Abstract
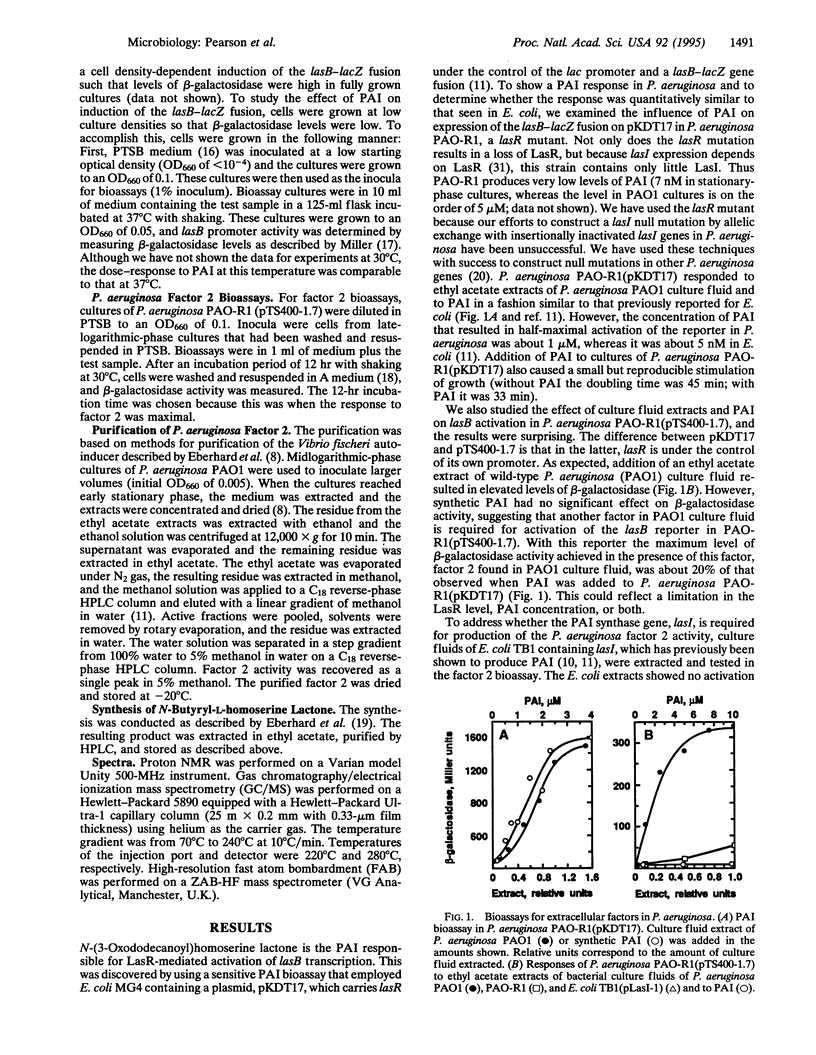
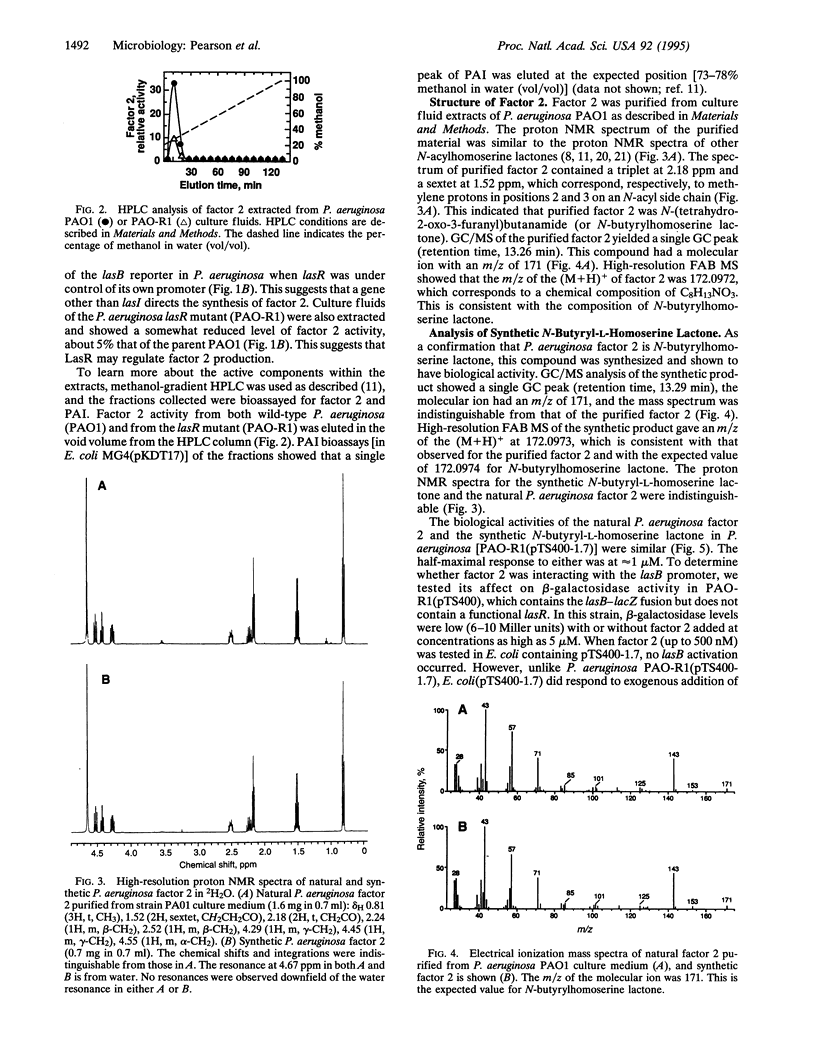
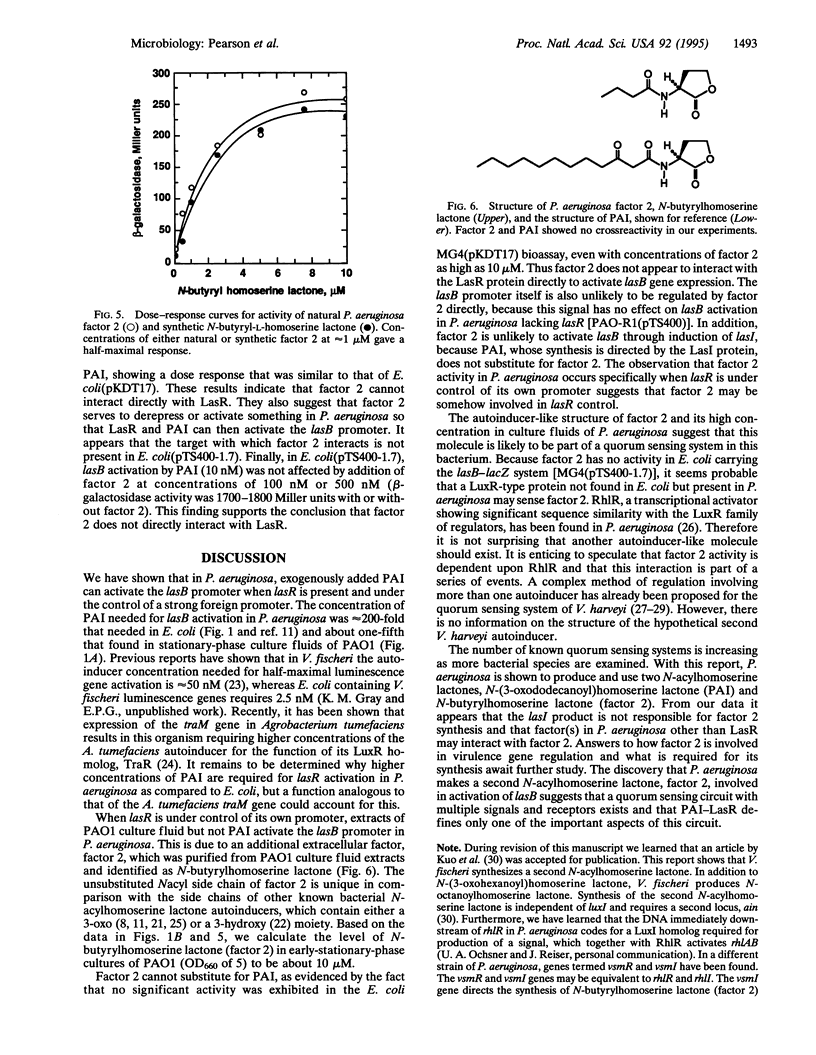
Quorum sensing systems are used by a number of Gram-negative bacterial species to regulate specific sets of genes in a cell density-dependent manner. Quorum sensing involves synthesis and detection of extracellular signals termed autoinducers. As shown in recombinant Escherichia coli, the Pseudomonas aeruginosa autoinducer (PAI) N-(3-oxododecanoyl)homoserine lactone, together with the lasR gene product, activate the P. aeruginosa lasB gene. In this study, PAI was shown to activate lasB-lacZ expression in a P. aeruginosa lasR mutant containing a plasmid with lasR under the control of the lac promoter. The concentration of PAI necessary for half-maximal activation of the lasB-lacZ fusion was approximately 1 microM, which is within the range of PAI levels found in P. aeruginosa culture fluids. The effect of PAI on a P. aeruginosa lasR mutant containing a plasmid with lasR under the control of its own promoter and containing the lasB-lacZ fusion was also tested. Although extracts of culture fluid activated the lasB promoter in this construct, concentrations of PAI as high as 10 microM did not. This indicates the presence of a second extracellular factor (factor 2) that is required for lasB activation in P. aeruginosa when lasR is controlled by its own promoter but not when lasR is controlled by a strong foreign promoter. Factor 2 was shown to be N-butyrylhomoserine lactone. Although recombinant E. coli cells containing the PAI synthase gene, lasI, produce PAI, these cells do not produce factor 2. Furthermore, a P. aeruginosa mutant that produced about 0.1% of the wild-type level of PAI made about 5% of the wild-type level of factor 2. This indicates that factor 2 synthesis results from the activity of a gene product other than PAI synthase. The role of factor 2 in virulence gene regulation remains to be determined, but this compound may affect the expression of lasR, which in turn activates transcription of numerous virulence genes in the presence of sufficient PAI. Apparently, multiple quorum sensing systems can occur and interact with each other in a single bacterial species.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainton N. J., Stead P., Chhabra S. R., Bycroft B. W., Salmond G. P., Stewart G. S., Williams P. N-(3-oxohexanoyl)-L-homoserine lactone regulates carbapenem antibiotic production in Erwinia carotovora. Biochem J. 1992 Dec 15;288(Pt 3):997–1004. doi: 10.1042/bj2880997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassler B. L., Wright M., Showalter R. E., Silverman M. R. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol. 1993 Aug;9(4):773–786. doi: 10.1111/j.1365-2958.1993.tb01737.x. [DOI] [PubMed] [Google Scholar]
- Bassler B. L., Wright M., Silverman M. R. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol Microbiol. 1994 Jul;13(2):273–286. doi: 10.1111/j.1365-2958.1994.tb00422.x. [DOI] [PubMed] [Google Scholar]
- Bassler B. L., Wright M., Silverman M. R. Sequence and function of LuxO, a negative regulator of luminescence in Vibrio harveyi. Mol Microbiol. 1994 May;12(3):403–412. doi: 10.1111/j.1365-2958.1994.tb01029.x. [DOI] [PubMed] [Google Scholar]
- Bever R. A., Iglewski B. H. Molecular characterization and nucleotide sequence of the Pseudomonas aeruginosa elastase structural gene. J Bacteriol. 1988 Sep;170(9):4309–4314. doi: 10.1128/jb.170.9.4309-4314.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumlik M. J., Storey D. G. Zinc and iron regulate translation of the gene encoding Pseudomonas aeruginosa elastase. Mol Microbiol. 1992 Feb;6(3):337–344. doi: 10.1111/j.1365-2958.1992.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Cao J. G., Meighen E. A. Purification and structural identification of an autoinducer for the luminescence system of Vibrio harveyi. J Biol Chem. 1989 Dec 25;264(36):21670–21676. [PubMed] [Google Scholar]
- Eberhard A., Burlingame A. L., Eberhard C., Kenyon G. L., Nealson K. H., Oppenheimer N. J. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981 Apr 28;20(9):2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- Eberhard A., Widrig C. A., McBath P., Schineller J. B. Analogs of the autoinducer of bioluminescence in Vibrio fischeri. Arch Microbiol. 1986 Oct;146(1):35–40. doi: 10.1007/BF00690155. [DOI] [PubMed] [Google Scholar]
- Engebrecht J., Nealson K., Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983 Mar;32(3):773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- Engebrecht J., Silverman M. Identification of genes and gene products necessary for bacterial bioluminescence. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4154–4158. doi: 10.1073/pnas.81.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua W. C., Winans S. C., Greenberg E. P. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994 Jan;176(2):269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambello M. J., Iglewski B. H. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J Bacteriol. 1991 May;173(9):3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiby N. Pseudomonas aeruginosa infection in cystic fibrosis. Relationship between mucoid strains of Pseudomonas aeruginosa and the humoral immune response. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Aug;82(4):551–558. [PubMed] [Google Scholar]
- Jensen S. E., Fecycz I. T., Campbell J. N. Nutritional factors controlling exocellular protease production by Pseudomonas aeruginosa. J Bacteriol. 1980 Nov;144(2):844–847. doi: 10.1128/jb.144.2.844-847.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H. B., Greenberg E. P. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J Bacteriol. 1985 Sep;163(3):1210–1214. doi: 10.1128/jb.163.3.1210-1214.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo A., Blough N. V., Dunlap P. V. Multiple N-acyl-L-homoserine lactone autoinducers of luminescence in the marine symbiotic bacterium Vibrio fischeri. J Bacteriol. 1994 Dec;176(24):7558–7565. doi: 10.1128/jb.176.24.7558-7565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner U. A., Koch A. K., Fiechter A., Reiser J. Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. J Bacteriol. 1994 Apr;176(7):2044–2054. doi: 10.1128/jb.176.7.2044-2054.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman D. E., Cryz S. J., Iglewski B. H. Isolation and characterization of Pseudomonas aeruginosa PAO mutant that produces altered elastase. J Bacteriol. 1980 Jun;142(3):836–842. doi: 10.1128/jb.142.3.836-842.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passador L., Cook J. M., Gambello M. J., Rust L., Iglewski B. H. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science. 1993 May 21;260(5111):1127–1130. doi: 10.1126/science.8493556. [DOI] [PubMed] [Google Scholar]
- Pearson J. P., Gray K. M., Passador L., Tucker K. D., Eberhard A., Iglewski B. H., Greenberg E. P. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralling G., Bodrug S., Linn T. Growth rate-dependent regulation of RNA polymerase synthesis in Escherichia coli. Mol Gen Genet. 1985;201(3):379–386. doi: 10.1007/BF00331327. [DOI] [PubMed] [Google Scholar]
- Swift S., Bainton N. J., Winson M. K. Gram-negative bacterial communication by N-acyl homoserine lactones: a universal language? Trends Microbiol. 1994 Jun;2(6):193–198. doi: 10.1016/0966-842x(94)90110-q. [DOI] [PubMed] [Google Scholar]
- Toder D. S. Gene replacement in Pseudomonas aeruginosa. Methods Enzymol. 1994;235:466–474. doi: 10.1016/0076-6879(94)35162-7. [DOI] [PubMed] [Google Scholar]
- Zhang L., Murphy P. J., Kerr A., Tate M. E. Agrobacterium conjugation and gene regulation by N-acyl-L-homoserine lactones. Nature. 1993 Apr 1;362(6419):446–448. doi: 10.1038/362446a0. [DOI] [PubMed] [Google Scholar]



