Abstract
Objective
Long-term follow-up studies documenting maintenance of treatment effects are few in adolescent anorexia nervosa (AN). This exploratory study reports relapse from full remission and attainment of remission during a four-year open follow-up period using a convenience sample of a subgroup of 65% (n=79) from an original cohort of 121 participants who completed a randomized clinical trial comparing family based therapy (FBT) and adolescent focused individual therapy (AFT).
Method
Follow-up assessments were completed up to four years posttreatment (average of 3.26 years). Available participants completed the Eating Disorder Examination as well as self-report measures of self-esteem and depression at two to four years posttreatment.
Results
Two participants (6.1%) relapsed (FBT: n = 1, 4.5%; AFT: n = 1, 9.1%), on average 1.98 years (SD = 0.14) after remission was achieved at one-year follow-up. Ten new participants (22.7%) achieved remission (FBT: n = 1, 5.9%; AFT: n = 9, 33.3%). Mean time to remission for this group was 2.01 years (SD = 0.82) from one-year follow-up. There were no differences based on treatment group assignment in either relapse from full remission or new remission during long-term follow-up. Other psychopathology was stable over time.
Conclusion
There were few changes in the clinical presentation of participants who were assessed at long-term follow-up. These data suggest that outcomes are generally stable posttreatment regardless of treatment type once remission is achieved.
Clinical trial registration information—Effectiveness of Family-Based Versus Individual Psychotherapy in Treating Adolescents With Anorexia Nervosa; http://www.clinicaltrials.gov/; NCT00149786.
Keywords: anorexia nervosa, adolescence, long-term follow-up, family-based treatment, adolescentfocused treatment
Introduction
Despite high rates of morbidity and mortality for adolescents with anorexia nervosa(AN),1 relatively few randomized controlled trials (RCTs) for this patient population have been conducted.2-8 Even fewer studies have examined the long-term maintenance of treatment effects in RCTs.9-11
Using a relatively low standard for remission (weight >85% of expected body weight [EBW]), colleagues at the Maudsley Hospital in London demonstratedremission rate for family therapy was stable over time at 90% for both one-year andfive-year follow-up assessment.9 In contrast, theyshowed that the remission rate for individual therapy significantly improved from 18% atone-year follow-up to 60% at five-year follow-up. Nonetheless, the remission rate in family therapy remained superior over that of individual therapy. Another four-year follow-up study that compared two doses of family therapy found no differences in remission rates by dose. Using various standard definitions of remission, rates ranged from 60-90%.11 A study that compared conjoint family therapy to separated family therapy found no differences in remission at four-year follow-up (78% and 90% respectively, using Morgan Russell Categories).10 Taken together these studies suggest that longer-term outcome in adolescents who were successfully treated in family therapy are likely to be maintained at follow-up regardless of the definition of remission utilized.12-13
The current study examined relapse from remission, with the latter defined as >95% EBW for age, height, and gender, and a global Eating Disorder Examination (EDE) score within one SD of the community mean of 1.54 for adolescents. The sample was drawn from the original cohort of 121 participants in an RCT that compared two manual-based treatments, family-based treatment (FBT) and adolescent focused therapy (AFT).6 Based on the findings from previous studies showing stability in outcomes over time, we predicted that those who were remitted at one-year follow-up were likely to remain so at longer-term follow-up regardless of treatment type received.
Method
Participants and Procedures
The design of the original two-site RCT (The University of Chicago and Stanford University) was described in detail in our main report.6 Briefly, 121 adolescents with DSM-IV AN,14 except for the amenorrhea criterion, were randomized to either FBT or AFT. Participants were enrolled if they were 12-18 years of age, lived with parents or legal guardians, and were medically stable for outpatient treatment.15 Written informed consent was obtained after a study coordinator provided a description of the study to the participant and their parents. The institutional review boards of the two clinical sites provided approval for the study. The participants in the current study are a convenience sample who agreed to provide follow-up at two, three, and four years posttreatment after the original study was completed. Participant outcomes at the end of treatment and one-year follow-up have been previously reported.6
Treatments
The two manual-based treatments utilized in the original study are described in detail elsewhere: AFT16 and FBT.17 Briefly, AFT is an individual therapy and focuses on amending eating disorder symptoms in the context of examining common themes in adolescent development. FBT, on the other hand, is familyfocused and leverages parental support of eating-related behaviors in their child. Participants were seen as outpatients for 24 contact hours over a one-year period.
Assessments
For the present study, independent assessors not involved in treatment delivery conducted assessments at two, three, and four years posttreatment. Utilizing the last available data point from two- to four-year follow-up, mean time to long-term follow-up from the end of treatment for the full sample was 3.26 years (SD = 1.29; range: 0.87, 5.40). Mean follow-up time was comparable between FBT (M = 3.30, SD = 1.33) and AFT (M = 3.21, SD = 1.26). Variables examined at these various time points included: 1) weight (percentile body mass index [BMI] using Centers for Disease Control and Prevention [CDC] norms for age and gender);18 2) eating disorder psychopathology (EDE-Global score);19 3) depressive symptoms (Beck Depression Inventory, or BDI);20 4) self-esteem (Rosenberg Self-Esteem Scale, or RSES);21 5) obsessivecompulsive features of eating symptoms and behaviors (YBC-ED);22 and 6) use of psychiatric medications.
Our main outcome of interest is relapse (defined as meeting study entry weight criteria of 87% of expected mean BMI for age, height, and gender) from full remission (defined as weight greater than or equal to 95% of expected BMI for age, height, and gender and an EDE-Global score within 1 SD of community means (i.e., 1.54), and new remissions using these same criteria. Secondary outcomes of interest are changes in eating pathology, depressive symptoms, self-esteem, and obsessive-compulsive symptoms. The prevalence of other eating disorder diagnoses, mood disorders, and anxiety disorders were also examined at long-term follow-up.
Statistical Analyses
SPSS Version 19.0 was used for all analyses. Independent t- and chi-square tests were used to compare individuals with and without missing data. Evaluating time to relapse and remissionfrom one-year follow-up was performed using survival analyses,23-24 with time calculated as the difference between the assessment date at one-year follow-up and the assessment date at which the event (i.e., relapse or remission) occurred. For the survival curve of time to relapse (27.3%, n=33), participants with missing data (23.1%, n=28) and those who were not remitted (49.6%, n=60) at one-year follow-up were excluded. Participants with no follow-up data and those who remained recovered at all measured follow-up time points were treated as ″censored″ observations, indicating that relapse did not occur prior to termination of the measurement period. For the survival curve of time to remission (49.6%, n=60), participants with missing data (23.1%, n=28) and those who had remitted (27.3%, n=33) at one-year follow-up were excluded. Participants with no follow-up data and those who remained nonremitted at all measured follow-up time points were treated as ″censored″ observations, indicating that remission did not occur prior to termination of the measurement period.
Indicator variables, coded as -.5 and +.5 were created to represent treatment group and treatment center. Baseline (i.e., age, duration of illness, psychiatric comorbidity, psychotropic medication use, prior hospitalization, %EBW, EDE global score, YBC-ED score, and BDI score) and end of treatment (i.e., EDE global score, YBC-ED score, or BDI score, %EBW) predictors were examined in separate survival analyses, controlling for the effects of treatment group and center. A Cox proportional hazard model was then fitted using a log logistic distribution.
Results
Comparison of follow-up sample and non-follow-up sample
One-year follow-up data were available for 77% of the original cohort (93/121). For the present longer-term follow-up study, data were available for 65.3% (79/121) of the original cohort, i.e., at least one data point two to four years after the end of treatment. Of these, 33 (41.8%) had only one assessment— 6 (18.2%) at two years, 10 (30.3%) at three years, and 17 (51.5%) at four years. The sample sizes for follow-ups at two-, three-, and four-year follow-ups were 37, 50, and 52. Compared to adolescents who did not complete any long-term follow-up assessments (i.e., no data available between two- and four-year follow-up), those who completed at least one follow-up assessment between two and four years did not differ with respect to treatment (FBT versus AFT), site, baseline demographic characteristics (i.e., age, gender, racial/ethnic minority, family income, parent education, and intact family), or baseline clinical characteristics (i.e., AN subtype, duration of illness, prior hospitalization, psychiatric comorbidity, and psychotropic medication use). In addition, they did not differ on %EBW or global EDE score at baseline.
Time to relapse after remission at one-year follow-up
The clinical characteristics of the sample available for analysis at the long-term outcome assessment are summarized in Table 1. Taking the last completed assessment from two to four years posttreatment, mean %EBW at long-term follow-up was 94.11 (SD = 11.11). Eating disorder symptoms (assessed with global EDE score) at long-term follow-up were minimal (M = 1.00, SD = 1.25). Approximately one third of participants achieved full remission at long-term follow-up (n = 25, 31.6%), while more than a quarter was still symptomatic (i.e., %EBW ≤87) at long-term follow-up (n = 21, 26.6%). Using DSM-IV diagnoses, two (2.5%) participants met criteria for bulimia nervosa (FBT: n = 1; AFT: n = 1), and five (6.3%) met criteria for eating disorder not otherwise specified (FBT: n = 1; AFT: n = 4). Eight (10.1%) met criteria for a mood disorder (FBT: n = 3; AFT: n = 5), and 12 (15.2%) met criteria for an anxiety disorder (FBT: n = 6; AFT: n = 6).
Table 1. Participant Characteristics at One-Year and Long-Term Follow-Up, Based on Observed Means and Frequencies.
| One-Year Follow-Upa | Long-Term Follow-Upb | ||||||
|---|---|---|---|---|---|---|---|
| Family-Based Treatment (n=44) |
Adolescent- Focused Treatment (n=49) |
Full Sample (N=93) |
Family-Based Treatment (n=36) |
Adolescent- Focused Treatment (n=43) |
Full Sample (n=79) |
||
| Percent (%), or Mean (SD) | Percent (%), or Mean (SD) | Test statistic | |||||
| Age | 16.59 (1.62) | 17.36 (1.43) | 17.00 (1.56) | 19.54 (1.82) | 19.83 (1.36) | 19.70 (1.59) | t = -0.80, p = .43 |
| Full Remission (%) | 50.0% | 22.4% | 35.5% | 27.80% | 34.90% | 31.60% | φ = 0.08, p = .50 |
| %EBW | 94.23 (9.49) | 93.06 (13.72) | 93.62 (11.84) | 94.43 (12.10) | 93.84 (10.34) | 94.11 (11.11) | t = 0.24, p = .82 |
| EDE | 0.68 (1.13) | 1.03 (1.06) | 0.87 (1.10) | 0.82 (1.06) | 1.16 (1.38) | 1.00 (1.25) | t = -1.21, p = .24 |
| YBC-ED | 3.25 (6.57) | 4.69 (7.31) | 4.01 (6.97) | 3.31 (5.88) | 5.54 (7.40) | 4.51 (6.79) | t = -1.43, p = .15 |
| BDI | 4.38 (7.35) | 8.70 (9.06) | 6.64 (8.52) | 6.11 (8.00) | 7.75 (7.59) | 6.99 (7.77) | t = -0.91, p = .37 |
| RSES | 19.52 (7.31) | 21.87 (6.87) | 20.74 (7.14) | 29.11 (7.56) | 28.70 (7.11) | 28.89 (7.28) | t = 0.24, p = .81 |
| Medication | 22.7% | 37.5% | 30.4% | 41.70% | 34.90% | 38.00% | φ = -0.07, p = .54 |
Note: BDI = Beck Depression Inventory; %EBW = Percent Expected Body Weight; EDE = Eating Disorder Examination; RSES = Rosenberg Self-Esteem Scale; YBC-ED = Yale-Brown-Cornell Eating Disorder Scale.
One-year follow-up data reported in Lock et al. (2010).6
Comparison between treatment groups at long-term follow-up.
For those who had achieved full remission at one-year posttreatment (n=33; n=22 in FBT, n=11 in AFT), a survival curve examined time to relapse. Seven participants were dropped from the survival curve (21.2%), including six participants with no follow-up data and one participant with follow-up data prior to the earliest event in the stratum. The remaining 26 participants were assessed on average 2.68 years (SD = 0.60) after one-year follow-up. Of these, 24 (72.7%) were “censored,” indicating that relapse did not occur prior to termination of the measurement period, while two (6.1%) relapsed (FBT: n = 1, 4.5%; AFT: n = 1, 9.1%). For those who were remitted at one-year follow-up, mean time to relapse was 1.98 years (SD = 0.14) after one-year follow-up.
The treatment group by center interaction was not included in the model due to non-convergence; therefore, only the main effects for treatment group and center were included in the main survival analysis. There was no significant main effect of treatment (Wald chi-square=0.320, df=1, p=.57, OR = 0.443) or center (Wald chi-square=0.001, df=1, p=.98, OR = 0.967; see Figure 1). Controlling for treatment, there were no significant effects (main effects or interactions with treatment) for age, duration of illness, initial and end of treatment %EBW, initial EDE global score, initial YBC-ED score, and initial BDI score (p's > >.10). The impact of EDE global score, YBC-ED score, and BDI score at EOT on time to relapse could not be examined due to model non-convergence. Additionally, the impact of psychiatric comorbidity, psychotropic medication use, and prior hospitalization could not be examined due to model non-convergence.
Figure 1.

Time to relapse by treatment from one-year follow-up (n=26; treatment: Wald chisquare=0.320, df=1, p=.57, OR = 0.443). Note: AFT = adolescent focused therapy; FBT = family-based treatment.
Time to remission from one-year follow-up
For those who had not achieved full remission at one-year posttreatment (n=60; n=22 in FBT, n=38 in AFT), a survival curve examined time to remission. Sixteen participants were not included in the survival curve (26.7%), including 15 participants with no follow-up data and one participant with follow-up data prior to the earliest event in the stratum. The remaining 44 participants were assessed on average 2.49 years (SD = 0.75) after one-year follow-up. Of these, 35 (79.5%) were “censored,” indicating that full remission did not occur prior to termination of the measurement period, while ten new participants (22.7%) achieved first time remission (FBT: n = 1, 5.9%; AFT: n = 9, 33.3%). For those who were not remitted at one-year follow-up, mean time to remission was 2.01 years (SD = 0.82) after one-year follow-up.
The treatment group by center interaction was not included in the model due to non-convergence; therefore, only the main effects for treatment group and center were included in the main survival analysis. There were no significant main effects for treatment (Wald chi-square=3.047, df=1, OR = 0.156, p=.081) or center (Wald chi-square=0.282, df=1, OR = 1.435, p=.60; see Figure 2). Controlling for treatment, there were no significant effects (main effects or interactions with treatment) for duration of illness, initial %EBW, initial EDE global score, initial YBC-ED score, or initial BDI score (p's >.10). There were also no significant effects (main effects or interactions with treatment) for EDE global score, YBC-ED score, or BDI score at the end of treatment (p's >.10). The impact of age, psychiatric comorbidity, psychotropic medication use, prior hospitalization, and %EBW at end of treatment could not be examined due to model non-convergence. Figure 2 depicts time to remission, where the vertical axis represents the survival function, or the proportion of adolescents who were not in remission.
Figure 2.
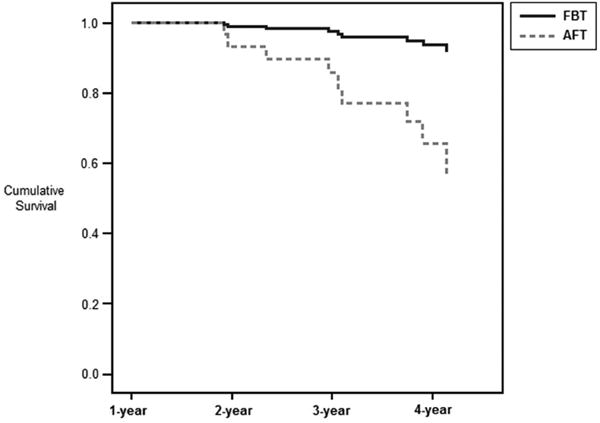
Time to remission by treatment from one-year follow-up (n=44; treatment: Wald chi-square=3.047, df=1, OR = 0.156, p=.081). Note: AFT = adolescent focused therapy; FBT = family-based treatment.
Discussion
This exploratory follow-up study examined relapse and remission rates in adolescents with AN using a convenience sub-sample available for assessment from a sample treated in an RCT comparing FBT to AFT. We predicted that there would be stability in terms of remission status from the initial one-year follow-up through the follow-up period, regardless of treatment group.
Indeed, only two participants who were remitted at the one year follow-up point relapsed (one in each treatment group). This finding supports our main hypothesis about the stability of remission, once achieved, regardless of treatment assignment. Furthermore, an additional one-quarter of participants that were not remitted at one year follow-up achieved remission during longer term follow-up. Findings from the present study also indicate that approximately one third of participants were remitted at long-term follow-up, which is around the same proportion that was remitted at one-year follow-up across treatment groups. Eating disorder symptom severity and %EBW also remained relatively stable over time. Moreover, there were low rates of bulimia nervosa, other eating disorders, and mood or anxiety disorders at follow-up. Taken together, treatment outcomes were relatively stable over time in the subsample used in this study.
Several strengths as well as limitations to this study are worth mentioning. Our initial study was designed with adequate power to detect a difference on our main outcome variable (i.e., remission). Participants were randomized to one of two manual-based treatments, which supported the consistent and accurate application of these therapies. However, our follow-up study suffers at least three important limitations. We were able to retain only 65% of the original cohort for any follow-up assessments beyond one year, as this was an open and unplanned follow-up. Consequently, long-term follow-up participants represent a convenience sample rather than a random sample, and generalizability to the full sample is not appropriate. Of those who did complete a long-term follow-up assessment, approximately two-fifths participated in only one follow-up interview, which precludes a more detailed assessment of variability in psychiatric status over time. As a result, these data were limited in their ability to capture events (e.g., new remissions or relapses) and record the timing of events with relative accuracy. Furthermore, these assessments only captured remission and relapse at specific points in time, rather than during more comprehensive windows of time. In addition, we also relied on phone interviews in some instances, which may have led to imprecise information, especially in terms of weight outcomes. A possible confounding factor is the fact that some of the study participants may have sought additional treatment during the follow-up period, and this information was not systematically captured in this follow-up.
Taken together, these findings suggest that once achieved, full remission is stable regardless of treatment type, as there were few relapses during the longer-term follow-up period. This study also suggests that, when efficacious, FBT works relatively quickly and has sustained effects. AFT participants made additional gains over time, but it is unclear if these improvements were related to late response to AFT or other factors. Nonetheless, new remissions during the follow-up period were few, suggesting that additional recovery during follow-up was unlikely. This finding is consistent with studies of adolescents and adults with AN with a longer duration of the disorder, for which no treatments have yet demonstrated substantial differential benefits. Future research is critically needed to investigate potential ways to enhance outcomes. Findings from the present study, keeping the caveats of the limitations described in mind, add support to the importance of early and effective intervention with adolescents with AN.25 They also highlight the challenges inherent to conducting longitudinal treatment studies with this patient population. There clearly is a need to improve the outcomes for this clinical population, and for future innovative research to examine, for example, new treatments or a targeted combination of individual and family treatments.
Acknowledgments
This research was supported by grants R01-MH-070620 (D.L.G.), R01-MH-070621, and K24 MH-074467 (J.L.).
Ms. Bryson served as the statistical expert for this research.
The authors wish to thank Kali Ludwig, MA, and Sara La Casse, PhD, of the University of Chicago, for assistance in data collection.
Dr. Le Grange has received grant or research support from the University of Melbourne/Baker Foundation, the National Eating Disorders Association, and Insight Behavioral Health Centers. He has owned or held stock in the Training Institute for Child and Adolescent Eating Disorders. He has received royalties from Guilford Press and Routledge. Dr. Lock has received grant or research support from the Davis Foundation and the Global Foundation for Eating Disorders. He has served as a consultant for United Behavioral Health and the Centers for Discovery. He has owned or held stock in the Training Institute for Child and Adolescent Eating Disorders. He has received royalties from Guilford Press and Oxford University Press. He has presented expert testimony for Sedgwick LLP.
Footnotes
Disclosure: Dr. Agras has received royalties from Oxford University Press. Drs. Accurso, Darcy, Forsberg, and Ms. Bryson report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Daniel Le Grange, The University of Chicago.
James Lock, Stanford University, Stanford, CA.
Erin C. Accurso, The University of Chicago.
W. Stewart Agras, Stanford University, Stanford, CA.
Alison Darcy, Stanford University, Stanford, CA.
Sarah Forsberg, Stanford University, Stanford, CA.
Susan W. Bryson, Stanford University, Stanford, CA.
References
- 1.Swanson S, Crow S, Le Grange D, Swendsen J, Merikangas K. Prevalence and Correlates of Eating Disorders in Adolescents: Results from the National Comorbidity Survey Replication Adolescent Supplement. Arch Gen Psychiatry. 2011;67:714–723. doi: 10.1001/archgenpsychiatry.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisler I, Dare C, Hodes M, Russell G, Dodge E, Le Grange D. Family therapy for adolescent anorexia nervosa: the results of a controlled comparison of two family interventions. J Child Psychol Psychiatry. 2000;41:727–736. [PubMed] [Google Scholar]
- 3.Gowers SG, Clark A, Roberts C, et al. Clinical effectiveness of treatments for anorexia nervosa in adolescents: Randomised controlled trial. Br J Psychiatry. 2007;191:427–435. doi: 10.1192/bjp.bp.107.036764. [DOI] [PubMed] [Google Scholar]
- 4.Le Grange D, Eisler I, Dare C, Russell G. Evaluation of family treatments in adolescent anorexia nervosa: a pilot study. Int J Eat Disord. 1992;12:347–357. [Google Scholar]
- 5.Lock J, Agras WS, Bryson S, Kraemer H. A comparison of short- and long-term family therapy for adolescent anorexia nervosa. J Am Acad Child Adolesc Psychiatry. 2005;44:632–639. doi: 10.1097/01.chi.0000161647.82775.0a. [DOI] [PubMed] [Google Scholar]
- 6.Lock J, Le Grange D, Agras WS, Moye A, Bryson S, Jo B. A randomized clinical trial comparing family based treatment to adolescent focused individual therapy for adolescents with anorexia nervosa. Arch Gen Psychiatry. 2010;67:1025–1032. doi: 10.1001/archgenpsychiatry.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robin A, Siegal P, Moye A, et al. A controlled comparison of family versus individual therapy for adolescents with anorexia nervosa. J Am Acad Child Adolesc Psychiatry. 1999;38:1482–1489. doi: 10.1097/00004583-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Russell GFM, Szmukler GI, Dare C, Eisler I. An evaluation of family therapy in anorexia nervosa and bulimia nervosa. Arch Gen Psychiatry. 1987;44:1047–56. doi: 10.1001/archpsyc.1987.01800240021004. [DOI] [PubMed] [Google Scholar]
- 9.Eisler I, Dare C, Russell GFM, Szmukler GI, Le Grange D, Dodge E. Family and individual therapy in anorexia nervosa: A five-year follow-up. Arch Gen Psychiatry. 1997;54:1025–1030. doi: 10.1001/archpsyc.1997.01830230063008. [DOI] [PubMed] [Google Scholar]
- 10.Eisler I, Simic M, Russell G, Dare C. A randomized controlled treatment trial of two forms of family therapy in adolescent anorexia nervosa: a five-year follow-up. J Child Psychol Psychiatry. 2007;48:552–60. doi: 10.1111/j.1469-7610.2007.01726.x. [DOI] [PubMed] [Google Scholar]
- 11.Lock J, Couturier J, Agras WS. Comparison of long-term outcomes in adolescents with anorexia nervosa treated with family therapy. Am J Child Adolesc Psychiatry. 2006;45:666–672. doi: 10.1097/01.chi.0000215152.61400.ca. [DOI] [PubMed] [Google Scholar]
- 12.Couturier J, Lock J. What constitutes remission in adolescent anorexia nervosa: a review of various conceptualizations and a quantitative analysis. Int J Eat Disord. 2006;39:175–183. doi: 10.1002/eat.20224. [DOI] [PubMed] [Google Scholar]
- 13.Bardone-Cone AM, Harney MB, Maldonado CR, et al. Defining recovery from an eating disorder: Conceptualization, validation, and examination of psychosocial functioning and psychiatric comorbidity. Beh Res Therapy. 2010;48:194–202. doi: 10.1016/j.brat.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington: American Psychiatric Association Press; 2000. [Google Scholar]
- 15.Golden NH, Katzman DK, Kreipe RE, et al. Eating disorders in adolescents: position paper of the society for adolescent medicine. J Adolesc Health. 2003;33:496–503. doi: 10.1016/s1054-139x(03)00326-4. [DOI] [PubMed] [Google Scholar]
- 16.Fitzpatrick K, Moye A, Hoste R, Le Grange D, Lock J. Adolescent Focused Therapy for Adolescent Anorexia Nervosa. J Contemp Psychotherapy. 2010;40:31–39. [Google Scholar]
- 17.Lock J, Le Grange D. Treatment manual for anorexia nervosa: A family-based approach. Second. New York: Guilford Publications, Inc; 2013. [Google Scholar]
- 18.Center for Disease Control and Prevention. CDC Growth Charts for the United States: Development and Methods. Atlanta: Center for Disease Control; 2002. [Google Scholar]
- 19.Cooper Z, Cooper PJ, Fairburn CG. The validity of the eating disorder examination and its subscales. Br J Psychiatry. 1989;154:807–812. doi: 10.1192/bjp.154.6.807. [DOI] [PubMed] [Google Scholar]
- 20.Beck A, Steer R, Brown G. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 21.Rosenberg M. Conceiving the Self. New York: Basic Books; 1979. [Google Scholar]
- 22.Mazure S, Halmi CA, Sunday S, Romano S, Einhorn A. The Yale-Brown-Cornell Eating Disorder Scales: Development, use, reliability, and validity. J Psychiatric Res. 1994;28:425–445. doi: 10.1016/0022-3956(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 23.Cox DR, Oakes D. Analysis of Survival Data. New York: Chapman and Hall/CRC; 1984. [Google Scholar]
- 24.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New Jersey: John Wiley and Sons; 1980. [Google Scholar]
- 25.Treasure J, Russell G. The case for early intervention in anorexia nervosa: theoretical exploration of maintaining factors. Br J Psychiatry. 2012;199:5–7. doi: 10.1192/bjp.bp.110.087585. [DOI] [PubMed] [Google Scholar]


