Summary
A major gap in our understanding of sensation is how a single sensory neuron can differentially respond to a multitude of different stimuli (polymodality), such as propio- or noci- sensation. The prevailing hypothesis is that different stimuli are transduced through ion channels with diverse properties and subunit composition. In a screen for ion channel genes expressed in polymodal nociceptive neurons we identified Ppk26, a member of the trimeric Degenerin/Epithelial Sodium (DEG/ENaC) channel family as being necessary for proper locomotion behavior in Drosophila larvae in a mutually dependent fashion with co-expressed Ppk1, another member of the same family. Mutants lacking Ppk1 and Ppk26 were defective in mechanical but not thermal nociception behavior. Mutants of Piezo, a channel involved in mechanical nociception in the same neurons, did not show a defect in locomotion, suggesting distinct molecular machinery for mediating locomotor feedback and mechanical nociception.
Introduction
The somatosensory system serves to integrate multiple modalities, including temperature sensation, mechanical cues, body posture (proprioception), and pain (nociception) (Lumpkin and Caterina, 2007). Stimuli can be either unimodal, responding to only one type of stimulus, or polymodal, responding to multiple stimuli. Nociceptors in the skin are an important class of somatosensory neurons, either unimodally or polymodally dedicated to painful sensation of high threshold mechanical stimuli, noxious temperatures, chemical insult or tissue damage. Mammalian nociceptors typically have multiple bare sensory dendrites with a highly elaborate dendritic pattern, with sensory receptors for each modality thought to reside in dendrites. Nociceptors of similar morphology and function are present in Drosophila, providing evidence for evolutionary conservation of this type of sensory neurons (Tracey et al., 2003).
Proprioceptors are another important class of sensory neurons, involved in sensing body position during movement. Although poorly understood, peripheral proprioceptive mechanosensory input provides ongoing information on body position, necessary for tuning central mechanisms driving locomotion (Proske and Gandevia, 2012). Sensory ion channels, primary candidates mediating this process (Corey, 2006), reside in dendrites of multidendritic and ciliated sensory neurons (Desai et al., 2014).
Although some sensory neurons function only as proprioceptors, there is growing evidence that polymodal nociceptive neurons can also convey information about body position. Patients with congenital insensitivity to pain, arising from deficits in Aδ and C fiber function but not from myelinated proprioceptive fibers, also have impaired proprioception (Axelrod and Hilz, 2003; Iijima and Haga, 2009; Rosemberg et al., 1994). “Sleeping” nociceptors in humans do not respond to painful stimuli unless sensitized by chronic injury (Ørstavik et al., 2003), and these could contribute proprioceptive or kinesthetic information in non-sensitized conditions. In C. elegans, PVD and FLP neurons are high-threshold mechanosensors mediating mechanical nociceptive behaviors, but also acting to coordinate body posture, locomotion, and temperature sensing (Way and Chalfie, 1989; Chatzigeorgiou et al., 2010; Arnadottir et al., 2011; Liu et al., 2012). An open question is how a single neuron can distinguish between mechanosensory modalities, for example those involved in proprioception and nociception.
The dendritic arborization (da) neurons in Drosophila larvae constitute a useful genetic system for the study of mechanisms underlying dendrite development and function via ion channels. Da neurons are a set of four classes of well-defined segmentally repeated sensory neurons. Each class has dendritic arbors with a unique stereotypic pattern of arborization, presumably reflecting its functional requirement (Bodmer and Jan, 1987; Grueber et al., 2003). Of these, Class III and Class IV da neurons have dendrites that tile the epidermis.
Class III da neurons have actin-rich spine-like filopodia and are sensitive to gentle touch. These neurons express the TRPN channel NompC, localized to dendrites and involved in mechanosensation of gentle touch (Tsubouchi et al., 2012; Yan et al., 2013; Kernan et al., 1994). Class I da and bd neurons also express NompC and are important for coordinating the appropriate timing of peristaltic locomotion; loss of NompC function in these neurons results in profound slowing and paralysis (Cheng et al., 2010).
Class IV da neurons express the Degenerin/Epithelial Sodium Channel (DEG/ENaC), Pickpocket (Ppk1), and play an essential role in coordinating turning behavior (Adams et al., 1998; Ainsley et al., 2003). The DEG/ENaC or Ppk channel superfamily are voltage insensitive and are assembled as either homomeric or heteromeric trimers. Interestingly, Ppk1 also plays an essential role in mechanical nociception behavior (Zhong et al., 2010), suggesting that these neurons can process multiple stimulus modalities. Class IV da neurons resemble mammalian nociceptors morphologically (Caterina and Julius, 1999; Tracey et al., 2003) and in their polymodal sensitivity to a variety of sensory stimuli (Ohyama et al., 2013). In addition to sensing mechanical nociception and coordinating locomotion, Class IV da neurons are also sensitive to temperature (Hwang et al., 2012), light (Xiang et al., 2010), and chemical stimuli (Kang et al., 2010; Xiang et al., 2010). Recent studies show that the newly discovered mechanosensory ion channel, Piezo (Coste et al., 2010), functions in mechanical nociception in Class IV da neurons (Kim et al., 2012) in a pathway that is parallel to Ppk1 with respect to mechanical nociception. How Ppk1 functions in parallel to Piezo during this process, is still unclear.
DEG/ENaC-type channels are often co-expressed in various parts of the nervous system. Of these, Acid-Sensing Ion Channels (ASICs) are gated by protons and are targets of potent analgesics (Diochot et al., 2012). Although gated by protons, this stimulus activates only a small fraction of the maximal ASIC conductance, and mounting evidence suggests the existence of more potent stimuli in vivo, acting independently or coincidently to protons (Bagriantsev and Minor, 2010; Bohlen et al., 2011; Yu et al., 2010). During tissue injury and acidosis, ASIC channels function in behavior and physiology directly related to pH change (Ziemann et al., 2009). However, other stimuli may regulate the channels at physiologic pH (Wemmie et al., 2013). In heterologous systems, differential ASIC subunit composition can result in distinct response properties, but heteromutimerization might also affect surface trafficking of these channels in vivo (Sherwood et al., 2011). In C. elegans, many MEC channels involved in mechanotransduction are co-expressed, such as the DEG/ENaC pore-forming subunits mec-4 and mec-10. These subunits, along with deg-1 act as mechanosensory ion channels for touch sensation in vivo (Geffeney et al., 2011; O’Hagan et al., 2004). However, loss of mec-10 does not result in complete loss of touch sensitivity, but rather in an atypical mechanoreceptor current (Arnadottir et al., 2011).
In Drosophila, our knowledge about DEG/ENaC hetero-oligomerization is limited to co-expression of Ppk23 and Ppk29 in contact-chemoreceptors (Thistle et al., 2012), Ppk11 and Ppk16 in motor neurons (Younger et al., 2013), and Ppk11 and Ppk19 in taste bristles (Liu et al., 2003). Ppk23 and Ppk29 play non-redundant and essential roles in contact chemoreceptors, with each mutant showing loss of calcium response to cuticular hydrocarbons and defective courtship. Ppk11 and Ppk16 are co-expressed in motor neurons as a single transcript in an operon-like fashion, and mutations in each or both subunits result in the same defect in homeostatic plasticity (Younger et al., 2013). Disrupting Ppk11 and Ppk19 also affects the ability to taste salt (Liu et al., 2003). Functional dependency on heteromeric channels could explain the non-redundancies observed in these cases, a possibility that remains to be tested.
Here we report that the previously uncharacterized DEG/ENaC channel Ppk26/CG8546 is specifically expressed in Class IV da neurons, which have been implicated in both mechanical nociception and proprioception. We find that Ppk26 and Ppk1 proteins co-localize in dendrites of Class IV da neurons and that they interact biochemically. Analysis of specific Ppk1 and Ppk26 null mutants reveals a reciprocal dependence for plasma membrane localization in dendrites. Behavioral analysis of mutants and knockdowns suggest that Ppk26 and Ppk1 are likely to function together during locomotor behavior, presumably through the transduction or processing of a proprioceptive cue. We also report that both Ppk1 and Ppk26 play a role in mechanical but not thermal nociception behavior. Surprisingly, we found that Piezo null mutants have normal locomotion, suggesting that while Piezo might function in parallel with Ppk1 in mechanical nociception, they participate in a separate pathway during locomotion. The identification of Ppk26 as a DEG/ENaC subunit that is co-expressed with Ppk1 possibly in the same channel complex, as well as the reciprocal requirement for their dendritic plasma membrane localization, provides insights into the function of DEG/ENaC channels during mechanosensitive behaviors.
Results
Ppk26 is a member of the DEG/ENaC family
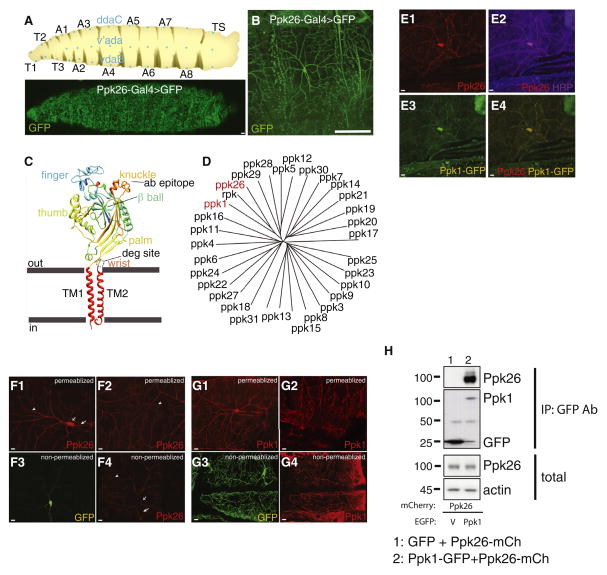
Sensory modality is thought to be in large part determined by the complement of sensory ion channels expressed in a neuron. In a screen for ion channels specifically expressed in larval Class IV da neurons, we identified Ppk26 as a strong candidate. By fusing a 0.8 kB enhancer sequence upstream of the ppk26 transcriptional start sequence to a minimal promoter and the Gal4 element, we generated the ppk26-Gal4 driver, which was found to express Gal4 exclusively in Class IV da neurons (Figure 1A,B). A similar enhancer of 2.2kB upstream of Ppk26 was independently observed to drive Class IV da neuron-specific expression (Zelle et al., 2013). The ppk26 gene is predicted to encode a member of the DEG/ENaC family of ion channels, which are thought to be trimeric based on crystallographic evidence (Jasti et al., 2007). Each subunit contains two transmembrane helices flanking a large extracellular domain (Figure 1C). The closest relatives of Ppk26 in Drosophila are Ripped pocket (Rpk) and Pickpocket (Ppk1), as well as Ppk5, Ppk12, Ppk28, and Ppk29 – all members of the DEG/ENaC family (Figure 1D).
Figure 1. Ppk26 is a newly identified DEG/ENaC subunit expressed in Class IV neuron dendrites and interacting with Ppk1.
A: Location of Class IV neurons on the larval body wall. B: Expression pattern of ppk26-Gal4 (Grueber et al., 2007). Scale bar = 5μm. C: Homology model of a single Ppk26 subunit based on ASIC2A crystal structure. Peptide antibodies were raised against the knuckle region. D: Phylogenetic tree showing relationship between members of the Ppk family. E: Endogenous Ppk26 immunoreactivity was colocalized with Ppk1-EGFP immunoreactivity in Class IV dendrites. Scale bar = 10μm. F: Ppk26 immunoreactivity was present in the cell body and dendrites of Class IV neurons under permeablizing conditions (F1, F2). Surface immunoreactivity was present throughout the dendritic compartment including proximal and distal dendrites. Small arrowheads = terminal dendrites; open arrow = cell body; short arrow = axon. G: Ppk1 immunoreactivity was present in the cell body and terminal dendrites of Class IV neurons (G1, G2), and surface immunoreactivity was present in the cell body and terminal dendrites of Class IV neurons (G3, G4). Scale bar = 20μm. H: Immunoprecipitation of EGFP from cells co-transfected with Ppk26-mCherry and Ppk1-EGFP or EGFP. Coprecipitation of Ppk26-mCherry was observed when co-transfecting with Ppk1-EGFP (lane 2; ), but no when co-transfecting with EGFP (lane 1; V= EGFP vector). Note that that IgG heavy chain is observed at ~ 50kD in lanes 1 and 2, and that a EGFP degradation or cleavage product at ~ 27kDa can be observed in lane 2. See also Figure S1.
A homology model of monomeric Ppk26, based on ASIC1 structure, demonstrates conservation of many structural features, including intracellular N and C termini, of 96 and 20 amino acids respectively, two helical transmembrane domains, and a large extracellular loop structure comprising an arm and hand holding a ball (Jasti et al., 2007) (Figure 1C). Key conserved components of this extracellular loop are the wrist, palm, thumb, finger, knuckle and beta ball domains (Jasti et al., 2007) (Figure 1C). Conservation extends to the pore region of the predicted Ppk26 channel, where TM2 lines the pore and TM1 contacts the lipid bilayer (Gonzales et al., 2009, Baconguis et al., 2012) (Figure S1). This conservation includes an alanine residue proximal to the pore that is thought to constitute the Degenerin site, namely, a residue which when mutated from alanine to valine leads to tissue degeneration as well as increased channel open time and open probability in C. elegans (Brown et al., 2007). Thus, our screen identified Ppk26 as a DEG/ENaC ion channel expressed in Class IV da neurons, which may form part of a functional channel with Ppk1.
Ppk26 protein is specifically expressed in Class IV da neurons
To confirm that ppk26-Gal4 reflects the endogenous expression pattern, we generated anti-peptide antibodies to a Ppk26 epitope (amino acids 532–539) located on the exposed and structurally rigid knuckle region (Figure 1C) (Jasti et al., 2007, Gonzalez et al., 2009, Baconguis et al., 2012). In third-instar body walls, we found that anti-Ppk26 immunoreactivity is specifically localized to Class IV da neurons in the PNS (Figure 1E). anti-Ppk26 signal was found in the cell body as well as in the entire complement of dendritic processes, including higher and lower order branches, and the axon initial segment (Figure 1F1, F2). We also raised an antibody against a peptide (amino acids 506–523) inside the knuckle region of Ppk1. Similar to anti-Ppk26, anti-Ppk1 signal was found exclusively in Class IV da neurons - within the cell body, the entire complement of dendritic processes, and the axon initial segment (Figure 1G1–2). The specificity of both antibodies was validated by immunocytochemistry of Ppk1-EGFP and Ppk26-mCherry expressed in heterologous systems (Figure S1), as well as null mutants (Figure 3A1–A4).
ENaC channel surface trafficking is important for proper physiological function and mutants affecting trafficking result in aberrant sodium transport, as is the case in the renal tubules of Liddle’s syndrome patients (Snyder et al., 1995). However, very little is known about Ppk channel family localization in neurons, besides channel function in sensory bristles (Thistle et al., 2012) and motor neuron presynaptic terminals (Younger et al., 2013). To determine the likely site of Ppk26 function in Class IV da neurons, we ascertained the cell surface localization of the channel under permeabilizing versus non-permeabilizing conditions (Figure 1F). Under permeabilizing conditions, Ppk26 was detected throughout the neuron including the dendritic arbor (Figure 1F1, F2). However, under non-permeabilizing conditions, Ppk26 immunoreactivity was absent from the surface of the cell body and segments of primary dendrites proximal to the cell body (Figure 1F3, F4). Instead, Ppk26 immunoreactivity was observed inside more distal regions of primary dendrites as well as in lower order dendrites. Similarly, we found that under permeabilizing conditions, Ppk1 was detectable throughout the neuron including the dendritic arbor (Figure 1G1, G2). However, under non-permeabilizing conditions, Ppk1 immunoreactivity was absent from the surface of the cell body and segments of primary dendrites proximal to the cell body. Instead, Ppk1 immunoreactivity was observed inside more distal regions of primary dendrites and well as in lower order dendrites (Figure 1G3, G4). Thus, both Ppk26 and Ppk1 are specifically expressed in Class IV da neurons and are inserted in the plasma membrane of dendrites at a distance from the cell body. However, the possibility that glial membrane wrapping of the soma (Han et al., 2011) may prevent antibody penetration to the soma cannot be ruled out.
Ppk26 and Ppk1 likely co-exist in the same protein complex
To test whether Ppk1 and Ppk26 could function together, we first examined if they had overlapping localization in Class IV da neurons. Double labeling ppk1>Ppk1-EGFP expressing animals with antibodies against GFP and Ppk26 revealed that both proteins are colocalized in these neurons (Figure 1E). We also performed immunoprecipitation experiments to determine if Ppk1 and Ppk26 are found in the same protein complex. We co-transfected HEK293 cells with either Ppk26-mCherry and Ppk1-EGFP or Ppk26-mCherry and EGFP as control, and used anti-EGFP antibodies to immunoprecipitate proteins in complex with Ppk1-EGFP. We found that Ppk26 co-immunoprecipitated with Ppk1-EGFP when Ppk26-mCherry and Ppk1-EGFP were cotransfected (Figure 1H, lane 2), suggesting that these channel subunits can form a complex in heterologous cells. This co-immunoprecipitation was specific, since Ppk26 was not detectable in control immunoprecipitation from cells expressing Ppk26-mCherry and EGFP (Figure 1H, lane 1). The finding that Ppk26 can form a complex with Ppk1 and that both proteins colocalize in the same dendritic regions of Class IV da neurons supports the notion that these channel subunits may assemble in vivo to form a functional heteromultimeric channel.
Interestingly, examination of RNA seq data through development (Daines et al., 2011) revealed that ppk26 and ppk1 transcripts have similar developmental expression dynamics (Figure S1C, bottom) with the absolute ppk26 RNA levels about twice that of ppk1 RNA expression (Figure S1C, top).
Generation of ppk26 and ppk1 mutants
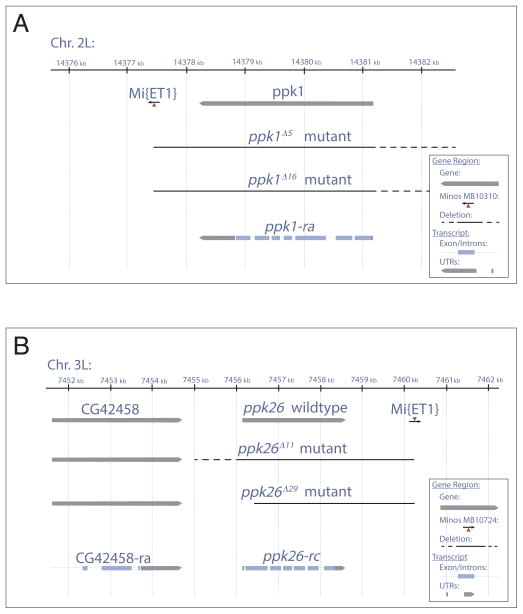
Thus far studies of Ppk1 in Drosophila have relied on analyses of a combination of RNAi and large deficiencies removing genes in addition to ppk1 (Ainsley et al., 2003; Zhong et al., 2010; Boiko et al., 2012). To determine the function of Ppk26 and its relation to Ppk1, we generated specific ppk26 (Figure 2B) and ppk1 (Figure 2A) null mutants by “super-sized” Minos element excision, using the Minos-element strains MB01724 and MB10310 respectively to generate specific deficiencies uncovering each gene (Witsell et al., 2009). We excised each of these Minos elements, which were located at the 3′ end of the ppk26 or ppk1 gene (Figure 2). Since imprecise excisions of Minos elements rarely generate large deletions, we combined respective Minos element strains for ppk1 and ppk26, in a mutant background for mus309, the Drosophila homolog of Bloom Syndrome Helicase, to generate “super-sized” deletions. This mus309 mutation interferes with double stranded break repair, resulting in large and frequent deletions upon Minos element excision (Witsell et al., 2009). Using this strategy, we were able to generate large but specific excisions of each gene. Imprecise excision of MB01724 resulted in Df(3R)ppk26rv11 and Df(3R)ppk26rv29, referred to as ppk26Δ11 and ppk26Δ29. The ppk26Δ11 deletion removes the entire gene but retains a piece of the Minos element (Figure 2B), while ppk26Δ29 removes all except for a piece of the first coding exon. To test for specificity, CG42458 and CG42660 located 1.4kB upstream and 6.9kB downstream respectively were assayed for genomic integrity (Figure S2A). Imprecise excision of MB10310 resulted in Df(2L)ppk1rv5 and Df(2L)ppk1rv16, referred to as Ppk1Δ5 and Ppk1Δ16. The Ppk1Δ5 deletion removes the gene through exon 7 UTR, and retains a piece of the Minos element (Figure 2A), and Ppk1Δ16 removes the entire gene, also leaving behind a piece of the Minos element. To test for specificity, elbowB and spel1 genes, located 9.4kB upstream and 11.3kB downstream of ppk1 respectively were assayed for integrity in each case. Both genes were found to be intact (Figure S2B). As genetic background controls, we also isolated precise excisions of MB01724 and MB10310, resulting in the revertants Mi{ET1}MB10724rv214 for Ppk26 and Mi{ET1}MB10310rv1 for Ppk1. To our knowledge these are the first specific null alleles of ppk26 and ppk1, which will be useful for future functional studies of these co-expressed DEG/ENaC channel subunits, as well as analysis of genetic interactions.
Figure 2. Generation of specific ppk1 and ppk26 null mutants.
A: Schematic showing excision of the Mi{ET1}MB10310 element at the 3′ of ppk1/CG3478 produced ppk1Δ5 and ppk1Δ16 mutants, which delete the entire open-reading frame of Ppk1, leaving behind a piece of the Mi{ET1} element. B: Schematic showing excision of the Mi{ET1}MB10724 element at the 3′ of ppk26/CG8546 gene produced ppk26Δ11 and ppk26Δ29 alleles. ppk26Δ11 deletes the entire open-reading frame of ppk26 leaving a piece of the Mi{ET1} element behind. ppk26Δ29 deletes exons 3 through 6 as well as the 3′ UTR and polyadenylation sequence, leaving intact the non-coding exon 1 and part of the coding exon 2. Dashed lines indicates the region containing the break point, which does not affect upstream or downstream genes. Solid line indicates deletions confirmed by PCR. See also Figure S2.
Ppk1 and Ppk26 exhibit mutual dependence for dendritic plasma membrane localization
Ion channels and receptors composed of multiple subunits are often assembled in the endoplasmic reticulum (ER), and traffic as multimers to their site of function (Heusser et al., 2005, Muth et al., 2003). Thus, lack of one of the subunits may prevent the others from reaching their destination. For example, the vanilloid receptor (TRPV) channel subunits, Nanchung and Inactive, which are critical for hearing in the adult fly (Gong, 2004) and larval sound response (Wu et al., 2011; Zhang et al., 2013) have interdependent trafficking in chordotonal organs. Mutants in either nanchung or inactive result in the apparent absence of the other subunit in cilia, possibly accounting for the hearing defect in either mutant (Gong et al., 2004), and indicating that expression is either co-regulated or that the proteins become degraded in single mutants. In C. elegans, OSM-9 and OCR-2 channels also have mutually dependent transport and function in the ciliated ASH neuron (Tobin et al., 2002), although surface expression has not been assayed.
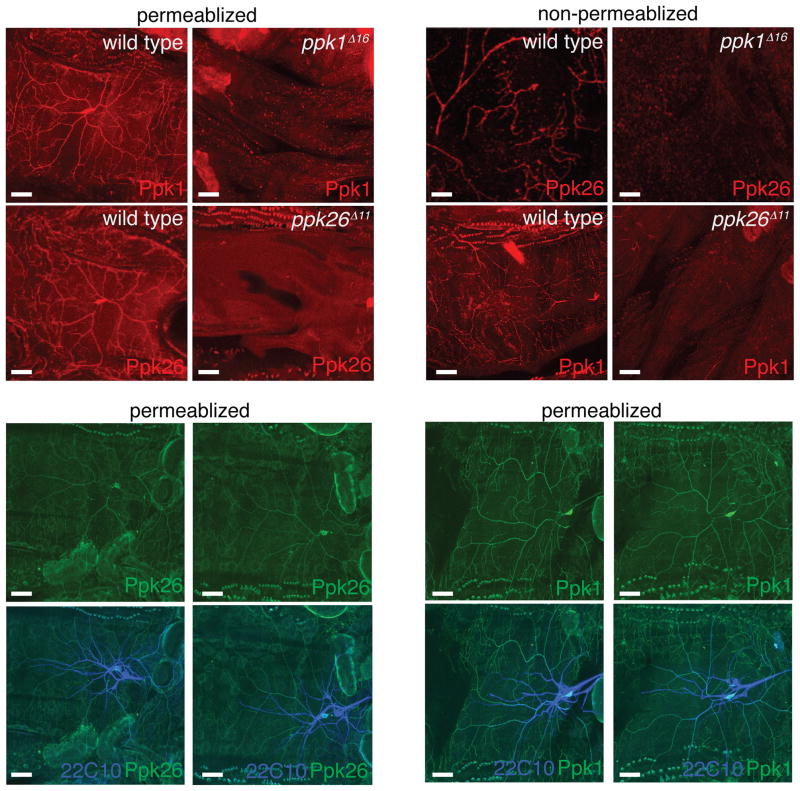
If Ppk1 and Ppk26 form part of the same ion channel, then their trafficking to the cell surface might be linked. To test this hypothesis, we examined the surface expression of Ppk26 on the dendritic plasma membrane under non-permeabilizing conditions, in both wild type larvae and in ppk1Δ16 mutants. Strikingly, in the absence of Ppk1, Ppk26 failed to be inserted at the plasma membrane of dendrites (Figure 3B1, B2). Similarly, we found that the removal of Ppk26 prevented Ppk1 from localizing to the plasma membrane of dendrites (Figure 3B3, B4). Thus, Ppk1 and Ppk26 are mutually required for localization to the surface of dendrites – presumably their site of function. Interestingly, although surface localization was mutually dependent, under permeablizing conditions both Ppk1 and Ppk26 immunoreactivities were still found in the dendrites of Ppk26 and Ppk1 mutants, (Figure 3C, D) suggesting that it is plasma membrane integration which is affected. Although Ppk1 and Ppk26 are exclusively expressed in Class IV da neurons, we also confirmed that other da neuron classes were present in ppk26 and ppk1 mutants by labeling preparations with Futsch/22C10 antibody, which marks a neuronal epitope (Figure 3C, D).
Figure 3. Ppk1 and Ppk26 show mutually dependent surface expression on Class IV neuron dendrites.
A: ppk1 and ppk26 null mutants lack immunoreactivity under permeablizing conditions. B: Ppk26 was present on the surface of Class IV neuron dendrites in wild type, and absent or severely reduced in mutants lacking Ppk1 (B1, B2). Ppk1 was present on the surface of Class IV neuron dendrites in wild type, and absent or severely reduced in mutants lacking Ppk26 (B3, B4). C: Immunostaining under permeablizing conditions showed mutants that while lacking Ppk1 still contained Ppk26 in Class IV neuron dendrites. D: Immunostaining under permeablizing conditions showed that mutants lacking Ppk26 still contained Ppk1 protein at Class IV dendrites. In C and D, Futsch/22C10 immunostaining confirmed the presence of the remaining neurons in the da neuron cluster in both in wild type and mutants. Scale bar = 30μm.
Ppk26 degenerin mutation leads to loss of class IV da neuron dendrites
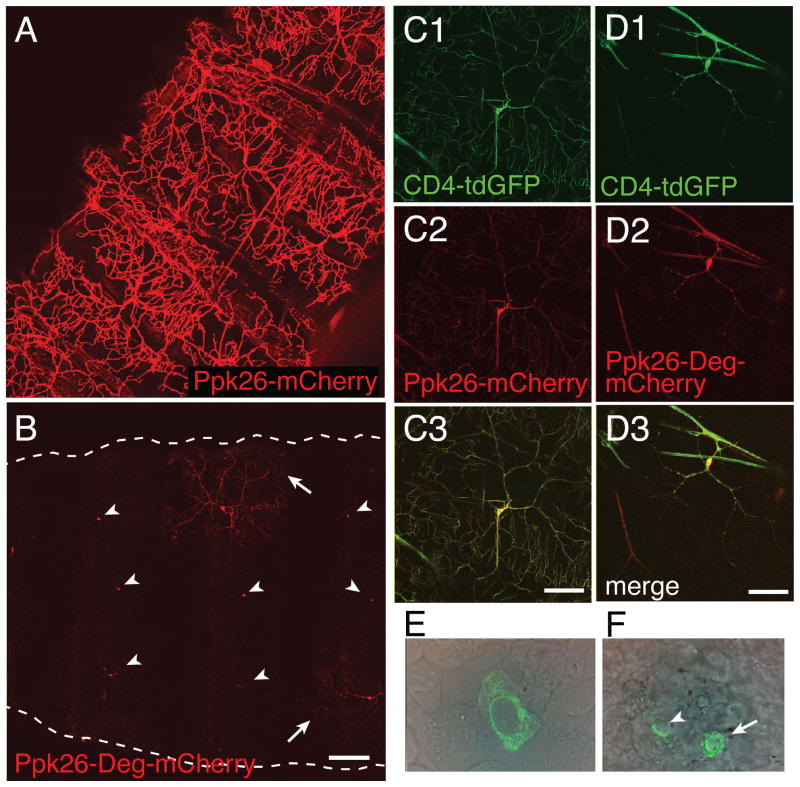
Mutations leading to increased activity of the human ENaC channel result in rare dominant hereditary diseases that impact kidney, colon and lung tissues (Kellenberger and Schild, 2002). In MEC channels it has been suggested that Degenerin mutations (substitution at the Degenerin residue; alanine for threonine, valine, or aspartate) increases calcium permeability (Bianchi et al., 2004), as well as channel open time and open probability (Brown et al., 2007), thereby increasing cytoplasmic calcium levels and leading to excitotoxicity. However, a degenerin phenotype has not been reported for Ppk channels, including animals over-expressing Ppk1-Deg (Wegman et al., 2010). Since Ppk26 contains a conserved Degenerin residue (Figure 1C), we sought to ascertain the effects of this mutation in Class IV da neurons. In particular, we tested a Ppk26-Deg-mCherry transgene containing the Degenerin mutation (A547V) in the peri-TM2 region of the protein for expression in Class IV neurons. Over-expressing a wild type Ppk26-mCherry transgene alone in Class IV da neurons did not result in any obvious abnormal phenotypes in these neurons (Figure 4A). In contrast, expressing a Ppk26-Deg-mCherry mutant transgene resulted in loss of dendritic coverage by many of the Class IV da neurons along the body wall (Figure 4B), due to a drastic reduction in both primary and secondary dendrites (Figure 4C). This diverged from the observation that, as previously reported (Wegman et al., 2010), expressing the Ppk1-Deg mutant did not elicit a strong dendritic arbor phenotype (Figure S4).
Figure 4. Overexpression of Ppk26-degenerin resulted in a reduction in Class IV dendritic arbors.
A: Overexpression of Ppk26-mCherry in Class IV neurons using the Ppk1 promoter resulted in strong localization to dendrites with elaborate primary and higher order arbors. B: Overexpression of Ppk26-degenerin-mCherry resulted in a dramatic reduction in dendritic arbors of mid-second instar larvae. Arrowheads= cell bodies; arrows= fragmented and reduced dendritic arbors. Scale bar = 300μm. C, D: Over-expression of Ppk26-degenerin-mCherry in a wild type background resulted in reduced arbor complexity of third instar Class IV neurons, as evidenced by the membrane marker CD4-tdGFP. Scale bar = 60μm.
Ppk26 regulates locomotor behavior
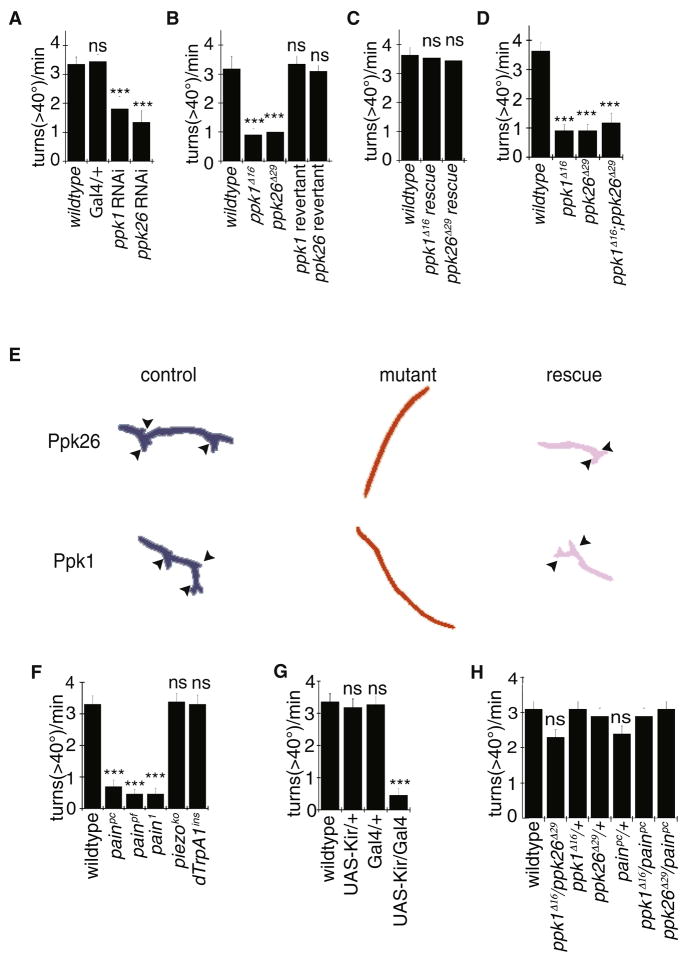
Class IV da neurons are implicated in locomotor behavior, presumably by acting as proprioceptors that modulate motor output in a Ppk1-dependent manner (Ainsley at el., 2003). To test whether Ppk1 and Ppk26 regulate locomotor behavior via their activity in Class IV da neurons in a cell-autonomous manner, we examined locomotor behavior in larvae expressing either Ppk26-RNAi or Ppk1-RNAi in Class IV da neurons using the ppk1-Gal4 driver (Grueber et al., 2007). Crawling behavior in Drosophila larvae consists of sequential contractions of circumferential and longitudinal muscles in each segment, which propagate from posterior to anterior segments (Fox et al., 2006; Hughes and Thomas, 2007; Song et al., 2007; Ainsley et al., 2003; Vogelstein et al., 2013). Knockdown of either Ppk1 or Ppk26 resulted in a decrease in turning frequency (Figure 5A), such that locomotion was predominately composed of directional crawling. Similar abnormal behavior was observed in ppk1 and ppk26 null mutants (Figure 5B, E), but not in their respective revertants. This defect was rescued by expressing a wild type Ppk1-EGFP or Ppk26-mCherry transgene in Class IV neurons of the mutants (Figure 5C, E), thus confirming the critical role of these channel subunits in controlling locomotion. To look for any additive effect, we examined the phenotype of ppk1; ppk26 double mutants, and found that turning was reduced to a similar extent as the single null mutant of either ppk1 or ppk26 (Figure 5D), supporting the notion that the two genes function together in the same pathway. Silencing the Class IV neurons by over-expressing Kir2.1 showed a reduction in turning, consistent with Class IV sensory neurons themselves contributing to this locomotion behavior (Figure 5G). Interestingly, ppk1 and ppk26 transheterozygotes did not differ from wild type controls (Figure 5H).
Figure 5. Ppk26 and Ppk1 are required for locomotion behavior.
A: RNAi mediated knockdown of Ppk26 or Ppk1in Class IV neurons using ppk1-Gal4 driver (Grueber et al., 2007) results in reduced turn frequency. B: ppk1 and ppk26 null mutant, but not revertant strains, have a reduced frequency of turns. C: Expression of Ppk1-EGFP or Ppk26-mCherry in Class IV neurons, using one copy of a direct ppk1 promoter fusion, rescued the turning defects in ppk1 and ppk26 mutants. D: ppk26 and ppk1 but not piezo mutants had a reduced turning frequency. E: Representative traces of wild type, mutant, and rescue animals for Ppk26 and Ppk1. Arrowheads = turning. F: Locomotor turning behavior of painless, dTrpA1, and piezo mutants. G: Effect of silencing Class IV neurons. Error bars represent ±SEM, *=p<0.01. In violin plots, white circles show the medians; box limits indicate the 25th and 75th percentiles as determined by R software; whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles; polygons represent density estimates of data and extend to extreme values. H. Turning behavior of heterozygotes and transheterozygotes. Error bars denote ±SEM, n ≥10; ***= p < 0.001, Student’s t-test. See also Figure S5.
Since Piezo is a mechanosensitive channel required in Class IV da neurons for harsh mechanical touch sensation (Kim et al., 2012), we also examined turning behavior in these mutants. In contrast to ppk1 and ppk26 mutants, there was no significant difference in piezo mutant turning behavior (Figure 5F). Because painless and dTrpA1 have also been implicated in Class IV da neuron function, we also tested mutations in these genes for turning behavior. Because a bonafide null allele for Painless was not available, we generated painpc and painpf alleles missing the coding region and the entire gene region respectively (Figure S3), to be used in conjunction with existing alleles. While dTrpA1 mutants showed no significant defect in locomotion, pain1, painpc and painpf alleles showed significant defects in turning behavior (Figure 5F). While pain1 showed defects in both thermal and mechanical nociception, painpc and painpf mutants showed less severe phenotypes (Figure S6).
Ppk26 serves mechanical but not thermal nociception behavior
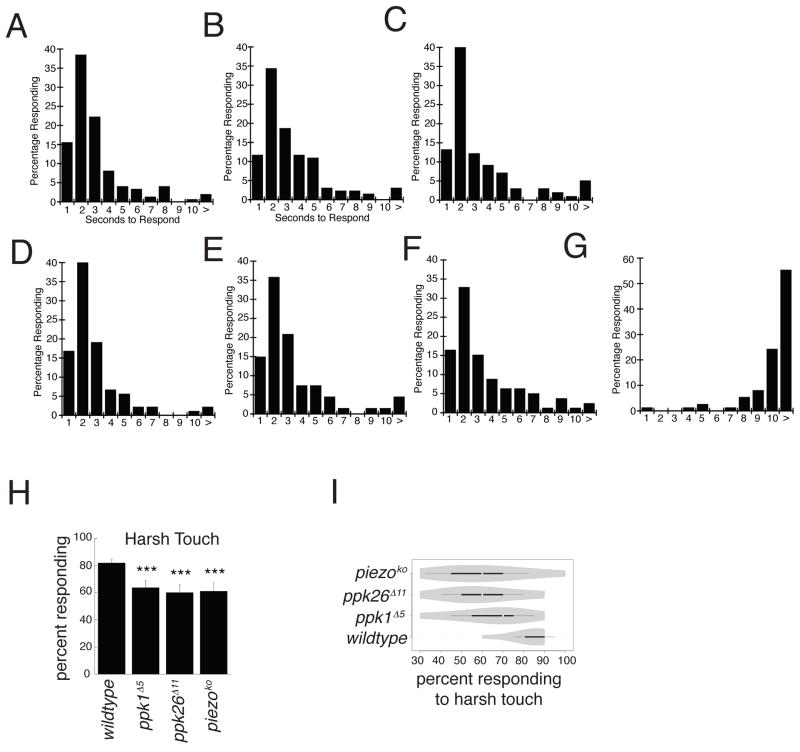
Class IV neurons have been implicated in both proprioceptive as well as multimodal nociceptive behaviors. Since Class IV neurons are important for thermal nociception behavior (Chattopadhyay et al., 2012; Hwang et al., 2012), we examined thermal response in mutant animals. While animals expressing Kir2.1 in Class IV neurons had a significant rightward shift in temperature response to a 46°C heated probe as compared to wild type (Figure 6D–G), ppk1 and ppk26 mutants showed no significant difference from wild type (Figure 6A–C). Since Ppk1 as well as Piezo have been implicated in Class IV da neurons for harsh touch sensation (Kim et al., 2012), we also tested harsh touch behavior in ppk1, ppk26 and piezo mutants. We found significant defects in harsh touch behavior in all of the above mutants, consistent with previous work, as well as our finding that Ppk26 is the likely partner of Ppk1.
Figure 6. Ppk26 and Ppk1 are required for mechanical but not thermal nociception.
A–C: Thermal nociception of ppk1 and ppk26 mutants were assessed by applying a thermal probe and monitoring the time to nocifestive response (n≥100). D–G: Silencing of Class IV neurons using ppk-Gal4 (Grueber et al., 2007) resulted in a strong defect in thermal nociception response time (n≥100). H, I: ppk1, ppk26, and piezo mutant animals showed significant defects in harsh mechanical nociception response. Error bars = ± SEM, n ≥10, ***= p < 0.001, Student’s t-test and Fisher’s exact test. See also Figure S6.
Discussion
We have identified Ppk26, an DEG/ENaC channel subunit that is co-expressed with Ppk1in Class IV da neurons. Consistent with the model that Ppk26 and Ppk1 may be subunits of the same channel, we found that Ppk1 and Ppk26 co-localize in Class IV da neurons, that they form a complex in heterologous expression systems, and that they show non-additive and non-redundant mutant phenotypes in vivo. Ppk26 protein was found in somatic, dendritic and axonal compartments, and plasma membrane insertion was observed in terminal dendrites. Ppk1 and Ppk26 were reciprocally required for normal trafficking and/or insertion to the plasma membrane, further supporting the notion that these two channel subunits interact in vivo. We show that, as is the case for Mec Degenerin channels, mutations at the Degenerin position of Ppk26 lead to loss of Class IV da neuron integrity. We also found that Ppk26 function plays essential roles in normal larval locomotion, particularly in turning behavior.
Proposed role of Ppk1 and Ppk26 in mechanosensation by class IV da neuron dendrites
Overexpression of EGFP-tagged MEC channels in C. elegans has been reported to result in a punctate localization, leading to suggestions that each of these puncta represents a mechanosensory apparatus (O’Hagan et al., 2004). In light of the speculation that contact of dendrites with subcuticular epidermis is part of the apparatus that senses mechanical stimuli (Chalfie 2009; Chen and Chalfie, 2014; Han et al., 2012), it is intriguing that we find both Ppk1 and Ppk26 on the surface of distal and higher order dendrites (Figure 1), consistent with channel function in this compartment. Mutants in Ppk1 and Ppk26 showed defects in the frequency of turning of freely crawling larvae (Figure 5). Moreover, loss of function of either or both Ppk26 and Ppk1 had the same effect on larval locomotion. These findings support the notion that Ppk26 and Ppk1 may act in the same pathway – perhaps in the same channel complex – in mechanosensation that is important for proper locomotion.
Is Ppk26 associated with Ppk1 in a multimeric channel?
Class IV da neurons are known to express the DEG/ENaC channel subunit Ppk1 (Adams et al., 1998), an observation that was confirmed in our study. Given that DEG/ENaC channels are trimeric ion channels that typically require the assembly of different subunits for proper function, the specific co-expression of Ppk26 in Class IV da neurons raises the possibility that Ppk1 and Ppk26 may correspond to different subunits of the same mechanosensory channel in vivo. This is supported by our findings of biochemical interactions between Ppk1 and Ppk26 (Figure 1), their null mutant phenotypes that indicate non-redundancy (Figure 5), and by the reciprocal requirements of Ppk1 and Ppk26 for normal trafficking or insertion to the plasma membrane (Figure 3). The mechanism for this mutual dependence is currently unknown.
Notably, inspection of the developmental expression profile of ppk1 RNA and ppk26 RNA revealed a similar time course, with the absolute levels of RNA for ppk26 twice as high as those of RNA for ppk1 throughout development (Figure S1). Although we cannot be certain that this quantitative difference in RNA levels reflects a similar quantitative difference in the levels of Ppk1 and Ppk26 subunits, it is tempting to speculate that two Ppk26 subunits and one Ppk1 subunit may be assembled to form a surface-expressed trimeric channel.
Tendency of the Degenerin mutation to be involved in neuronal degeneration
Studies in many systems have suggested that mutations in the Degenerin domain of DEG/ENaC lead to loss of cell integrity and perhaps degeneration, however this behavior has not yet been observed in Drosophila Pickpocket family members. The Degenerin position is localized in the wrist region, close to the mouth of the pore. Thus, it has been suggested that Degenerin mutations change the properties of the channel, increasing open time probability and perhaps shifting its ion selectivity from Na+ to Ca2+ (Bianchi et al., 2004; Brown et al, 2007). Given the tight regulation of Ca2+ within cells and its involvement in critical cellular processes, this increase in Ca2+ permeability may lead to loss of cellular homeostasis, in a process that has been dubbed excitotoxicity. Consistent with these findings, we also observed that when a Degenerin mutation was introduced into Ppk26 that was overexpressed in Class IV da neurons, it resulted in a marked reduction in dendritic arbor size (Figure 5). This suggests that the Ppk26-Deg mutation leads to toxicity in the sensory neurons, as has been observed in DEG/ENaC channels in C. elegans, but not in Ppk1 (Wegman et al., 2010). Whereas it is likely that the function of the pore structure of these channels is evolutionarily conserved, it is unclear why Ppk26-Deg has a more potent effect than Ppk1-Deg. One possibility is that the Ppk26-Deg residue makes a larger contribution to pore structure than Ppk1-Deg, due to its intrinsic structure or due to a potential 2:1 stoichiometry in vivo.
Role of Ppk26 and Ppk1 in larval locomotion
Larval locomotion is likely regulated by sensory input provided by sensory neurons in the body wall, which may in turn modulate the motoneurons innervating the body wall. The C. elegans mechanosensitive TRPN channel TRP-4 acts in the DVA neuron to coordinate bending behavior and body posture through positive and negative modulation (Li et al., 2006). It seems likely that Class IV da neurons likewise provide some information for sensory modulation of locomotion through a mechanosensory mechanism, but future work will need to determine if this is indeed the case.
Role of Class IV da neurons in proprioception
Proprioception is a mechanosensory process involving sensory neurons that transduce the mechanical information related to body position or characteristics of the environment for the generation of appropriate behavioral output, such as the turning locomotor behavior that is essential for foraging larvae (Chalfie et al., 2009; Proske and Gandevia, 2012). Here we have identified a member of the DEG/ENaC family of proteins, Ppk26, which acts together with Ppk1 likely as subunits of a channel important for mechanosensation. Our results suggest that perhaps a major site of mechanosensory transduction is located in Class IV da neuron dendritic processes. The behavioral phenotypes of larvae with Ppk1 and Ppk26 knockdown in Class IV da neurons as well as the respective null mutants or double mutants suggest that a deficit in these channels interferes with the ability of the animal to execute proper turning behavior, raising the possibility that the two subunits could be involved in proprioceptively sensing the deformation of the cuticle. Whether Class IV neurons function as proprioceptors still needs to be directly demonstrated, and future experiments will be needed to address the relationship between Class IV neural activity and body position.
Role of Class IV da neurons in nociception
Numerous studies have implicated the Class IV da neurons in both thermal and mechanical nociception behavior (Zhong et al., 2010, Zhong et al., 2012, Kim et al., 2012). We found that while Ppk1 and Ppk26 are important for mechanical nociception behavior, they are dispensable for thermal nociception behavior. While Ppk1 and Ppk26 channels indeed moonlight during two processes in the same neuron, namely mechanical nociception and proprioception, these channels must be playing a specific role as they are not involved in thermal response by the same neuron (Figure 4, Figure 6).
Different ion channels may serve different mechanosensory modalities in the same neuron
Whereas the Class I da neurons and bd neurons are implicated in proprioception for the regulation of sequential contractions use the TRPN channel NompC as the sensor (Cheng et al., 2010), Class IV da neurons rely on the DEG/ENaC channel likely composed of Ppk26 and Ppk1 for the regulation of turning behavior as well as mechanical nociception, perhaps through sensing a mechanical signal at the cuticle. Interestingly, we found that Piezo, a bonafide mechanotransducing ion channel involved in Class IV da neuron mechanotransduction and required for mechanical nociception, does not appear to be involved in turning behavior, suggesting that different combinations of ion channels may serve different mechanosensory functions in the same neuron.
Experimental Procedures
For more details in procedures, see Supplemental Experimental Procedures
Molecular Biology and Cloning
Standard cloning techniques were used to generate the C-terminal tagged Ppk26-mCherry and Ppk1-EGFP. Ppk1>Ppk26-mCherry and Ppk1>Ppk1-EGFP lines used for rescue experiments were cloned into an APPHIH vector containing the Ppk enhancer. UAS expression constructs were generated by cloning the tagged genes into pTW and pUAST. UAS-Ppk26 Degenerin-mCherry was generated by site directed mutagenesis [A547V] of Ppk26-mCherry/pmCherry-N1.
Locomotion Behavior Assay
Wandering 3rd instar larvae were placed on a fresh agarose plate, equilibrated for 2–3 minutes, then transferred to an additional agarose plate for imaging. The plate was illuminated tangentially using 850nm infrared LED strips 120 degree beam angle, #3258 (EnvironmentalLights.com). The LED light was passed through a diffuser to form an arena with high contrast. The larvae were tracked using OpenCV software.
Mechanical and Thermal Nociception Assays
Mechanical Nociception Assay was performed as in Zhong et al., 2010. Each trial corresponds to the percentage of responding larvae. Thermal Nociception Assay was performed as in Hwang et al., 2012.
Immunocytochemistry and Immunofluorescence Imaging
Larvae were dissected in PBS and fixed in 4% Paraformaldehyde. For permeablizing conditions, larvae were washed with 0.1% Triton-X 100 in PBS and for non-permeablized conditions, Triton-X 100 was omitted. Rabbit anti-Ppk26 was used at 1:10,000 and Rabbit anti-Ppk1 at 1:3000.
Antibody Generation
Rabbit anti-peptide antibodies were generated against synthetic peptides representing the knuckle region of PPK1 (AA 506–523) and PPK26 (AA 532–539) (YenZym, South San Francisco).
Fly Genetics and Mutant Generation
The following fly stocks were used: Mi{ET1}MB10724, Mi{ET1}MB10310, P{hsILMiT}2.4, mus309[N1], and mus309[D2] (Bloomington Stock Center); UAS-Ppk26-mCherry and Ppk1-EGFP (generated via germline transformation); ppk1-RNAi (Xu et al., 2004); ppk26-RNAi (GD5110; VDRC); was obtained from VDRC; painpc and painfc (generated as in Gao et al., 2008); dTrpA1ins (Dr. Paul Garrity); piezoko (Dr. Ardem Patapoutian). A 1kB ppk1-Gal4 was used to label Class IV neurons (Grueber et al., 2007). Mi{ET1} excisions were performed in a mus309 mutant background as in Witsell et al,. 2009.
Immunoprecipitation and Westerns
Immunoprecipitations were performed from transfected HEK293 cell homogenates using protein A-conjugated Sepharose beads (Invitrogen). Proteins separated by SDS/PAGE were transferred to nitrocellulose membranes and after immnolabeling examined by chemiluminescence.
Supplementary Material
Figure S1, related to Figure 1:
A. To determine anti-Ppk1 antibody specificity, HEK293 cells were transfected with Ppk26-mCherry (A1) or Ppk1-EGFP (A2) and probed with anti-Ppk1 antibody. B. To determine anti-Ppk26 antibody specificity, HEK293 cells were transfected with Ppk26-mCherry (B1) or Ppk1-EGFP (B2) and probed with anti-Ppk26 antibody. Scale bar = 10μm. C. Expression levels from RNA Seq data, plotted as absolute expression level through development, and normalized expression level through development. D. Alignment of Ppk1 and Ppk26 protein sequences, as well as the peri-TM2 region of C. elegans Mec-4, Mec-10, D. melanogaster Ppk1, Ppk26, Rpk, as well as human ENaC α, β and γ proteins.
Figure S2, related to Figure 2:
A. Genomic locus for ppk26, showing upstream (CG42458) and downstream (CG42661) genes as well as the Minos element (m symbol). PCRs of various ppk26 exons, the first being distal to the Minos element, the last being proximal to the minos element. PCRs of the last exon of CG42458 in wild type and ppk26 mutants indicate CG42458 is intact. PCRs of the first exon of CG42661 in wild type and ppk26 mutants indicate CG42661 is intact. A water negative control was used to rule out potential contamination with wild type DNA in our reagents. B. Genomic locus for ppk1, showing downstream (spe1) and upstream (elbowB) genes as well as the Minos element (m symbol). Several of the candidates from our original screen are shown, with ppk1Δ5 and ppk1Δ16 showing deletion of the first exon distal to the minos element, indicating the gene is deleted. PCRs of spe1 last exon show that the gene is intact in the ppk1Δ5 and ppk1Δ16 mutants. PCRs of elbowB last exon show that the gene is intact in the ppk1Δ5 and ppk1Δ16 mutants. A water negative control was used to rule out potential contamination with wild type DNA in our reagents.
Figure S3, related to Figure 3:
A, B. Morphology of ppk1Δ16 (A) and ppk26Δ11 (B) mutants were examined by confocaling live animals expressing a Ppk>CD4tdTomato marker in Class IV neurons. Scale bar = 40μm
Figure S4, related to Figure 4:
A, B. Morphology of Ppk1-Deg over-expressed in wild type Class IV neurons did not show a dramatic phenotype. Scale bar = 50μm (A) and 40μm (B). Outline of the larva is denoted with a white dashed line (A). Dendrites were marked with the Ppk>CD4tdGFP marker.
Figure S5, related to Figure 5:
A, B. Schematic diagram of painless gene region is shown, with deletion of the coding and full length regions. NaCP60E 3′ UTRs are shown upstream and CG30427 is shown downstream. C. PCR was used to verify the deletion of painless coding and painless full length mutants (isogenic, w-), with primers upstream and downstream of the large locus. D. Normalized painless expression in the mutants was determined using qPCR.
Figure S6, related to Figure 6:
A, B. Mechanical nociception response of painless mutants to a 50mN Von Frey filament. Error bars = ± SEM, n ≥10, ***= p < 0.001, Student’s t-test. F–H. Thermal nociception response of painless mutants using a 46°C heatprobe (C–F) (n≥50).
Acknowledgments
We would like to thank Jill Wildonger for her assistance in the painless mutant generation. We would like to thank all members of the Jan Lab for their useful discussion and comments on the manuscript, particularly Smita Yadav and Christian Peters. We would like to thank Matthew Klassen and Wei Zhang for their technical assistance throughout this work. We would like to thank Dr. Miriam Goodman for her helpful discussion. We thank the Bloomington Stock Center, VDRC, as well as Dr. Fen Biao Gao for making fly stocks available for this work. This work is supported by NIHR37NS040929 to YNJ, T32 GM007449 Pre-doctoral Training Grant in Neurobiology to UCSF Neuroscience Graduate Program, and NIGMS Graduate Fellowship to DG. LYJ and YNJ are HHMI investigators.
Footnotes
Author Contributions: DG YN and LJ designed the experiments. DG, SY, SK, LC, HL, WS, all contributed to the experiments. SY and DG made the ppk1 and ppk26 mutants. SY, SM, and LC made the painless mutants. DG and SM did the behavioral experiments and analyzed the data. WS made ppk1 transgenic lines and ppk26-Gal4. DG made ppk26 transgenic lines. DG and YN wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams CM, Anderson MG, Motto DG, Price MP, Johnson WA, Welsh MJ. Ripped Pocket and Pickpocket, Novel Drosophila DEG/ENaC Subunits Expressed in Early Development and in Mechanosensory Neurons. J Cell Biol. 1998;140:143–152. doi: 10.1083/jcb.140.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsley JA, Pettus JM, Bosenko D, Gerstein CE, Zinkevich N, Anderson MG, Adams CM, Welsh MJ, Johnson WA. Enhanced Locomotion Caused by Loss of the Drosophila DEG/ENaC Protein Pickpocket1. Curr Biol. 2003;13:1557–1563. doi: 10.1016/s0960-9822(03)00596-7. [DOI] [PubMed] [Google Scholar]
- Arnadottir J, O’Hagan R, Chen Y, Goodman MB, Chalfie M. The DEG/ENaC Protein MEC-10 Regulates the Transduction Channel Complex in Caenorhabditis elegans Touch Receptor Neurons. J Neurosci. 2011;31:12695–12704. doi: 10.1523/JNEUROSCI.4580-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod FB, Hilz MJ. Inherited autonomic neuropathies. Semin Neurol. 2003;23:381–390. doi: 10.1055/s-2004-817722. [DOI] [PubMed] [Google Scholar]
- Bagriantsev SN, Minor DL. Small molecule ion channel match making: a natural fit for new ASIC ligands. Neuron. 2010;68:1–3. doi: 10.1016/j.neuron.2010.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi L, Gerstbrein B, Frøkjær-Jensen C, Royal DC, Mukherjee G, Royal MA, Xue J, Schafer WR, Driscoll M. The neurotoxic MEC-4(d) DEG/ENaC sodium channel conducts calcium: implications for necrosis initiation. Nat Neurosci. 2004;7:1337–1344. doi: 10.1038/nn1347. [DOI] [PubMed] [Google Scholar]
- Bodmer R, Jan Y. Morphological differentiation of the embryonic peripheral neurons in Drosophila. Roux’s Arch Dev Biol. 1987;196:69–77. doi: 10.1007/BF00402027. [DOI] [PubMed] [Google Scholar]
- Bohlen CJ, Chesler AT, Sharif-Naeini R, Medzihradszky KF, Zhou S, King D, Sánchez EE, Burlingame AL, Basbaum AI, Julius D. A heteromeric Texas coral snake toxin targets acid-sensing ion channels to produce pain. Nature. 2011;479:410–414. doi: 10.1038/nature10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiko N, Kucher V, Stockand JD, Eaton BA. Pickpocket1 is an ionotropic molecular sensory transducer. J Biol Chem 2012. 2012;287:39878–86. doi: 10.1074/jbc.M112.411736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AL, Fernandez-Illescas SM, Liao Z, Goodman MB. Gain-of-Function Mutations in the MEC-4 DEG/ENaC Sensory Mechanotransduction Channel Alter Gating and Drug Blockade. J Gen Physiol. 2007;129:161–173. doi: 10.1085/jgp.200609672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. Sense and specificity: a molecular identity for nociceptors. Curr Op Neurobiol. 1999;9:525–530. doi: 10.1016/S0959-4388(99)00009-4. [DOI] [PubMed] [Google Scholar]
- Chalfie M. Neurosensory Mechanotransduction. Nat Rev Mol Cell Biol. 2009;10:44–52. doi: 10.1038/nrm2595. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A, Gilstrap AV, Galko MJ. Local and global methods of assessing thermal nociception in Drosophila larvae. J Vis Exp. 2012 May;18(63):e3837. doi: 10.3791/3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Chalfie M. Modulation of C. elegans Touch Sensitivity Is Integrated at Multiple Levels. J Neurosci. 2014;34:6522–6536. doi: 10.1523/JNEUROSCI.0022-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Jeffries O, Rowe IC, Liang Z, Knaus HG, Ruth P, Shipston MJ. Membrane trafficking of large conductance calcium-activated potassium channels is regulated by alternative splicing of a transplantable, acidic trafficking motif in the RCK1-RCK2 linker. J Biol Chem. 2010;285:23265–75. doi: 10.1074/jbc.M110.139758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng LE, Song W, Looger LL, Jan LY, Jan YN. The Role of the TRP Channel NompCin Drosophila Larval and Adult Locomotion. Neuron. 2010;67:373–380. doi: 10.1016/j.neuron.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey DP. What is the hair cell transduction channel? J Physiol. 2006;576:23–28. doi: 10.1113/jphysiol.2006.116582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 Are Essential Components of Distinct Mechanically Activated Cation Channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daines B, Wang H, Wang L, Li Y, Han Y, Emmert D, Gelbart W, Wang X, Li W, Gibbs R, et al. The Drosophila melanogaster transcriptome by paired-end RNA sequencing. Genome Research. 2011;21:315–324. doi: 10.1101/gr.107854.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai BS, Chadha A, Cook B. The stum Gene Is Essential for Mechanical Sensing in Proprioceptive Neurons. Science. 2014;343:1256–1259. doi: 10.1126/science.1247761. [DOI] [PubMed] [Google Scholar]
- Diochot S, Baron A, Salinas M, Douguet D, Scarzello S, Dabert-Gay AS, Debayle D, Friend V, Alloui A, Lazdunski M, et al. Black mamba venom peptides target acid-sensing ion channels to abolish pain. Nature. 2012;490:552–555. doi: 10.1038/nature11494. [DOI] [PubMed] [Google Scholar]
- Fox LE, Soll DR, Wu CF. Coordination and modulation of locomotion pattern generators in Drosophila larvae: effects of altered biogenic amine levels by the tyramine beta hydroxlyase mutation. J Neurosci. 2006;26:1486–98. doi: 10.1523/JNEUROSCI.4749-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffeney SL, Cueva JG, Glauser DA, Doll JC, Lee THC, Montoya M, Karania S, Garakani AM, Pruitt BL, Goodman MB. DEG/ENaC but Not TRP Channels Arethe Major Mechanoelectrical Transduction Channels in a C. elegans Nociceptor. Neuron. 2011;71:845–857. doi: 10.1016/j.neuron.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–78. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- Grueber WB, Ye B, Yang CH, Younger S, Borden K, Jan LY, Jan YN. Projections of Drosophila multidendritic neurons in the central nervous system: links with peripheral dendrite morphology. Development. 2007;134:55–64. doi: 10.1242/dev.02666. [DOI] [PubMed] [Google Scholar]
- Gong Z. Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. J Neurosci. 2004;24:9059–9066. doi: 10.1523/JNEUROSCI.1645-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Jan LY, Jan YN. Enhancer-driven membrane markers for analysis of nonautonomous mechanisms reveal neuron-glia interactions in Drosophila. Proc Natl Acad Sci U S A. 2011;108:9673–9678. doi: 10.1073/pnas.1106386108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Wang D, Soba P, Zhu S, Lin X, Jan LY, Jan YN. Integrins regulate repulsion-mediated dendritic patterning of drosophila sensory neurons by restricting dendrites in a 2D space. Neuron. 2012;73:64–78. doi: 10.1016/j.neuron.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CL, Thomas JB. A sensory feedback circuit coordinates muscle activity in Drosophila. Mol Cell Neurosci. 2007;35:383–396. doi: 10.1016/j.mcn.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang RY, Stearns NA, Tracey WD. The Ankyrin Repeat Domain of the TRPA Protein Painless Is Important for Thermal Nociception but Not Mechanical Nociception. PLoS ONE. 2012;7:e30090. doi: 10.1371/journal.pone.0030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima M, Haga N. Evaluation of nonnociceptive sensation in patients with congenital insensitivity to pain with anhidrosis. Childs Nerv Syst. 2009;26:1085–1089. doi: 10.1007/s00381-009-1057-6. [DOI] [PubMed] [Google Scholar]
- Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL, Garrity PA. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 2010;464:597–600. doi: 10.1038/nature08848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- Kernan M, Cowan D, Zuker C. Genetic dissection of mechanosensory transduction: mechanoreception-defective mutations of Drosophila. Neuron. 1994;12:1195–1206. doi: 10.1016/0896-6273(94)90437-5. [DOI] [PubMed] [Google Scholar]
- Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. The role of Drosophila Piezo in mechanical nociception. Nature. 2012;483:209–212. doi: 10.1038/nature10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Feng Z, Sternberg PW, Shawn Xu XZ. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature. 2006;440:684–687. doi: 10.1038/nature04538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Leonard AS, Motto DG, Feller MA, Price MP, Johnson WA, Welsh MJ. Contribution of Drosophila DEG/ENaC genes to salt taste. Neuron. 2003;39:133–46. doi: 10.1016/s0896-6273(03)00394-5. [DOI] [PubMed] [Google Scholar]
- Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445:858–865. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- O’Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat Neurosci. 2004;8:43–50. doi: 10.1038/nn1362. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Jovanic T, Denisov G, Dang TC, Hoffmann D, Kerr RA, Zlatic M. High-throughput analysis of stimulus-evoked behaviors in Drosophila larva reveals multiple modality-specific escape strategies. PLoS ONE. 2013;20:e71706. doi: 10.1371/journal.pone.0071706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, Gandevia SC. The Proprioceptive Senses: Their Roles in Signaling Body Shape, Body Position and Movement, and Muscle Force. Physiol Rev. 2012;92:1651–1697. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- Rosemberg S, Marie SK, Kliemann S. Congenital insensitivity to pain with anhidrosis (hereditary sensory and autonomic neuropathy type IV) Pediatr Neurol. 1994;11:50–56. doi: 10.1016/0887-8994(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Sherwood TW, Lee KG, Gormley MG, Askwith CC. Heteromeric Acid-Sensing Ion Channels (ASICs) Composed of ASIC2b and ASIC1a Display Novel Channel Properties and Contribute to Acidosis-Induced Neuronal Death. J Neurosci. 2011;31:9723–9734. doi: 10.1523/JNEUROSCI.1665-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder PM, Price MP, McDonald FJ, Adams CM, Volk KA, Zeiher BG, Stokes JB, Welsh MJ. Mechanism by which Liddle’s syndrome mutations increase activity of a human epithelial Na+ channel. Cell. 1995;83:969–978. doi: 10.1016/0092-8674(95)90212-0. [DOI] [PubMed] [Google Scholar]
- Thistle R, Cameron P, Ghorayshi A, Dennison L, Scott K. Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell. 2012;149:1140–1151. doi: 10.1016/j.cell.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin DM, Madsen DM, Kahn-Kirby A, Peckol EL. Combinatorial Expression of TRPV Channel Proteins Defines Their Sensory Functions and Subcellular Localization in C. elegans Neurons. Neuron. 2002;35:307–318. doi: 10.1016/s0896-6273(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila Gene Essential for Nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- Tsubouchi A, Caldwell JC, Tracey WD. Dendritic Filopodia, Ripped Pocket, NOMPC, and NMDARs Contribute tothe Sense of Touch in Drosophila Larvae. Curr Biol. 2012;22:2124–2134. doi: 10.1016/j.cub.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein JT, Park Y, Ohyama T, Kerr RA, Truman JW, Priebe CE, Zlatic M. Discovery of brainwide neural-behavioral maps via multiscale unsupervised structure learning. Nature. 2014;344:386–392. doi: 10.1126/science.1250298. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Taugher RJ, Kreple CJ. Acid-sensing ion channels in pain and disease. Nat Rev Neurosci. 2013;14:461–471. doi: 10.1038/nrn3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegman LJ, Ainsley JA, Johnson WA. Developmental timing of a sensory-mediated larval surfacing behavior correlates with cessation of feeding and determination of final adult size. Developmental Biology. 2010;345:170–179. doi: 10.1016/j.ydbio.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witsell A, Kane DP, Rubin S, McVey M. Removal of the Bloom Syndrome DNA Helicase Extends the Utility of Imprecise Transposon Excision for Making Null Mutations in Drosophila. Genetics. 2009;183:1187–1193. doi: 10.1534/genetics.109.108472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Sweeney LB, Ayoob JC, Chak K, Andreone BJ, Ohyama T, Kerr R, Luo L, Zlatic M, Kolodkin AL. A Combinatorial Semaphorin Code Instructs the Initial Steps of Sensory Circuit Assembly in the Drosophila CNS. Neuron. 2011;70:281–298. doi: 10.1016/j.neuron.2011.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, Jan YN. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 2010;468:921–926. doi: 10.1038/nature09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Bogert BA, Li W, Su K, Lee A, Gao FB. The fragile X-related gene affects crawling behavior of Drosophila larvae by regulating the mRNA level of the DEG/ENaC protein pickpocket1. Curr Biol. 2004;12:1025–34. doi: 10.1016/j.cub.2004.05.055. [DOI] [PubMed] [Google Scholar]
- Yan Z, Zhang W, He Y, Gorczyca D, Xiang Y, Cheng LE, Meltzer S, Jan LY, Jan YN. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature. 2013;493:221–225. doi: 10.1038/nature11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger MA, Müller M, Tong A, Pym EC, Davis GW. A Presynaptic ENaC Channel Drives Homeostatic Plasticity. Neuron. 2013;79:1183–1196. doi: 10.1016/j.neuron.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Chen Z, Li WG, Cao H, Feng EG, Yu F, Liu H, Jiang H, Xu TL. A nonproton ligand sensor in the acid-sensing ion channel. Neuron. 2010;68:61–72. doi: 10.1016/j.neuron.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Zelle KM, Lu B, Pyfrom SC, Ben-Shahar Y. The genetic architecture of degenerin/epithelial sodium channels in Drosophila. G3. 2013;3:441–50. doi: 10.1534/g3.112.005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yan Z, Jan LY, Jan YN. Sound response mediated by the TRP channels NOMPC, NANCHUNG, and INACTIVE in chordotonal organs of Drosophila larvae. Proc Natl Acad Sci U S A. 2013;110:13612–13617. doi: 10.1073/pnas.1312477110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Hwang RY, Tracey WD. Pickpocket Is a DEG/ENaC Protein Required for Mechanical Nociception in Drosophila Larvae. Curr Biol. 2010;20:429–434. doi: 10.1016/j.cub.2009.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Bellemer A, Yan H, Ken H, Jessica R, Hwang RY, Pitt GS, Tracey WD. Thermosensory and nonthermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat-sensor domains of a thermoTRP Channel. Cell Rep. 2012;1:43–55. doi: 10.1016/j.celrep.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, Welsh MJ, et al. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørstavik K, Weidner C, Schmidt R, Schmelz M, Hilliges M, Jørum E, Handwerker H, Torebjörk E. Pathological C-fibres in patients with a chronic painful condition. Brain. 2003;126:567–578. doi: 10.1093/brain/awg060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1, related to Figure 1:
A. To determine anti-Ppk1 antibody specificity, HEK293 cells were transfected with Ppk26-mCherry (A1) or Ppk1-EGFP (A2) and probed with anti-Ppk1 antibody. B. To determine anti-Ppk26 antibody specificity, HEK293 cells were transfected with Ppk26-mCherry (B1) or Ppk1-EGFP (B2) and probed with anti-Ppk26 antibody. Scale bar = 10μm. C. Expression levels from RNA Seq data, plotted as absolute expression level through development, and normalized expression level through development. D. Alignment of Ppk1 and Ppk26 protein sequences, as well as the peri-TM2 region of C. elegans Mec-4, Mec-10, D. melanogaster Ppk1, Ppk26, Rpk, as well as human ENaC α, β and γ proteins.
Figure S2, related to Figure 2:
A. Genomic locus for ppk26, showing upstream (CG42458) and downstream (CG42661) genes as well as the Minos element (m symbol). PCRs of various ppk26 exons, the first being distal to the Minos element, the last being proximal to the minos element. PCRs of the last exon of CG42458 in wild type and ppk26 mutants indicate CG42458 is intact. PCRs of the first exon of CG42661 in wild type and ppk26 mutants indicate CG42661 is intact. A water negative control was used to rule out potential contamination with wild type DNA in our reagents. B. Genomic locus for ppk1, showing downstream (spe1) and upstream (elbowB) genes as well as the Minos element (m symbol). Several of the candidates from our original screen are shown, with ppk1Δ5 and ppk1Δ16 showing deletion of the first exon distal to the minos element, indicating the gene is deleted. PCRs of spe1 last exon show that the gene is intact in the ppk1Δ5 and ppk1Δ16 mutants. PCRs of elbowB last exon show that the gene is intact in the ppk1Δ5 and ppk1Δ16 mutants. A water negative control was used to rule out potential contamination with wild type DNA in our reagents.
Figure S3, related to Figure 3:
A, B. Morphology of ppk1Δ16 (A) and ppk26Δ11 (B) mutants were examined by confocaling live animals expressing a Ppk>CD4tdTomato marker in Class IV neurons. Scale bar = 40μm
Figure S4, related to Figure 4:
A, B. Morphology of Ppk1-Deg over-expressed in wild type Class IV neurons did not show a dramatic phenotype. Scale bar = 50μm (A) and 40μm (B). Outline of the larva is denoted with a white dashed line (A). Dendrites were marked with the Ppk>CD4tdGFP marker.
Figure S5, related to Figure 5:
A, B. Schematic diagram of painless gene region is shown, with deletion of the coding and full length regions. NaCP60E 3′ UTRs are shown upstream and CG30427 is shown downstream. C. PCR was used to verify the deletion of painless coding and painless full length mutants (isogenic, w-), with primers upstream and downstream of the large locus. D. Normalized painless expression in the mutants was determined using qPCR.
Figure S6, related to Figure 6:
A, B. Mechanical nociception response of painless mutants to a 50mN Von Frey filament. Error bars = ± SEM, n ≥10, ***= p < 0.001, Student’s t-test. F–H. Thermal nociception response of painless mutants using a 46°C heatprobe (C–F) (n≥50).