Synopsis
In this article we discuss the growing problem of ROP worldwide, treatments for severe ROP including standard of care laser treatment, and the need for new treatments. We will also discuss the reasons to consider inhibiting the VEGF signaling pathway in severe ROP and the concerns about broad VEGF inhibition. Finally, we will cover the potential role of VEGF in ROP based on studies in animal models of oxygen-induced retinopathy (OIR), the effects of anti-VEGF based on basic research data, and the clinical relevance of these data.
Keywords: vascular endothelial growth factor, physiologic retinal vascular development (PRVD), intravitreal neovascularization (IVNV), bevacizumab, angiogenesis, oxygen-induced retinopathy (OIR)
Introduction
Over the past several decades, vascular endothelial growth factor (VEGF) has become recognized as an important pathologic angiogenic factor in a number of eye diseases, including age-related macular degeneration (AMD),1–3 diabetic retinopathy,4,5 retinal vein occlusion,4 and retinopathy of prematurity (ROP). Prior to FDA approval of anti-VEGF agents for AMD, a disease affecting elderly adults, preclinical studies tested VEGF inhibitors in animal models of angiogenesis, including models of oxygen-induced retinopathy (OIR) in which blood vessels grow into the vitreous cavity similar to what occurs in diabetic retinopathy and ROP.6,7 After proven efficacy that anti-VEGF agents reduced intravitreal angiogenesis in preclinical testing in models of OIR and aberrant angiogenesis in clinical trials for neovascular AMD and adult eye diseases, a clinical trial was performed to test the effect of inhibiting the bioactivity of VEGF using the monoclonal antibody, bevacizumab, in severe ROP.8 Success was reported in a subgroup of preterm infants with zone I, stage 3 ROP with plus disease. However, concerns remain.
No dosing studies were performed to determine an effective and safe dose or optimal agent for ROP. VEGF is an important angiogenic factor in development, a survival factor of newly formed capillaries, and also plays a role in the homeostasis of already developed vasculature.9,10 In adults, repeated treatment with anti-VEGF agents, the standard of care for AMD, has been associated with geographic atrophy, another cause of vision loss in AMD.11 VEGF is also a neuroprotective agent for retinal neurons.12 These were the concerns for the preterm infant retina. In addition, anti-VEGF agents injected into the vitreous cavity reduced serum VEGF for several weeks,13,14 raising additional concern of the effects of removing systemic VEGF on the development of organs, particularly kidney, brain and lung in the preterm infant. Following the publication of the clinical trial, complications were reported after a single intravitreal injection of bevacizumab. These complications included persistent avascular retina, recurrent intravitreal angiogenesis and stage 5 retinal detachment.15,16 Therefore, before considering anti-VEGF agents in preterm infants, more studies are needed to determine long-term effects on safety, proper doses, or even the type of anti-VEGF agent or other drug.
In this article, we will discuss the growing problem of ROP worldwide, the standard of care laser treatment in severe ROP and the need for new treatments. We will also discuss the reasons to consider inhibiting the VEGF signaling pathway in ROP and the concerns about broad inhibition. Finally, we will cover the potential role of VEGF in ROP based on studies in OIR models, the effects of anti-VEGF based on basic research data, and the clinical relevance of these data.
The Problem: ROP is Increasing Worldwide and has Different Phenotypes
With increases in preterm births, ROP has become one of the leading causes of childhood blindness world-wide.17 In the US, ~14% of childhood blindness is attributed to ROP and in some developing nations, estimates are >20%.18 In addition, some countries have developed the ability to save preterm infants but lack resources to regulate oxygen and are experiencing not only cases of ROP from extreme prematurity, but also additional cases of ROP in larger and older infants from high oxygen-induced damage to newly formed retinal capillaries similar to what occurred in the 1940’s and 1950’s in the US, UK and Canada.15,19,20 Compounding these increases in ROP cases throughout the world, the number of adequately trained ophthalmologists to diagnose and treat ROP is not increasing to meet the need.19 There also appears to be a heritable component to ROP,20 and genetic pools differ throughout the world. Thus, ROP phenotypes may vary throughout the world based on environmental factors and potentially, differences in genetic variants. These considerations are important when comparing outcomes from clinical reports after anti-VEGF therapy.
Current Treatment for ROP and Reasons for Better Therapies
When ROP was first diagnosed as retrolental fibroplasia in the 1940’s in the US, studies in animal models were performed which revealed that high oxygen at birth was a cause.21 Oxygen damaged newly formed retinal capillaries and led to broad areas of avascular retina.22,23 When the infant was moved from high oxygen to a relatively hypoxic environment, cells within the avascular retina were stimulated by hypoxia and believed to increase the expression of angiogenic growth factors.24 Now several mechanisms are proposed, including the stabilization and nuclear translocation of hypoxia inducible factors that cause transcription of angiogenic factors, including VEGF.25 However, rather than vascular growth into the avascular retina to relieve the hypoxic stimulus, vessels grow into the vitreous. Efforts were made to reduce oxygen, which drastically reduced cases of ROP but also increased infant morbidity and mortality. With advances in neonatal care and the ability to regulate oxygen, ROP re-emerged as smaller and younger preterm infants were surviving.
In the 1990’s, the Supplemental Therapeutic Oxygen for Prethreshold ROP (STOP-ROP) multicenter trial tested the hypothesis that increased supplemental oxygen treatment would reduce the hypoxic stimulus for vasoproliferation and thereby prevent severe ROP [Table 1]. However, only in a post-hoc analysis was reduced progression to severe ROP noted in a subgroup with higher oxygen saturation (94–99% SaO2) compared to conventional oxygen saturation (89–94% SaO2).26 Other smaller studies reported that supplemental oxygen later in the neonatal course in the nursery reduced severe ROP.26,27 More recent studies tested the hypotheses that lower than conventional oxygen saturation targets would reduce severe ROP.28–30 In two studies, the Surfactant, Positive Airway Pressure, Pulse Oximetry Randomized Trial (SUPPORT)31 and Benefits of Oxygen Saturation Targeting (BOOST),31 low oxygen saturation targets were associated with lower incidence of ROP but higher mortality, whereas in the Canadian Oxygen Trial (COT),32 there was no effect on ROP or mortality.31 However, the studies had important differences, including regional differences in the phenotype of ROP of enrolled infants [Table 1].33 The conclusion from these studies is that even when oxygen is regulated, ROP can still occur in the smallest and youngest preterm infants.
Table 1.
List of Major Oxygen Trials
| Trial | Dates | Enrolled | Exclusion | % not meeting inclusion criteria | Geography | Birth weight (mean or range) | Intervention | Outcome | Mortality |
|---|---|---|---|---|---|---|---|---|---|
| STOP-ROP | 1994–1999 | 30–48 weeks GA with prethreshold ROP in one eye and median SaO2 <94% | Median SaO2>94% or congenital abnormality | 34% | United States | 726 grams | Infants randomized to SaO2 ranges of 89–94% or 96–99% | No significant difference in threshold ROP. In subgroup, threshold ROP < infants without Plus disease in 96–99% SaO2 group | Not assessed formally 7 infants in lower oxygen tension group vs. 9 infants in the higher tension group died) |
| Support | 2005–2009 | 24–27 weeks GA 6 days at birth who underwent full resuscitation | Infants with major congenital abnormalities | 6.6% | United States | 825–836 grams |
|
Decreased ROP in 85–89% SaO2 group | Increased mortality in 85–89% SaO2 group |
| BOOST II | 2006–2011 | <28 weeks GA |
|
9% | Australia, UK, New Zealand | 826–837 grams | Randomized to SaO2 of 85–89% or 91–95% | Decreased ROP in 85–89% SaO2 group | Increased mortality when targeting SaO2 <90% |
| COT | 2006–2012 | >23 weeks to 27 weeks and 6 days GA |
|
16% | Canada, United States, Argentina Finland, Germany Israel | 827–845 grams | Randomized to SaO2 of 85–89% or 91–95% | No significant difference between SaO2 targets on ROP at 18 months | No significant difference between SaO2 targets on death or disability at 18 months |
GA-gestational age; SaO2 – oxygen saturation; STOP-ROP - Supplemental Therapeutic Oxygen for Prethreshold Retinopathy of Prematurity; BOOST – Benefits of Oxygen Saturation Targeting; SUPPORT - Surfactant, Positive Airway Pressure, Pulse Oximetry Randomized Trial; COT – Canadian Oxygen Trial
Fluctuations in oxygenation have also been associated with severe ROP.34 There is speculation that strict control of oxygen levels may lead to more fluctuations in oxygenation in preterm infants because of the difficulty in maintaining oxygen saturation levels within a tight range.35 Repeated fluctuations in oxygen delivery in animal models leads to increased oxidative compounds,36 over-expression of VEGF and VEGF receptor 2 and also over-activation of signaling cascades involving VEGFR2.3,37 Besides fluctuations in oxygenation, risks for human ROP have included associations with poor infant growth38 and increased oxidative stress.39–41
The current standard of care for ROP of all causes is treatment of the peripheral avascular retina with laser, preferably to cryotherapy, when a level of severity (type 1 ROP) develops42 [Tables 2 and 3]. There are several considerations for successful treatment. First, timing of treatment is critical. Early treatment for type 1 ROP before threshold ROP42 occurs led to prevention of blindness in over 90% of enrolled infants compared to about a 75% success rate from the multicenter study, Cryotherapy for ROP [Tables 2 and 4].43 Eyes that advance in severity of ROP beyond threshold do not respond well to laser, cryotherapy or surgery.44 Diagnosis and treatment with laser or cryotherapy require skill using an indirect ophthalmoscope and a scleral depressor.45 Although wide-angle retinal imaging is being investigated as an aid in diagnosis,46 treatment with laser or cryotherapy still requires ability with an indirect ophthalmoscope in order to target the appropriate areas in the retina. Subsequently, it is essential to have adequately trained ophthalmologists and staff who can diagnose47 and treat severe ROP. Unfortunately, there is a shortage of suitably trained ophthalmologists who are willing to diagnose and treat preterm infants for ROP.17,46 Adequate treatment and ability to determine when retreatment is needed are important, and only a few studies have addressed the factors associated with progression of retinal detachment following laser treatment and the window of surgical opportunity.48,49 Finally, laser treatment for severe ROP may take 2 or more hours to perform, whereas the time to perform an intravitreal injection is often less than 30 minutes. This adds an incentive to find methods of treatment besides laser for ROP. However, adequate research and testing for safety and efficacy are needed.
Table 2.
Prethreshold and Threshold ROP
| Type 1 ROP - (high risk) prethreshold ROP: | |
| zone I | Any stage with plus disease |
| zone I, stage 3 | Without plus disease |
| zone II, stage 2 or 3 | With plus disease |
| Type 2 ROP - (low risk) prethreshold ROP: | |
| zone I, stage 1 or 2 | Without plus disease |
| zone II, stage 3 | Without plus disease |
Table 3.
Current Management of ROP
| Screening Guidelines | |
| US | < 30 weeks GA or ≤1,500-gram BW (and preterm infants with an unstable clinical course)47 |
| UK | ≤ 31 weeks GA or ≤ 1500 gram BW |
| Canada | ≤ 30 6/7 weeks GA or ≤ 1250 gram |
| Timing of Screening and Examinations | |
| First examination at 4–6 weeks chronologic age or 31 weeks post-gestational age | |
| Repeated examinations recommended by examining ophthalmologist based on retinal findings and suggested schedule | |
| Type of Examination | |
| Dilated binocular indirect ophthalmoscopy | |
| Ongoing studies of validation and reliability of retinal imaging as a potential telemedicine alternative for screening | |
| Parameters of ROP Determined in Examinations | |
| zone – Area of retinal vascularization | I: Retinal vasculature extends within a circle centered on the optic nerve, the radius of which is twice the optic nerve-to-macula distance |
| II: Retinal vasculature extends beyond zone I within the limits of a circular area, the radius of which is the distance from the optic nerve to the nasal ora serrata | |
| III: The remaining area outside zones I or II | |
| stage – Disease severity (Fig. 1) | 1: Line |
| 2: Ridge (with volume) | |
| 3: intravitreal angiogenesis or neovascularization (IVNV) | |
| 4: Partial retinal detachment | |
| 5: Total retinal detachment | |
| Plus disease – dilation and tortuosity of retinal vessels | |
| Treatment | |
| Application of laser to peripheral avascular retina for type 1 ROP (high-risk prethreshold) | zone I: stage 3 |
| zone I: any stage with plus disease | |
| zone II: stage 2 or 3 with plus disease | |
| In some cases, anti-VEGF for stage 3 and plus disease in zone I | Additional study needed to determine dose, safety, and type of anti-VEGF |
| Visual Rehabilitation | Refractive correction very often needed for associated refractive errors (ametropia and anisometropia); protective eyewear and low vision aids |
GA-gestational age; BW-birth weight
Table 4.
Major Clinical Trials in ROP
| Trial (Enrollment) | Criteria | Number Enrolled | Endpoint (Follow-up) | Outcome Measures | Results |
|---|---|---|---|---|---|
| CRYO-ROP (1/1/86–1/22/88) | <1251 grams BW; survived 28 days of life; no major systemic or ocular anomalies | 4099 (291 with threshold ROP randomized to cryo or observationa, 254 analyzed at 15 yr) | Reports at 3 mo, 1 through 15 yrs. | Visual functionb; structural findings; | At 15 years; 44.7% cryo vs. 64.3% observation had <20/200 and 30.0% vs. 51.9% had unfavorable structural outcomes; p<0.001 |
| ETROP (10/9–10/02) | <1251 grams BW; prethreshold ROP | 828 infants (730 studied); 401 high risk prethreshold (≥ 15% risk of unfavorable outcome=type 1 ROP), 329 low risk (< 15% risk=type 2 ROP) | 9 months, 6 years early treatment (prethreshold) vs. conventional (threshold) ROP | Vision at 9 mos (Teller Acuity Cards) and 6 years follow up; secondary outcomes retinal structure, myopia, amblyopia, strabismus | Early treatment - reduced unfavorable visual outcome (19.8% to 14.3%, p=.01) at 9 months and for type 1 ROP at 6 years (16.4% early vs.25.2% conventional); significantly reduced unfavorable outcome (15.6% vs. 9.0%; p<.001 at 9 mo and 15.2% to 8.9% at 6 years) |
| BEAT-ROP (3/13/08–8/4/10) | ≤ 1500 g BW, ≤ 30 weeks, stage 3+ ROP in zone I or zone II | 150 infants (67 with zone I, 83 with posterior zone II) enrolled, 143 survived; 75 randomized to conventional laser, 75 to intravitreal bevacizumab | 54 weeks PMA | Recurrence of ROP (primary outcome), interval from treatment to recurrence, need for surgery | Reduced recurrence of stage 3 ROP in zone I ROP after intravitreal bevacizumab (4%) compared to laser (22%); no effect for zone II disease |
Toward a Treatment Solution
It is important to review new evidence regarding the pathophysiology of ROP that has been realized since early studies by Ashton,50 Patz,22 and preclinical studies before the FDA approval of anti-VEGF agents in adult diseases. Since it is not possible to safely obtain tissue or vitreous samples from the preterm infant eye in order to study ROP without risks of bleeding, cataract, or inoperable retinal detachment,44 animal models of oxygen-induced retinopathy (OIR) have been used. It is important to know strengths and limitations of different models when reviewing the evidence. First, all models use newborn, and not premature, animals, unlike the human infant with ROP. Second, most studies have used the mouse OIR model,51 which exposes newborn mice to high oxygen levels. This model may reflect ROP in places that lack resources to regulate oxygen52 or ROP that occurred in the US and UK in the 1950’s53,54 but is not as representative of ROP in places where oxygen is regulated. [BOX 1]. A benefit of the mouse OIR model is the ability to use transgenic mice to study mechanisms of angiogenesis, high oxygen and relative hypoxia. In contrast, the rat 50/10 OIR model (“rat ROP model”)55,56 reflects the conditions associated with the pathogenesis of human ROP because it reproduces arterial oxygen levels of preterm infants with severe ROP34,58. It also causes extrauterine growth restriction.57 It is the most representative model of ROP today.54 Previously, mechanistic studies relied mainly on pharmacologic manipulations, but recently, methods have been developed using a gene therapy approach that permits knockdown of VEGF in specific cells in the retina that overexpress VEGF.59,60 These new approaches have been valuable in understanding the mechanisms whereby overexpressed VEGF causes aberrant intravitreal angiogenesis, as well as the effect of various methods to inhibit VEGF bioactivity on developing retinal vasculature and developing systemic organs (Table 5).
Box 1. Animal Models.
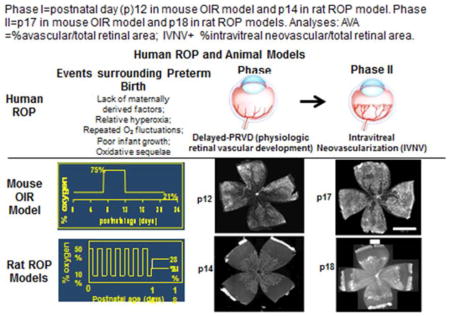
Table 5.
Guidelines for Use of Anti-VEGF in ROP47
| Indications for use | Informed consent | Log | Follow up |
|---|---|---|---|
| Clinical trial BEAT-ROP only found effect in zone I, Stage 3+ disease | Agents not FDA approved, | Age, date, eye treated, dose and agent used, volume | Monitor weekly after treatment until vascularization is complete to ora serrata |
| No clinical trial evidence of effect with less severe ROP | Questions remain regarding long-term safety, dose, timing, visual outcomes and long-term effects, including systemically | Communicate with neonatologist and ophthalmologist during transfer or discharge | Longer follow up is needed because recurrences following bevacizumab occurred later than laser treatment |
| Communication with parents is essential including the need for follow up examinations and risks, and documentation must be performed | Unknown what to do if avascular retina persists after 60 weeks chronologic age when awake examinations are difficult; consider ablative treatment |
Data from Ophthalmology AAOPSo, OPHTHALMOLOGY AAO, OPHTHALMOLOGY AAFP, STRABISMUS, ORTHOPTISTS AAOC. Screening Examination of Premature Infants for Retinopathy of Prematurity. Pediatrics 2013;131:189–95.
The BEAT-ROP Study
Although several clinical series had been reported previously, the Bevacizumab Eliminates the Angiogenic Threat of Retinopathy of Prematurity (BEAT-ROP) was the first published clinical trial that tested intravitreal anti-VEGF antibody, bevacizumab, (0.625 mg in 0.025mL) compared to laser treatment in 150 infants.61 Infants rather than eyes were enrolled to reduce confounding from crossover effects of the antibody. A benefit for infants with zone I/posterior zone II, stage 3+ ROP was reported in patients (p=0.003). In many of these eyes, physiologic vascularization progressed into zone II after intravitreal angiogenesis was inhibited. The study was too small to assess safety and effects on future development of brain and other tissues. The study also did not address dose or anti-VEGF agent. The study follow up was continued through 54 weeks post-gestational age. However, later studies of infants treated with intravitreal bevacizumab and followed through 60 weeks post-gestational age reported associated complications, including persistent peripheral avascular retina, new intravitreal neovascularization, retinal detachment, and macular dragging.16,62 Other studies reported reduced serum VEGF in infants who received bevacizumab or ranibizumab even two weeks following the intravitreal injection.13,14 Although there is some promise with anti-VEGF treatment, there is clinical risk of poor outcome and safety concerns potentially from systemic reduction of VEGF. Better treatments are needed.
Consideration of Anti-VEGF Agents in Severe ROP: Knowledge from Animal Models
PRO
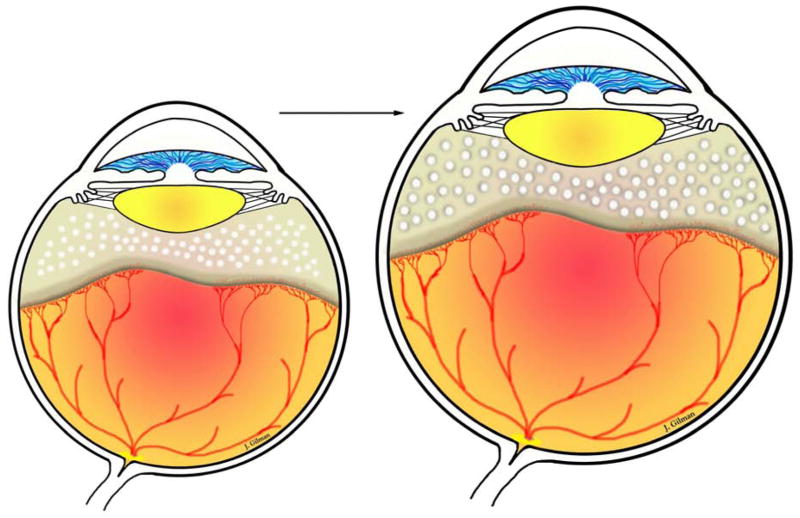
Evidence that Inhibiting VEGF Inhibits intravitreal angiogenesis in Severe ROP ROP has been characterized by two phases based on clinical observations and animal models.50,63,64 The first “epidemic” of ROP occurred in the US and UK in the 1950’s. Using a model in kittens, Ashton described Phase 1 as high oxygen-induced vaso-obliteration and Phase 2 as later hypoxia-induced vasoproliferation.50 Since then, with changes in neonatal practices and technologic improvements in oxygen regulation and monitoring, the 2 –phase description has been refined.54 In Phase I ROP, mainly peripheral avascular retina occurs from a delay in physiologic retinal vascular development (PRVD)54 and, in places with insufficient resources to regulate oxygen, hyperoxia-induced vaso-attenuation.52 With delayed PRVD but continued eye growth, the peripheral avascular retina can increase in area. (Eye growth can also expand avascular retina between laser spots and is hypothesized to create an additional hypoxic stimulus for the development of recurrent intravitreal angiogenesis after laser treatment in very small immature preterm infants) (Figure 1).65
Figure 1.
As the eye grows there is increased avascular retina peripherally and thus an increase in area between laser spots. Avascular retina leads to hypoxia, which can stimulate angiogenic signaling through hypoxia inducible factors that translocate to the nucleus and bind to DNA promoter to cause transcription of multiple angiogenic factors, like VEGF, erythropoietin, angiopoietins, as examples.
In Phase II ROP, intravitreal neovascularization (IVNV) occurs from hypoxia or potentially oxidative stress or metabolic demands.51,54,66,67 VEGF is an important angiogenic factor in both PRVD and in IVNV, and inhibition of its bioactivity would be predicted to inhibit both normal (PRVD) as well as pathologic (IVNV) angiogenesis. However, evidence from OIR models suggests that inhibition of VEGF signaling to a certain degree may not adversely inhibit PRVD and still reduce IVNV (Table 6). From the mouse OIR model, initial observations suggested that the central vaso-obliterated retina induced by high oxygen was not increased after an anti-VEGF neutralizing antibody was delivered into the vitreous at a dose that was found to effectively inhibit IVNV.68 Later, studies by Geisen et al69 and Budd et al70 used quantitative methods in the rat 50/10 OIR model and found that neither a neutralizing antibody to VEGF nor a VEGF receptor 2 tyrosine kinase inhibitor increased the avascular retinal area at doses that significantly inhibited IVNV. Since a single allele knockout of VEGF or one of its splice variants or receptors is lethal in mice, studies done in an embryonic stem cell model were performed to understand the mechanisms whereby inhibition of an angiogenic factor would reduce intravitreal, but not systemic, intraretinal angiogenesis. Using a knockout of VEGFR1 (flt1) in the embryonic stem cell model permitted VEGF to trigger signaling mainly through VEGF receptor 2 (VEGFR2) by over activating the receptor. This model demonstrated that over activation of VEGFR2 disordered angiogenesis and caused a pattern of growth similar in appearance to IVNV. The pattern of growth could be rescued and physiologic vascularization restored with the addition of a transgene of VEGFR1 containing a CD31 promoter to target endothelial cells.71 This work demonstrated that not only over activated VEGFR2, but also VEGFR2 specifically in endothelial cells, was responsible for aberrant angiogenesis. Then, in the rat 50/10 OIR model, the relationship between the long axis of lectin-labeled retinal vessels and the anti-phospho-histone H3 labeled cleavage planes of dividing endothelial cells to a tortuosity index in lectin stained arteries and veins were determined following treatment with a neutralizing antibody to rat VEGF compared to a non-immune IgG control. The neutralizing antibody was found to reduce dilation and tortuosity in the OIR model.72 This study supported the development of the hypothesis that over activation of VEGFR2 disordered dividing endothelial cells allowing them to grow in a pattern similar to IVNV and that by down-regulating VEGFR2 signaling, intraretinal vascularization occurred. More recently, a lentivector gene therapy approach was developed in the rat 50/10 OIR model to reduce overexpressed VEGF in Müller cells, where the VEGF signal was found.59 A short hairpin RNA to knockdown VEGFA in Müller cells only was introduced into the model and found to reduce VEGFR2 signaling in endothelial cells59 and significantly inhibit IVNV, but not PRVD. Also, down-regulating over activated VEGFR2 in endothelial cells ordered the cleavage planes of dividing endothelial cells into a physiologic pattern promoting vessel elongation.73 Thus, experimental evidence supports the premise that inhibiting the VEGF/VEGFR2 signaling cascade not only inhibits IVNV, but also permits PRVD by restoring the normal orientation to dividing endothelial cells. This suggests that regulating VEGFR2 to physiologic signaling may be a promising approach to reduce IVNV without interfering with PRVD. But VEGF is also important in physiologic development and homeostasis of retinal neurons and glial cells,10,60 so efforts to target signaling effectors downstream of VEGF/VEGFR2 activation appear important.
Table 6.
Pros and Cons for inhibiting VEGF signaling
| Pros | Cons |
|---|---|
| Orders retinal angiogenesis | Reduces serum VEGF in infants |
| Preclinical studies showing effects in models of oxygen-induced retinopathy | Recurrent NV in preterm infants often much later than after laser |
| Survival factor in adult homeostasis and in developing vascular and neural beds | |
| Animal models show reduced body weight gain, loss of retinal capillary support, cell death in photoreceptors, reduced serum VEGF, recurrent IVNV |
CON
Evidence that VEGF inhibition can lead to harm. Most studies regarding retinal vascular development have been done in animals. Evidence concerning vascular development exists up through 22 weeks gestation in human preterm infant eyes. Based on careful immunohistochemical studies, retinal vascularization occurred through a process of vasculogenesis at about 12 weeks’ gestation in the human embryonic retina and continued through at least 22 weeks gestation allowing for inner retinal plexus vascularization through zone I.74 Vasculogenesis is the formation of blood vessels de novo from endothelial precursor cells or angioblasts.
After 22 weeks, it is less clear how the retinal vasculature extends to the ora serrata because of the difficulty in obtaining human eyes in adequate condition for study, but based on mice and other animals that vascularize their retinas after birth, vascularization is believed to occur through angiogenesis, i.e., the budding of new vessels from existing blood vessels. Both processes appear to involve VEGF.75 Besides its role in angiogenesis, VEGF is also a survival factor for other cells of the retina including neurons and is important in other organ development.12 These are important considerations when considering anti-VEGF agents in the developing preterm infant. However, many infants at risk of severe ROP also have delayed central nervous system development. Therefore, sorting out the effects of anti-VEGF treatment for ROP from prematurity and periventricular leukomalacia may be difficult.
Since the BEAT-ROP study, reports of reduced serum VEGF levels have been reported for at least 2 weeks following intravitreal anti-VEGF agents.13,14 There have also been numerous reports on associations of intravitreal anti-VEGF agents8 with prolonged, persistent avascular retina, recurrent IVNV, and even blindness from retinal detachment.16,76 Early studies reported that the risk of ROP following laser or cryotherapy generally was removed after about 45 weeks post-gestational age.77 However, recurrences after anti-VEGF were reported at 60 weeks post-gestational age.62 The causes of the recurrences remain unclear. In one study using the rat 50/10 OIR model, investigators found recurrence after higher doses of anti-VEGF agents in association with other angiogenic factors, including erythropoietin.78
In another study, PRVD in retinal flat mounts was determined in retinal plexi by two methods. The first method was the ratio of the areas of vascularized retina, determined by extent of coverage of lectin-stained retinal vessels, to total retina. In the second method, the number of pixels of fluorescence from lectin-stained vessels was determined and a ratio was created between pixels of lectin fluorescence to total retinal area. The latter measurement took into account both extent of retinal vascularization and capillary density. Two treatments were used; a broad intravitreal antibody to VEGF or a lentivector gene therapy approach to reduce overexpressed VEGF with an shRNA to VEGFA in Müller cells. Each treatment was compared to its respective control, either intravitreal IgG for the intravitreal anti-VEGF antibody, or a control shRNA to the non-mammalian gene, luciferase, for the lentivector gene therapy strategy. Compared to respective controls, each treatment reduced IVNV 4-fold and did not adversely affect PRVD determined by extent of lectin stained vascular coverage. However, the broad intravitreal anti-VEGF antibody reduced pixels of lectin fluorescence and, therefore, capillary density in retinal vascular plexi, whereas targeted VEGF knockdown did not. In addition, intravitreal anti-VEGF antibody also reduced body weight gain78 and serum VEGF levels, whereas targeted VEGF knockdown in Müller cells did not.59 These results suggest that targeted VEGF knockdown in Müller cells may be safer than broad anti-VEGF inhibition. Also, VEGF appeared to be important for already developed retinal capillaries, and these studies suggested that capillary dropout may be a possible mechanism for late recurrent IVNV reported after intravitreal anti-VEGF antibody.78
In an additional study, the question was posed whether knockdown of a VEGF splice variant might be safer that knockdown of the full VEGFA sequence. In the rat 50/10 OIR model, a gene therapy approach was used to knockdown VEGF or a splice variant in Müller cells using shRNAs to VEGFA, VEGF164 splice variant, or luciferase as a control.60 Initially, shRNAs to either VEGFA or VEGF164 significantly reduced IVNV compared to control but only the VEGF164 knockdown maintained IVNV inhibition at the later time point studied. Also, targeted Müller cell knockdown of VEGFA caused increased TUNEL+ cell death and retinal thinning of the outer nuclear layer where the photoreceptor nuclei are located. From these studies, targeted knockdown of VEGFA in Müller cells following repeated fluctuations in oxygenation appears safer than broad intravitreal anti-VEGF antibody, but may still cause photoreceptor loss. (Photoreceptors, i.e., rods and cones, are essential to visual development.) Targeting Müller cell VEGF164 may therefore be potentially safer. However, more studies are needed to determine the long-term effects of targeted knockdown of VEGF splice variants on visual and retinal function and structure. Also, it appears that measurements of body weight gain, vascular coverage or persistent avascular retina, and recurrence of IVNV are insufficient to determine safety in preterm infant retinas receiving anti-VEGF treatment since none of these was adversely affected by knockdown of either VEGFA or VEGF164 in Müller cells.
As infants become older, examination of the peripheral retina without anesthesia becomes more difficult and less accurate. A number of clinicians may preclude laser treatment of the peripheral avascular retina after anti-VEGF agents with signs of recurrence or when the infant grows large enough that the ability to perform an adequate retinal examination without anesthesia. However, the question whether persistent avascular retina should be treated is difficult to address, because all OIR models have regression of IVNV and vascularization of the avascular retina. It is possible that some human preterm retinas may be incapable of supporting retinal vasculature, but in other eyes vascularization might occur or avascular retina persist without ever causing IVNV.
Considerations Regarding Adult/Preterm Infant Size and Dose Considerations
No anti-VEGF agent has been FDA-approved for treatment of ROP. Ranibizumab (Lucentis, Genentech) or aflibercept (Eylea, Regeneron) are the FDA approved agents for adult eye diseases. The BEAT-ROP clinical trial used bevacizumab, which has not been FDA approved for any eye disease, but has been tested head-to-head with ranibizumab in a clinical trial for adult AMD and shown to be non-inferior.79 Bevacizumab is a humanized monoclonal antibody to VEGF that was tested as an anti-cancer agent but was not formally tested or formulated for use in the eye. Ranibizumab was tested for use in the eye and is the Fab fragment of a monoclonal anti-VEGF antibody. Treatment with bevacizumab is approximately 1/20th the cost of ranibizumab. The dose of either ranibizumab (0.5 mg) or bevacizumab (1.25 mg) is injected into the adult eye in a volume of 0.05 mL. Because no pharmacologic formulation is available for preterm infant eyes, the same dosing and volume have often been chosen because of the difficulty in drawing up smaller volumes accurately and the lack of knowledge as to appropriate dose in the preterm infant eye.
It is unknown what a normal VEGF level is in the blood or vitreous in a preterm infant who does not develop ROP. There also is no way to safely measure VEGF in the vitreous of a preterm infant, so the dose to neutralize VEGF cannot currently be determined for individual infants or eyes. Both ranibizumab and bevacizumab can reduce serum VEGF levels in preterm infants13,14 even though in adults, a study comparing bevacizumab, ranibizumab and pegaptanib (an aptamer to VEGF splice variant, VEGF165) found that only bevacizumab reduced serum VEGF.80 The different concentrations of serum VEGF between adults and preterm infants may in part reflect the differences in eye/blood volumes. A preterm infant’s vitreous volume is about 1 mL, whereas an adult’s is approximately 4 mLs. However, a preterm infant’s blood volume is about 120 mL at a post-gestational age of 35 to 39 weeks when severe ROP occurs, and an adult’s blood volume is usually over 5000 mLs. Therefore, even though the concentration of active VEGF is not known, there is less effect from dilution of the drug in the preterm infant’s blood volume compared to the adult’s. In addition, ROP develops often 2 or 3 months after birth. In the US, an infant that develops severe ROP is often much smaller (and blood volume less) than an infant with severe ROP in other countries where ROP occurs in larger and older infants. Therefore, the safety profile from studies that test anti-VEGF in severe ROP from these countries may not be comparable to that of the US. Anti-angiogenic treatment may need to be individualized based on the eye and infant. These are potential considerations that are rarely discussed when comparing anti-VEGF treatment outcomes or side effects in infants from developing nations or the US.
Guidelines If Considering anti-VEGF Treatment [BOX 1]
Bevacizumab is not FDA approved. However, there are more studies reported on bevacizumab than on ranibizumab, for which there is no clinical trial for ROP reported to date. However, there have been opinions as to which anti-VEGF agent is optimal. Bevacizumab causes longer-term reduction in systemic VEGF levels in adults compared to ranibizumab and, therefore, may be more damaging to the preterm infant. However, in preterms, ranibizumab also reduced serum VEGF. Ranibizumab penetrates more deeply into the eye, and there is concern this might affect the choroidal circulation, which provides oxygen to the developing retina and is believed important in the pathophysiology of ROP.81 The American Academy of Pediatrics (AAP) and the American Academy of Ophthalmology (AAO) have developed guidelines for ROP and the consideration of anti-VEGF treatment.82 Because no clinical trial has been performed for ranibizumab at the time of this writing, the recommendations are based on bevacizumab. If bevacizumab is contemplated in cases in which corneal, lenticular, or vitreous opacities preclude treatment with laser, it should only be used for stage 3+ ROP in zone I and not for zone II ROP. Also, a detailed informed consent outlining the potential risks is required. If bevacizumab is used, infants must be examined weekly until full vascularization of the retina occurs. Follow up must be performed for a longer period of time than after conventional laser treatment, because recurrent stage 3 ROP has been reported at later time points than after conventional laser (16 +/− 4.6 weeks vs. 6.2 +/− 5.7 weeks). Also, a log of infants treated and dates of treatment is recommended. Good and clear communication between the treating ophthalmologist and neonatologist and the new team is essential upon transfer or discharge.
Future
Studies are ongoing to determine pharmacologic approaches that target signaling downstream of VEGF receptors to safely and effectively inhibit pathologic angiogenesis without interfering with ongoing retinal vascular development. More clinical studies are needed to determine potential safe doses of anti-VEGF agents and dose escalation studies are being considered. In addition, studies to promote normal retinal vascular development may be considered through the use of nutrients, such as peptides83, omega-3 fatty acids84 and growth factors, including insulin-like growth factor 1 (IGF-1).85 Clinical studies are also being performed testing early use of erythropoietin on later cognitive function. There is some evidence that erythropoietin can be angiogenic in preterm infants.86 A recent clinical in preterm infants study testing darbepoietin, a form of erythropoietin, reported no increased, but also no reduced risk of severe ROP, but numbers were small.87 Further studies are warranted. Since heritability may be associated with ROP,20 studies on the association of severe ROP and genetic variants are needed. Potentially phenotype/genotype studies may identify infants at great risk of severe ROP and also help in understanding the pathophysiology of disease so as to develop new treatments.
Key Points.
Before considering anti-VEGF agents in preterm infants, more studies are needed to determine long-term effects on safety, proper doses, or even the type of anti-VEGF agent or other drug.
ROP phenotypes may vary throughout the world based on environmental factors and potentially, differences in genetic variants. These considerations are important when comparing outcomes from clinical reports after anti-VEGF therapy.
Although there is some promise with anti-VEGF treatment, there is clinical risk of poor outcome and safety concerns potentially from systemic reduction of VEGF. Better treatments are needed.
Footnotes
Disclosure: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Churchill AJ, Carter JG, Lovell HC, et al. VEGF polymorphisms are associated with neovascular age-related macular degeneration. Human Molecular Genetics. 2006;15:2955–61. doi: 10.1093/hmg/ddl238. [DOI] [PubMed] [Google Scholar]
- 2.Bird AC. Therapeutic targets in age-related macular disease. The Journal of Clinical Investigation. 2010;120:3033–41. doi: 10.1172/JCI42437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Z, Wang H, Jiang Y, Hartnett ME. VEGFA Activates Erythropoietin Receptor and Enhances VEGFR2-Mediated Pathological Angiogenesis. Am J Pathol. 2014;184:1230–9. doi: 10.1016/j.ajpath.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. New Eng J Med. 1994;331:1480–7. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 5.Nicholson B, Schachat A. A review of clinical trials of anti-VEGF agents for diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010;248:915–30. doi: 10.1007/s00417-010-1315-z. [DOI] [PubMed] [Google Scholar]
- 6.Cooke RWI, Drury JA, Mountford R, Clark D. Genetic Polymorphisms and Retinopathy of Prematurity. Investigative Ophthalmology Visual Science. 2004;45:1712–5. doi: 10.1167/iovs.03-1303. [DOI] [PubMed] [Google Scholar]
- 7.Mititelu M, Chaudhary KM, Lieberman RM. An Evidence-Based Meta-analysis of Vascular Endothelial Growth Factor Inhibition in Pediatric Retinal Diseases: Part 1. Retinopathy of Prematurity. Journal of pediatric ophthalmology and strabismus. 2012:1–9. doi: 10.3928/01913913-20120821-03. [DOI] [PubMed] [Google Scholar]
- 8.Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of Intravitreal Bevacizumab for Stage 3+ Retinopathy of Prematurity. New England Journal of Medicine. 2011;364:603–15. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D’Amore PA. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proceedings of the National Academy of Sciences. 2009;106:18751–6. doi: 10.1073/pnas.0905010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saint-Geniez M, Maharaj AS, Walshe TE, et al. Endogenous VEGF is required for visual function: evidence for a survival role on muller cells and photoreceptors. PLoS One. 2008;3:e3554. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K. Seven-Year Outcomes in Ranibizumab-Treated Patients in ANCHOR, MARINA, and HORIZON: A Multicenter Cohort Study (SEVEN-UP) Ophthalmology. 2013 doi: 10.1016/j.ophtha.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 12.Nishijima K, Ng YS, Zhong L, et al. Vascular Endothelial Growth Factor-A Is a Survival Factor for Retinal Neurons and a Critical Neuroprotectant during the Adaptive Response to Ischemic Injury. American Journal of Pathology. 2007;171:53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato T, Wada K, Arahori H, et al. Serum Concentrations of Bevacizumab (Avastin) and Vascular Endothelial Growth Factor in Infants With Retinopathy of Prematurity. American Journal of Ophthalmology. 2012;153:327–33. doi: 10.1016/j.ajo.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Hoerster R, Muether P, Dahlke C, et al. Serum concentrations of vascular endothelial growth factor in an infant treated with ranibizumab for retinopathy of prematurity. Acta Ophthalmol. 2013;91:e74–5. doi: 10.1111/j.1755-3768.2012.02469.x. [DOI] [PubMed] [Google Scholar]
- 15.Hu J. REactivation of retinopathy of prematurity after bevacizumab injection. Archives of Opthalmology. 2012;130:1000–6. doi: 10.1001/archophthalmol.2012.592. [DOI] [PubMed] [Google Scholar]
- 16.Patel RD, Blair MP, Shapiro MJ, Lichtenstein SJ. Significant treatment failure with intravitreous bevacizumab for retinopathy of prematurity. Arch Ophthalmol. 2012;130:801–2. doi: 10.1001/archophthalmol.2011.1802. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert C. Retinopathy of prematurity: A global perspective of the epidemics, population of babies at risk and implications for control. Early Human Development. 2008;84:77–82. doi: 10.1016/j.earlhumdev.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Kong L, Fry M, Al-Samarraie M, Gilbert C, Steinkuller PG. An update on progress and the changing epidemiology of causes of childhood blindness worldwide. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus / American Association for Pediatric Ophthalmology and Strabismus. 2012;16:501–7. doi: 10.1016/j.jaapos.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Kemper AR, Freedman SF, Wallace DK. Retinopathy of prematurity care: patterns of care and workforce analysis. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus / American Association for Pediatric Ophthalmology and Strabismus. 2008;12:344–8. doi: 10.1016/j.jaapos.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bizzarro MJ, Hussain N, Jonsson B, et al. Genetic susceptibility to retinopathy of prematurity. Pediatrics. 2006;118:1858–63. doi: 10.1542/peds.2006-1088. [DOI] [PubMed] [Google Scholar]
- 21.Patz A. Studies on retinal neovascularization. Friedenwald Lecture Investigative Ophthalmology & Visual Science. 1980;19:1133–8. [PubMed] [Google Scholar]
- 22.Patz A. Studies on retinal neovascularisation. Investigative Ophthalmology & Visual Science. 1980;19:1133–8. [PubMed] [Google Scholar]
- 23.Ashton N, Cook C. Direct observation of the effect of oxygen on developing vessels: preliminary report. Br J Ophthalmol. 1954;38:433–40. doi: 10.1136/bjo.38.7.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashton N. Editorial: Retrolental fibroplasia now retinopathy of prematurity. Br J Ophthalmol. 1984;68:689. [Google Scholar]
- 25.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–5. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 26.Group TS-RMS. Supplemental Therapeutic Oxygen for Prethreshold Retinopathy of Prematurity (STOP-ROP), a randomized, controlled trial. I: primary outcomes. Pediatrics. 2000;105:295–310. doi: 10.1542/peds.105.2.295. [DOI] [PubMed] [Google Scholar]
- 27.Gaynon MW. RETHINKING STOP-ROP: IS IT WORTHWHILE TRYING TO MODULATE EXCESSIVE VEGF LEVELS IN PRETHRESHOLD ROP EYES BY SYSTEMIC INTERVENTION?: A Review of the Role of Oxygen, Light Adaptation State, and Anemia in Prethreshold ROP. Retina. 2006:26. doi: 10.1097/01.iae.0000244292.86627.1e. [DOI] [PubMed] [Google Scholar]
- 28.Early CPAP versus Surfactant in Extremely Preterm Infants. New England Journal of Medicine. 2010;362:1970–9. doi: 10.1056/NEJMoa0911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stenson BJ, Tarnow-Mordi WO, Darlow BA, et al. Oxygen saturation and outcomes in preterm infants. N Engl J Med. 2013;368:2094–104. doi: 10.1056/NEJMoa1302298. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt B, Whyte RK, Asztalos EV, et al. Effects of targeting higher vs lower arterial oxygen saturations on death or disability in extremely preterm infants: a randomized clinical trial. JAMA. 2013;309:2111–20. doi: 10.1001/jama.2013.5555. [DOI] [PubMed] [Google Scholar]
- 31.Hartnett ME, Lane RH. Effects of oxygen on the development and severity of retinopathy of prematurity. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus / American Association for Pediatric Ophthalmology and Strabismus. 2013;17:229–34. doi: 10.1016/j.jaapos.2012.12.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt B, Whyte RK, Asztalos EV, et al. Effects of targeting higher vs lower arterial oxygen saturations on death or disability in extremely preterm infants: a randomized clinical trial. JAMA. 2013;309:2111–20. doi: 10.1001/jama.2013.5555. [DOI] [PubMed] [Google Scholar]
- 33.Owen L, Hartnett ME. Current Concepts of Oxygen Management and ROP. Journal of Ophthalmic & Vision Research. In Press. [PMC free article] [PubMed] [Google Scholar]
- 34.York JR, Landers S, Kirby RS, Arbogast PG, Penn JS. Arterial oxygen fluctuation and retinopathy of prematurity in very-low-birth-weight infants. Journal of Perinatology. 2004;24:82–7. doi: 10.1038/sj.jp.7211040. [DOI] [PubMed] [Google Scholar]
- 35.Hauspurg AK, Allred EN, Vanderveen DK, et al. Blood Gases and Retinopathy of Prematurity: The ELGAN Study. Neonatology. 2011;99:104–11. doi: 10.1159/000308454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saito Y, Geisen P, Uppal A, Hartnett ME. Inhibition of NAD(P)H oxidase reduces apoptosis and avascular retina in an animal model of retinopathy of prematurity. Molecular Vision. 2007;13:840–53. [PMC free article] [PubMed] [Google Scholar]
- 37.Budd SJ, Thompson H, Hartnett ME. Association of Retinal Vascular Endothelial Growth Factor With Avascular Retina in a Rat Model of Retinopathy of Prematurity. Archives of Ophthalmology. 2010;128:1014–21. doi: 10.1001/archophthalmol.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hellstrom A, Hard AL, Engstrom E, et al. Early Weight Gain Predicts Retinopathy in Preterm Infants: New, Simple, Efficient Approach to Screening. Pediatrics. 2009;123:e638–e45. doi: 10.1542/peds.2008-2697. [DOI] [PubMed] [Google Scholar]
- 39.Penn JS. Oxygen-induced retinopathy in the rat: possible contribution of peroxidation reactions. Documenta Ophthalmologica. 1990;74:179–86. doi: 10.1007/BF02482607. [DOI] [PubMed] [Google Scholar]
- 40.Phelps DL. Vitamin E and retinopathy of prematurity: The clinical investigator’s perspective on antioxidant therapy: Side effects and balancing risks and benefits. Birth Defects. 1988;24:209–18. [PubMed] [Google Scholar]
- 41.Raju TNK, Langenberg P, Bhutani V, Quinn GE. Vitamin E prophylaxis to reduce retinopathy of prematurity: A reappraisal of published trials. Journal of Pediatrics. 1997;131:844–50. doi: 10.1016/s0022-3476(97)70031-3. [DOI] [PubMed] [Google Scholar]
- 42.Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Archives of Ophthalmology. 2003;121:1684–94. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 43.Group CfRoPC. Multicenter trial of cryotherapy for retinopathy of prematurity: Snellen visual acuity and structural outcome at 51/2 years after randomization. Archives of Ophthalmology. 1996;114:417–24. doi: 10.1001/archopht.1996.01100130413008. [DOI] [PubMed] [Google Scholar]
- 44.Hartnett ME. Features associated with surgical outcome in patients with stages 4 and 5 retinopathy of prematurity. Retina. 2003;23:322–9. doi: 10.1097/00006982-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Wong RK, Ventura CV, Espiritu MJ, et al. Training fellows for retinopathy of prematurity care: a Web-based survey. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus / American Association for Pediatric Ophthalmology and Strabismus. 2012;16:177–81. doi: 10.1016/j.jaapos.2011.12.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ells AL, Holmes JM, Astle WF, et al. Telemedicine approach to screening for severe retinopathy of prematurity: a pilot study. Ophthalmology. 2003;110:2113–7. doi: 10.1016/S0161-6420(03)00831-5. [DOI] [PubMed] [Google Scholar]
- 47.Ophthalmology AAOPSo, OPHTHALMOLOGY AAO OPHTHALMOLOGY AAFP STRABISMUS ORTHOPTISTS AAOC. Screening Examination of Premature Infants for Retinopathy of Prematurity. Pediatrics. 2013;131:189–95. doi: 10.1542/peds.2012-2996. [DOI] [PubMed] [Google Scholar]
- 48.DKC. Retinopathy of prematurity: involution, factors predisposing to retinal detachment, and expected utility of preemptive surgical reintervention. Trans Am Ophthalmol Soc. 2005;103:281–312. [PMC free article] [PubMed] [Google Scholar]
- 49.Hartnett ME, McColm JR. Retinal features predictive of progressive stage 4 retinopathy of prematurity. Retina. 2004;24:237–41. doi: 10.1097/00006982-200404000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ashton N, Ward B, Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. British Journal of Ophthalmology. 1954;38:397–430. doi: 10.1136/bjo.38.7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith LEH, Wesolowski E, McLellan A, et al. Oxygen induced retinopathy in the mouse. Investigative Ophthalmology & Visual Science. 1994;35:101–11. [PubMed] [Google Scholar]
- 52.Shah PK, Narendran V, Kalpana N. Aggressive posterior retinopathy of prematurity in large preterm babies in South India. Archives of disease in childhood Fetal and neonatal edition. 2012;97:F371–5. doi: 10.1136/fetalneonatal-2011-301121. [DOI] [PubMed] [Google Scholar]
- 53.Patz A. Oxygen studies in retrolental fibroplasia. American Journal of Ophthalmology. 1954;38:291–308. doi: 10.1016/0002-9394(54)90845-4. [DOI] [PubMed] [Google Scholar]
- 54.Hartnett ME, Penn JS. Mechanisms and Management of Retinopathy of Prematurity. New England Journal of Medicine. 2012;367:2515–26. doi: 10.1056/NEJMra1208129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Penn JS, Henry MM, Tolman BL. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr Res. 1994;36:724–31. doi: 10.1203/00006450-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Berkowitz BA, Zhang W. Significant reduction of the panretinal oxygenation response after 28% supplemental oxygen recovery in experimental ROP. Investigative Ophthalmology & Visual Science. 2000;41:1925–31. [PubMed] [Google Scholar]
- 57.Holmes JM, Duffner LA. The effect of postnatal growth retardation on abnormal neovascularization in the oxygen exposed neonatal rat. Curr Eye Res. 1996;15:403–9. doi: 10.3109/02713689608995831. [DOI] [PubMed] [Google Scholar]
- 58.Cunningham S, Fleck BW, Elton RA, Mclntosh N. Transcutaneous oxygen levels in retinopathy of prematurity. Lancet. 1995;346:1464–5. doi: 10.1016/s0140-6736(95)92475-2. [DOI] [PubMed] [Google Scholar]
- 59.Wang H, Smith GW, Yang Z, et al. Short Hairpin RNA-Mediated Knockdown of VEGFA in Muller Cells Reduces Intravitreal Neovascularization in a Rat Model of Retinopathy of Prematurity. Am J Pathol. 2013 doi: 10.1016/j.ajpath.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang Y, Wang H, Culp D, et al. Targeting Muller cell-derived VEGF164 to reduce intravitreal neovascularization in the rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2014;55:824–31. doi: 10.1167/iovs.13-13755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mintz-Hittner HA. Treatment of retinopathy of prematurity with vascular endothelial growth factor inhibitors. Early Human Development. 2012;88:937–41. doi: 10.1016/j.earlhumdev.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 62.Hu J, Blair MP, Shapiro MJ, Lichtenstein SJ, Galasso JM, Kapur R. Reactivation of retinopathy of prematurity after bevacizumab injection. Arch Ophthalmol. 2012;130:1000–6. doi: 10.1001/archophthalmol.2012.592. [DOI] [PubMed] [Google Scholar]
- 63.Smith LE. Through the eyes of a child: understanding retinopathy through ROP the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2008;49:5177–82. doi: 10.1167/iovs.08-2584. [DOI] [PubMed] [Google Scholar]
- 64.An International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity Revisited. Archives of Ophthalmology. 2005;123:991–9. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 65.Hartnett . Retinopathy of prematurity: a template for studying retinal vascular disease. In: Chalupa JSWaLM., editor. The New Visual Neurosciences. Cambridge MA: MIT Press; 2014. pp. 1483–502. [Google Scholar]
- 66.Byfield G, Budd S, Hartnett ME. The role of supplemental oxygen and JAK/STAT signaling in intravitreous neovascularization in a ROP rat model. Invest Ophthalmol Vis Sci. 2009;50:3360–5. doi: 10.1167/iovs.08-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saito Y, Uppal A, Byfield G, Budd S, Hartnett ME. Activated NAD(P)H Oxidase from Supplemental Oxygen Induces Neovascularization Independent of VEGF in Retinopathy of Prematurity Model. Investigative Ophthalmology Visual Science. 2008;49:1591–8. doi: 10.1167/iovs.07-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sone H, Kawakami Y, Kumagai AK, et al. Effects of intraocular or systemic administration of neutralizing antibody against vascular endothelial growth factor on the murine experimental model of retinopathy. Life Sciences. 1999;65:2573–80. doi: 10.1016/s0024-3205(99)00526-3. [DOI] [PubMed] [Google Scholar]
- 69.Geisen P, Peterson L, Martiniuk D, Uppal A, Saito Y, Hartnett M. Neutralizing antibody to VEGF reduces intravitreous neovascularization and does not interfere with vascularization of avascular retina in an ROP model. Molecular Vision. 2008;14:345–57. [PMC free article] [PubMed] [Google Scholar]
- 70.Budd S, Byfield G, Martiniuk D, Geisen P, Hartnett ME. Reduction in endothelial tip cell filopodia corresponds to reduced intravitreous but not intraretinal vascularization in a model of ROP. Experimental Eye Research. 2009;89:718–27. doi: 10.1016/j.exer.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeng G, Taylor SM, McColm JR, et al. Orientation of endothelial cell division is regulated by VEGF signaling during blood vessel formation. Blood. 2007;109:1345–52. doi: 10.1182/blood-2006-07-037952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hartnett ME, Martiniuk D, Byfield G, Geisen P, Zeng G, Bautch VL. Neutralizing VEGF decreases tortuosity and alters endothelial cell division orientation in arterioles and veins in a rat model of ROP: relevance to plus disease. Invest Ophthalmol Vis Sci. 2008;49:3107–14. doi: 10.1167/iovs.08-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang H, Yang Z, Jiang Y, et al. Quantitative analyses of retinal vascular area and density after different methods to reduce VEGF in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2014;55:737–44. doi: 10.1167/iovs.13-13429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hasegawa T, McLeod DS, Prow T, Merges C, Grebe R, Lutty GA. Vascular Precursors in Developing Human Retina. Investigative Ophthalmology & Visual Science. 2008;49:2178–92. doi: 10.1167/iovs.07-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chan-Ling T, Gock B, Stone J. The effect of oxygen on vasoformative cell division: Evidence that ‘physiological hypoxia’ is the stimulus for normal retinal vasculogenesis. Investigative Ophthalmology & Visual Science. 1995;36:1201–14. [PubMed] [Google Scholar]
- 76.Jalali S, Balakrishnan D, Zeynalova Z, Padhi TR, Rani PK. The Indian Twin Cities Retinopathy of Prematurity Screening database Report number 5. Archives of Disease in Childhood - Fetal and Neonatal Edition. 2012. Serious adverse events and visual outcomes of rescue therapy using adjunct bevacizumab to laser and surgery for retinopathy of prematurity. [DOI] [PubMed] [Google Scholar]
- 77.Reynolds JD, Dobson V, Quinn GE, et al. Evidence-based screening criteria for retinopathy of prematurity: natural history data from the CRYO-ROP and LIGHT-ROP studies. Arch Ophthalmol. 2002;120:1470–6. doi: 10.1001/archopht.120.11.1470. [DOI] [PubMed] [Google Scholar]
- 78.McCloskey M, Wang H, Jiang Y, Smith GW, Strange J, Hartnett ME. Anti-VEGF Antibody Leads to Later Atypical Intravitreous Neovascularization and Activation of Angiogenic Pathways in a Rat Model of Retinopathy of Prematurity. Invest Ophthalmol Vis Sci. 2013;54:2020–6. doi: 10.1167/iovs.13-11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zehetner C, Kirchmair R, Huber S, Kralinger MT, Kieselbach GF. Plasma levels of vascular endothelial growth factor before and after intravitreal injection of bevacizumab, ranibizumab and pegaptanib in patients with age-related macular degeneration, and in patients with diabetic macular oedema. Br J Ophthalmol. 2013;97:454–9. doi: 10.1136/bjophthalmol-2012-302451. [DOI] [PubMed] [Google Scholar]
- 81.Chemtob S, Hardy P, Abran D, et al. Peroxide-cyclooxygenase interactions in postasphyxial changes in retinal and choroidal hemodynamics. Journal of Applied Physiology. 1995;78:2039–46. doi: 10.1152/jappl.1995.78.6.2039. [DOI] [PubMed] [Google Scholar]
- 82.Fierson WM. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131:189–95. doi: 10.1542/peds.2012-2996. [DOI] [PubMed] [Google Scholar]
- 83.Neu J, Afzal A, Pan H, Gallego E, et al. The Dipeptide Arg-Gln Inhibits Retinal Neovascularization in the Mouse Model of Oxygen-Induced Retinopathy. Investigative Ophthalmology Visual Science. 2006;47:3151–5. doi: 10.1167/iovs.05-1473. [DOI] [PubMed] [Google Scholar]
- 84.Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of [omega]-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–73. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hellström A, Smith LEH, Dammann O. Retinopathy of prematurity. The Lancet. 382:1445–57. doi: 10.1016/S0140-6736(13)60178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brown MS, Baron AE, France EK, Hamman RF. Association between higher cumulative doses of recombinant erythropoietin and risk for retinopathy of prematurity. Journal of American Association for Pediatric Ophthalmology and Strabismus. 2006;10:143–9. doi: 10.1016/j.jaapos.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 87.Ohls RK, Christensen RD, Kamath-Rayne BD, et al. A Randomized, Masked, Placebo-Controlled Study of Darbepoetin Alfa in Preterm Infants. Pediatrics. 2013 doi: 10.1542/peds.2013-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]