Abstract
The vast majority of HIV-1 infections occur at mucosa during sexual contact. It may therefore be advantageous to provide mucosal barrier protection against this entry by mucosal vaccination. While a number of mucosal routes of vaccination are possible, many like enteric oral vaccines or intranasal vaccines have significant impediments that limit vaccine efficacy or pose safety risks. In contrast, immunogens applied to the sublingual region of the mouth could provide a simple route for mucosal vaccination. While sublingual immunization is appealing, this site does not always drive strong immune responses, particularly when using protein antigens. To address this issue, we have tested the ability of two mucosal adjuvants: alpha-galactosylceramide (αGalCer) that is a potent stimulator of natural killer T cells and CpG-oligodeoxynucleotide (CpG-ODN) a TLR9 agonist for their ability to amplify immune responses against clade C gp140 HIV-1 envelope protein antigen. Immunization with envelope protein alone resulted in a weak T cell and antibody responses. In contrast, CD4+ and CD8+ T cells responses in systemic and mucosal tissues were significantly higher in mice immunized with gp140 in the presence of either αGalCer or CpG-ODN and these responses were further augmented when the two adjuvants were used together. While both the adjuvants effectively increased gp140-specific serum IgG and vaginal IgA antibody levels, combining both significantly improved these responses. Memory T cell responses 60 days after immunization revealed αGalCer to be more potent than CpG-ODN and the combination of the αGalCer and CpG-ODN adjuvants was more effective than either alone. Serum and vaginal washes collected 60 days after immunization with gp140 with both αGalCer and CpG-ODN adjuvants had significant neutralization activity against Tier 1 and Tier 2 SHIVs. These data support the utility of the sublingual route for mucosal vaccination particularly in combination with αGalCer and CpG-ODN adjuvants.
Introduction
Genital tissues constitute the major portals of human immunodeficiency virus type 1 (HIV-1) infection and clade C strains are the most prevalent HIV-1 subtype globally [1-3]. Vaccination strategies generating antigen-specific antibody and T cells mediated immune responses against these strains are essential for protection [4-6]. Given that the mucosal surface is the predominant entry route for HIV-1, there has been an increasing interest in the development of vaccines that can generate robust antiviral antibody and cellular responses at mucosal surfaces [3, 5].
Observations from the RV144 trial in Thailand have demonstrated that canary pox vector vaccine ALVAC-HIV (vCP1521) for priming combined with the gp120 protein vaccine AIDSVAX B/E for boosting resulted in 31% vaccine efficacy [7]. Specifically, data from this trial suggested a protective role for anti-envelope antibodies thereby providing proof-of-principle for further exploration of vaccine strategies employing the HIV-1 envelope protein [8].
Adjuvants are important for the use of recombinant envelope immunogens, since these proteins by themselves generate only weak immune responses [9, 10]. Historically, selection of vaccine adjuvants has not focused on specifically amplifying mucosal immunity. For potent vaccine formulations delivered by mucosal routes, incorporation of adjuvants that harness the potential of innate immune modulators is important for overcoming immune tolerance and enhancing the immunogenicity of co-administered antigens[11-13]. The RV144 trial used alum as an adjuvant, which was then the only licensed vaccine adjuvant. However, alum is not thought to support robust cellular immune responses [14, 15].
Bacterial toxins are by far the most potent mucosal adjuvant candidates, but concerns remain regarding their safety even when mutated to reduce toxicity [16, 17]. In contrast, ligands for TLRs 7/8 and 9 serve as potent adjuvants for parenteral and mucosal vaccines based on plasmid DNA, viral vectors and recombinant proteins[11, 12, 18]. In particular, CpG-containing synthetic oligodeoxynucleotides (CpG-ODN) that activate TLR9 on dendritic cells (DCs) appear potent in stimulating antigen presentation and induction of antigen-specific immune responses [12, 18].
The synthetic glycolipid alpha-galactosylceramide (αGalCer) has been tested primarily in cancer immunotherapy studies because of its capacity to serve as a ligand and potent activator of invariant natural killer T (NKT) [19, 20]. The NKT cells are a highly conserved T cell lineage activated by a variety of CD1d-restricted microbial antigens. As an important component of the innate immune system, NKT cells are recognized for their ability to “jump-start” adaptive immune responses through their unique ability to activate DCs and play pivotal roles in the innate immune response to many pathogens including viruses even if the particular infectious agent does not itself encode CD1d-restricted antigens[19]. We previously reported that αGalCer amplifies systemic and mucosal immune responses to antigens including HIV envelope peptides [21, 22]. In addition, we found that repeated mucosal delivery of αGalCer adjuvant in primary and booster immunizations resulted in repeated activation of NKT cells and DC to progressively increase adaptive immune responses[22].
Based on the concept of the common mucosal immune system, delivering vaccines by the more practical nasal and oral/sublingual routes affords induction of broadly disseminated antigen-specific immune responses in multiple mucosal and systemic tissues[23, 24]. Sublingual immunization, relative to the other mucosal routes offers an effective, safer, inexpensive, and non-invasive practical option for vaccine delivery[25-28]. This is due to direct absorption of antigens into the bloodstream from oral mucosa bypassing gastrointestinal processing and limiting proteolytic degradation[25]. In the present study we tested the effectiveness of sublingual route in generating strong effector and memory immune responses to the clade C HIV-1 gp140 envelope protein using αGalCer alone or in combination with CpG-ODN as adjuvants.
Materials and methods
Animals
Female C57BL/6 X BALB/c (CB6F1) mice aged 6-10 weeks were purchased from the National Cancer Institute (Frederick, MD). The animals were maintained in specific pathogen-free environment at the institutional animal facility. The animal facility is fully accredited by the Association for Assessment and Accreditation of Laboratory Animals Care International. All animal procedures were conducted in compliance with the institutionally approved protocols.
Reagents
The HIV/Clade C gp140 (gp140) protein was purchased from Immune Technology Corp. (New York, NY). The alpha-galactosylceramide (αGalCer) was purchased from Diagnocine LLC (Hackensack, NJ) and dissolved in dimethyl sulfoxide, (Sigma, St. Louis, MO) at a concentration of 1 mg/ml. The CpG-ODN (5′- TCCATGACGTTCCTGACGTT-3′) (motif # 1826) was purchased from Invivogen (San Diego, CA) and resuspended in endotoxin-free water (Life Technologies, Grand Island, NY).
Cells
The human cell line HeLa CD4-LTR/β-gal was obtained from the AIDS Research and Reference Reagent Program (Germantown, MD) and were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco-Invitrogen, Grand Island NY, USA) supplemented in each case with heat inactivated 10% fetal bovine serum (FBS) at 37°C and 5% CO2.
Immunizations
For sublingual immunization, mice were first anesthetized by intraperitoneal (i.p.) injection of ketamine and xylazine hydrochloride (100mg/kg and 10mg/kg respectively). Each animal received an administration of 5μg of gp140 protein either alone (Group 1) or with 2μg of αGalCer (Group 2) or with 10μg of CpG-ODN (Group 3) or with 2μg of αGalCer and 10μg of CpG-ODN (Group 4) under the tongue using the previously described procedure [26]. These doses for the antigen and adjuvants were chosen based on our own past studies and literature reports [29, 30]. To avoid swallowing, the total volume of the inoculum was limited to 10μl/animal and the animals were maintained with their heads in ante-flexion till they regained consciousness. Mice received two immunizations at 7 day intervals and adaptive immune responses in different tissues were determined at various times post immunization.
IFN-γ ELISpot Assay
Antigen-specific responses of CD4+ and CD8+ T lymphocytes isolated from cervical lymph nodes, lungs, and spleens of the immunized animals at different times post immunization were determined by IFN-γ ELISpot assay as described previously[21, 22]. The cells were stimulated by incubating with either medium alone or gp140 protein (1μM) or Concavalin A (5μg/ml) for 48 h before secondary antibody treatment and color development of IFN-γ spot forming cells (SFC) using the commercial reagent kit (BD Biosciences, San Jose, CA). Enumeration of spots representing individual cells producing IFN-γ was done by Zellnet Consulting Inc., Fort Lee NJ using KS-ELISPOT automatic system (Carl Zeiss Inc., Thornwood, NY). Responses were considered positive only when they were above 50 SFC/106 input cells and at least twice the number obtained in cells cultured with medium alone.
Analyses of antigen specific T lymphocytes
Presence of activated antigen-specific CD8+ and CD4+ T cells after immunization was determined by flow cytometric analysis of cells after intracellular cytokine staining assay. Cell were stimulated overnight with gp140 protein and then incubated further with GolgiPlug reagent (BD Biosciences, San Jose, CA) for 6 hours prior to cellular staining. Cells were first stained for surface markers and then permeablized for staining with PE-conjugated IFN-γ antibody (BD Biosciences, San Jose, CA) in 1× Perm/Wash Buffer (BD Biosciences, San Jose, CA)(17). Samples were run on the LSRII flow cytometer and analyses were performed using FlowJo software (Tree Star Inc, Ashland, OR). The monoclonal antibodies used were PB-anti-CD3 (clone 500A2), PerCPCy5.5-anti-CD8 (clone 53-6.7) APC-anti-CD4 (clone RM4-5) and PE-anti-IFN-γ (clone XMG1.2), all purchased from BD Biosciences, San Jose, CA. For exclusion of cells with background fluorescence for PE, an aliquot of cells used for fluorescence-minus-one (FMO) from each tissue was stained with anti-CD3, anti-CD8 and anti-CD4 antibodies and used as control for establishing gates for CD4+IFN-γ+ and CD8+IFN-γ+ cell population. Percentage of antigen specific activated cells within CD4+ and CD8+ lymphocyte subsets was determined for animals receiving immunization with either gp140 protein alone or gp140 + adjuvants.
Antigen specific antibody response
Antigen specific antibody responses were evaluated in the blood, saliva and vaginal washes of immunized animals. Blood samples were collected from the retro-orbital sinuses. For collection of saliva, the anaesthetized animals were administered pilocarpine (200mg/kg) i.p. and then saliva was collected using a micropipette. Vaginal washes were collected by repeated flushing with PBS. Serum (dilution 1:100) and mucosal secretions (dilution 1:5) were assayed for antibody levels to gp140 by ELISA using standard protocols (9). HRP-conjugated goat antibodies to mouse IgG or IgA (KPL Inc., Gaithersburg, MD) were used for detection. The gp140 specific antibody concentration in the sample was determined by subtracting the absorbance of the pre-immunization samples from post-immunization sample for individual animals. For each group of immunized mice, results were expressed as average absorbance ± SD.
Neutralizing antibody response
Serum and vaginal washes from all the mice in this study were tested for neutralizing antibodies against SHIV-1157ipEL-p, a tier 1 [31] and SHIV-2873Nip, a tier 2 [32] virus and neutralization titers were measured using the TZM-bl reporter cell line-based neutralization assay as described previously [33, 34]. In brief, TZB-bl cells (5,000 cells/well) were seeded in 100 μl DMEM/10% FCS (Gibco-Invitrogen, Grand Island NY, USA). Serial 2-fold dilutions of immune sera were prepared in triplicates in 96-well flat bottom culture plates. In parallel, the pre-immune sera were serially diluted and used as controls as described previously [33, 34]. Since, limited volumes of serum and vaginal wash samples were available, the samples from each of the experimental groups were pooled and data from replicates were represented for each group as follows: Group 1, received gp140 protein only; Group 2, gp140 + αGalCer; Group 3, gp140 + CpG-ODN; and Group 4, gp140 + αGalCer +CpG-ODN.
Statistical Analysis
The immune responses and tumor foci were expressed as averages of 3-6 animals/group. Non-parametric test (Mann-Whitney Test) was used to determine the significance of difference between different immunization groups. All analyses were performed using GraphPad prism (version 6) and p≤0.05 was considered statistically significant.
Results
αGalCer alone or in combination with CpG amplifies T cell responses against clade C HIV-1 gp140 envelope protein after sublingual immunization
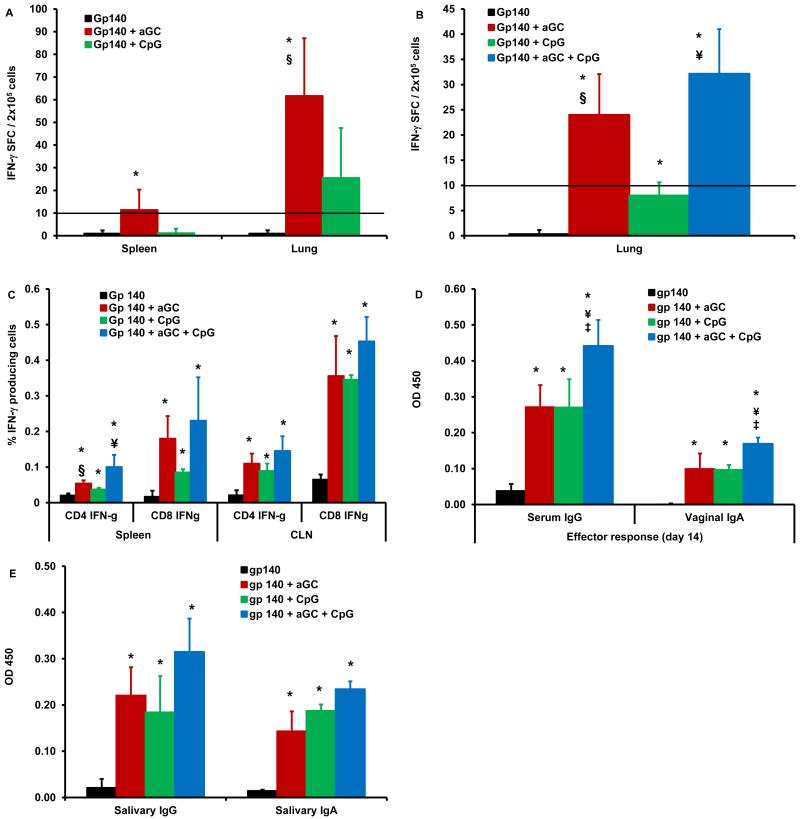
Protein immunogens are typically relatively weak immune stimulators when applied alone to mucosal surfaces. To test this for vaccination against clade C HIV-1, female CB6F1 mice were immunized by sublingual route with two doses of gp140 envelope protein (Group 1) at weekly intervals followed 7 days later by sacrificing and collecting different tissues for immune assays (Fig. 1). Single cell suspensions from spleen and lung tissues analyzed for antigen-specific IFN-γ producing cells by ELISpot demonstrated detectable, but weak T cell responses in both the systemic (spleen) and mucosal (lung) compartments (Fig. 2). In contrast, when gp140 was combined with αGalCer (Group 2) or with CpG-ODN (Group 3) during sublingual immunization, αGalCer (Group 2) drove significantly higher gp140-specific IFN-γ responses than gp140 alone (Group 1) or gp140 with CpG-ODN (Group 3) (Fig. 2A). In mesenteric lymph nodes, antigen-specific IFN-γ response (67 SFU/2×105 cells) generated by gp140 combined with αGalCer (Group 2) were greater than the response (1 SFU/2×105 cells) generated by gp140 alone (Group 1). Furthermore, in a follow-up experiment we observed significantly higher number of gp140-specific IFN-γ producing T cells in the lungs of mice immunized with gp140 in the presence of combination of αGalCer and CpG adjuvants (Group 4), relative to gp140 alone (Group 1) (Fig. 2B). In fact, αGalCer alone (Group 2) as well as in combination with CpG-ODN (Group 4) was significantly more effective than the CpG-ODN alone (Group 3) as an adjuvant for inducing gp140-specific IFN-γ producing cells. Similarly, flow cytometry analyses revealed that αGalCer (Group 2) and CpG-ODN (Group 3) individually were effective in significantly increasing gp140-specific intracellular IFN-γ producing CD4+ as well as CD8+ T cells in multiple tissues in mice compared to gp140 alone (Group 1) (Fig. 2C). This adjuvant effect was further augmented when the two adjuvants (Group 4) were administered together with gp140, but the differences did not reach significance. Also with sublingual immunization with either adjuvant no gross pathological affect in the oral cavity or draining lymph nodes was observed.
Fig. 1. Scheme for immunization and immune assays.
Groups of female CB6F1 mice (n=4-6) were immunized by sublingual route with two doses of the clade C HIV-1 gp140 envelope protein alone or along with αGalCer +/− CpG-ODN at weekly intervals and were sacrificed either at day 14 or day 60 for determining antigen-specific humoral and cellular immune responses during the effector and memory phases.
Fig. 2. Sublingual immunization with clade C HIV-1 gp140 envelope protein using αGalCer and/or CpG-ODN adjuvant induces cellular and humoral immune responses.
Separate groups of mice (n=4-6) were immunized by sublingual route with two doses of gp140 either alone or with αGalCer or CpG-ODN or αGalCer and CpG-ODN at 7 days interval and sacrificed 7 days after second immunization to determine effector adaptive immune responses. Single cells suspensions from spleen and lung tissues were analyzed for antigen-specific IFN-γ producing cells using a standard IFN-γ ELISpot assay (A and B). Responses specific to HIV-1 gp140 envelope protein were determined by subtracting the background values of medium stimulation from that of gp140 protein stimulation and expressed as mean ± S.D. (C) Intracellular cytokine flow cytometry analysis was performed to determine the gp140 protein specific IFN-γ production by CD4+ and CD8+ T cells isolated from spleens and CLN of immunized mice. Using a standard ELISA, serum (diluted 1:100), vaginal wash samples (diluted 1:5) and saliva samples (diluted 1:5) were assayed for HIV-1 gp140 protein specific systemic IgG and mucosal IgA antibody responses, respectively (D and E). Concentration of antigen specific antibodies is expressed as optical density at 450nm (mean ± S.D). Data are representative of two separate experiments (n=4-6 mice). Statistical analyses using the student t-test revealed significant differences (p≤0.05) between groups of mice immunized with gp140 alone and gp140 + αGalCer or CpG-ODN adjuvants (*), gp140 + CpG-ODN and gp140 + αGalCer (§), gp140 + αGalCer and gp140 + αGalCer + CpG-ODN (‡), and gp140 + CpG-ODN and gp140 + αGalCer + CpG-ODN (¥).
αGalCer alone or in combination with CpG amplifies systemic and mucosal antibody responses against clade C HIV-1 gp140 envelope protein after sublingual immunization
The induction of humoral immune response against envelope was analyzed by determining gp140-specific IgG in the serum and IgA in the vaginal wash samples. We observed that αGalCer or CpG adjuvants individually were equally potent in inducing strong gp140-specific serum IgG as well as vaginal IgA antibody responses (Fig. 2D). When the two adjuvants were combined, significant improvement in both IgG and IgA responses was observed relative to either adjuvant alone. We also observed similar enhancement of IgG as well as IgA antibody levels within the salivary secretions representing local immune responses in these mice immunized by the sublingual route (Fig. 2E). Since the Ig subtypes in mice differ from those of humans in relation to functional significance to anti-HIV immunity, current studies did not include such analyses.
Establishment of persistent humoral and cellular immune responses induced against clade C HIV-1 gp140 envelope by sublingual immunization employing αGalCer and CpG-ODN adjuvants
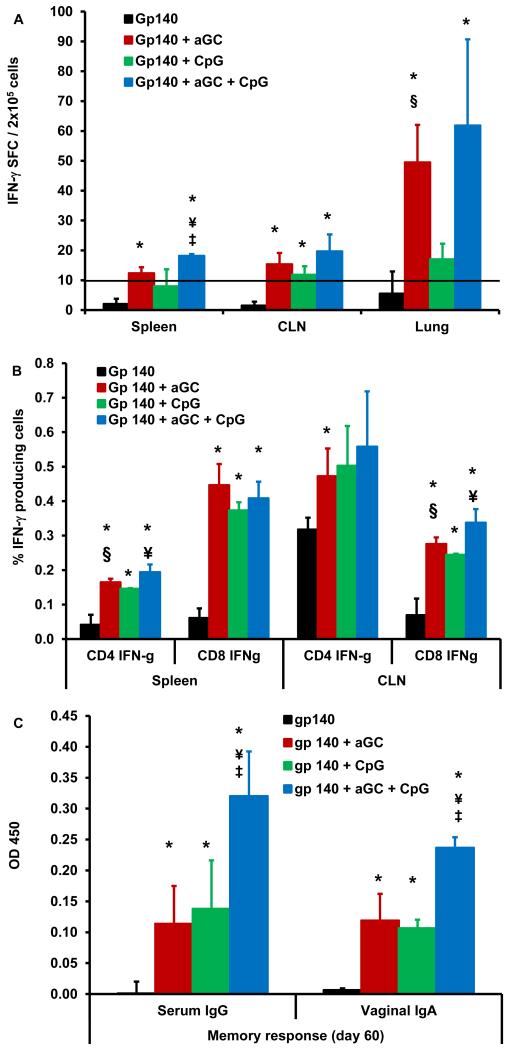
To determine the long-term persistence of these immune responses, mice were vaccinated with gp140 in the presence of αGalCer and/or CpG adjuvants by the sublingual route and were sacrificed 60 days later for cellular and humoral immune assays. Based on literature reports related to T cell memory and our own earlier studies, the 60d time point is valued to represent persistent memory response characterized by the expression of memory cell markers such as CD44 [35-37]. Results from the ELISpot analyses revealed that immunization using gp140 with αGalCer was more effective than gp140 with CpG or gp140 alone at inducing gp140-specific IFN-γ producing cells in the spleen, cervical lymph nodes (CLN), lung (Fig. 3A), and mesenteric lymph nodes (MLN) (data not shown). Furthermore, combining αGalCer and CpG was more effective than either adjuvant alone. This trend was also observed in intracellular cytokine staining for IFN-γ in CD4+ and CD8+ T cells (Fig. 3B). Thus, stronger gp140-specific memory T cells responses were generated by the combination of the two adjuvants for sublingual vaccination. We also observed strong and persisting gp140-specific serum IgG and vaginal IgA antibody levels in mice immunized with either adjuvant alone, which further increased when both adjuvants were combined (Fig. 3C).
Fig. 3. Persistence of antigen-specific humoral and cellular responses after sublingual immunization with clade C HIV-1 gp140 envelope protein using αGalCer and/or CpG-ODN adjuvant.
Separate groups of mice (n=4-6) were immunized by sublingual route with two doses of gp140, gp140 + αGalCer or CpG-ODN or gp 140 + αGalCer + CpG-ODN at 7 days interval. The mice were sacrificed after 60 days and single cell suspensions from spleen, CLN and lungs were analyzed for gp140-specific IFN-γ producing cells using a standard IFN-γ ELISpot assay (A); gp140-specific intracellular IFN-g producing CD4+ and CD8+ T lymphocytes from spleen and CLN were determined by flow cytometry (B); and HIV-1 gp140 protein specific serum IgG and vaginal mucosal IgA effector antibody immune responses were determined by ELISA (C). The concentration of antigen specific antibodies is expressed as mean ± S.D. of optical density at 450nm. Data are representative of two separate experiments (n=4-6 mice). Statistical analyses using the student t-test revealed significant differences (p≤0.05) between groups of mice immunized with gp140 alone and gp140 + αGalCer or CpG-ODN adjuvants (*), gp140 + CpG-ODN and gp140 + αGalCer (§), gp140 + αGalCer and gp140 + αGalCer + CpG-ODN (‡), and gp140 + CpG-ODN and gp140 + αGalCer + CpG-ODN (¥).
Generation of SHIV neutralizing antibodies by sublingual immunization with αGalCer and CpG adjuvants
Serum and vaginal wash samples were collected at the effector and memory phases after last immunization (days 14 and 60, respectively). These were analyzed for neutralizing antibodies against clade C SHIV strains SHIV-1157ipEL-p and SHIV-2873Nip, tier 1 and tier 2 viruses, respectively [31, 32]. This is because; SHIV infection of rhesus macaques is the available best animal model for testing the HIV neutralizing activity of the vaccine-generated antibodies. At the 60 day time point, neutralizing antibodies against these heterologous clade C viruses were observed in plasma for tier 1 virus and in the vaginal washes against both tier 1 and tier 2 viruses, only in the animals that received gp140 in combination with both αGalCer and CpG (Table 1). No neutralizing activity was observed in groups immunized with gp140 alone or with the single adjuvants. Mean IC50 values ranged from 1:40 to 1:53 in plasma and vaginal wash samples, respectively for tier 1 virus and 1:30 in vaginal wash samples for tier 2 virus. For tier 2 virus, the mean IC50 value of serum antibodies was less than 1:20. The data presented is from two independent experiments and because most of the titers were lower than the initial dilutions of plasma (1:20) and vaginal washes (1:10), statistical analysis could not be performed.
Table 1.
Neutralization titers of sera and vaginal washes from mice immunized with Gp140 with various adjuvants
| Neutralizing Antibody IC50 (1/serum dilution)* | ||||
|---|---|---|---|---|
|
| ||||
| SHIV-1157ipEL-p (Tier 1) | SHIV-11572873Nip (Tier 2) | |||
| Immunization Group | Day 14 | Day 60 | Day 14 | Day 60 |
| Gp 140 | <20 | <20 | <20 | <20 |
| Gp140 + α-Galcer | <20 | <20 | <20 | <20 |
| Gp140 + CpG | <20 | <20 | <20 | <20 |
| Gp140 + CpG + α-aGalcer | <20 | 40 | <20 | <20 |
|
| ||||
|
| ||||
| Neutralizing Antibody IC50 (1/vaginal wash dilution) * | ||||
|
| ||||
| SHIV-1157ipEL-p (Tier 1) | SHIV-11572873Nip (Tier 2) | |||
|
| ||||
| Immunization Group | Day 14 | Day 60 | Day 14 | Day 60 |
| Gp 140 | <10 | <10 | <10 | <10 |
| Gp140 + α-Galcer | <10 | <10 | <10 | <10 |
| Gp140 + CpG | <10 | <10 | <10 | <10 |
| Gp140 + CpG + α-aGalcer | <10 | 53 | <10 | 30 |
50% inhibitory concentration given as reciprocal serum dilution for 50% neutralization
Discussion
Many HIV vaccine approaches now use prime-boost strategies employing gene-based vaccines for priming and envelope protein for the booster immunization(s) [38-40]. The RV144 trial is one example of this approach where canary pox gene-based vaccines delivered one HIV envelope gene and booster immunization delivered gp120 protein adjuvanted with alum [7]. It is hypothesized that vaccination at mucosal sites may drive better barrier protection against pathogens that enter at mucosal surfaces. Therefore, efforts to maximize mucosal immunization by gene-based as well as protein-based vaccines are needed. In this work, we have explored the utility of the well-known TLR9 ligand CpG as an adjuvant for protein immunization. We also explored the use of the novel NKT cell agonist αGalCer as a single or combination mucosal adjuvant to address this need.
Results from this investigation support the effectiveness of sublingual route of vaccination provided one or more potent adjuvants are applied with the protein immunogen. As a single adjuvant, αGalCer was generally equal or superior to CpG at inducing strong systemic and mucosal antibody responses as well as cell-mediated immunity against the envelope protein gp140, corresponding to clade C HIV-1 strain. These data are consistent with previous observations of the potency of the αGalCer as a systemic or mucosal adjuvant [21, 22, 35]. This is also in accordance with earlier reports for vaccines against influenza virus and cytomegalovirus where αGalCer potentiated memory immunity attributed to increased expression of bcl-2, an anti-apoptotic pro-survival gene [41, 42]. We further demonstrate that the combination of this NKT agonist with the well-studied TLR9 ligand as adjuvants are even more potent at driving systemic and mucosal immune responses against clade C HIV-1 envelope protein.
The persistence of antigen-specific immunity observed after sublingual immunization using αGalCer adjuvant alone or in combination with the CpG adjuvant is a highly desirable trait in general for efficacious HIV vaccination campaigns. The sublingual route, similar to nasal and oral mucosal administrations, is non-invasive and effective in inducing strong antigen-specific immunity. It is important however to note that sublingual route of vaccination offers the added advantages over oral route in potentially avoiding degradation by digestive enzymes and low pH conditions and over intranasal route, which is known for concerns related to retrograde transport of antigen and/or adjuvant to the brain resulting in some cases cerebral palsy [25, 27]. Anatomically, the sublingual mucosal tissues are well suited for vaccine antigen delivery because they contain MHC class II expressing antigen presenting cells such as the dendritic cells and macrophages, important for efficient antigen uptake and subsequent migration to draining lymph nodes for priming of adaptive immune responses in systemic as well as mucosal compartments [27, 43-45]. Indeed, our results showing strong antigen-specific T cells responses in the draining cervical lymph nodes and lungs as well as serum IgG and vaginal IgA responses support these potential underlying mechanisms for the potency of sublingual immunization regimen. Although the magnitude of the cell mediated immune response in the spleen is lower than that of lungs, the advantage of sublingual immunization is that both mucosal and systemic immunity is induced unlike systemic vaccination that primarily generates systemic immunity [46]. This difference in the localization of the immune response is a function of the induction site of immune responses that regulates the homing of T cells preferably to mucosal locations such as lungs compared to systemic locations such as spleen [47].
Notably, only the combined use of αGalCer and CpG generated neutralizing antibodies against tier 1 and tier 2 clade C SHIVs. More importantly, we observed cross clade neutralization suggesting that a combination of these adjuvants can enhance generation of broadly neutralizing antibodies in mucosal tissues. Interestingly, the response was more in the mucosal compartments compared to systemic (vaginal washes vs. serum). Based on the observations from the current study, it is of interest to purse this vaccination strategy in non-human primate model involving challenge studies to evaluate the protective response against infection. While other studies have used oligomeric gp140 or gp160 or trimeric gp160 as immunogens, they could only demonstrate limited breadth of neutralizing antibody response against heterologous viruses in macaques [48, 49]. Additionally, both these studies did not evaluate mucosal antibody responses. In contrast, our study provided better response in vaginal washes, suggesting that this response may be protective since most of the HIV infections occurs through genital mucosa. Given the burgeoning interest in the production of V1/V2 binding antibodies and HIV neutralizing antibodies, these data suggest that αGalCer and CpG-ODN may have utility to augment these responses for mucosal protein vaccines.
Given the importance of envelope based vaccine components potentially contributing to the observed partial protective efficacy in the RV144 DNA-protein vaccine trial and the prevalence of clade C HIV-1 strain for global HIV pandemic, our results showing efficient induction and persistence of both humoral and cellular immune response to the clade C HIV-1 envelope protein gp140 in the systemic and mucosal compartments are highly significant. The effectiveness of sublingual route of vaccine delivery combined with the use of the synthetic molecules αGalCer and CpG-ODN as adjuvants demonstrated in this investigation offer a safer vaccination regimen that is practical for mass scale vaccination campaigns essential for resource limited areas of the world where curbing this epidemic is the leading objective of the HIV research community.
Highlights.
Sublingual immunization with gp140 and αGalCer or CpG-ODN induces systemic and mucosal immunity.
Combination of both adjuvants significantly improves gp140-specific IgG and IgA antibody levels.
Combination of both adjuvants together induces significant neutralization of clade C SHIV.
Acknowledgment
This work was supported in part by grants from the NIH R01AI96967 (to MAB and KJS) and R21 AI098581 (to SNB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that no conflicts of interest exist.
References
- [1].Shaw GM, Hunter E. HIV transmission. Cold Spring Harbor perspectives in medicine. 2012;2 doi: 10.1101/cshperspect.a006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hemelaar J, Gouws E, Ghys PD, Osmanov S. Global trends in molecular epidemiology of HIV-1 during 2000-2007. AIDS. 2011;25:679–89. doi: 10.1097/QAD.0b013e328342ff93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yu M, Vajdy M. Mucosal HIV transmission and vaccination strategies through oral compared with vaginal and rectal routes. Expert opinion on biological therapy. 2010;10:1181–95. doi: 10.1517/14712598.2010.496776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Strbo N, Vaccari M, Pahwa S, Kolber MA, Doster MN, Fisher E, et al. Cutting edge: novel vaccination modality provides significant protection against mucosal infection by highly pathogenic simian immunodeficiency virus. J Immunol. 2013;190:2495–9. doi: 10.4049/jimmunol.1202655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kaufman DR, Barouch DH. Translational Mini-Review Series on Vaccines for HIV: T lymphocyte trafficking and vaccine-elicited mucosal immunity. Clinical and experimental immunology. 2009;157:165–73. doi: 10.1111/j.1365-2249.2009.03927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Srivastava IK, Ulmer JB, Barnett SW. Neutralizing antibody responses to HIV: role in protective immunity and challenges for vaccine design. Expert review of vaccines. 2004;3:S33–52. doi: 10.1586/14760584.3.4.s33. [DOI] [PubMed] [Google Scholar]
- [7].Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. The New England journal of medicine. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- [8].Montefiori DC, Karnasuta C, Huang Y, Ahmed H, Gilbert P, de Souza MS, et al. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. The Journal of infectious diseases. 2012;206:431–41. doi: 10.1093/infdis/jis367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. The Journal of infectious diseases. 2006;194:1661–71. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- [10].Nkolola JP, Cheung A, Perry JR, Carter D, Reed S, Schuitemaker H, et al. Comparison of multiple adjuvants on the stability and immunogenicity of a clade C HIV-1 gp140 trimer. Vaccine. 2014;32:2109–16. doi: 10.1016/j.vaccine.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gebril A, Alsaadi M, Acevedo R, Mullen AB, Ferro VA. Optimizing efficacy of mucosal vaccines. Expert review of vaccines. 2012;11:1139–55. doi: 10.1586/erv.12.81. [DOI] [PubMed] [Google Scholar]
- [12].Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nature reviews Immunology. 2012;12:592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- [13].Lawson LB, Norton EB, Clements JD. Defending the mucosa: adjuvant and carrier formulations for mucosal immunity. Current opinion in immunology. 2011;23:414–20. doi: 10.1016/j.coi.2011.03.009. [DOI] [PubMed] [Google Scholar]
- [14].Oleszycka E, Lavelle EC. Immunomodulatory properties of the vaccine adjuvant alum. Current opinion in immunology. 2014;28C:1–5. doi: 10.1016/j.coi.2013.12.007. [DOI] [PubMed] [Google Scholar]
- [15].Kool M, Fierens K, Lambrecht BN. Alum adjuvant: some of the tricks of the oldest adjuvant. Journal of medical microbiology. 2012;61:927–34. doi: 10.1099/jmm.0.038943-0. [DOI] [PubMed] [Google Scholar]
- [16].Holmgren J, Czerkinsky C, Eriksson K, Mharandi A. Mucosal immunisation and adjuvants: a brief overview of recent advances and challenges. Vaccine. 2003;21(Suppl 2):S89–95. doi: 10.1016/s0264-410x(03)00206-8. [DOI] [PubMed] [Google Scholar]
- [17].Rhee JH, Lee SE, Kim SY. Mucosal vaccine adjuvants update. Clinical and experimental vaccine research. 2012;1:50–63. doi: 10.7774/cevr.2012.1.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Harandi AM, Holmgren J. CpG DNA as a potent inducer of mucosal immunity: implications for immunoprophylaxis and immunotherapy of mucosal infections. Curr Opin Investig Drugs. 2004;5:141–5. [PubMed] [Google Scholar]
- [19].Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nature reviews Immunology. 2009;9:28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- [20].Seino K, Taniguchi M. Functional roles of NKT cell in the immune system. Frontiers in bioscience: a journal and virtual library. 2004;9:2577–87. doi: 10.2741/1418. [DOI] [PubMed] [Google Scholar]
- [21].Courtney AN, Nehete PN, Nehete BP, Thapa P, Zhou D, Sastry KJ. Alpha-galactosylceramide is an effective mucosal adjuvant for repeated intranasal or oral delivery of HIV peptide antigens. Vaccine. 2009;27:3335–41. doi: 10.1016/j.vaccine.2009.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Courtney AN, Thapa P, Singh S, Wishahy AM, Zhou D, Sastry J. Intranasal but not intravenous delivery of the adjuvant alpha-galactosylceramide permits repeated stimulation of natural killer T cells in the lung. European journal of immunology. 2011;41:3312–22. doi: 10.1002/eji.201041359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Holmgren J, Svennerholm AM. Vaccines against mucosal infections. Current opinion in immunology. 2012;24:343–53. doi: 10.1016/j.coi.2012.03.014. [DOI] [PubMed] [Google Scholar]
- [24].Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nature medicine. 2005;11:S45–53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- [25].Czerkinsky C, Cuburu N, Kweon MN, Anjuere F, Holmgren J. Sublingual vaccination. Human vaccines. 2011;7:110–4. doi: 10.4161/hv.7.1.13739. [DOI] [PubMed] [Google Scholar]
- [26].Cuburu N, Kweon MN, Song JH, Hervouet C, Luci C, Sun JB, et al. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine. 2007;25:8598–610. doi: 10.1016/j.vaccine.2007.09.073. [DOI] [PubMed] [Google Scholar]
- [27].Kweon MN. Sublingual mucosa: A new vaccination route for systemic and mucosal immunity. Cytokine. 2011;54:1–5. doi: 10.1016/j.cyto.2010.12.014. [DOI] [PubMed] [Google Scholar]
- [28].Pedersen GK, Ebensen T, Gjeraker IH, Svindland S, Bredholt G, Guzman CA, et al. Evaluation of the sublingual route for administration of influenza H5N1 virosomes in combination with the bacterial second messenger c-di-GMP. PloS one. 2011;6:e26973. doi: 10.1371/journal.pone.0026973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shim BS, Choi YK, Yun CH, Lee EG, Jeon YS, Park SM, et al. Sublingual immunization with M2-based vaccine induces broad protective immunity against influenza. PloS one. 2011;6:e27953. doi: 10.1371/journal.pone.0027953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Buffa V, Klein K, Fischetti L, Shattock RJ. Evaluation of TLR agonists as potential mucosal adjuvants for HIV gp140 and tetanus toxoid in mice. PloS one. 2012;7:e50529. doi: 10.1371/journal.pone.0050529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Siddappa NB, Watkins JD, Wassermann KJ, Song R, Wang W, Kramer VG, et al. R5 clade C SHIV strains with tier 1 or 2 neutralization sensitivity: tools to dissect env evolution and to develop AIDS vaccines in primate models. PloS one. 2010;5:e11689. doi: 10.1371/journal.pone.0011689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Siddappa NB, Song R, Kramer VG, Chenine AL, Velu V, Ong H, et al. Neutralization-sensitive R5-tropic simian-human immunodeficiency virus SHIV-2873Nip, which carries env isolated from an infant with a recent HIV clade C infection. Journal of virology. 2009;83:1422–32. doi: 10.1128/JVI.02066-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Humbert M, Rasmussen RA, Ong H, Kaiser FM, Hu SL, Ruprecht RM. Inducing cross-clade neutralizing antibodies against HIV-1 by immunofocusing. PloS one. 2008;3:e3937. doi: 10.1371/journal.pone.0003937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Siddappa NB, Hemashettar G, Wong YL, Lakhashe S, Rasmussen RA, Watkins JD, et al. Development of a tier 1 R5 clade C simian-human immunodeficiency virus as a tool to test neutralizing antibody-based immunoprophylaxis. Journal of medical primatology. 2011;40:120–8. doi: 10.1111/j.1600-0684.2010.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Singh S, Yang G, Schluns KS, Anthony SM, Sastry KJ. Sublingual vaccination induces mucosal and systemic adaptive immunity for protection against lung tumor challenge. PloS one. 2014;9:e90001. doi: 10.1371/journal.pone.0090001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lau LL, Jamieson BD, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–52. doi: 10.1038/369648a0. [DOI] [PubMed] [Google Scholar]
- [37].Cerwenka A, Morgan TM, Dutton RW. Naive, effector, and memory CD8 T cells in protection against pulmonary influenza virus infection: homing properties rather than initial frequencies are crucial. J Immunol. 1999;163:5535–43. doi: 10.4049/jimmunol.163.10.5535. [DOI] [PubMed] [Google Scholar]
- [38].Newman MJ. Heterologous prime-boost vaccination strategies for HIV-1: augmenting cellular immune responses. Curr Opin Investig Drugs. 2002;3:374–8. [PubMed] [Google Scholar]
- [39].Ratto-Kim S, Currier JR, Cox JH, Excler JL, Valencia-Micolta A, Thelian D, et al. Heterologous prime-boost regimens using rAd35 and rMVA vectors elicit stronger cellular immune responses to HIV proteins than homologous regimens. PloS one. 2012;7:e45840. doi: 10.1371/journal.pone.0045840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Paris RM, Kim JH, Robb ML, Michael NL. Prime-boost immunization with poxvirus or adenovirus vectors as a strategy to develop a protective vaccine for HIV-1. Expert review of vaccines. 2010;9:1055–69. doi: 10.1586/erv.10.106. [DOI] [PubMed] [Google Scholar]
- [41].Guillonneau C, Mintern JD, Hubert FX, Hurt AC, Besra GS, Porcelli S, et al. Combined NKT cell activation and influenza virus vaccination boosts memory CTL generation and protective immunity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3330–5. doi: 10.1073/pnas.0813309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Reilly EC, Thompson EA, Aspeslagh S, Wands JR, Elewaut D, Brossay L. Activated iNKT cells promote memory CD8+ T cell differentiation during viral infection. PloS one. 2012;7:e37991. doi: 10.1371/journal.pone.0037991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Song JH, Kim JI, Kwon HJ, Shim DH, Parajuli N, Cuburu N, et al. CCR7-CCL19/CCL21-regulated dendritic cells are responsible for effectiveness of sublingual vaccination. J Immunol. 2009;182:6851–60. doi: 10.4049/jimmunol.0803568. [DOI] [PubMed] [Google Scholar]
- [44].Mascarell L, Lombardi V, Zimmer A, Louise A, Tourdot S, Van Overtvelt L, et al. Mapping of the lingual immune system reveals the presence of both regulatory and effector CD4+ T cells. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2009;39:1910–9. doi: 10.1111/j.1365-2222.2009.03337.x. [DOI] [PubMed] [Google Scholar]
- [45].Hovav AH. Dendritic cells of the oral mucosa. Mucosal immunology. 2013 doi: 10.1038/mi.2013.42. [DOI] [PubMed] [Google Scholar]
- [46].Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nature reviews Immunology. 2006;6:148–58. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- [47].Qimron U, Paul L, Bar-Haim E, Bloushtain N, Eisenbach L, Staats HF, et al. Non-replicating mucosal and systemic vaccines: quantitative and qualitative differences in the Ag-specific CD8(+) T cell population in different tissues. Vaccine. 2004;22:1390–4. doi: 10.1016/j.vaccine.2003.11.043. [DOI] [PubMed] [Google Scholar]
- [48].Forsell MN, Schief WR, Wyatt RT. Immunogenicity of HIV-1 envelope glycoprotein oligomers. Current opinion in HIV and AIDS. 2009;4:380–7. doi: 10.1097/COH.0b013e32832edc19. [DOI] [PubMed] [Google Scholar]
- [49].Lakhashe SK, Wang W, Siddappa NB, Hemashettar G, Polacino P, Hu SL, et al. Vaccination against heterologous R5 clade C SHIV: prevention of infection and correlates of protection. PloS one. 2011;6:e22010. doi: 10.1371/journal.pone.0022010. [DOI] [PMC free article] [PubMed] [Google Scholar]