Abstract
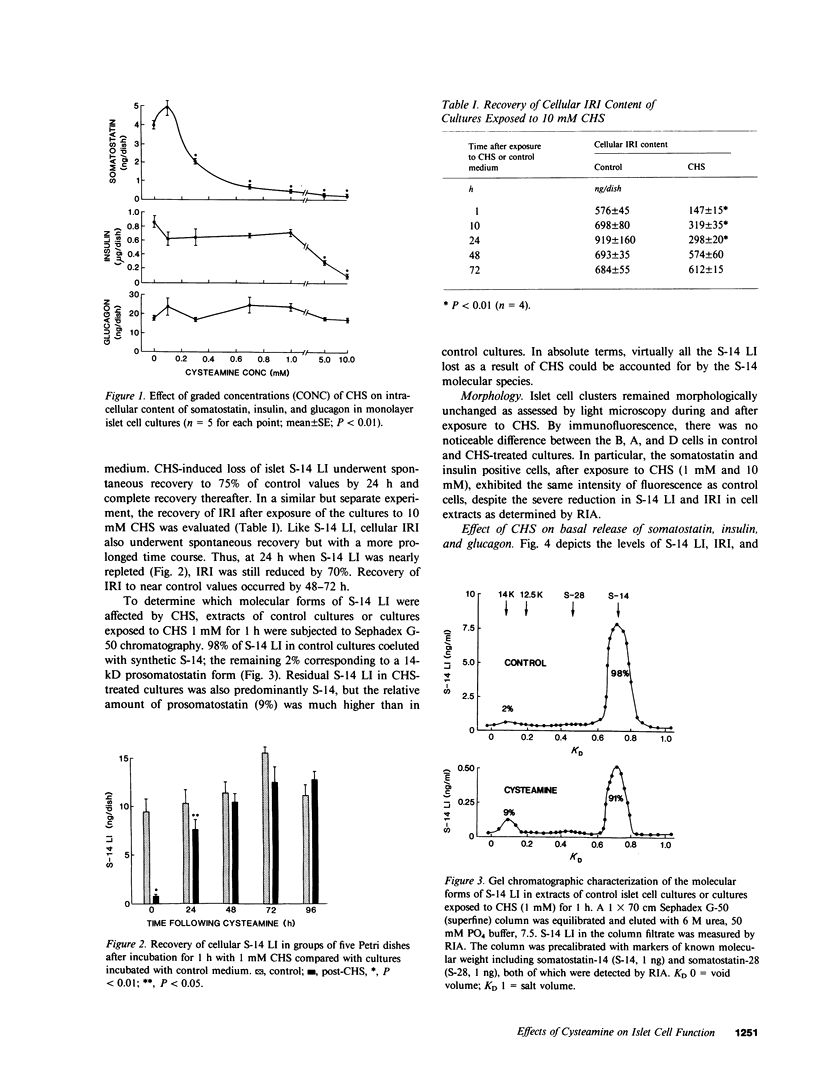
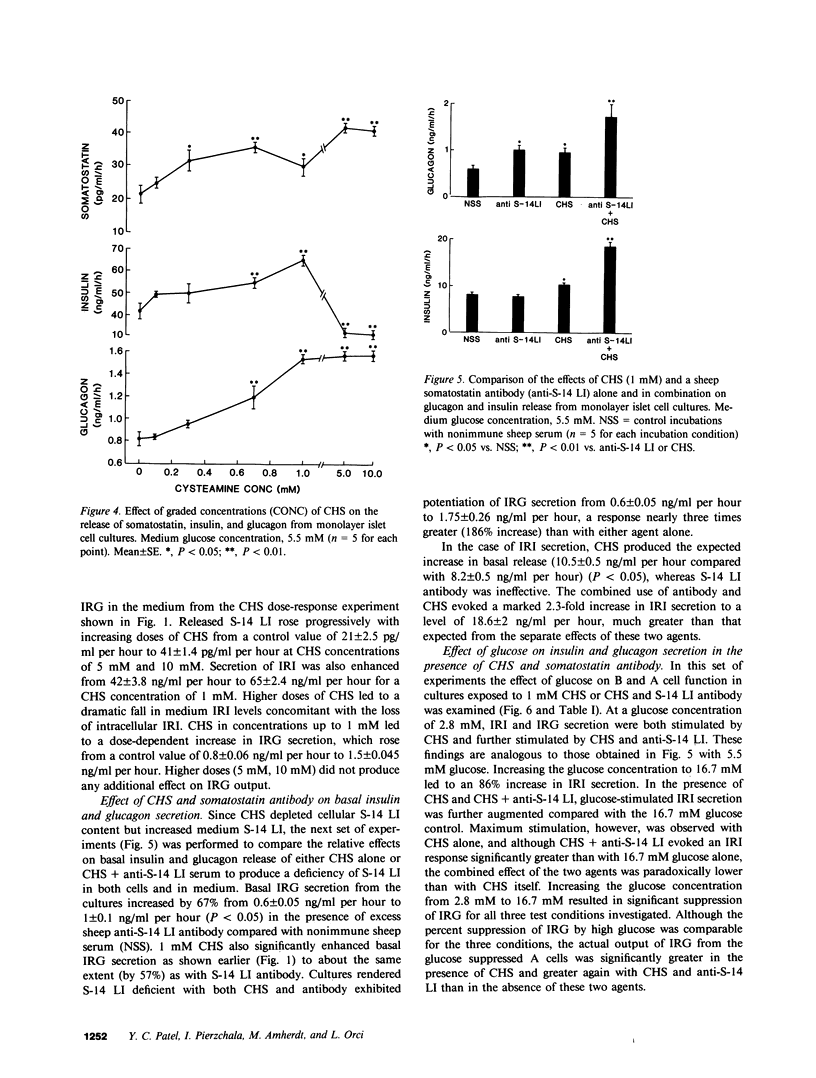
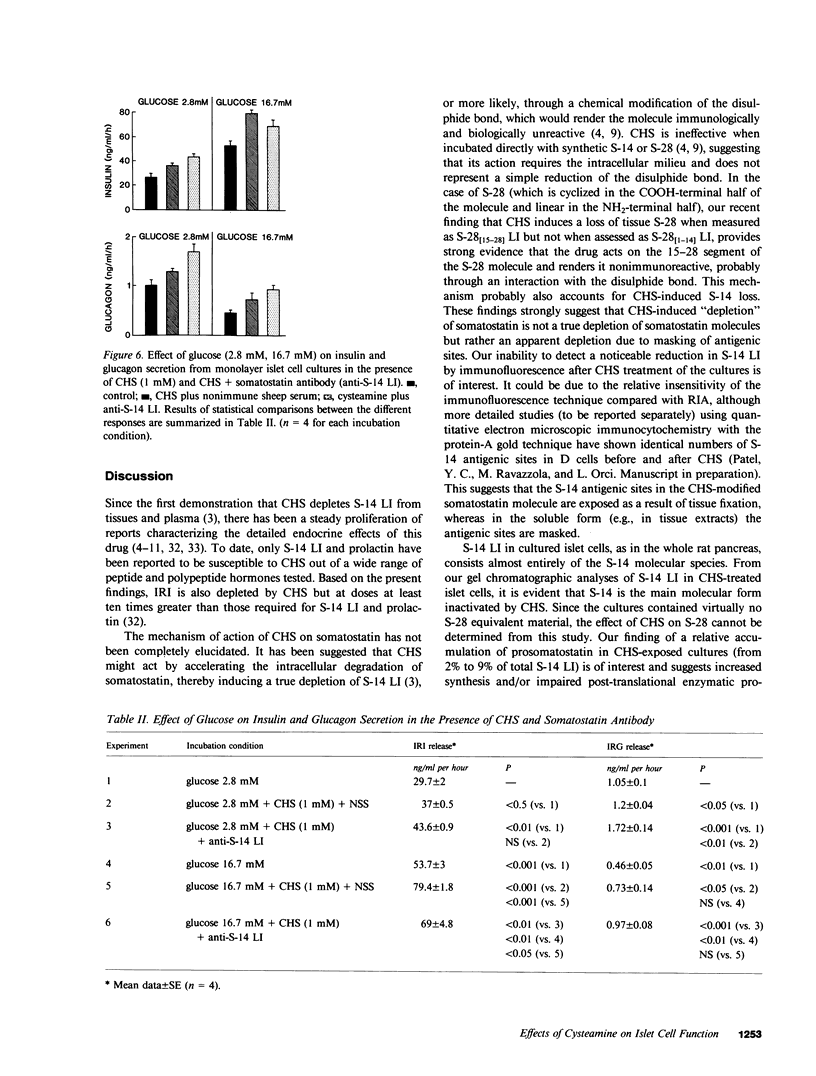
In this study we have characterized the effects of cysteamine (CHS) on the cellular content and release of immunoreactive somatostatin (S-14 LI), insulin (IRI), and glucagon (IRG) from monolayer cultures of neonatal rat islets. Incubation of cultures with 0.1-10 mM CHS for 1 h led to an apparent, dose-dependent reduction of cellular S-14 LI that was 50% of control at 0.3 mM, 87% at 1 mM, and 95% at 10 mM. IRI content was unaffected by CHS up to 1 mM, but at 10 mM 90% loss of IRI occurred. All concentrations were without effect on IRG content. The loss of S-14 LI and IRI was completely reversible with time, but with different recovery rates for the two hormones (48 h for S-14 LI, and 72 h for IRI). Released S-14 LI rose progressively with increasing doses of CHS from 21 +/- 2.5 pg/ml per hour to 41 +/- 1.4 pg/ml per hour at CHS concentrations of 5 mM and 10 mM. IRI and IRG secretion were both also significantly enhanced (by 55% and 88%, respectively), despite the elevated medium S-14 LI. Since CHS reduced cellular S-14 LI but augmented medium S-14 LI, the relative effects of CHS (1 mM) and immunoneutralization with antibody to S-14 LI on IRI and IRG secretion were tested. Anti S-14 LI alone stimulated basal IRG (67%) but not IRI. Cultures rendered S-14 LI deficient with both CHS and anti-S-14 LI exhibited threefold and 2.3-fold potentiation of IRG and IRI secretions, respectively, greater than that expected from the separate effects of the two agents. Increasing medium glucose from 2.8 mM to 16.7 mM stimulated IRI release by 86% and suppressed IRG by 53%. CHS (1 mM) and anti-S-14 LI further augmented stimulated IRI release, by 30%; although 16.7 mM glucose suppression of IRG was still maintained under these conditions, the quantitative IRG response was significantly greater. These results suggest that CHS induces an apparent loss of islet S-14 LI, and at high doses, of IRI as well, but has no effect on A cells. Complete islet S-14 LI deficiency augments IRI and IRG secretion over a wide range of glucose concentrations, suggesting a physiological role of D cells on B cell and A cell regulation. D cell modulation of B cells requires cellular but not extracellular S-14 LI, being mediated probably though direct intracellular communication, whereas the A cells seem to be regulated by both direct contact as well as through locally secreted S-14 LI.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barden N., Cote J. P., Lavoie M., Dupont A. Secretion of somatostatin by rat islets of Langerhans and gastric mucosa and a role for pancreatic somatostatin in the regulation of glucagon release. Metabolism. 1978 Sep;27(9 Suppl 1):1215–1218. doi: 10.1016/0026-0495(78)90045-8. [DOI] [PubMed] [Google Scholar]
- Brown M. R., Fisher L. A., Sawchenko P. E., Swanson L. W., Vale W. W. Biological effects of cysteamine: relationship to somatostatin depletion. Regul Pept. 1983 Jan;5(2):163–179. doi: 10.1016/0167-0115(83)90124-6. [DOI] [PubMed] [Google Scholar]
- Fujimoto W. Y., Kawazu S., Ikeuchi M., Kanazawa Y. In vitro paracrine regulation of islet B-cell function by A and D cells. Life Sci. 1983 Apr 18;32(16):1873–1878. doi: 10.1016/0024-3205(83)90066-8. [DOI] [PubMed] [Google Scholar]
- Itoh M., Mandarino L., Gerich J. E. Antisomatostatin gamma globulin augments secretion of both insulin and glucagon in vitro: evidence for a physiologic role for endogenous somatostatin in the regulation of pancreatic A- and B-cell function. Diabetes. 1980 Sep;29(9):693–696. doi: 10.2337/diab.29.9.693. [DOI] [PubMed] [Google Scholar]
- Kawai K., Ipp E., Orci L., Perrelet A., Unger R. H. Circulating somatostatin acts on the islets of Langerhans by way of a somatostatin-poor compartment. Science. 1982 Oct 29;218(4571):477–478. doi: 10.1126/science.6126931. [DOI] [PubMed] [Google Scholar]
- Kohen E., Kohen C., Rabinovitch A. Cell-to-cell communication in rat pancreatic islet monolayer cultures is modulated by agents affecting islet-cell secretory activity. Diabetes. 1983 Jan;32(1):95–98. doi: 10.2337/diab.32.1.95. [DOI] [PubMed] [Google Scholar]
- Kohen E., Kohen C., Thorell B., Mintz D. H., Rabinovitch A. Intercellular communication in pancreatic islet monolayer cultures: a microfluorometric study. Science. 1979 May 25;204(4395):862–865. doi: 10.1126/science.35828. [DOI] [PubMed] [Google Scholar]
- Meda P., Kohen E., Kohen C., Rabinovitch A., Orci L. Direct communication of homologous and heterologous endocrine islet cells in culture. J Cell Biol. 1982 Jan;92(1):221–226. doi: 10.1083/jcb.92.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels R. L., Sheridan J. D. Islets of Langerhans: dye coupling among immunocytochemically distinct cell types. Science. 1981 Nov 13;214(4522):801–803. doi: 10.1126/science.6117129. [DOI] [PubMed] [Google Scholar]
- Millard W. J., Sagar S. M., Landis D. M., Martin J. B. Cysteamine: a potent and specific depletor of pituitary prolactin. Science. 1982 Jul 30;217(4558):452–454. doi: 10.1126/science.7089575. [DOI] [PubMed] [Google Scholar]
- Millard W. J., Sagar S. M., Simpkins J. W., Owens R. E., Badger T. M., Friesen H. G., Martin J. B. Cysteamine induces depletion of both immunological and biological prolactin activity in the anterior pituitary and blood of the rat. Endocrinology. 1983 Dec;113(6):2161–2167. doi: 10.1210/endo-113-6-2161. [DOI] [PubMed] [Google Scholar]
- Orci L. Macro- and micro-domains in the endocrine pancreas. Diabetes. 1982 Jun;31(6 Pt 1):538–565. doi: 10.2337/diab.31.6.538. [DOI] [PubMed] [Google Scholar]
- Pace C. S., Tarvin J. T. Somatostatin: mechanism of action in pancreatic islet beta-cells. Diabetes. 1981 Oct;30(10):836–842. doi: 10.2337/diab.30.10.836. [DOI] [PubMed] [Google Scholar]
- Palkovits M., Brownstein M. J., Eiden L. E., Beinfeld M. C., Russell J., Arimura A., Szabo S. Selective depletion of somatostatin in rat brain by cysteamine. Brain Res. 1982 May 20;240(1):178–180. doi: 10.1016/0006-8993(82)90660-6. [DOI] [PubMed] [Google Scholar]
- Patel Y. C., Amherdt M., Orci L. Quantitative electron microscopic autoradiography of insulin, glucagon, and somatostatin binding sites on islets. Science. 1982 Sep 17;217(4565):1155–1156. doi: 10.1126/science.6126003. [DOI] [PubMed] [Google Scholar]
- Patel Y. C., Amherdt M., Orci L. Somatostatin secretion from monolayer cultures of neonatal rat pancreas. Endocrinology. 1979 Mar;104(3):676–679. doi: 10.1210/endo-104-3-676. [DOI] [PubMed] [Google Scholar]
- Patel Y. C., Reichlin S. Somatostatin in hypothalamus, extrahypothalamic brain, and peripheral tissues of the rat. Endocrinology. 1978 Feb;102(2):523–530. doi: 10.1210/endo-102-2-523. [DOI] [PubMed] [Google Scholar]
- Patel Y. C., Wheatley T., Ning C. Multiple forms of immunoreactive somatostatin: comparison of distribution in neural and nonneural tissues and portal plasma of the rat. Endocrinology. 1981 Dec;109(6):1943–1949. doi: 10.1210/endo-109-6-1943. [DOI] [PubMed] [Google Scholar]
- Rorstad O. P., Epelbaum J., Brazeau P., Martin J. B. Chromatographic and biological properties of immunoreactive somatostatin in hypothalamic and extrahypothalamic brain regions of the rat. Endocrinology. 1979 Nov;105(5):1083–1092. doi: 10.1210/endo-105-5-1083. [DOI] [PubMed] [Google Scholar]
- Rufener C., Amherdt, DuBois M. P., Orci L. Letter: Ultrastructural immunocytochemical localization of somatostatin in rat pancreatic monolayer culture. J Histochem Cytochem. 1975 Nov;23(11):866–869. doi: 10.1177/23.11.1194672. [DOI] [PubMed] [Google Scholar]
- Sagar S. M., Landry D., Millard W. J., Badger T. M., Arnold M. A., Martin J. B. Depletion of somatostatin-like immunoreactivity in the rat central nervous system by cysteamine. J Neurosci. 1982 Feb;2(2):225–231. doi: 10.1523/JNEUROSCI.02-02-00225.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar S. M., Martin J. B. The effect of cysteamine on immunoreactive somatostatin in the rabbit retina. Neurosci Lett. 1982 Dec 31;34(3):265–269. doi: 10.1016/0304-3940(82)90186-0. [DOI] [PubMed] [Google Scholar]
- Scammell J. G., Dannies P. S. Depletion of pituitary prolactin by cysteamine is due to loss of immunological activity. Endocrinology. 1984 Mar;114(3):712–716. doi: 10.1210/endo-114-3-712. [DOI] [PubMed] [Google Scholar]
- Schusdziarra V., Zyznar E., Rouiller D., Boden G., Brown J. C., Arimura A., Unger R. H. Splanchnic somatostatin: a hormonal regulator of nutrient homeostasis. Science. 1980 Feb 1;207(4430):530–532. doi: 10.1126/science.7352262. [DOI] [PubMed] [Google Scholar]
- Schwedes U., Usadel K., Szabo S. Somatostatin prevents cysteamine-induced duodenal ulcer. Eur J Pharmacol. 1977 Jul 15;44(2):195–196. doi: 10.1016/0014-2999(77)90107-8. [DOI] [PubMed] [Google Scholar]
- Selye H., Szabo S. Experimental model for production of perforating duodenal ulcers by cysteamine in the rat. Nature. 1973 Aug 17;244(5416):458–459. doi: 10.1038/244458a0. [DOI] [PubMed] [Google Scholar]
- Sorenson R. L., Grouse L. H., Elde R. P. Cysteamine blocks somatostatin secretion without altering the course of insulin or glucagon release. A new model for the study of islet function. Diabetes. 1983 Apr;32(4):377–379. doi: 10.2337/diab.32.4.377. [DOI] [PubMed] [Google Scholar]
- Sorenson R. L., Lindell D. V., Elde R. P. Glucose stimulation of somatostatin and insulin release from the isolated, perfused rat pancreas. Diabetes. 1980 Sep;29(9):747–751. doi: 10.2337/diab.29.9.747. [DOI] [PubMed] [Google Scholar]
- Srikant C. B., Patel Y. C. Cysteamine-induced depletion of brain somatostatin is associated with up-regulation of cerebrocortical somatostatin receptors. Endocrinology. 1984 Sep;115(3):990–995. doi: 10.1210/endo-115-3-990. [DOI] [PubMed] [Google Scholar]
- Szabo S., Reichlin S. Somatostatin in rat tissues is depleted by cysteamine administration. Endocrinology. 1981 Dec;109(6):2255–2257. doi: 10.1210/endo-109-6-2255. [DOI] [PubMed] [Google Scholar]
- Taniguchi H., Utsumi M., Hasegawa M., Kobayashi T., Watanabe Y., Murakami K., Seki M., Tsutou A., Makimura H., Sakoda M. Physiologic role of somatostatin. Insulin release from rat islets treated by somatostatin antiserum. Diabetes. 1977 Jul;26(7):700–702. doi: 10.2337/diab.26.7.700. [DOI] [PubMed] [Google Scholar]
- Tannenbaum G. S., Epelbaum J., Colle E., Brazeau P., Martin J. B. Antiserum to somatostatin reverses starvation-induced inhibition of growth hormone but not insulin secretion. Endocrinology. 1978 Jun;102(6):1909–1914. doi: 10.1210/endo-102-6-1909. [DOI] [PubMed] [Google Scholar]



