak early annular diastolic velocity, cm/sec | 75 | 8.64 ± 2.92 | 88 | 9.10 ± 2.69 | 0.30 |
| Peak atrial annular diastolic velocity, cm/sec | 63 | 6.98 ± 2.49 | 68 | 6.80 ± 1.67 | 0.64 |
| E/E′ ratio | 71 | 10.36 ± 5.47 | 83 | 8.85 ± 3.23 | 0.04 |
| Flow reversal during atrial systole | 77 | 98 | 0.24 | ||
| Yes, n (%) | 5 (6.5%) | 2 (2.0%) | |||
| No, n (%) | 72 (93.5%) | 96 (98.0%) | |||
| RV global function | |||||
| MPI, Inflow Doppler calculation | 95 | 0.45 ± 0.17 | 92 | 0.45 ± 0.19 | 0.93 |
| MPI, DTI calculation | 46 | 0.63 ± 0.27 | 58 | 0.59 ± 0.19 | 0.46 |
| Neo-aortic valve size and function | |||||
| Neo-aortic annular area/BSA | 108 | 3.85 ± 0.87 | 111 | 3.69 ± 1.08 | 0.22 |
| Neo-aortic annular area, z-score | 108 | 5.59 ± 2.54 | 111 | 5.12 ± 3.12 | 0.22 |
| Severity of neoaortic valve regurgitation | 121 | 130 | 0.73 | ||
| None, n (%) | 74 (61.2%) | 78 (60.0%) | |||
| Mild, n (%) | 44 (36.4%) | 46 (35.4%) | |||
| Moderate, n (%) | 3 (2.5%) | 6 (4.6%) | |||
| Severe, n (%) | 0 (0.0%) | 0 (0.0%) | |||
| ≥Moderate neo-aortic valve regurgitation | 121 | 130 | 0.50 | ||
| Yes, n (%) | 3 (2.5%) | 6 (4.6%) | |||
| No, n (%) | 118 (97.5%) | 124 (95.4%) | |||
| Tricuspid valve size and function | |||||
| Tricuspid indexed annular area/BSA | 113 | 5.51 ± 1.83 | 120 | 5.71 ± 1.62 | 0.37 |
| Tricuspid indexed annular area for BSA, z-score | 113 | 1.87 ± 2.23 | 120 | 2.11 ± 1.96 | 0.38 |
| Severity of tricuspid valve regurgitation | 121 | 131 | 0.79 | ||
| None, n (%) | 14 (11.6%) | 19 (14.5%) | |||
| Mild, n (%) | 84 (69.4%) | 91 (69.5%) | |||
| Moderate, n (%) | 17 (14.0%) | 17 (13.0%) | |||
| Severe, n (%) | 6 (5.0%) | 4 (3.1%) | |||
| Multiple, n (%) | 0 (0.0%) | 0 (0.0%) | |||
| ≥Moderate tricuspid valve regurgitation | 121 | 131 | 0.62 | ||
| Yes, n (%) | 23 (19.0%) | 21 (16.0%) | |||
| No, n (%) | 98 (81.0%) | 110 (84.0%) | |||
| Aortic size and patency | |||||
| Ascending aortic maximal diameter, cm | 102 | 1.95 ± 0.38 | 111 | 1.90 ± 0.40 | 0.37 |
| Ascending aortic maximal diameter for BSA, z-score | 102 | 3.43 ± 2.32 | 111 | 3.10 ± 2.41 | 0.31 |
| Distal arch to descending aorta CWD peak velocity, m/sec | 91 | 1.67 ± 0.51 | 96 | 1.81 ± 0.58 | 0.08 |
BSA = body surface area; CWD = continuous wave Doppler; DTI = Doppler tissue imaging; MPI = myocardial performance index; SD = standard deviation. Other abbreviations as in Table 1.
In comparing the 14-month and pre-Fontan studies, we found no significant change in RVEF or systolic volume in the MBTS group. However, RV indexed end-systolic area increased and fractional area change decreased between the two time periods. All other systolic indices were similar (Table 4). In the RVPAS group, the RV indexed end-systolic area and end-systolic volume both increased while fractional area change and ejection fraction (44 ± 7% vs. 41 ± 7%; p = 0.004) both decreased over time (Table 5, Figure 1). This interval change in RVEF differed significantly between groups (-3.24 for the RVPAS vs. + 0.99 for the MBTS; p = 0.009). No other significant changes in systolic indices were identified from 14 months to the pre-Fontan study for the RVPAS group.
Table 4. Summary Statistics for 14-month and Pre-Fontan Echocardiographic Measures (MBTS Group).
|
n |
14 Months Mean ± SD |
Pre-Fontan Mean ± SD |
p Value |
|
|---|---|---|---|---|
| RV systolic size and function | ||||
| End systolic volume, ml/BSA1.3 | 56 | 49.39 ± 16.62 | 51.23 ± 15.45 | 0.35 |
| End systolic area, cm2/BSA0.8 | 93 | 15.52 ± 3.54 | 16.45 ± 3.37 | 0.01 |
| Ejection fraction, % | 56 | 44 ± 8 | 45 ± 6 | 0.40 |
| Fractional area change, % | 93 | 33 ± 7 | 31 ± 5 | 0.02 |
| Peak systolic velocity, cm/sec | 70 | 5.56 ± 1.38 | 5.67 ± 1.51 | 0.51 |
| Isovolumic contraction acceleration, cm/sec2 | 29 | 35.34 ± 9.94 | 31.90 ± 8.14 | 0.17 |
| RV diastolic size and function | ||||
| End diastolic volume, mL/BSA1.3 | 56 | 87.07 ± 23.41 | 92.17 ± 24.60 | 0.07 |
| End diastolic area, cm2/BSA0.8 | 93 | 23.13 ± 4.35 | 23.94 ± 4.43 | 0.09 |
| Transverse valve peak early velocity, m/sec | 95 | 0.85 ± 0.24 | 0.80 ± 0.23 | 0.05 |
| Transverse valve peak atrial velocity, m/sec | 48 | 0.74 ± 0.21 | 0.69 ± 0.18 | 0.21 |
| E/A ratio | 48 | 1.11 ± 0.37 | 1.17 ± 0.34 | 0.36 |
| Peak early diastolic velocity, cm/sec | 70 | 8.89 ± 3.59 | 8.57 ± 2.99 | 0.47 |
| Peak atrial diastolic velocity, cm/sec | 35 | 7.01 ± 2.25 | 7.35 ± 2.81 | 0.43 |
| E/E′ ratio | 65 | 11.42 ± 5.12 | 10.67 ± 5.60 | 0.23 |
| Flow reversal during atrial systole | 69 | >0.99 | ||
| Yes, n (%) | 5 (7.2%) | 4 (5.8%) | ||
| No, n (%) | 64 (92.8%) | 65 (94.2%) | ||
| RV global function | ||||
| MPI, Inflow Doppler calculation | 84 | 0.44 ± 0.16 | 0.45 ± 0.17 | 0.58 |
| MPI, DTI calculation | 30 | 0.57 ± 0.20 | 0.62 ± 0.28 | 0.37 |
| Valvular size and function | ||||
| Neo-aortic annular area/BSA | 96 | 4.35 ± 1.05 | 3.89 ± 0.83 | <0.001 |
| Neo-aortic annular area for BSA, z-score | 96 | 6.80 ± 2.94 | 5.70 ± 2.44 | <0.001 |
| Severity of neo-aortic valve regurgitation | 110 | <0.001 | ||
| None, n (%) | 45 (40.9%) | 69 (62.7%) | ||
| Mild, n (%) | 64 (58.2%) | 39 (35.5%) | ||
| Moderate, n (%) | 1 (0.9%) | 2 (1.8%) | ||
| Severe, n (%) | 0 (0.0%) | 0 (0.0%) | ||
| ≥ Moderate neo-aortic valve regurgitation | 110 | >0.99 | ||
| Yes, n (%) | 1 (0.9%) | 2 (1.8%) | ||
| No, (%) | 109 (99.1%) | 108 (98.2%) | ||
| Tricuspid valve size and function | ||||
| Tricuspid indexed annular area/BSA | 105 | 6.16 ± 1.88 | 5.59 ± 1.84 | <0.001 |
| Tricuspid indexed annular area for BSA, z-score | 105 | 2.61 ± 2.24 | 1.96 ± 2.24 | 0.002 |
| Severity of tricuspid valve regurgitation | 111 | 0.78 | ||
| None, n (%) | 17 (15.3%) | 12 (10.8%) | ||
| Mild, n (%) | 72 (64.9%) | 78 (70.3%) | ||
| Moderate, n (%) | 16 (14.4%) | 15 (13.5%) | ||
| Severe, n (%) | 6 (5.4%) | 6 (5.4%) | ||
| Multiple, n (%) | 0 (0.0%) | 0 (0.0%) | ||
| ≥Moderate tricuspid valve regurgitation | 111 | 0.99 | ||
| Yes, n (%) | 22 (19.8%) | 21 (18.9%) | ||
| No, n (%) | 89 (80.2%) | 90 (81.1%) | ||
| Aortic size and patency | ||||
| Ascending aortic maximal diameter, cm | Not collected at 14 months | |||
| Ascending aortic maximal diameter for BSA, z-score | Not collected at 14 months | |||
| Distal arch to descending aorta CWD peak velocity, m/sec | 71 | 1.67±0.55 | 1.72±0.52 | 0.25 |
Table 5. Summary Statistics for 14-Month and Pre-Fontan Echocardiographic Measures (RVPAS Group).
|
n |
14 months Mean ± SD |
Pre-Fontan Mean ± SD |
p Value |
|
|---|---|---|---|---|
| RV systolic size and function | ||||
| End systolic volume, ml/BSA1.3 | 58 | 49.11 ± 14.47 | 55.00 ± 17.84 | 0.004 |
| End systolic area, cm2/BSA0.8 | 98 | 15.96 ± 3.64 | 17.09 ± 3.96 | 0.004 |
| Ejection fraction, % | 58 | 44 ± 7 | 41 ± 7 | 0.004 |
| Fractional area change, % | 98 | 33 ± 6 | 31 ± 6 | <0.001 |
| Peak systolic velocity, cm/sec | 72 | 5.06 ± 1.40 | 5.59 ± 1.60 | 0.02 |
| Isovolumic contraction acceleration, cm/sec2 | 37 | 35.86 ± 11.90 | 37.31 ± 10.11 | 0.50 |
| RV diastolic size and function | ||||
| End diastolic volume, ml/BSA1.3 | 58 | 87.73 ± 23.58 | 92.59 ± 25.82 | 0.09 |
| End diastolic area, cm2/BSA0.8 | 98 | 23.92 ± 4.97 | 24.53 ± 4.95 | 0.22 |
| Transverse valve peak early velocity, m/sec | 98 | 0.81 ± 0.23 | 0.76 ± 0.20 | 0.02 |
| Transverse valve peak atrial velocity, m/sec | 55 | 0.70 ± 0.22 | 0.65 ± 0.22 | 0.01 |
| E/A ratio | 55 | 1.11 ± 0.35 | 1.17 ± 0.35 | 0.23 |
| Peak early diastolic velocity, cm/sec | 72 | 7.79 ± 2.80 | 9.09 ± 2.76 | <0.001 |
| Peak atrial diastolic velocity, cm/sec | 40 | 6.53 ± 2.03 | 6.89 ± 1.71 | 0.30 |
| E/E′ ratio | 67 | 11.28 ± 4.12 | 8.75 ± 3.16 | <0.001 |
| Flow reversal during atrial systole | 82 | 0.44 | ||
| Yes, n (%) | 5 (6.1%) | 2 (2.4%) | ||
| No, n (%) | 77 (93.9%) | 80 (97.6%) | ||
| RV global function | ||||
| MPI, Inflow Doppler calculation | 79 | 0.42 ± 0.14 | 0.46 ± 0.19 | 0.07 |
| MPI, DTI calculation | 33 | 0.59 ± 0.17 | 0.58 ± 0.11 | 0.79 |
| Valvular size and function | ||||
| Neo-aortic annular area/BSA | 95 | 4.20 ± 1.15 | 3.67 ± 1.01 | <0.001 |
| Neo-aortic annular area for BSA, z-score | 95 | 6.37 ± 3.21 | 5.07 ± 2.91 | <0.001 |
| Severity of neo-aortic valve regurgitation | 121 | 0.97 | ||
| None, n (%) | 70 (57.9%) | 72 (59.5%) | ||
| Mild, n (%) | 46 (38.0%) | 44 (36.4%) | ||
| Moderate, n (%) | 5 (4.1%) | 5 (4.1%) | ||
| Severe, n (%) | 0 (0.0%) | 0 (0.0%) | ||
| ≥Moderate neo-aortic valve regurgitation | 121 | >0.99 | ||
| Yes, n (%) | 5 (4.1%) | 5 (4.1%) | ||
| No, n (%) | 116 (95.9%) | 116 (95.9%) | ||
| Tricuspid valve size and function | ||||
| Tricuspid indexed annular area/BSA | 110 | 6.47 ± 2.11 | 5.69 ± 1.63 | <0.001 |
| Tricuspid indexed annular area for BSA, z-score | 110 | 2.97 ± 2.51 | 2.09 ± 1.98 | <0.001 |
| Severity of tricuspid valve regurgitation | 122 | 0.88 | ||
| None, n (%) | 21 (17.2%) | 17 (13.9%) | ||
| Mild, n (%) | 81 (66.4%) | 84 (68.9%) | ||
| Moderate, n (%) | 15 (12.3%) | 17 (13.9%) | ||
| Severe, n (%) | 5 (4.1%) | 4 (3.3%) | ||
| Multiple, n (%) | 0 (0.0%) | 0 (0.0%) | ||
| ≥Moderate tricuspid valve regurgitation | 122 | >0.99 | ||
| Yes, n (%) | 20 (16.4%) | 21 (17.2%) | ||
| No, n (%) | 102 (83.6%) | 101 (82.8%) | ||
| Aortic size and patency | ||||
| Ascending aortic maximal diameter, cm | Not collecte d at 14 months | |||
| Ascending aortic maximal diameter for BSA, z-score | Not collec ted at 14 months | |||
| Distal arch to descending aorta CWD peak velocity, m/sec | 75 | 1.80 ± 0.66 | 1.81 ± 0.63 | 0.93 |
Figure 1. Comparison of RVEF between the MBTS and RVPAS Groups at the 14-month and Pre-Fontan Echocardiograms.
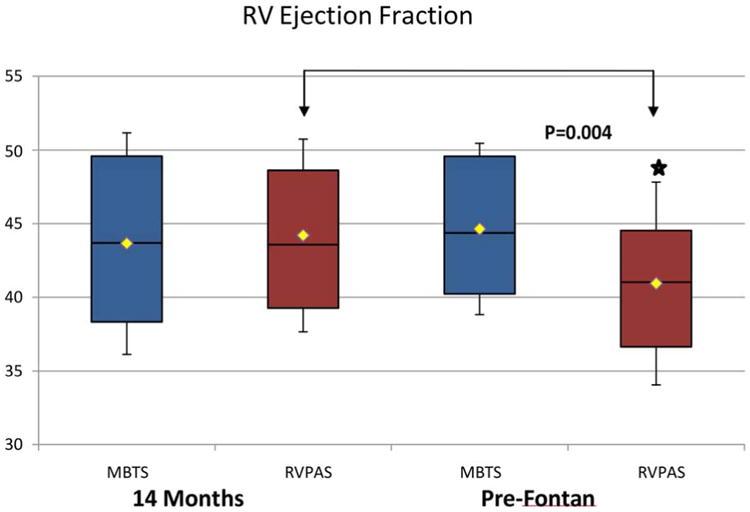
There was no significant change in RVEF ejection fraction in the MBTS group, while the RVPAS group had significantly decreased ejection fraction compared to 14-month study (bracket).
MBTS = modified Blalock-Taussig shunt; RVEF = right ventricular ejection fraction; RVPAS = right ventricle-to-pulmonary artery shunt.
Right Ventricular Diastolic Size and Function
Diastolic indices, including RV indexed end-diastolic volume, indexed end-diastolic area, tricuspid peak E and A velocities and E/A ratio, tricuspid annular E′ and A′ velocities, E/E′ ratio, and presence of pulmonary vein flow reversal during atrial systole were similar between shunt groups at the pre-Fontan study (Table 3). In addition, the presence of pulmonary vein reversal of flow with atrial systole was rarely identified in either group (6.5% in MBTS; 2% in RVPAS; p = 0.24). When compared with measures obtained at 14 months, there was no significant change in diastolic indices over time for the MBTS group (Table 4). In the RVPAS group, tricuspid peak E and A velocities decreased and the tricuspid annular E′ velocity increased from 14 months to the pre-Fontan study, which resulted in a significantly decreased E/E′ ratio at the pre-Fontan study, bringing this measure into the normal range (E/E′ ratio for age z-score 3.0 ± 2.6 at 14 months vs. 1.7 ± 2.0 pre-Fontan). All other diastolic indices were stable between the 2 intervals for the RVPAS group (Table 5).
Right Ventricular Global Function
The myocardial performance index, as assessed by both blood flow Doppler and annular Doppler tissue imaging, was not significantly different between the shunt groups at the pre-Fontan study (Table 3). Additionally, we found no significant change in the index as calculated by either method from 14 months to the pre-Fontan study for either group (Tables 4 and 5).
Neo-aortic Valve Size and Function
Indexed neo-aortic annular areas were similar in both shunt groups at the pre-Fontan study with marked neo-aortic annular dilatation, as evidenced by mean z-scores of 5.6 ± 2.5 for MBTS and 5.1 ± 3.1 for RVPAS (p = 0.22) (Table 3). Both shunt groups showed a significant decrease in indexed neo-aortic valve size from the 14-month to the pre-Fontan study, however, with an average change in z-score of -1.1 for the MBTS and -1.3 for the RVPAS (Figure 2). This change over time was not significantly different between the shunt groups. The degree of neo-aortic regurgitation was similar in the 2 groups and did not vary significantly between time points. At the pre-Fontan study, 2.5% of the MBTS and 4.6% of the RVPAS cohort (p = 0.73) had moderate regurgitation and no subject had severe regurgitation.
Figure 2. Comparison of Indexed Neo-aortic Annular z-Scores between the MBTS and RVPAS Groups at the 14-month and Pre-Fontan Echocardiograms.
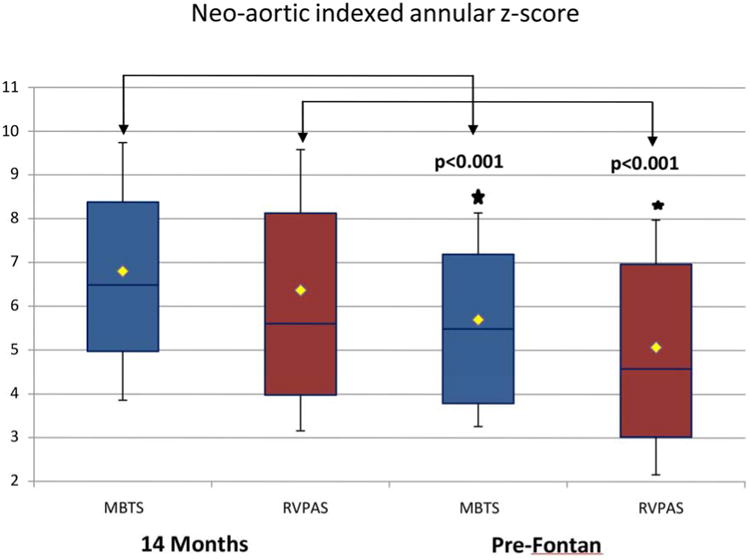
Indexed neo-aortic annular z-scores were similar in both groups at the pre-Fontan study with marked neo-aortic annular dilatation and with a significant decrease in valve size for both groups from the 14-month study (brackets).
Abbreviations as in Figure 1.
Tricuspid Valve Size and Function
Indexed tricuspid annular area and z-score were similar in both groups at the pre-Fontan study, with a mean z-score of 1.9 ± 2.2 for MBTS and 2.1 ± 2.0 for RVPAS (p = 0.38; Table 3). Both groups demonstrated a significant decrease in indexed tricuspid valve size from the 14-month to the pre-Fontan study. The degree of change over time was similar for both groups, with an average change in z-score of -0.7 for the MBTS and -0.9 for the RVPAS (Figure 3). The degree of tricuspid regurgitation was comparable in the two groups, with 19% of the MBTS and 16% of the RVPAS cohort (p = 0.62) having moderate or severe regurgitation at the pre-Fontan study. The percentage of subjects with moderate or greater tricuspid regurgitation was stable when compared to the 14-month assessment; it was present in <20% in both groups at both intervals.
Figure 3. Comparison of Indexed Tricuspid Annular z-Scores between the MBTS and RVPAS Groups at the 14-month and Pre-Fontan Echocardiograms.
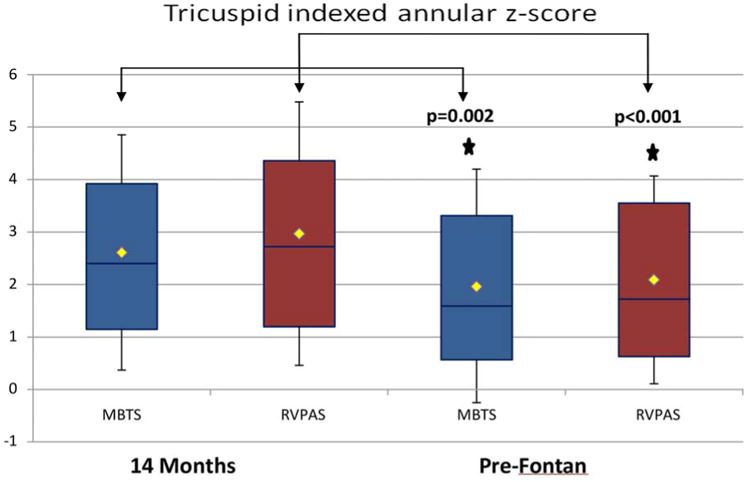
Indexed tricuspid annular area and z-score were similar in both shunt groups at the pre-Fontan study, with a significant decrease in both groups from the 14-month study (brackets).
Abbreviations as in Figure 1.
Aortic Size and Patency
The ascending aortic diameter was similar in both shunt groups at the pre-Fontan study with moderate ascending aortic dilatation, as evidenced by mean z-scores of 3.4 ± 2.3 for MBTS and 3.1 ± 2.4 for RVPAS (p = 0.31) (Table 3). As the ascending aorta was not quantified at the 14-month study, information about change over time was unavailable. We saw similarities in peak velocity through the distal aortic arch between the shunt groups at the pre-Fontan study (1.7 ± 0.5 for MBTS vs 1.8 ± 0.6 m/s for RVPAS; p = 0.08). The peak velocity was also stable when compared to the 14-month assessment, with average peak velocities <2 m/s for both groups at both intervals (Tables 4 and 5).
Transplant-free Survival to Fontan
Eighteen subjects died or required heart transplantation from the 14-month echocardiographic study to the Fontan procedure. In univariate analyses, a larger RV indexed end-systolic volume and area, lower RVEF, larger RV indexed diastolic volume and area, higher peak early diastolic tricuspid filling velocity, higher myocardial performance index (MPI), larger indexed tricuspid indexed annular area and z-score for BSA, and at least moderate tricuspid valve regurgitation were associated with an increased risk of transplant or death between the 14-month echocardiogram to Fontan palliation (Table 6). Neo-aortic valve size, degree of neo-aortic regurgitation, and aortic arch gradient at 14 months were not associated with transplant-free survival to Fontan.
Table 6. Univariate Analysis of 14-Month Echocardiographic Indices as Predictors of Transplant-free Survival from the Time of Study to Fontan Surgery.
| Transplant-free Survivors | Transplanted or Died | Hazard Ratio | p Value | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||||
| n | N | |||||
| RV systolic size and function | ||||||
| End systolic volume, ml/BSA1.3 | 221 | 49.88 ± 16.74 | 12 | 99.61 ± 53.29 | 1.05 | <0.001 |
| End systolic area, cm2/BSA0.8 | 284 | 15.82 ± 4.07 | 17 | 20.98 ± 8.09 | 1.16 | <0.001 |
| Ejection fraction, % | 221 | 43 ± 7 | 12 | 34 ± 9 | 0.85 | <0.001 |
| Area fraction, % | 284 | 33 ± 7 | 17 | 26 ± 8 | 0.89 | <0.001 |
| Peak systolic velocity, cm/sec | 291 | 5.33±1.44 | 17 | 5.81±1.23 | 1.24 | 0.18 |
| Isovolumic contraction acceleration, cm/sec2 | 156 | 35.24 ± 11.57 | 8 | 32.50 ± 8.80 | 0.98 | 0.58 |
| RV diastolic size and function | ||||||
| End diastolic volume, ml/BSA1.3 | 221 | 87.54 ± 24.64 | 12 | 144.88 ± 57.24 | 1.05 | <0.001 |
| End diastolic area, cm2/BSA0.8 | 284 | 23.50 ± 5.02 | 17 | 28.16 ± 8.52 | 1.15 | <0.001 |
| Transverse valve peak early velocity, m/sec | 300 | 0.86 ± 0.25 | 17 | 0.96 ± 0.24 | 4.46 | 0.08 |
| Transverse valve peak atrial velocity, m/sec | 172 | 0.72 ± 0.20 | 3 | 0.67 ± 0.31 | 0.23 | 0.62 |
| E/A ratio | 172 | 1.09 ± 0.39 | 3 | 1.21 ± 0.34 | 1.78 | 0.54 |
| Peak early diastolic velocity, cm/sec | 294 | 8.56 ± 3.60 | 17 | 11.41 ± 4.57 | 1.16 | 0.002 |
| Peak atrial diastolic velocity, cm/sec | 168 | 6.82 ± 2.24 | 5 | 7.50 ± 1.93 | 1.14 | 0.50 |
| E/E′ ratio | 288 | 11.32 ± 4.82 | 16 | 9.81 ± 5.43 | 0.93 | 0.22 |
| Flow reversal during atrial systole | 291 | 18 (6.2%) | 17 | 3 (17.6%) | 0.29 | 0.05 |
| RV global function | ||||||
| MPI, Inflow Doppler/ET calculation | 281 | 43.47 ± 16.33 | 15 | 55.53 ± 22.27 | 1.03 | 0.006 |
| MPI DTI calculation | 169 | 0.60 ± 0.20 | 5 | 0.63 ± 0.23 | 2.42 | 0.68 |
| Valvular size and function | ||||||
| Neo-aortic annular area/BSA | 287 | 4.24 ± 1.09 | 18 | 4.30 ± 1.10 | 1.05 | 0.82 |
| Neo-aortic annular area for BSA, z-score | 287 | 6.50 ± 3.06 | 18 | 6.65 ± 3.08 | 1.02 | 0.83 |
| At least moderate neo-aortic valve regurgitation | 312 | 7 (2.2%) | 18 | 1 (5.6%) | 0.34 | 0.29 |
| Tricuspid valve size and function | ||||||
| Tricuspid indexed annular area/BSA | 306 | 6.36 ± 2.00 | 18 | 7.53 ± 2.51 | 1.29 | 0.02 |
| Tricuspid indexed annular area for BSA, z-score | 306 | 2.85 ± 2.38 | 18 | 4.25 ± 3.02 | 1.24 | 0.02 |
| At least moderate tricuspid valve regurgitation | 312 | 65 (20.8%) | 18 | 8 (44.4%) | 0.34 | 0.02 |
| Neo-aortic size and patency | ||||||
| Distal arch to descending aorta CWD peak velocity, m/sec | 246 | 1.67 ± 0.55 | 15 | 1.73 ± 0.56 | 1.25 | 0.63 |
Discussion
The SVR trial provides a unique opportunity for longitudinal assessment of a large, high-risk cohort of children born with single right ventricle anomalies as they progress through staged surgical palliation. In previous work, initial shunt type used with the Norwood procedure was found to influence transplant-free survival at 1 year, with lower mortality in those subjects who received a RVPAS compared with a MBTS (3). However, this survival benefit appears to dissipate with time after the first year, so the best surgical strategy remains in question. In the current manuscript, we describe changes in echocardiographic indices of RV, neo-aortic, and tricuspid valve size and function from completion of the initial trial (14 months, which marked the end of the primary SVR study visit) until subsequent Fontan surgery was performed.
Preservation of single RV function from the time of the Norwood onward is paramount for children undergoing staged palliation as the resultant physiology depends on well-maintained RV function to avoid chronic cardiovascular morbidities including heart failure, frequent hospitalizations, arrhythmias, and transplant, as well as early mortality. Here, we found that initial shunt type appears to influence RV remodeling during the second and third years of life prior to Fontan surgery with deterioration in RVEF in those with an RVPAS (Central Illustration). This change is associated with poorer transplant-free survival during the same time period for subjects with an RVPAS (10), raising concerns that this surgical strategy may result in progressive deleterious effects on RV systolic function that could have important implications for long-term morbidity and mortality. Although previous single-center studies have also shown improved early outcomes with the RVPAS compared with the MBTS (11-14), concerns about poorer qualitative (15,16) and quantitative (17) RV systolic function prior to and after the final stage Fontan operation have been described in patients who had the RVPAS. It has been hypothesized that the ventriculotomy required for the RVPAS may cause myocardial injury and/or scar with aneurysm formation, and that this injury may ultimately affect ventricular performance (18). If true, it is possible that the effects of this early myocardial injury take time to manifest in a way that echocardiography can measure. No difference was detected in RV fractional area change, but this method does not evaluate the outflow tract, which could potentially be the area most affected by the ventriculotomy scar. RVEF, as calculated here by the biplane pyramidal method (19), is likely a more sensitive tool for RV systolic function in this cohort.
Central Illustration. Flow Diagram of the Results of the SVR Extension Trial.
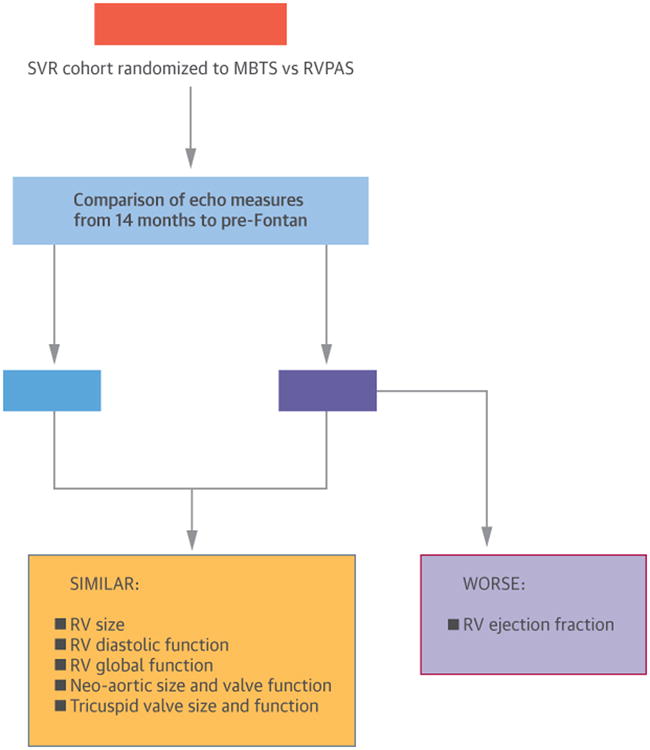
At 14 months, patients in the SVR Trial showed no differences in RV function whether they had received an RVPAS or MBTS, but longer-term results in the SVR Extension Trial demonstrated greater RVEF deterioration after RVPAS. Encouragingly, other indices of RV function remained stable prior to Fontan regardless of shunt type.
MBTS = modified Blalock-Taussig shunt; RV = right ventricle/ventricular; RVPAS = right ventricle-to-pulmonary artery shunt; SVR = Single Ventricle Reconstruction
Review of other echocardiographic indices that characterize ventricular function in this cohort did not provide a clear picture of RV failure in the RVPAS survivors prior to Fontan, which suggests that conclusions about the shunt-influenced health of the RV may require additional longitudinal follow-up. Indexed RV end-diastolic volumes and areas were stable and did not show the expected progressive chamber dilation of a failing ventricular pump in either shunt group between 14 months and the pre-Fontan studies. Global indices of function were also remarkably stable and similar for both shunt groups over time as assessed by the myocardial performance index, with the RV MPI (as calculated by inflow Doppler) well below (i.e., much better than) previously published values for children with single ventricle anomalies after staged palliation using the same MPI technique (20). Additionally, RV diastolic indices, which typically show the earliest pathologic changes with progressive ventricular dysfunction, are also stable with little evidence of progressive restrictive disease in either group as evidenced by the absence of subjects who exhibited late diastolic pulmonary venous reversal of flow. In fact, diastolic function appears to improve over time in the RVPAS survivors from the 14-month to the pre-Fontan evaluation, with a significantly decreased tricuspid E/E′; ratio that falls within 2 standard deviations of age-matched normal controls.
Neo-aortic and tricuspid valve size and function also exhibited reassuring trends from 14 months to the pre-Fontan echocardiogram. Although the neo-aortic annulus remained markedly dilated compared with normative controls at both intervals, there was a significant decrease in indexed annular size and z-score over time in both shunt groups. Despite the significant neo-aortic annular and ascending aortic dilatation, more than mild valve regurgitation was rare (<5%) and similar at both intervals for the two shunt groups. Neo-aortic valve size and integrity will be important to track in this cohort over time, given reports of significant neo-aortic root dilatation and neo-aortic valve regurgitation later in childhood after Norwood palliation with MBTS (21). In our cohort, it is similarly encouraging that indexed tricuspid annular size also decreased at the pre-Fontan study compared with the 14-month assessment in both shunt groups, nearly normalizing in size with z-scores approximating 2. The incidence of moderate or greater tricuspid regurgitation was stable (<20% at both intervals for both groups), a finding that differs from a recently published report that identified worse tricuspid valve (TV) function in the MBTS patients after Fontan (22).
After their first year, children with single RV anomalies remain at risk for death or transplant prior to Fontan surgery. Not surprisingly, transplant-free survival to Fontan was associated with significantly smaller RV indexed end-diastolic and end-systolic volumes and areas, higher RV percent fractional area change and RVEF, lower myocardial performance index, smaller TV indexed annular area, and a lower incidence of moderate or worse TV regurgitation at 14 months. This suggests that patients with significantly larger RV volumes, decreased RV systolic and global functional indices, TV annular dilation, and significant TV regurgitation warrant closer surveillance.
Study Limitations
Unlike for the left ventricle, 2D echocardiographic tools used to assess RV volume and systolic function remain limited due to the chamber's complex geometry. Analysis of regional RV wall motion was not performed as part of this protocol, so the impact of focal scarring/dyskinesis was not specifically assessed. Careful training in protocol image acquisition was provided and reinforced at all sites with regular quality assurance feedback from the core lab to optimize appropriate image capture; consequently, >98% of the submitted studies were found to be acceptable in providing images that allowed data extraction. However, while data extraction was possible in nearly all studies, not all measurements were achievable in the majority of studies because of incomplete or inadequate image acquisition. The impact of this limitation in the characterization of RV function for this cohort is unclear but emphasizes the challenge of multi-institutional echocardiographic trials that require extensive data acquisition, particularly when obtaining those data from young children with complex heart disease.
Conclusions
Initial shunt type used with the Norwood procedure may influence RV remodeling during the second and third years of life prior to Fontan surgery in survivors with single RV anomalies, as evidenced by decreased RVEF after the RVPAS that is not seen after MBTS. Other echocardiographic measures of RV function, however, appear to remain stable in both shunt groups. In addition, neo-aortic and tricuspid valve size decrease in both shunt groups over time, and both valves continue to function well for most survivors without progressive regurgitation before the Fontan. Factors associated with poorer transplant-free survival to the Fontan operation included echocardiographic evidence of markedly enlarged RV volumes, severely abnormal RV systolic and global indices of function, significant tricuspid annular dilation, and at least moderate tricuspid regurgitation at 14 months.
Perspectives.
Competency in Medical Knowledge
Initial shunt type used for the Norwood procedure may influence right ventricular (RV) remodeling during the second and third years of life prior to Fontan surgery in survivors with single RV anomalies, as evidenced by decreased RVE ejection fraction after the right ventricle-to-pulmonary shunt procedure that is not seen in those after use of the modified Blalock-Taussig shunt. Other echocardiographic measures of RV and valve function, however, appear to remain stable in both shunt groups.
Competency in Patient Care
The best choice of initial shunt type in infants requiring the Norwood procedure for single RV anomalies remains unclear based on current data from the Single Ventricle Reconstruction (SVR) trial.
Translational Outlook
Longitudinal follow-up of the SVR cohort is ongoing and will likely provide further insights into the long-term effect of initial shunt type on clinical outcome and echocardiographic indices of cardiac size and function.
Acknowledgments
Funding Sources: Supported by U01 grants from the National Heart, Lung, and Blood Institute (HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288, HL085057, HL109781, HL109737). The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute
Abbreviations
- BSA
body surface area
- MBTS
modified Blalock-Taussig shunt
- MPI
myocardial performance index
- RV
right ventricular
- RVEF
right ventricular ejection fraction
- RVPAS
right ventricle-to-pulmonary artery shunt
- SVR
Single Ventricle Reconstruction trial
Footnotes
Disclosures: none
Clinical Trial Registration #: NCT00115934
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mahony L, Sleeper LA, Anderson PA, et al. The Pediatric Heart Network: a primer for the conduct of multicenter studies in children with congenital and acquired heart disease. Pediatr Cardiol. 2006;27:191–8. doi: 10.1007/s00246-005-1151-9. [DOI] [PubMed] [Google Scholar]
- 2.Ohye RG, Gaynor JW, Ghanayem NS, et al. Design and rationale of a randomized trial comparing the Blalock-Taussig and right ventricle-pulmonary artery shunts in the Norwood procedure. J Thorac Cardiovasc Surg. 2003;136:968–75. doi: 10.1016/j.jtcvs.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohye RG, Sleeper LA, Mahony L, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–92. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frommelt PC, Guey LL, Minich LL, et al. Does Initial Shunt Type for the Norwood Procedure Impact Echocardiographic Measures of Cardiac Size and Function during Infancy? The Single Ventricle Reconstruction Trial. Circulation. 2012;125:2630–8. doi: 10.1161/CIRCULATIONAHA.111.072694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghanayem NS, Allen KR, Tabbutt S, et al. Interstage mortality after the Norwood procedure: Results of the multicenter Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:896–906. doi: 10.1016/j.jtcvs.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tabbutt S, Ghanayem N, Ravishankar C, et al. Risk factors for hospital morbidity and mortality after the Norwood procedure: A report from the Pediatric Heart Network Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:882–95. doi: 10.1016/j.jtcvs.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tweddell JS, Sleeper LA, Ohye RG, et al. Intermediate-term mortality and cardiac transplantation in infants with single-ventricle lesions: Risk factors and their interaction with shunt type. J Thorac Cardiovasc Surg. 2012;144:152–9. doi: 10.1016/j.jtcvs.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hourihan M, Colan SD, Wernovsky G, et al. Growth of the aortic anastomosis, annulus, and root after the arterial switch procedure performed in infancy. Circulation. 1993;88:615–20. doi: 10.1161/01.cir.88.2.615. [DOI] [PubMed] [Google Scholar]
- 9.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99:445–57. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 10.Newburger JW, Sleeper LA, Frommelt PC, et al. Transplant-free survival and interventions at three years in the single ventricle reconstruction trial. Circulation. 2014;129:2013–20. doi: 10.1161/CIRCULATIONAHA.113.006191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pizarro C, Malec E, Maher KO, et al. Right ventricle to pulmonary artery conduit improves outcome after stage I Norwood for hypoplastic left heart syndrome. Circulation. 2003;150:55–60. doi: 10.1161/01.cir.0000087390.94142.1d. [DOI] [PubMed] [Google Scholar]
- 12.Sano S, Ishino K, Kado H, et al. Outcome of right ventricle-to-pulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome: a multi-institutional study. Ann Thorac Surg. 2004;78:1951–8. doi: 10.1016/j.athoracsur.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 13.Mair R, Tulzer G, Sames E, et al. Right ventricular to pulmonary artery conduit instead of modified Blalock-Taussig shunt improves postoperative hemodynamics in newborns after the Norwood operation. J Thorac Cardiovasc Surg. 2003;126:1378–84. doi: 10.1016/s0022-5223(03)00389-1. [DOI] [PubMed] [Google Scholar]
- 14.Malec E, Januszewska K, Kolcz J, Mroczek T. Right ventricle-to-pulmonary artery shunt versus modified Blalock-Taussig shunt in the Norwood procedure for hypoplastic left heart syndrome—influence on early and late haemodynamic status. Eur J Cardiothorac Surg. 2003;23:728–34. doi: 10.1016/s1010-7940(03)00072-1. [DOI] [PubMed] [Google Scholar]
- 15.Ballweg JA, Dominguez TE, Ravishankar C, et al. A contemporary comparison of the effect of shunt type in hypoplastic left heart syndrome on the hemodynamics and outcome at Fontan completion. J Thorac Cardiovasc Surg. 2010;140:537–44. doi: 10.1016/j.jtcvs.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 16.Graham EM, Zyblewski SC, Phillips JW, et al. Comparison of Norwood shunt types: do the outcomes differ 6 years later? Ann Thorac Surg. 2010;90:31–5. doi: 10.1016/j.athoracsur.2010.03.078. [DOI] [PubMed] [Google Scholar]
- 17.Tanoue Y, Kado H, Shiokawa Y, et al. Midterm Ventricular Performance After Norwood Procedure With Right Ventricular–Pulmonary Artery Conduit. Ann Thorac Surg. 2004;78:1965–71. doi: 10.1016/j.athoracsur.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Padalino MA, Castellani C, Toffoli S, et al. Pathological changes and myocardial remodeling related to the mode of shunting following surgical palliation for hypoplastic left heart syndrome. Cardiol Young. 2008;18:415–22. doi: 10.1017/S1047951108002461. [DOI] [PubMed] [Google Scholar]
- 19.Helbing WA, Bosch HG, Maliepaard C, et al. Comparison of echocardiographic methods with magnetic resonance imaging for assessment of right ventricular function in children. Am J Cardiol. 1995;76:589–94. doi: 10.1016/s0002-9149(99)80161-1. [DOI] [PubMed] [Google Scholar]
- 20.Williams RV, Ritter S, Tani LY, et al. Quantitative assessment of ventricular function in children with single ventricles using the Doppler myocardial performance index. Am J Cardiol. 2000;86:1106–10. doi: 10.1016/s0002-9149(00)01168-1. [DOI] [PubMed] [Google Scholar]
- 21.Cohen MS, Marino BS, McElhinney DB, et al. Neo-aortic root dilatation and valve regurgitation up to 21 years after staged reconstruction for hypoplastic left heart syndrome. J Am Coll Cardiol. 2003;42:533–40. doi: 10.1016/s0735-1097(03)00715-0. [DOI] [PubMed] [Google Scholar]
- 22.Bautista-Hernandez V, Scheurer M, Thiagarajan R, et al. Right ventricle and tricuspid valve function at midterm after the Fontan operation for hypoplastic left heart syndrome: Impact of shunt type. Pediatr Cardiol. 2011;32:160–6. doi: 10.1007/s00246-010-9835-1. [DOI] [PubMed] [Google Scholar]


