Abstract
Heart failure patients are classified by ejection fraction (EF) into distinct groups: heart failure with preserved EF (HFpEF) or heart failure with reduced EF (HFrEF). Although patients with heart failure commonly have multiple comorbidities that complicate management and may adversely affect outcomes, their role in the HFpEF and HFrEF groups is not well-characterized. This review summarizes the role of noncardiac comorbidities in patients with HFpEF versus HFrEF, emphasizing prevalence, underlying pathophysiologic mechanisms, and outcomes. Pulmonary disease, diabetes mellitus, anemia, and obesity tend to be more prevalent in HFpEF patients, but renal disease and sleep-disordered breathing burdens are similar. These comorbidities similarly increase morbidity and mortality risk in HFpEF and HFrEF patients. Common pathophysiologic mechanisms include systemic and endomyocardial inflammation with fibrosis. We also discuss implications for clinical care and future HF clinical trial design. The basis for this review was discussions between scientists, clinical trialists, and regulatory representatives at the 10th Global CardioVascular Clinical Trialists Forum.
Keywords: comorbidities, ejection fraction, heart failure
INTRODUCTION
Heart failure (HF) patients often have multiple concomitant diseases that complicate management and may adversely affect outcomes (1,2). Recent data from the Center for Medicare Services demonstrate that 55% of Medicare patients coded with HF have 5 or more chronic comorbidities (3). Data from the European Society of Cardiology Heart Failure Pilot Survey indicate that the majority of chronic HF patients (74%) had at least 1 comorbidity, of which the most common are renal disease, anemia, and diabetes mellitus (DM)(4). In general, more than a quarter of HF patients have comorbid pulmonary or renal dysfunction, which are associated with increased morbidity and mortality in the overall HF population (5–8). Patients are commonly classified by ejection fraction (EF) into heart failure with preserved EF (HFpEF; EF ≥50%) or heart failure with reduced EF (HFrEF; EF <50%). The role of comorbidities has not been well-characterized in these HF types. In this review, we summarize the role of noncardiac comorbidities in patients with HFpEF versus HFrEF with particular emphasis on prevalence, underlying pathophysiologic mechanisms, and association with outcomes. We focus on chronic obstructive pulmonary disease (COPD), anemia, DM, renal disease, sleep-disordered breathing (SDB), and obesity. We briefly discuss other noncardiac comorbidities, including frailty and arthritis, and highlight the need for future research on these topics, as well as on depression, myopathy, and liver disease. We describe the implications of these data for clinical care and for the design of future HF clinical trials. Cardiac comorbidities including hypertension, coronary artery disease, and atrial fibrillation were recently discussed elsewhere (9) and are beyond the scope of this review. This review is based on discussions between scientists, clinical trialists, and regulatory and industry representatives at the 10th Global CardioVascular Clinical Trialists (CVCT) Forum in Paris, France on December 6, 2013.
Overview of Data Sources
To identify additional relevant articles not discussed at the 10th annual CVCT, we searched MEDLINE (via PubMed) from January 1994 to July 2014 (see Online Appendix for the full search strategy). We used Medical Subject Headings (MeSH) and key words, focusing on the most relevant terms for this topic. We manually searched reference lists of pertinent reviews, including studies and background articles to find any relevant citations that our searches might have missed. We imported all citations into an EndNote X7 database. One reviewer (J.P.K.) screened and evaluated the retrieved records to select relevant studies. In order to focus on studies with representative patient samples, our search strategy required that publications included more than 500 patients and reported data from multiple sites. Given that entry criteria for clinical trials tend to exclude those with significant comorbidities, we focused on data from large HF registries and cohort studies. We required that the primary papers or supplemental materials included data on noncardiac comorbidities of interest.
In general, the prevalence of comorbidities was high across all studies, as demonstrated in the 3 largest U.S. HF registries (2,10,11), as well as ambulatory HF populations in the United States (12,13), as discussed later in this review. Other world regions demonstrated findings similar to those seen in the United States (14–16). Table 1 presents comorbidity prevalence data from several representative HF registries and epidemiologic cohorts.
Table 1.
Demographics and Prevalence of Noncardiovascular Comorbidities in Patients With Acute Heart Failure
| ADHERE Registry (2) | OPTIMIZE-HF Registry (10) | GWTG Registry (11)† | Ather S et al. (24) | Olmsted County 1987–2001 |
Olmsted County 2003–2005 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Reduced N = 25,865 50 % | Preserved N = 26,322 50 % | LVSD N = 20,118 49% | Preserved N = 21,149 51% | EF <40% N = 55,083 50% | EF ≥50% N = 40,354 36% | Reduced N = 6,599 70% | Preserved N = 2843 30% | Reduced N = 2,429 53% | Preserved N = 2167 47% | Reduced N = 248 45% | Preserved N = 308 55% | |
| Population | Acute HF, 274 hospitals*, 2001– 2004 | Acute HF, 259 hospitals, 2003–2004 | Acute HF, 275 hospitals, 2005– 2010 | Ambulatory HF, US Veterans, 2000–2002 | Acute HF, 1987–2001 | Inpatients and outpatients with HF | ||||||
| Age, yrs. | 70 ± 14 | 74 ± 13 | 70 ± 14 | 75 ± 13 | 70 (58– 80) | 78 (67– 85) | 70 ± 10 | 71 ± 10 | 72 ± 12 | 74 ± 14 | 73 ± 14 | 77 ± 13 |
| Female | 40% | 62% | 38% | 62% | 36% | 63% | 4% | 9% | 35% | 56% | 42% | 57% |
| African American | 22% | 17% | 21% | 15% | 25% | 16% | 13% | 10% | - | - | - | - |
| Medical History | ||||||||||||
| COPD or asthma | 27% | 31% | - | - | 27% | 33% | 27% | 34% | - | - | 30% | 38% |
| Renal insufficiency | 26% | 26% | - | - | 48% | 52% | 52% | 49% | - | - | - | - |
| Anemia | - | - | - | - | 14% | 22% | 28% | 33% | - | - | 49% | 53% |
| Diabetes mellitus | 40% | 45% | 24% non- insulin/ 15% Insulin | 26% non- insulin/ 17% insulin | 22% oral therapy/ 18% insulin | 24% oral therapy/ 22% insulin | 40% | 45% | 34% | 33% | 38% | 36% |
| Obesity (%)or body weight, kg | - | - | 78.5 (65.8– 94.0) | 78.9 (64.0– 97.5) | 25% | 33% | 35% | 51% | 36% | 41% | - | - |
| Laboratory Data | ||||||||||||
| BMI, kg/m2 | - | - | - | - | - | - | - | - | 28.6 ± 7.0 | 29.7 ± 7.8 | 29.1 ± 7.8 | 29.6 ± 7.5 |
| Hemoglobin, g/dL | - | - | 12.5 ± 2.0 | 11.9 ± 2.0 | 12.4 (11– 13.8) | 11.5 (10.2– 12.9) | - | - | 12.5 ± 2.0 | 11.8 ± 2.1 | - | - |
| Creatinine, mg/dL | 1.6 ± 1.3 | 1.7 ± 1.5 | 1.4 (1.1–1.9) | 1.3 (1.0– 1.8) | 1.3 (1– 1.8) | 1.3 (1– 1.9) | - | - | 1.6 ± 1.0 | 1.6 ± 1.1 | - | - |
Presented as mean ± standard deviation, median (interquartile range) or %.
274 hospitals included in the analysis cited.
Patients with an LVEF between 40–49% were excluded from this table.
Abbreviations: ADHERE = Acute Decompensated Heart Failure National Registry; BMI = body mass index; COPD = chronic obstructive pulmonary disease; EF = ejection fraction; GWTG = Get With The Guidelines; HF = heart failure; LVSD = left ventricular systolic dysfunction; OPTIMIZE-HF = Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure..
HFpEF versus HFrEF: Demographic Differences
Overall, the data suggest that patients hospitalized with HFpEF tend to be 4 to 8 years older than those with HFrEF and are more often female (Table 1). These observations are supported by an analysis from the Framingham Heart Study, which demonstrated that female sex was independently associated with a >2-fold increased risk for HFpEF versus HFrEF (17). Increasing age predicts both HFpEF and HFrEF, but the risk is significantly greater for HFpEF (18). The sex differences in HF phenotype appear largely due to increased HFrEF in men related to previous myocardial infarction (18). Another explanation for these findings relates to a differential response to hypertension in men versus women. Men tend to develop eccentric left ventricular (LV) hypertrophy in response to hypertension, while women tend to develop concentric LV hypertrophy (19). Studies also demonstrate that African Americans have HFpEF less often than HFrEF (2,10,11). These findings are counterintuitive due to the high prevalence of hypertension and LV hypertrophy in this population. The specific reasons for these observations are unknown and require further investigation, but may be related, in part, to ascertainment of the HFpEF diagnosis. It is unclear whether these findings are due to increased complexity in HFpEF detection, particularly in regions where echocardiography or natriuretic peptides are less available. In general, there is a relative paucity of large-scale comparative data regarding the burden of comorbidities in racial and ethnic minority populations with HF (20).
Outcomes Overview
Evidence regarding the outcomes of HFpEF versus HFrEF patients varied in different populations, but overall, the data suggest that HFpEF is associated with substantial morbidity and mortality that approaches or matches that of HFrEF (13,21,22). Interestingly, 1 recent analysis demonstrated that when similar B-type natriuretic peptide (BNP) levels were compared across EF values, the risk for adverse outcomes was similar in HFpEF and HFrEF patients (23). The overall incidence of hospital admission is similar between the 2 groups, but HFpEF patients have a higher incidence of non-HF hospitalizations, while HFrEF patients have a higher incidence of HF hospitalizations (24). Comorbidities, such as COPD, renal disease and DM, are strongly associated with adverse outcomes in HF patients (25). HFpEF is not merely a disease of old age and multiple comorbidities, but is a distinct entity associated with poor prognosis and severe cardiovascular dysfunction (26,27). However, few studies have explored the differential association between comorbidities and outcomes in HFrEF and HFpEF patients (24,28–30). In general, the increased risk for morbidity and mortality associated with these comorbidities is similar in those with HFpEF and HFrEF.
Pathophysiology
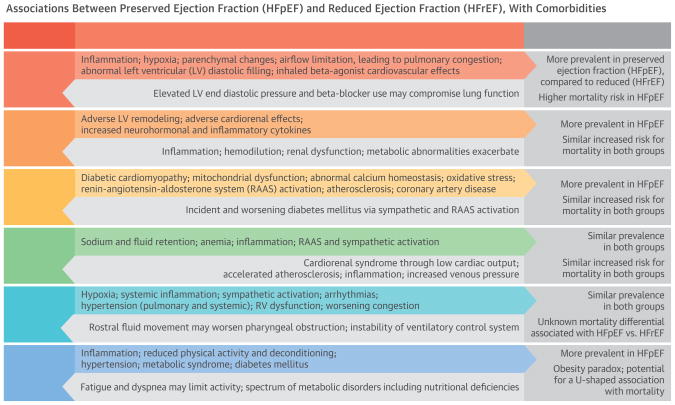
The Central Illustration presents pathways linking several comorbidities to disease progression in both HFpEF and HFrEF. These comorbidities interrelate by several common mechanisms, including inflammation and activation of the sympathetic and renin-angiotensin-aldosterone systems (RAAS), as discussed later in this review.
Comorbidities in HFrEF versus HFpEF Patients: Prevalence, Outcomes and Treatments
Chronic Obstructive Pulmonary Disease
COPD occurs in approximately a third of HF patients, with a slightly higher prevalence in HFpEF patients compared with HFrEF patients (5), although the specific rationale for the increased prevalence in HFpEF patients is unclear. Comorbidities such as COPD were suggested to induce a proinflammatory state that causes endothelial and cardiomyocyte dysfunction, with resultant myocardial fibrosis and clinical HFpEF (31). Ongoing smoking was also identified as an independent predictor of HFpEF, but not HFrEF, in epidemiologic studies (18), which supports the inflammatory hypothesis. However, further research is needed because smoking would also be expected to increase risk for coronary events and resultant ischemic cardiomyopathy with reduced EF. An alternative explanation for the close association between COPD and HFpEF involves coupling between impaired LV filling and pulmonary venous changes due to lung parenchymal abnormalities (32). Moreover, HF may result in pulmonary function changes and patient symptoms that mimic COPD. Since they do not have the alternative diagnosis of low EF, patients with preserved EF may be more likely to receive a COPD diagnosis as an explanation for dyspnea (33,34). Despite the potential for bias related to increased COPD diagnosis in HFpEF patients, the consistent observation of increased COPD prevalence in HFpEF patients suggests that concomitant pulmonary and cardiac dysfunction may be particularly important in the preserved EF group.
The primary effect of COPD appears to be increased noncardiovascular mortality during HF hospitalization (6), with similar outcomes in the early period following discharge (35). COPD is associated with increased long-term morbidity (36) and mortality (25). HF patients with COPD are less likely to receive beta-blockers compared with those without COPD (36), possibly due to clinicians’ concerns about precipitating bronchoconstriction (33,37,38). The overlapping symptom of dyspnea with both diseases may lead to misapplication of therapy. Given the discordant beta-receptor effects of the different disease treatments, a patient’s symptoms and outcome could be adversely affected by the treatment of the comorbid disease. HF patients with COPD also tend to have lower blood pressure, higher creatinine, and underuse of angiotensin converting enzyme (ACE) inhibitors and mineralocorticoid receptor antagonists (6,35). These characteristics likely contribute to the increased mortality risk associated with COPD in HF patients.
In a recent analysis of the differential association between comorbidities and outcomes in HFrEF and HFpEF patients, COPD was the only comorbidity for which there was a significant interaction (p = 0.01) with EF group and outcomes. COPD contributed to a higher hazard for mortality in patients with HFpEF compared with HFrEF (24). Notably, COPD was an independent predictor of mortality in both groups. These between-group differences are supported by findings from another small study where COPD was highly predictive of death in those with HFpEF, but not HFrEF(39). Data from the Framingham Study support a potential causal role between airflow limitation and HFpEF, but not HFrEF (40). The link between even mild airflow limitation and abnormal LV diastolic filling (41) may explain, in part, the stronger association between COPD and HFpEF.
COPD treatment recommendations focus on prevention, including vaccinations and smoking cessation. Pending randomized trial data, chronic therapy with long-acting anticholinergics is recommended preferentially over inhaled beta-agonists (37). Early intervention in the setting of exacerbations and a multidisciplinary approach may be indicated to balance therapies for both diseases (33). Intravascular volume management may represent an area of particular focus, with the goal of minimizing LV filling pressures and pulmonary interstitial fluid, even in the presence of agents that adversely affect volume status, such as corticosteroids. With respect to beta-blocker use, there is a mechanistic rationale to consider the preferential use of cardioselective agents, such as metoprolol succinate or bisoprolol, rather than carvedilol (37). Evidence from several small studies supports this approach (38,42,43). However, observational studies suggested that there is no differential benefit with cardioselective agents compared with non-cardioselective agents (36,44). Thus, adequately powered prospective studies are needed to investigate the optimal beta-blocker approach in HF patients with COPD.
Anemia
Comorbid anemia is more frequent in HFpEF patients than in HFrEF patients. Prevalence reports vary depending on the specific anemia definition used, but the trends are comparable. Contemporary studies tend to use the WHO classification of anemia as hemoglobin <13 g/dL in men and <12 g/dL in women (24). In the Get With The Guidelines (GWTG) Registry, there was an association between higher EF and increased prevalence of anemia (11). The prevalence of anemia was 22% in patients with an EF ≥50%, 20% with EF 40% to 49%, and 14% with EF <40%, and there were also sex-specific differences (45). The prevalence of anemia tended to be higher in women in the setting of either reduced or preserved EF. These findings are supported by an analysis from the Organized Program to Initiate Lifesaving Treatment in Patients with Heart Failure (OPTIMIZE-HF), which showed that HF patients (46) with low hemoglobin were older, more often female, and had preserved systolic function. In the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program, lower hemoglobin was associated with higher EF (47). Furthermore, female sex, DM, and worse renal function were several of the strongest predictors of anemia. These findings suggest a complex relationship between EF and anemia that likely involves inflammation, hemodilution, bone marrow deficiency, nutritional and metabolic factors, and nephropathy (48). In addition, studies suggest that anemia may increase cardiac output and reduce systemic resistance through nitric oxide-mediated vasodilation (49–51).
Anemia is associated with increased morbidity and mortality in HF patients (52–55). Potential explanations include adverse LV remodeling effects (56), increased neurohormonal and inflammatory cytokines (49), adverse cardiorenal effects (57), and the association with poor nutritional status (47).
Multiple previous studies demonstrated that there is no interaction between EF and outcomes related to anemia status. For instance, in 1 study, anemia was associated with an ~25% increased mortality risk in HFrEF and HFpEF patients (24). Similarly, Felker et al. demonstrated that anemia was independently associated with mortality, and there was no evidence of an interaction with systolic function (28). A recent analysis from the Study of the Effects of Nebivolol Intervention on OUtcomes and Rehospitalization in Seniors with Heart Failure (SENIORS) trial, which included HF patients irrespective of LVEF, extends these results to morbidity endpoints (58). Anemic patients were at increased risk for the composite of all-cause mortality or cardiovascular hospitalization and mortality, or HF hospitalization, irrespective of underlying HFrEF or HFpEF. Notably, the overall representation of patients with LVEF >50% in SENIORS was modest. The inability to demonstrate a differential relationship between anemia and outcomes in those with HFpEF versus HFrEF may be related, in part, to the number of different etiologies for anemia.
Given the neutral results of recent trials targeting anemia with erythropoietin-stimulating agents in HFrEF patients (59), the optimal treatment of underlying anemia in HF requires further study. Given iron deficiency’s relation to anemia status and its potential as a treatable target, this area is of particular interest (60). The prevalence of iron deficiency in HF patients is even greater than the prevalence of clinical anemia. Furthermore, iron deficiency may impact outcomes in HFpEF and HFrEF independent of anemia (61). Iron deficiency leads to worsening HF symptoms, HF progression, and poor outcomes (62). Ongoing and future studies will explore the effect of iron replacement in both HFpEF and HFrEF patients.
Diabetes mellitus
Registry and observational data consistently demonstrate the presence of DM in approximately 40% of HFrEF patients versus 45% of HFpEF patients (2,10,24). DM is associated with the development of myocardial dysfunction, even in the absence of significant coronary artery disease or hypertension (i.e., diabetic cardiomyopathy) (63). Myocardial changes result from insulin resistance and hyperglycemia through mechanisms including increased free fatty acid concentration, mitochondrial dysfunction, abnormal calcium homeostasis, RAAS activation, oxidative stress, and advanced glycation endproducts (63,64). Development of systolic dysfunction may be preceded by myocardial fibrosis and collagen deposition, resulting in diastolic dysfunction (65,66). Importantly, the relationship between DM and HF appears bidirectional, with HF also increasingly the risk for subsequent DM (67). The mechanisms underlying the effect of HF on incident DM or on DM progression are not completely known, but may involve sympathetic and RAAS activation, with subsequent lipolysis and increased cytokine production (68,69).
DM is associated with increased morbidity and mortality in chronic HF patients (29,70), but its influence as a predictor of long-term outcomes after HF hospitalization is less well-defined. Several acute HF registries have suggested that DM patients are at increased risk for mortality (71,72). However, in the OPTIMIZE-HF registry, DM patients were at increased short-term risk for rehospitalization, but at similar risk for in-hospital and short-term mortality (73). Similarly, in the EVEREST study, DM was associated with increased HF rehospitalization, but not all-cause mortality (74). DM may complicate the clinical course of HF patients through mechanisms including electrolyte disturbances, increased infection risk, and altered medication absorption, as well as through ischemia and other direct adverse effects on the myocardium (1).
The impact of DM in patients with HFpEF versus HFrEF is not well-defined. One recent analysis demonstrated that the point estimate of hazard for mortality associated with DM was greater in HFrEF patients than HFpEF patients, but formal statistical testing did not demonstrate an interaction between the DM and EF groups (24). An analysis from CHARM demonstrated that DM was associated with similarly increased risk for mortality in HFpEF and HFrEF patients (29). In contrast, DM was associated with increased cardiovascular death or HF hospitalization in HFpEF patients compared with HFrEF patients. This difference was due to an increased risk for HF hospitalization associated with DM in HFpEF patients. However, in an analysis from OPTIMIZE-HF, HFpEF patients with DM were not at increased risk for 60-day to 90-day mortality or rehospitalization, in contrast to the increased risk associated with DM in HFrEF patients (interaction p value = 0.0012) (73). Thus, the impact of DM on outcomes in different EF groups is not entirely clear, but may be related to predominant effects on long-term HF rehospitalization, rather than mortality. It is also possible that there are differential effects in patients who had a recent HF hospitalization compared to those with chronic stable HF. The optimal treatment of comorbid diabetes in HF patients is unclear. Several classes of antidiabetic agents such as thiazolidinediones (TZDs) and dipeptidyl peptidase 4 (DPP-4) inhibitors were shown to be associated with increased HF risk (75,76). Alternatively, ongoing studies are investigating whether antidiabetic agents, such as glucagon-like peptide-1 (GLP-1) agonists may improve outcomes in HF patients via benefits on cardiac metabolism (FIGHT; ClinicalTrials.gov Identifier: NCT01800968). Despite metformin’s package label warning about its use in HF patients, the occurrence of lactic acidosis is exceedingly low in clinical practice and recent observational data (77) suggest possible benefits on clinical outcomes associated with its use in HF. Ongoing large-scale diabetes studies investigating cardiovascular outcomes, including HF, will inform the management of DM in these patients (EXSCEL; ClinicalTrials.gov Identifier: NCT01144338). Pending the results of these studies, the treatment of DM in patients with HF should preferentially use agents with favorable safety profiles in patients with cardiovascular disease (78). Given the key roles of obesity and metabolic syndrome in the underlying pathophysiology (79), they may also represent important targets in DM patients with HF, particularly those with HFpEF.
Renal Dysfunction
Registry data indicate a similar extent of renal insufficiency in HF patients across the EF spectrum (2,11). The reports vary significantly in different datasets, depending on the specific criteria used, but the figures are similar in patients with HFrEF and HFpEF. The interdependence of heart and kidney dysfunction is captured by the recently described “cardiorenal syndrome” (7,80). Renal dysfunction may worsen HF through multiple mechanisms, including increased sodium and fluid retention, anemia, inflammation, and uremic toxins, as well as RAAS and sympathetic activation. A recent analysis demonstrated a significant association of an increase in urinary markers of renal dysfunction with the risk for new onset HFpEF, but not HFrEF (81). Conversely, HF may lead to renal dysfunction and cardiorenal syndrome through mechanisms related to low cardiac output, accelerated atherosclerosis, inflammation, and increased venous pressure. The multitude of mechanisms that may result in renal dysfunction may, in part, explain its similar prevalence in HFpEF and HFrEF patients. For instance, HFpEF patients may be more likely to have underlying renal dysfunction related to diabetic nephropathy, while atherosclerosis may contribute to renal function changes in patients with HFrEF due to ischemic/nephrosclerotic etiology (16).
Renal dysfunction is an established risk factor for adverse events in patients with HF (82,83). The ADHERE registry revealed that more than half of acute HF patients had at least moderate renal insufficiency on admission, which was associated with increased mortality (84). Importantly, recent data have demonstrated that the association between renal dysfunction and poor outcomes is complex. For instance, transient worsening renal function during acute HF hospitalization may not affect post-discharge outcomes (85,86) and aggressive fluid removal leading to hemoconcentration may be associated with lower mortality, despite evidence of worsening renal function (86). Thus, the underlying cause and trajectory of renal dysfunction may play a role in determining the impact on subsequent outcomes. Notably, HF patients with renal dysfunction tend to be older, with lower blood pressures, and higher plasma BNP levels (87). Kidney disease also affects guideline-directed medical therapy in HFrEF patients due to concerns about worsening GFR and hyperkalemia (87).
In addition to similar prevalence in HFrEF and HFpEF patients, the increased risk associated with the comorbidity is similar in both patient groups. Ather et al. demonstrated that renal insufficiency was associated with an approximately 25–30% increase in mortality (24). In a community-based HF patient cohort, worsening estimated glomerular filtration rate was associated with a graded increase in the risk for death and hospitalization, with similar findings in those with HFpEF and HFrEF(30).
The implications of renal insufficiency for treatment of HFpEF and HFrEF patients are severalfold. First, despite concerns related to hyperkalemia, therapies such as ACE inhibitors should be initiated and monitored in accordance with current guidelines (88–89). Recent data suggest that worsening renal function while on an RAAS inhibitor has a better prognosis than on placebo, suggesting that a RAAS inhibitor should not necessarily be discontinued in patients who develop worsening renal function (90). Renal insufficiency may also have important implications related to the management of volume status and titration of diuretic therapies. For instance, with more severe underlying renal disease, it may be necessary to consider alternative loop diuretics, such as torsemide, or the addition of a thiazide diuretic (91).
Sleep-Disordered Breathing
In recent years, the prevalence and impact of SDB in HF patients is increasingly recognized and multiple ongoing registries are collecting data in this regard (SchlaHF; ClinicalTrials.gov Identifier: NCT01500759). Prior studies demonstrated that SDB is prevalent in both those with HFpEF and those with HFrEF, occurring in upwards of 50 to 80% of patients (92–94). Two primary types of SDB occur and may coexist in HF patients: obstructive sleep apnea (OSA) and central sleep apnea (CSA). HFpEF patients tend to more often have OSA, in comparison to HFrEF patients who tend to have CSA to a greater extent (95). Women with HF are less likely to have SDB compared with men, and its severity may be lower (96). Risk factors for the development of both types of SDB in HF patients include male sex and increased age (97,98). Elevated body mass index is an additional risk factor for OSA, while severe LV impairment and atrial fibrillation increase the likelihood of CSA (97,99). SDB is proinflammatory, with effects on oxidative stress and sympathetic activation (94).
SDB has been associated with increased morbidity and mortality in the general population (100–102); however, its impact on HF patient outcomes is less well-defined. The majority of studies in HF patients focused on HFrEF patients, where SDB was an independent predictor of cardiac readmission (103). In 164 patients with chronic stable HFrEF, untreated OSA was associated with increased mortality on multivariable analysis (104). CSA was also shown to be a predictor of mortality in HFrEF (105). However, not all studies demonstrated a relationship between SDB and outcomes in HFrEF patients (106,107).
To our knowledge, no previous studies scrutinized a differential association between SDB and outcomes in HFrEF versus HFpEF patients. Studies to date in HFpEF patients generally assessed <200 patients, with the emphasis on describing prevalence and patient characteristics, rather than outcomes (95,108). Thus, future research is required to explore the impact of SDB on outcomes in HFpEF. SDB may represent a particularly important comorbidity in HFpEF patients, given the high prevalence of obesity in this patient population.
The primary treatment for OSA is nocturnal continuous positive airway pressure (CPAP). Observational studies in HF patients with OSA suggested potential benefits with CPAP on clinical outcomes (109), yet large-scale randomized studies are needed. The role of CPAP in CSA is even less well-defined. The largest randomized, prospective study of HF patients with CSA (N = 258) found no survival benefit with CPAP, despite improvements in EF and functional status (110). Compared with CPAP devices, minute ventilation-targeted adaptive servo-ventilation (ASV) may treat both CSA and OSA with improved tolerability. Studies have demonstrated benefits on surrogate endpoints with ASV in HF patients (111,112). Survival benefits with ASV in HF patients have not been demonstrated, and randomized trials are ongoing (SERVE-HF and CAT-HF; ClinicalTrials.gov Identifiers: NCT00733343, NCT01953874).
Other Comorbidities
Obesity is common in the general HF population with a higher prevalence in HFpEF patients (Table 1). While increased body weight has been associated with improved outcomes in cardiovascular disease populations (113), recent reports discussed a balanced reappraisal of this obesity paradox (114,115). A higher weight may be associated with better outcomes compared with HF patients with cardiac cachexia and/or nutritional deficiencies. Potential mechanisms for improved outcomes associated with obesity include increased metabolic reserve and lipoprotein pools to serve as scavengers for circulating endotoxins. However, the associations between obesity and metabolic syndrome, glucose intolerance, and diabetes are likely to explain, in part, the link between increased body weight and adverse events in certain circumstances. For instance, a recent study in 4,109 HFpEF patients found a U-shaped relationship between BMI and adverse clinical events. BMI <23.5 kg/m2 or ≥35 kg/m2 were each associated with a 27% increase in death or cardiovascular hospitalization, compared with the reference group BMI of 26.5 to 30.9 kg/m2 (116). Future studies are needed to assess whether treatment strategies targeting appropriate weight gain or weight reduction can improve outcomes in both HFpEF and HFrEF patients.
Additional important comorbidities in HF patients include frailty and arthritis (117). Frailty, limited mobility, and fall risk are increasingly recognized as important predictors of outcomes in HF patients (118). Osteoarthritis is of particular interest, given the inflammatory hypothesis linking comorbidities and adverse cardiovascular outcomes (119). Furthermore, NSAID treatment for osteoarthritis may have direct consequences on fluid retention and the precipitation of acute HF. There is a need for future research on these topics as well as on depression, myopathy, and liver disease, with respect to implications for the management and outcomes of HFpEF and HFrEF patients.
Clinical Practice and Implications for Future Trial Design
Although the presence of multiple comorbid diseases is almost universal in clinical practice, HF guidelines provide little discussion of this, and the evidence base is sparse and mostly observational. Compared to HFrEF patients, those with HFpEF tend to have an increased burden of COPD, DM, and anemia, which are associated with increased morbidity and mortality. Because the development of novel HF therapies has slowed in recent years and most contemporary HF trials have failed to improve outcomes above standard medical therapy (120,121), we suggest the need for a critical reappraisal of treatment strategies in HF where clinicians target comorbidities, in addition to targeting the underlying cardiac dysfunction (Table 2). This approach may be particularly relevant in HFpEF patients where there are no available therapies to reduce the substantial morbidity and mortality. In addition, on the basis of the recent data discussed earlier, improved management of specific comorbidities in HFpEF patients may have an even greater impact than in HFrEF. Most studies to date targeting comorbidities in HF patients focused on the HFrEF population, with few interventions on comorbidities empirically evaluated in HFpEF patients. Given that many of these conditions are closely interrelated and may potentiate each other, targeting comorbidities may represent an important component in the comprehensive management of HF patients.
Table 2.
Treatments for comorbidities in Patients With HFpEF and Those With HFrEF
| Comorbidity | Recommendations / Comments |
|---|---|
| COPD |
|
| Anemia |
|
| Diabetes mellitus |
|
| Renal dysfunction |
|
| Sleep disordered breathing |
|
Abbreviations: ACE = angiotensin converting enzyme; ARB = angiotensin receptor blocker; ASV = adaptive servo-ventilation; COPD = chronic obstructive pulmonary disease; CPAP = continuous positive airway pressure; DPP-4 = dipeptidyl peptidase 4; MRA = mineralocorticoid receptor antagonist; TZDs = thiazolidinediones.
Another major clinical implication of these data relates to polypharmacy, particularly in the elderly, in whom the prevalence of HF rises sharply. Elderly patients may exhibit variable responses to standard medical HF therapy and are also more prone to experience adverse effects. As the elderly also have more comorbidities requiring specific therapies, polypharmacy is commonplace. Medications with opposing actions may be used simultaneously (e.g., inhaled beta-agonists and beta-blockers) such that medications for non-HF comorbidities may exacerbate HF and vice versa. In general, polypharmacy increases the potential for drug interactions and reduces patients’ compliance. Moreover, frailty and cognitive impairment are more common among elderly HF patients and represent additional risks for nonadherence.
Data on the specific role of polypharmacy in HFrEF versus HFpEF are lacking. Given the greater burden of comorbidities in HFpEF, it is, however, reasonable to assume that the negative impact of polypharmacy is at least as great in HFpEF compared with HFrEF. At the same time, HF trials that established morbidity and mortality benefits were predominantly executed in nonelderly cohorts. Assumption of similar efficacy in geriatric populations is mostly on the basis of extrapolation of these data, but the true risk-benefit ratio may be less favorable.
These observations also may have important implications for future HF trial design. As the clinical trial landscape transitions towards more pragmatic trials with broad entry criteria, in some circumstances, study populations may (and perhaps, should) increasingly include those with multiple comorbid diseases. Noncardiologists managing HF patients cite comorbidity and lack of generalizability of prior trials to comorbid patients as a “reason” for not applying standard therapies. Improved generalizability of trial results could translate into increased uptake of evidence-based treatments for both HF and comorbid diseases. While this approach may be possible in HFrEF, given the heterogeneity of the patient population, broad entry criteria for trials in HFpEF may be more likely to fail. Strategies to promote success include use of natriuretic peptide levels for entry criteria, as highlighted by the results of the recent TOPCAT study (122). Importantly, data suggest that the use of different threshold levels for natriuretic peptides may be appropriate in HFrEF versus HFpEF patients, as well as in those with obesity and renal dysfunction (123). Considerations for trial design related to comorbidities are summarized in Figure 1. Importantly, the inclusion of patients with comorbidities in clinical trials may require intensified monitoring and safety evaluations. For instance, there are notable comorbidity-specific adverse effects associated with certain medications (e.g., hyperkalemia with RAAS inhibition in renal dysfunction patients). Finally, given the recent lack of success in HF clinical trials using add-on HF-directed therapies (e.g., direct renin inhibitors (124)), another potential trial approach is to specifically target underlying comorbidities in order to improve overall patient outcomes.
Figure 1. Clinical Trial Considerations Related to Comorbidities.
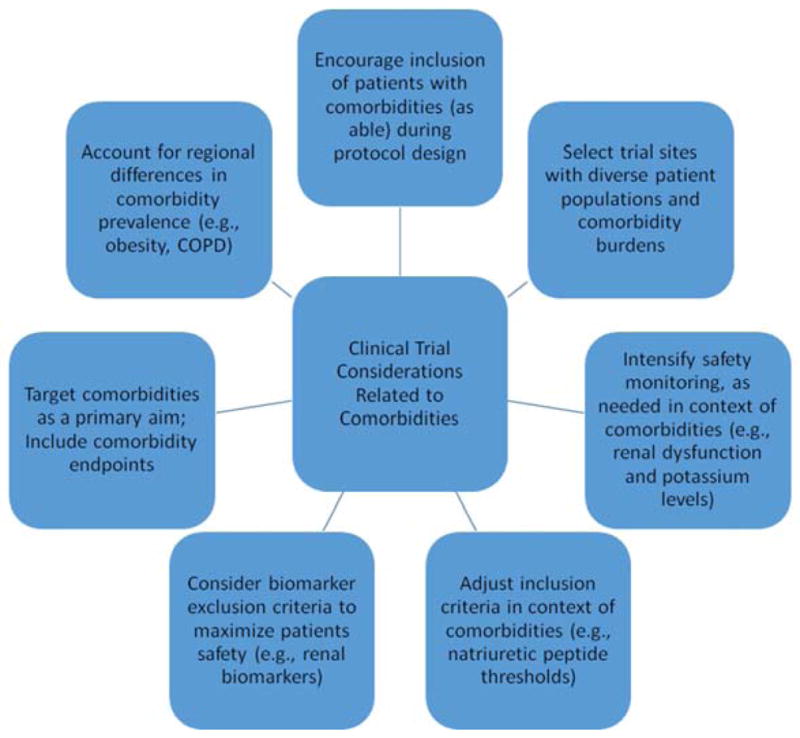
Trial protocols should consider feasibility of encouraging the inclusion of patients with comorbidities (as able). Trial sites should be selected on the basis, in part, of the comorbidity burden of the patient populations. Intensified safety monitoring may be needed in the context of comorbidities. Study entry criteria may need to be adjusted to acknowledge differences in biomarker thresholds in the context of comorbidities such as obesity and/or renal function. Exclusionary biomarker criteria (e.g., renal biomarkers) may offer mechanisms to support patient safety. As able, consider targeting comorbidities as a primary intervention and include comorbidity-specific trial endpoints (e.g., dyspnea relief and beta-blocker usage in context of COPD). Trialists should also take into account regional differences in the prevalence of comorbidities when performing sample size calculations and designing trials (e.g., differential event rates in the presence of comorbid diseases).
Conclusions
Compared with HFrEF, patients with HFpEF have an increased burden of COPD, DM, anemia, and obesity, but a similarly high prevalence of renal disease and SDB. In general, the increased risk for morbidity and mortality associated with these comorbidities is similar in those with HFpEF and HFrEF. Careful attention to the diagnosis and management of specific comorbidities in HF patients may help to improve patient outcomes, but further observational and interventional research are urgently required, particularly as noncardiac comorbidity is almost universal in the typical heart failure population.
Figure 2. Central Illustration. Associations Between Heart Failure and Comorbidities.
Pathways linking several common comorbidities to disease progression in both HFpEF and HFrEF are presented and factors exacerbating other comorbid conditions are highlighted. These comorbidities are interrelated by several common mechanisms, including inflammation and worsening congestion, as well as by sympathetic and renin-angiotensin aldosterone system activation. Heart failure influences each of the comorbidities, demonstrating the bidirectional association.
Abbreviations: COPD = chronic obstructive pulmonary disease; CV = cardiovascular; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; LV = left ventricular; RAAS = renin-angiotensin-aldosterone system; RV = right ventricular.
Acknowledgments
Dr. Mentz was supported by Grant #T32GM086330 from the National Institute of General Medical Sciences. Dr. Cowie is supported by the National Institute for Health Research Cardiovascular Biomedical Research Unit at the Royal Brompton Hospital. Dr. Butler reports research support from the National Institutes of Health, European Union, Health Resources and Services Administration.
ABBREVIATIONS
- COPD
chronic obstructive pulmonary disease
- DM
diabetes mellitus
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- SDB
sleep-disordered breathing
APPENDIX
Appendix.
Medline (via PubMed) search strategy.
| Search | Query | Items found |
|---|---|---|
| #2 | Search “heart failure”[MeSH Terms] OR “heart failure”[TIAB] | 139194 |
| #10 | Search “preserved ejection fraction”[tiab] | 983 |
| #11 | Search #2 AND #10 | 826 |
| #13 | Search “reduced ejection fraction”[tiab] | 808 |
| #14 | Search #2 AND #13 | 521 |
| #15 | Search #14 OR #11 | 1205 |
| #16 | Search (#15) AND (“1994/01/01”[Date - Publication] : “3000”[Date - Publication]) | 1167 |
| #19 | Search #16 NOT (“Animals”[Mesh] NOT “Humans”[Mesh]) | 1126 |
| #21 | Search “pulmonary disease, chronic obstructive”[MeSH Terms] OR “chronic obstructive pulmonary disease”[tiab] OR “COPD”[tiab] | 53449 |
| #25 | Search “diabetes mellitus”[MeSH Terms] OR “diabetes”[tiab] | 439784 |
| #28 | Search “sleep apnea syndromes”[MeSH Terms] OR “sleep apnea”[tiab] OR “sleep disordered breathing”[tiab] OR “SDB”[tiab] | 29082 |
| #30 | Search “kidney diseases”[MeSH Terms] OR kidney disease*[tiab] OR renal disease*[tiab] | 428081 |
| #32 | Search “anaemia”[tiab] OR “anemia”[MeSH Terms] OR “anemia”[tiab] | 179314 |
| #34 | Search “obesity”[MeSH Terms] OR “obesity”[tiab] | 195364 |
| #36 | Search “comorbidity”[MeSH Terms] OR “comorbidity”[tiab] OR “comorbidities” [tiab] | 105817 |
| #37 | Search #21 OR #25 OR #28 OR #30 OR #32 OR #34 OR #36 | 1280940 |
| #38 | Search #19 AND #37 | 283 |
| #39 | Search #19 AND #37 Filters: English | 266 |
Footnotes
Disclosures: Dr. Mentz receives research support from Gilead Sciences, AztraZeneca, BMS, GSK, Novartis, Otsuka, Amgen and ResMed, and honoraria from Thoratec. Dr. Cowie is a member of the speakers’ bureau for: Pfizer, Bayer, Novartis, Servier, ResMed, Medtronic, Boston Scientific, and St Jude Medical. Dr. Voors received consultancy fees and/or research grants from: Alere, AstraZeneca, Bayer, Boehringer Ingelheim, Cardio3Biosciences, Celladon, Johnson and Johnson, Merck/MSD, Novartis, Servier, Torrent, Trevena, Vifor. Dr. Butler reports serving as a consultant to Amgen, BG Medicine, Celladon, Gambro, Ono Pharma, Trevena, Takada, Bayer, Medtronic, CardioCell, Novartis, and GE Healthcare. Dr. Atar received consultancy honoraria from Novartis, Pfizer, Vifor Pharma, and Bayer Healthcare. Drs. Fiuzat and O’Connor received research support from ResMed. The remaining authors reported that they had no relevant relationships to the contents of this paper to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mentz RJ, Felker GM. Noncardiac comorbidities and acute heart failure patients. Heart Fail Clin. 2013;9:359–67. vii. doi: 10.1016/j.hfc.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yancy CW, Lopatin M, Stevenson LW, et al. ADHERE Scientific Advisory Committee and Investigators. Clinical presentation, management, and inhospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Medicare and Medicaid Services. Chronic Conditions Among Medicare Beneficiaries. Chartbook, 2012. Baltimore, MD: 2012. [Google Scholar]
- 4.van Deursen VM, Urso R, Laroche C, et al. Co-morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail. 2014;16:103–11. doi: 10.1002/ejhf.30. [DOI] [PubMed] [Google Scholar]
- 5.Hawkins NM, Petrie MC, Jhund PS, et al. Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. Eur J Heart Fail. 2009;11:130–9. doi: 10.1093/eurjhf/hfn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mentz RJ, Fiuzat M, Wojdyla DM, et al. Clinical characteristics and outcomes of hospitalized heart failure patients with systolic dysfunction and chronic obstructive pulmonary disease: findings from OPTIMIZE-HF. Eur J Heart Fail. 2012;14:395–403. doi: 10.1093/eurjhf/hfs009. [DOI] [PubMed] [Google Scholar]
- 7.Ronco C, Haapio M, House AA, et al. Cardiorenal Syndrome. J Am Coll Cardiol. 2008;52:1527–39. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 8.Mentz RJ, Lewis EF. Epidemiology of cardiorenal syndrome. Heart Fail Clin. 2010;6:333–46. doi: 10.1016/j.hfc.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Lau YC, Lane DA, Lip GYH. Atrial fibrillation and heart failure: a bad combination. Am J Cardiol. 2014;113:1196–7. doi: 10.1016/j.amjcard.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Fonarow GC, Stough WG, Abraham WT, et al. OPTIMIZE-HF Investigators. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–77. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 11.Steinberg BA, Zhao X, Heidenreich PA, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 12.Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–16. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 13.Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 14.Cleland JG, Swedberg K, Follath F, et al. The EuroHeart Failure survey programme—a survey on the quality of care among patients with heart failure in Europe: Part 1: patient characteristics and diagnosis. Eur Heart J. 2003;24:442–63. doi: 10.1016/s0195-668x(02)00823-0. [DOI] [PubMed] [Google Scholar]
- 15.Nieminen MS, Brutsaert D, Dickstein K, et al. EuroHeart Survey Investigators; Heart Failure Association, Europena Society of Cardiology. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–36. doi: 10.1093/eurheartj/ehl193. [DOI] [PubMed] [Google Scholar]
- 16.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317–27. doi: 10.1016/j.jacc.2003.07.046. [DOI] [PubMed] [Google Scholar]
- 17.Lee DS, Gona P, Vasan RS, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham Heart Study of the National Heart, Lung, and Blood Institute. Circulation. 2009;119:3070–7. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho JE, Lyass A, Lee DS, et al. Predictors of new-onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail. 2013;6:279–86. doi: 10.1161/CIRCHEARTFAILURE.112.972828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krumholz HM, Larson M, Levy D. Sex differences in cardiac adaptation to isolated systolic hypertension. Am J Cardiol. 1993;72:310–3. doi: 10.1016/0002-9149(93)90678-6. [DOI] [PubMed] [Google Scholar]
- 20.Klapholz M, Maurer M, Lowe AM, et al. New York Heart Failure Consortium. Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: results of the New York Heart Failure Registry. J Am Coll Cardiol. 2004;43:1432–8. doi: 10.1016/j.jacc.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 21.Senni M, Redfield MM. Heart failure with preserved systolic function: A different natural history? J Am Coll Cardiol. 2001;38:1277–82. doi: 10.1016/s0735-1097(01)01567-4. [DOI] [PubMed] [Google Scholar]
- 22.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–9. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 23.van Veldhuisen DJ, Linssen GCM, Jaarsma T, et al. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;61:1498–506. doi: 10.1016/j.jacc.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 24.Ather S, Chan W, Bozkurt B, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59:998–1005. doi: 10.1016/j.jacc.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42:1226–33. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 26.Campbell RT, Jhund PS, Castagno D, et al. What have we learned about patients with heart failure and preserved ejection fraction from DIG-PEF, CHARM-Preserved, and I-PRESERVE? J Am Coll Cardiol. 2012;60:2349–56. doi: 10.1016/j.jacc.2012.04.064. [DOI] [PubMed] [Google Scholar]
- 27.Mohammed SF, Borlaug BA, Roger VL, et al. Comorbidity and ventricular and vascular structure and function in heart failure with preserved ejection fraction: a community-based study. Circ Heart Fail. 2012;5:710–9. doi: 10.1161/CIRCHEARTFAILURE.112.968594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felker GM, Shaw LK, Stough WG, et al. Anemia in patients with heart failure and preserved systolic function. Am Heart J. 2006;151:457–62. doi: 10.1016/j.ahj.2005.03.056. [DOI] [PubMed] [Google Scholar]
- 29.MacDonald MR, Petrie MC, Varyani F, et al. CHARM Investigators. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:1377–85. doi: 10.1093/eurheartj/ehn153. [DOI] [PubMed] [Google Scholar]
- 30.Smith DH, Thorp ML, Gurwitz JH, et al. Chronic kidney disease and outcomes in heart failure with preserved versus reduced ejection fraction: the Cardiovascular Research Network PRESERVE Study. Circ Cardiovasc Qual Outcomes. 2013;6:333–42. doi: 10.1161/CIRCOUTCOMES.113.000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 32.Smith BM, Prince MR, Hoffman EA, et al. Impaired left ventricular filling in copd and emphysema: is it the heart or the lungs?: The Multi-Ethnic Study of Atherosclerosis COPD Study. Chest. 2013;144:1143–51. doi: 10.1378/chest.13-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mentz RJ, Fiuzat M, Kraft M, et al. Bronchodilators in heart failure patients with COPD: is it time for a clinical trial? J Cardiac Fail. 2012;18:413–422. doi: 10.1016/j.cardfail.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Caruana L, Petrie MC, Davie AP, et al. Do patients with suspected heart failure and preserved left ventricular systolic function suffer from “diastolic heart failure” or from misdiagnosis? A prospective descriptive study. BMJ. 2000;321:215–8. doi: 10.1136/bmj.321.7255.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mentz RJ, Schmidt PH, Kwasny MJ, et al. The impact of chronic obstructive pulmonary disease in patients hospitalized for worsening heart failure with reduced ejection fraction: an analysis of the EVEREST Trial. J Card Fail. 2012;18:515–23. doi: 10.1016/j.cardfail.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Mentz RJ, Schulte PJ, Fleg JL, et al. Clinical characteristics, response to exercise training, and outcomes in patients with heart failure and chronic obstructive pulmonary disease: findings from Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION) Am Heart J. 2013;165:193–9. doi: 10.1016/j.ahj.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hawkins NM, Petrie MC, Macdonald MR, et al. Heart failure and chronic obstructive pulmonary disease the quandary of Beta-blockers and Beta-agonists. J Am Coll Cardiol. 2011;57:2127–38. doi: 10.1016/j.jacc.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 38.Dungen HD, Apostolovic S, Inkrot S, et al. CIBIS-ELD Investigators and Project Multicentre Trials in the Competence Network Heart Failure. Titration to target dose of bisoprolol vs. carvedilol in elderly patients with heart failure: the CIBIS-ELD trial. Eur J Heart Fail. 2011;13:670–80. doi: 10.1093/eurjhf/hfr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berry C, Hogg K, Norrie J, et al. Heart failure with preserved left ventricular systolic function: a hospital cohort study. Heart. 2005;91:907–13. doi: 10.1136/hrt.2004.041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lam CS, Lyass A, Kraigher-Krainer E, et al. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circulation. 2011;124:24–30. doi: 10.1161/CIRCULATIONAHA.110.979203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362:217–27. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jabbour A, Macdonald PS, Keogh AM, et al. Differences between beta-blockers in patients with chronic heart failure and chronic obstructive pulmonary disease: a randomized crossover trial. J Am Coll Cardiol. 2010;55:1780–7. doi: 10.1016/j.jacc.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 43.Lainscak M, Podbregar M, Kovacic D, et al. Differences between bisoprolol and carvedilol in patients with chronic heart failure and chronic obstructive pulmonary disease: a randomized trial. Respir Med. 2011;105 (Suppl 1):S44–9. doi: 10.1016/S0954-6111(11)70010-5. [DOI] [PubMed] [Google Scholar]
- 44.Mentz RJ, Wojdyla D, Fiuzat M, et al. Association of beta-blocker use and selectivity with outcomes in patients with heart failure and chronic obstructive pulmonary disease (from OPTIMIZE-HF) Am J Cardiol. 2013;111:582–7. doi: 10.1016/j.amjcard.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 45.Hsich EM, Grau-Sepulveda MV, Hernandez AF, et al. Sex differences in in-hospital mortality in acute decompensated heart failure with reduced and preserved ejection fraction. Am Heart J. 2012;163:430–7. e3. doi: 10.1016/j.ahj.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 46.Young JB, Abraham WT, Albert NM, et al. Relation of low hemoglobin and anemia to morbidity and mortality in patients hospitalized with heart failure (insight from the OPTIMIZE-HF registry) Am J Cardiol. 2008;101:223–30. doi: 10.1016/j.amjcard.2007.07.067. [DOI] [PubMed] [Google Scholar]
- 47.O’Meara E, Clayton T, McEntegart MB, et al. CHARM Committees and Investigators. Clinical correlates and consequences of anemia in a broad spectrum of patients with heart failure: results of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Circulation. 2006;113:986–94. doi: 10.1161/CIRCULATIONAHA.105.582577. [DOI] [PubMed] [Google Scholar]
- 48.Anand IS. Anemia and chronic heart failure: implications and treatment options. J Am Coll Cardiol. 2008;52:501–11. doi: 10.1016/j.jacc.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 49.Anand IS, Chandrashekhar Y, Ferrari R, et al. Pathogenesis of oedema in chronic severe anaemia: studies of body water and sodium, renal function, haemodynamic variables, and plasma hormones. Br Heart J. 1993;70:357–62. doi: 10.1136/hrt.70.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anand IS, Chandrashekhar Y, Wander GS, et al. Endothelium-derived relaxing factor is important in mediating the high output state in chronic severe anemia. J Am Coll Cardiol. 1995;25:1402–7. doi: 10.1016/0735-1097(95)00007-Q. [DOI] [PubMed] [Google Scholar]
- 51.Katz SD, Rao R, Berman JW, et al. Pathophysiological correlates of increased serum tumor necrosis factor in patients with congestive heart failure. Relation to nitric oxide-dependent vasodilation in the forearm circulation. Circulation. 1994;90:12–6. doi: 10.1161/01.cir.90.1.12. [DOI] [PubMed] [Google Scholar]
- 52.Lindenfeld J. Prevalence of anemia and effects on mortality in patients with heart failure. Am Heart J. 2005;149:391–401. doi: 10.1016/j.ahj.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 53.Groenveld HF, Januzzi JL, Damman K, et al. Anemia and mortality in heart failure patients a systematic review and meta-analysis. J Am Coll Cardiol. 2008;52:818–27. doi: 10.1016/j.jacc.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 54.Tang YD, Katz SD. The prevalence of anemia in chronic heart failure and its impact on the clinical outcomes. Heart Fail Rev. 2008;13:387–92. doi: 10.1007/s10741-008-9089-7. [DOI] [PubMed] [Google Scholar]
- 55.van der Meer P, Groenveld HF, Januzzi JL, Jr, et al. Erythropoietin treatment in patients with chronic heart failure: a meta-analysis. Heart. 2009;95:1309–14. doi: 10.1136/hrt.2008.161091. [DOI] [PubMed] [Google Scholar]
- 56.Anand I, McMurray JJ, Whitmore J, et al. Anemia and its relationship to clinical outcome in heart failure. Circulation. 2004;110:149–54. doi: 10.1161/01.CIR.0000134279.79571.73. [DOI] [PubMed] [Google Scholar]
- 57.Scrutinio D, Passantino A, Santoro D, et al. The cardiorenal anaemia syndrome in systolic heart failure: prevalence, clinical correlates, and long-term survival. Eur J Heart Fail. 2011;13:61–7. doi: 10.1093/eurjhf/hfq167. [DOI] [PubMed] [Google Scholar]
- 58.von Haehling S, van Veldhuisen DJ, Roughton M, et al. Anaemia among patients with heart failure and preserved or reduced ejection fraction: results from the SENIORS study. Eur J Heart Fail. 2011;13:656–63. doi: 10.1093/eurjhf/hfr044. [DOI] [PubMed] [Google Scholar]
- 59.Swedberg K, Young JB, Anand IS, et al. RED-HF Committees; RED-HF Investigators. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med. 2013;368:1210–9. doi: 10.1056/NEJMoa1214865. [DOI] [PubMed] [Google Scholar]
- 60.Anker SD, Comin Colet J, Filippatos G, et al. FAIR-HF Trial Investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–48. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 61.Comín-Colet J, Enjuanes C, González G, et al. Iron deficiency is a key determinant of health-related quality of life in patients with chronic heart failure regardless of anaemia status. Eur J Heart Fail. 2013;15:1164–72. doi: 10.1093/eurjhf/hft083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klip IT, Comin-Colet J, Voors AA, et al. Iron deficiency in chronic heart failure: An international pooled analysis. Am Heart J. 2013;165:575–582. e3. doi: 10.1016/j.ahj.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 63.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–23. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 64.Aneja A, Tang WH, Bansilal S, et al. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med. 2008;121:748–57. doi: 10.1016/j.amjmed.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 65.Liu JE, Palmieri V, Roman MJ, et al. The impact of diabetes on left ventricular filling pattern in normotensive and hypertensive adults: the Strong Heart Study. J Am Coll Cardiol. 2001;37:1943–9. doi: 10.1016/s0735-1097(01)01230-x. [DOI] [PubMed] [Google Scholar]
- 66.Schannwell CM, Schneppenheim M, Perings S, et al. Left ventricular diastolic dysfunction as an early manifestation of diabetic cardiomyopathy. Cardiology. 2002;98:33–9. doi: 10.1159/000064682. [DOI] [PubMed] [Google Scholar]
- 67.Amato L, Paolisso G, Cacciatore F, et al. Congestive heart failure predicts the development of non-insulin-dependent diabetes mellitus in the elderly. The Osservatorio Geriatrico Regione Campania Group. Diabetes Metab. 1997;23:213–8. [PubMed] [Google Scholar]
- 68.Swan JW, Anker SD, Walton C, et al. Insulin resistance in chronic heart failure: relation to severity and etiology of heart failure. J Am Coll Cardiol. 1997;30:527–32. doi: 10.1016/s0735-1097(97)00185-x. [DOI] [PubMed] [Google Scholar]
- 69.Dhingra R, Vasan RS. Diabetes and the risk of heart failure. Heart Fail Clin. 2012;8:125–33. doi: 10.1016/j.hfc.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Groote P, Lamblin N, Mouquet F, et al. Impact of diabetes mellitus on long-term survival in patients with congestive heart failure. Eur Heart J. 2004;25:656–62. doi: 10.1016/j.ehj.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 71.Harjola VP, Follath F, Nieminen MS, et al. Characteristics, outcomes, and predictors of mortality at 3 months and 1 year in patients hospitalized for acute heart failure. Eur J Heart Fail. 2010;12:239–48. doi: 10.1093/eurjhf/hfq002. [DOI] [PubMed] [Google Scholar]
- 72.Parissis JT, Rafouli-Stergiou P, Mebazaa A, et al. Acute heart failure in patients with diabetes mellitus: clinical characteristics and predictors of in-hospital mortality. Int J Cardiol. 2012;157:108–13. doi: 10.1016/j.ijcard.2011.11.098. [DOI] [PubMed] [Google Scholar]
- 73.Greenberg BH, Abraham WT, Albert NM, et al. Influence of diabetes on characteristics and outcomes in patients hospitalized with heart failure: A report from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) Am Heart J. 2007;154:277, e1–8. doi: 10.1016/j.ahj.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 74.Sarma S, Mentz RJ, Kwasny MJ, et al. EVEREST Investigators. Association between diabetes mellitus and post-discharge outcomes in patients hospitalized with heart failure: findings from the EVEREST trial. Eur J Heart Fail. 2013;15:194–202. doi: 10.1093/eurjhf/hfs153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Komajda M, McMurray JJ, Beck-Nielsen H, et al. Heart failure events with rosiglitazone in type 2 diabetes: data from the RECORD clinical trial. Eur Heart J. 2010;31:824–31. doi: 10.1093/eurheartj/ehp604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scirica BM, Bhatt DL, Braunwald E, et al. SAVOR-TIMI 53 Steering Committee and Invesigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes Mellitus. N Engl J Med. 2013;369:1317–26. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 77.Weir DL, McAlister FA, Senthilselvan A, et al. Sitagliptin Use in Patients With Diabetes and Heart Failure: A Population-Based Retrospective Cohort Study. J Am Coll Cardiol HF. 2014 Jul 2; doi: 10.1016/j.jchf.2014.04.005. [E-pub ahead of print]; http://dx.doi:10.1016/j.jchf.2014.04.005. [DOI] [PubMed]
- 78.American Diabetes Association. Standards of Medical Care in Diabetes—2013. Diabetes Care. 2013;36 (Suppl 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Heerebeek L, Hamdani N, Falcão-Pires I, et al. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation. 2012;126:830–9. doi: 10.1161/CIRCULATIONAHA.111.076075. [DOI] [PubMed] [Google Scholar]
- 80.Waldum B, Os I. The cardiorenal syndrome: what the cardiologist needs to know. Cardiology. 2013;126:175–86. doi: 10.1159/000353261. [DOI] [PubMed] [Google Scholar]
- 81.Brouwers FP, de Boer RA, van der Harst P, et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J. 2013;34:1424–31. doi: 10.1093/eurheartj/eht066. [DOI] [PubMed] [Google Scholar]
- 82.Hillege HL, Nitsch D, Pfeffer MA, et al. Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Investigators. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671–8. doi: 10.1161/CIRCULATIONAHA.105.580506. [DOI] [PubMed] [Google Scholar]
- 83.Dries DL, Exner DV, Domanski MJ, et al. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:681–9. doi: 10.1016/s0735-1097(99)00608-7. [DOI] [PubMed] [Google Scholar]
- 84.Heywood JT, Fonarow GC, Costanzo MR, et al. ADHERE Scientific Advisory Committee and Investigators. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13:422–30. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 85.Aronson D, Burger AJ. The relationship between transient and persistent worsening renal function and mortality in patients with acute decompensated heart failure. J Card Fail. 2010;16:541–7. doi: 10.1016/j.cardfail.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 86.Testani JM, Chen J, McCauley BD, et al. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122:265–72. doi: 10.1161/CIRCULATIONAHA.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blair JE, Pang PS, Schrier RW, et al. EVEREST Investigators. Changes in renal function during hospitalization and soon after discharge in patients admitted for worsening heart failure in the placebo group of the EVEREST trial. Eur Heart J. 2011;32:2563–72. doi: 10.1093/eurheartj/ehr238. [DOI] [PubMed] [Google Scholar]
- 88.Yancy CW, Jessup M, Bozkurt B, et al. American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 89.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 90.Clark H, Krum H, Hopper I. Worsening renal function during renin-angiotensin-aldosterone system inhibitor initiation and long-term outcomes in patients with left ventricular systolic dysfunction. Eur J Heart Fail. 2014;16:41–8. doi: 10.1002/ejhf.13. [DOI] [PubMed] [Google Scholar]
- 91.Felker GM, Mentz RJ. Diuretics and ultrafiltration in acute decompensated heart failure. J Am Coll Cardiol. 2012;59:2145–53. doi: 10.1016/j.jacc.2011.10.910. [DOI] [PubMed] [Google Scholar]
- 92.Bitter T, Faber L, Hering D, et al. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail. 2009;11:602–8. doi: 10.1093/eurjhf/hfp057. [DOI] [PubMed] [Google Scholar]
- 93.Chan J, Sanderson J, Chan W, et al. Prevalence of sleep-disordered breathing in diastolic heart failure. Chest. 1997;111:1488–93. doi: 10.1378/chest.111.6.1488. [DOI] [PubMed] [Google Scholar]
- 94.Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol. 2011;57:119–27. doi: 10.1016/j.jacc.2010.08.627. [DOI] [PubMed] [Google Scholar]
- 95.Herrscher TE, Akre H, Overland B, et al. High prevalence of sleep apnea in heart failure outpatients: even in patients with preserved systolic function. J Card Fail. 2011;17:420–5. doi: 10.1016/j.cardfail.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 96.Paulino A, Damy T, Margarit L, et al. Prevalence of sleep-disordered breathing in a 316-patient French cohort of stable congestive heart failure. Arch Cardiovasc Dis. 2009;102:169–75. doi: 10.1016/j.acvd.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 97.Sin DD, Fitzgerald F, Parker JD, et al. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–6. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 98.Bradley TD, Floras JS. Sleep apnea and heart failure: Part I: obstructive sleep apnea. Circulation. 2003;107:1671–8. doi: 10.1161/01.CIR.0000061757.12581.15. [DOI] [PubMed] [Google Scholar]
- 99.Ferrier K, Campbell A, Yee B, et al. Sleep-disordered breathing occurs frequently in stable outpatients with congestive heart failure. Chest. 2005;128:2116–22. doi: 10.1378/chest.128.4.2116. [DOI] [PubMed] [Google Scholar]
- 100.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 101.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol. 2013;62:610–6. doi: 10.1016/j.jacc.2013.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khayat R, Abraham W, Patt B, et al. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail. 2012;18:534–40. doi: 10.1016/j.cardfail.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49:1625–31. doi: 10.1016/j.jacc.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 105.Javaheri S, Shukla R, Zeigler H, et al. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol. 2007;49:2028–34. doi: 10.1016/j.jacc.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 106.Yumino D, Wang H, Floras JS, et al. Relationship between sleep apnoea and mortality in patients with ischaemic heart failure. Heart. 2009;95:819–24. doi: 10.1136/hrt.2008.160952. [DOI] [PubMed] [Google Scholar]
- 107.Roebuck T, Solin P, Kaye DM, et al. Increased long-term mortality in heart failure due to sleep apnoea is not yet proven. Eur Respir J. 2004;23:735–40. doi: 10.1183/09031936.04.00060404. [DOI] [PubMed] [Google Scholar]
- 108.Sekizuka H, Osada N, Miyake F. Sleep Disordered Breathing in Heart Failure Patients with Reduced versus Preserved Ejection Fraction. Heart Lung Circ. 2013;22:104–9. doi: 10.1016/j.hlc.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 109.Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol. 2011;57:119–27. doi: 10.1016/j.jacc.2010.08.627. [DOI] [PubMed] [Google Scholar]
- 110.Bradley TD, Logan AG, Kimoff RJ, et al. CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–33. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 111.Sharma BK, Bakker JP, McSharry DG, et al. Adaptive servoventilation for treatment of sleep-disordered breathing in heart failure: a systematic review and meta-analysis. Chest. 2012;142:1211–21. doi: 10.1378/chest.12-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pepperell JC, Maskell NA, Jones DR, et al. A randomized controlled trial of adaptive ventilation for Cheyne-Stokes breathing in heart failure. Am J Respir Crit Care Med. 2003;168:1109–14. doi: 10.1164/rccm.200212-1476OC. [DOI] [PubMed] [Google Scholar]
- 113.Lavie CJ, McAuley PA, Church TS, et al. Obesity and cardiovascular diseases: implications regarding fitness, fatness, and severity in the obesity paradox. J Am Coll Cardiol. 2014;63:1345–54. doi: 10.1016/j.jacc.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 114.Doehner W. Critical appraisal of the obesity paradox in cardiovascular disease: How to manage patients with overweight in heart failure? Heart Fail Rev. 2014;19:637–44. doi: 10.1007/s10741-014-9425-z. [DOI] [PubMed] [Google Scholar]
- 115.Gaddam KK, Ventura HO, Lavie CJ. Metabolic syndrome and heart failure—the risk, paradox, and treatment. Curr Hypertens Rep. 2011;13:142–8. doi: 10.1007/s11906-011-0179-x. [DOI] [PubMed] [Google Scholar]
- 116.Haass M, Kitzman DW, Anand IS, et al. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure With Preserved Ejection Fraction (I-PRESERVE) Trial. Circ Heart Fail. 2011;4:324–31. doi: 10.1161/CIRCHEARTFAILURE.110.959890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63:747–62. doi: 10.1016/j.jacc.2013.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McNallan SM, Singh M, Chamberlain AM, et al. Frailty and healthcare utilization among patients with heart failure in the community. J Am Coll Cardiol HF. 2013;1:135–41. doi: 10.1016/j.jchf.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rahman MM, Kopec JA, Cibere J, et al. The relationship between osteoarthritis and cardiovascular disease in a population health survey: a cross-sectional study. BMJ Open. 2013;3:e002624. doi: 10.1136/bmjopen-2013-002624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Massie BM, O’Connor CM, Metra M, et al. PROTECT Investigators and Committees. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med. 2010;363:1419–28. doi: 10.1056/NEJMoa0912613. [DOI] [PubMed] [Google Scholar]
- 121.Mentz RJ, Felker GM, Ahmad T, et al. Learning from recent trials and shaping the future of acute heart failure trials. Am Heart J. 2013;166:629–35. doi: 10.1016/j.ahj.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pitt B, Pfeffer MA, Assmann SF, et al. TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–92. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 123.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–68. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 124.Gheorghiade M, Böhm M, Greene SJ, et al. ASTRONAUT Investigators and Coordinators. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA. 2013;309:1125–35. doi: 10.1001/jama.2013.1954. [DOI] [PubMed] [Google Scholar]