Abstract
BACKGROUND
Altered intestinal function is prevalent in patients with heart failure (HF), but its role in adverse outcomes is unclear.
OBJECTIVE
This study investigated the potential pathophysiologic contributions of intestinal microbiota in HF.
METHODS
We examined the relationship between fasting plasma trimethylamine-N-oxide (TMAO) and all-cause mortality over 5-year follow-up in 720 stable subjects with HF.
RESULTS
Median TMAO level was 5.0 μM, which was higher than in non-HF subjects (3.5 -μM; p < 0.001). There was modest but significant correlation between TMAO and B-type natriuretic peptide (BNP) (r = 0.23; p < 0.001). Higher plasma TMAO level was associated with a 3.4-fold increased mortality risk. Following adjustments for traditional risk factors and BNP, elevated TMAO levels remained predictive of 5-year mortality risk (hazard ratio [HR]: 2.2; 95% confidence interval [CI]: 1.42 to 3.43; p < 0.001), as well as following the addition of estimated glomerular filtration rate to the model (HR: 1.75; 95% CI: 1.07 to 2.86; p < 0.001).
CONCLUSION
High TMAO levels are observed in patients with HF and elevated TMAO levels portend higher long-term mortality risk independent of traditional risk factors.
Keywords: cardiorenal, C-reactive protein, intestinal microbiota, mortality
There is increasing recognition that the gastrointestinal system represents an often overlooked contributor to the pathogenesis of heart failure (HF) syndrome and its adverse complications (1,2). Normally, intestinal barrier function is maintained by a well-balanced intestinal microbial community, intact tight junctions in the mucosa, normal mucosal immunity, and normal sodium and water homeostasis. With HF and associated splanchnic circulation congestion, bacterial translocation can occur due to altered intestinal barrier function, bacterial overgrowth, and impaired host defense, leading to endotoxemia that in turn can contribute to systemic inflammation. Several of these features of altered intestinal function have been observed in patients with HF (3,4). Meanwhile, the impact of progressive venous congestion in subsets of patients with significant congestive signs and symptoms may lead to unwanted consequences of abdominal congestion, including adverse impact on drug absorption and pharmacokinetics (5,6), and renal glomerular and tubular dysfunction resulting from raised intra-abdominal pressures (7,8).
Intestinal microbiota are implicated in the regulation of multiple host metabolic pathways that contribute to phenotypes such as obesity and insulin resistance (9). Our group has recently described a mechanistic link between intestinal microbe-dependent generation of trimethylamine-N-oxide (TMAO) and increased risk for future cardiovascular events (death, myocardial infarction, and stroke) by a pathway involving dietary nutrients such as phosphatidylcholine, choline, and carnitine (10,11). In chronic systolic HF, the relationship between the intestinal microbiota-generated metabolite TMAO and both cardiorenal indices and long-term clinical prognosis have not been examined. We sought to investigate the relationship between fasting plasma TMAO levels and long-term clinical prognosis in stable patients with HF undergoing cardiac evaluation, particularly in relation with established prognostic markers (B-type natriuretic peptide [BNP] and estimated glomerular filtration rate [eGFR]).
METHODS
STUDY POPULATION
We prospectively enrolled stable cardiac patients seen at the Cleveland Clinic between 2001 and 2007 with a history of HF undergoing elective, nonurgent, coronary angiographic evaluation. We excluded those patients who had experienced acute coronary syndrome within the preceding 30 days (cardiac troponin I ≤0.03 ng/ml). All-cause mortality was prospectively tracked for 5 years by Social Security Death Index and medical chart review and confirmed by follow-up contact. We also performed a cross-sectional comparison of TMAO levels between our cohort of stable systolic HF patients and an independent set of 300 prospectively recruited, apparently healthy individuals without known cardiac disease from a health-screening program at various locations across Cleveland, Ohio. All subjects for all studies gave written informed consent approved by the Cleveland Clinic Institutional Review Board (IRB).
STUDY DESIGN AND ASSAY MEASUREMENTS
This is a single-center, prospective cohort study approved by the Cleveland Clinic IRB. After informed consent, fasting plasma blood samples were collected using ethylenediaminetetraacetic acid (widely known as EDTA) tubes, then immediately processed and frozen in −80°C until analysis. Quantification of fasting plasma TMAO levels was performed utilizing stable isotope dilution liquid chromatography with online tandem mass spectrometry (LC/MS/MS) on an APi 5000™ triple quadruple mass spectrometer (AB SCIEX, Framingham, MA) as previously described (10,11). BNP, high-sensitivity C-reactive protein (hsCRP), fasting lipid panel, uric acid, and serum creatinine were measured using the Architect ci8200 platform (Abbott Laboratories, Abbott Park, IL). Serum arylesterase activity was measured in the same platform as previously described (12).
STATISTICAL ANALYSES
Continuous variables were summarized as mean ± standard deviation (SD) if normally distributed and median (interquartile ranges [IQR]) if not normally distributed. The Student’s t-test or Wilcoxon-Rank sum test for continuous variables and chi-square test for categorical variables were employed to examine between-group differences. Spearman’s correlation was used to examine the associations between TMAO and other laboratory measurements. Kaplan-Meier survival plots and Cox proportional hazards analysis were used to determine hazard ratio (HR) and 95% confidence intervals (95% CI) for all-cause mortality stratified according to TMAO as a continuous variable (log transformed per SD increase) as well as in quartiles. Adjustments were made for individual traditional risk factors including age, sex, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol (HDL-C), smoking, and diabetes mellitus, as well as BNP to predict all-cause mortality risks. Net reclassification and area under Receiver Operator Characteristic curve (AUC) were calculated according to mortality risk estimated using Cox models adjusted for the above-mentioned traditional risk factors with versus without TMAO as previously described (13,14). All analyses performed used R 2.15.1 (Vienna, Austria). A p value <0.05 was considered statistically significant.
RESULTS
BASELINE CHARACTERISTICS
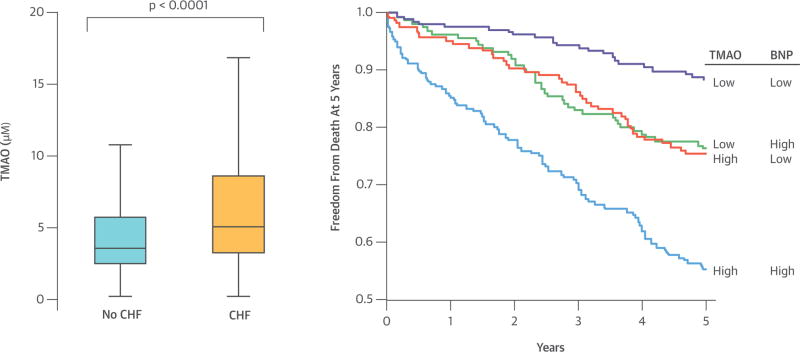
We analyzed a total of 720 subjects with a history of HF fulfilling inclusion and exclusion criteria. Baseline characteristics of our study cohort are provided in Table 1. The median TMAO level within the HF cohort was 5.0 μM (IQR 3.0 to 8.5 μM), which is higher than that in the healthy control cohort (3.5 μM [IQR 2.3 to 5.7 μM]; p < 0.001; Central Illustration). In our study cohort, patients with elevated TMAO levels tended to be older with a history of diabetes mellitus, renal insufficiency, and lower HDL-C levels. HF subjects with higher TMAO levels were also more likely to have higher BNP and diuretic use and lower beta-blocker use. In contrast, history of hypertension, ischemic etiology, left ventricular ejection fraction, smoking history, body mass index, and sex were similar across TMAO levels. Within the HF subject cohort, plasma TMAO levels were weakly but significantly inversely correlated with serum arylesterase activity (r = −0.135; p < 0.001), a measure of the antioxidant HDL-associated enzyme paraoxonase-1 (PON1) (12), and positively correlated with serum uric acid levels (r = 0.29; p < 0.001), which is associated with pro-oxidant function (15,16).
TABLE 1.
Baseline Characteristics of Heart Failure Cohort (n = 720)
| Characteristic | Overall | TMAO <5 μM | TMAO ≥5 μM | p Value |
|---|---|---|---|---|
| Age, yrs | 66 ± 10 | 64 ± 11 | 68 ± 10 | <0.001 |
| Male gender, % | 59 | 59 | 59 | 1.000 |
| Diabetes mellitus, % | 41 | 31 | 51 | <0.001 |
| Hypertension, % | 78 | 76 | 79 | 0.316 |
| Ischemic etiology, % | 64 | 63 | 65 | 0.673 |
| LV ejection fraction, % (units) | 35 (25–50) | 35 (25–51) | 40 (25–50) | 0.567 |
| Body mass index, kg/m2 | 28.4 (25.1–33.1) | 28.7 (25.2–33.3) | 28.1 (24.8–32.9) | 0.298 |
|
| ||||
| Baseline medications | ||||
| ACE inhibitors ARBs,(% | 69 | 71 | 66 | 0.206 |
| Beta-blockers, % | 69 | 73 | 65 | 0.031 |
| Loop diuretics, % | 59 | 53 | 65 | 0.001 |
| Statins, % | 61 | 66 | 57 | 0.013 |
| Aspirin, % | 64 | 66 | 63 | 0.505 |
|
| ||||
| LDL cholesterol, mg/dl | 91 (73–112) | 93 (75–113) | 88 (71–111) | 0.069 |
| HDL cholesterol, mg/dl | 32 (26–40) | 34 (27–42) | 30 (25–38) | <0.001 |
| BNP, pg/ml | 294 (114–658) | 226 (96–498) | 358 (150–907) | <0.001 |
| hsCRP, mg/l | 3.9 (1.6–9.0) | 2.9 (1.3–8.1) | 3.4 (1.3–8.9) | 0.585 |
| eGFR, ml/min/1.73 m2 | 72 (56–87) | 83 (70–93) | 60 (44–74) | <0.001 |
| TMAO, mM | 5 (3.0–8.5) | 3 (2.2–4) | 8.5 (6.6–13.6) | <0.001 |
Values expressed in mean ± standard deviation, percentages, median (interquartile ranges).
Abbreviations: ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; BNP = B-type natriuretic peptide; eGFR = stimated glomerular filtration rate; HDL = high-density lipoprotein; hsCRP = high-sensitivity C-reactive protein; LDL = low-density lipoprotein; LV = left ventricular; TMAO = trimethylamine N-oxide.
CENTRAL ILLUSTRATION. Comparison of fasting TMAO levels between patients with stable heart failure and apparently healthy normal controls.
Trimethylamine-N-oxide (TMAO) concentration was higher in patients with stable heart failure than healthy controls and portended poorer survival at higher levels regardless of B-type natriuretic peptide levels. Kaplan-Meier curves for 5-year all-cause mortality with trimethylamine-N-oxide (TMAO) with TMAO/B-type natriuretic peptide (BNP) stratified at median levels.
Fasting Plasma TMAO and Mortality Risk
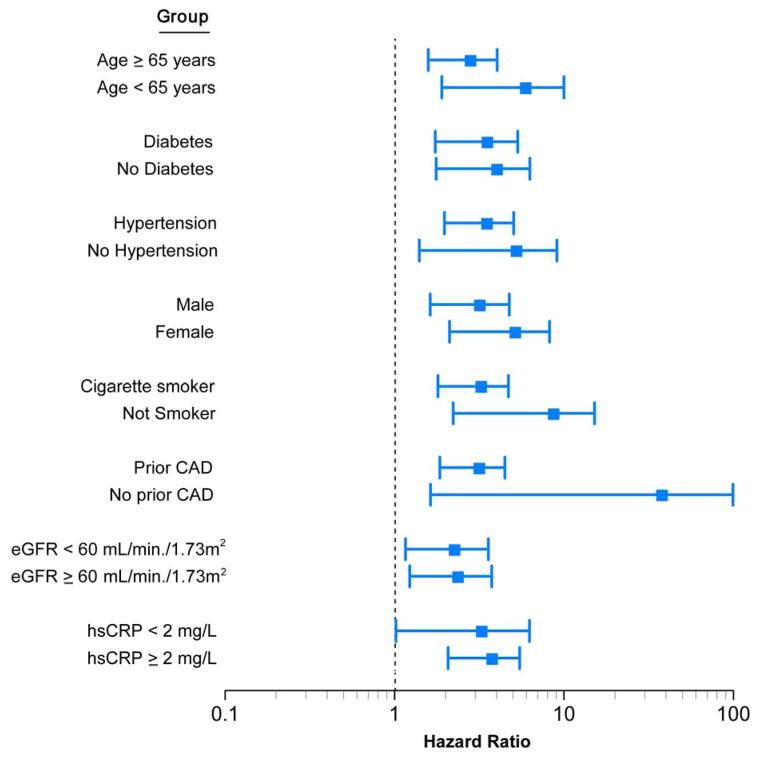
Over 5-year follow-up, a total of 207 deaths occurred in our study cohort. Figure 1 represents the Kaplan-Meier analysis of TMAO stratified by quartiles, which illustrates a graded increased mortality risk that becomes particularly apparent when TMAO levels rise higher than median levels. Importantly, elevated TMAO levels are associated with increased mortality risk within the HF cohort (quartile 4 vs. 1, HR: 3.42; 95% CI: 2.24 to 5.23; p < 0.001). As a continuous variable, an increase in TMAO levels was associated with increased mortality risk at 5 years after adjusting for traditional cardiac risk factors (HR: 1.18; 95% CI: 1.06 to 1.31 per SD; p < 0.01); moreover, mortality risks were similar among ischemic and nonischemic HF subjects, as well as other clinical subgroups (Figure 2). Addition of TMAO to a model of traditional cardiovascular risk factors showed elevated TMAO levels significantly improved net reclassification (integrated discrimination improvement 16.0%; p < 0.001; net reclassification index 10.9%; p < 0.001) and trended towards improvement in AUC (0.723 to 0.741, p = 0.096).
FIGURE 1. Kaplan-Meier estimates of risk of all-cause mortality according to quartiles of plasma levels of TMAO.
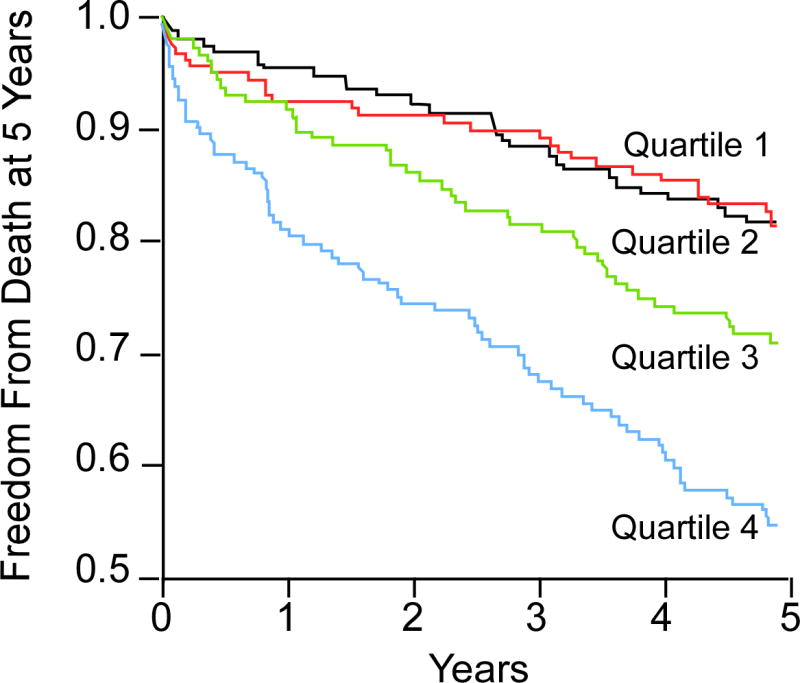
Kaplan-Meier curves for 5-year all-cause mortality with trimethylamine-N-oxide (TMAO) stratified as quartiles.
FIGURE 2. Relationship between Plasma TMAO Concentration and Mortality Risk Stratified According to Baseline Characteristics.
Forest plot of the hazard ratio (squares) of 5-year all-cause mortality risk comparing first and fourth quartiles of fasting plasma TMAO levels. Bars represent 95% confidence intervals. The wide confidence intervals in some subgroups are in part due to their small sample sizes and event rates. CAD = coronary artery disease; eGFR = estimated glomerular filtration rate; hsCRP = high-sensitivity C-reactive protein.
Association between TMAO and Cardiorenal Indices in Mortality Risk
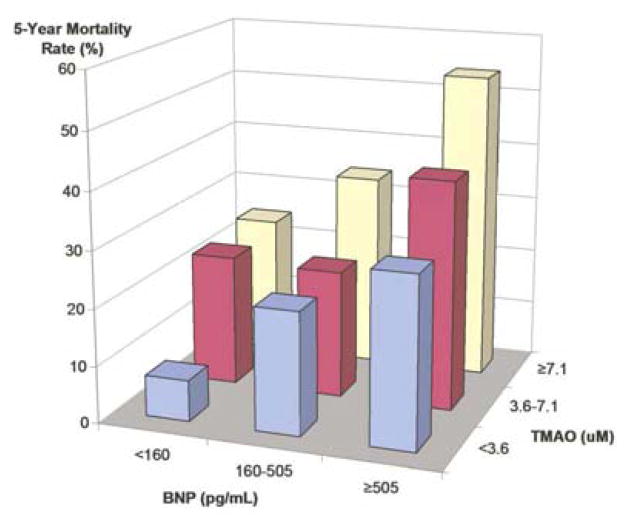
We found relatively modest but significant correlation between TMAO and BNP (r = 0.23; p < 0.001), and stronger inverse correlation between TMAO and eGFR (r = −0.55, p <0.001) in our study cohort. We further investigated the association between TMAO and cardiorenal indices by constructing a model that included BNP and eGFR. In this model, individuals at the highest quartile of TMAO (>8.5 μM) remained at a significantly higher mortality risk than those with lower TMAO levels. Specifically, elevated fasting TMAO levels were associated with a 2.2-fold increase in mortality risk after adjusting for traditional risk factors and BNP (HR: 2.20; 95% CI: 1.42 to 3.43; p < 0.01), and a 1.80-fold increase in mortality risk even after adjusting for traditional risk factors and BNP plus eGFR (HR: 1.75; 95% CI: 1.07 to 2.86; p < 0.05) and plus hsCRP (HR: 1.85; 95% CI: 1.14 to 3.00; p < 0.05). Interestingly within the intermediate BNP range (second tertile 160–505 pg/ml), the lower two tertiles of TMAO portend a 3.3-fold increase in mortality risk (95% CI: 1.4 to 8; p < 0.001), while the highest TMAO tertile (>7.2 μM) had a 5.7-fold increased mortality risk (95% CI: 2.5 to 13.2; p< 0.01) compared to both low BNP and TMAO levels.
DISCUSSION
The key finding to our study is the strong prognostic value of plasma TMAO levels in stable patients with HF incremental to traditional risk factors, cardiorenal indices (BNP and eGFR), and markers of systemic inflammation (hsCRP). It is intriguing that in the setting of elevated natriuretic peptides, which often represent significant myocardial disease progression, a relatively low fasting TMAO level is associated with far lower mortality risk than that seen with elevated levels of both markers. These observations further refine in a large HF patient population the notion of a “gut hypothesis,” and suggest a potential association between the gut microbiota pathway of known pro-atherogenic potential to adverse prognosis in HF subjects.
While TMAO has been shown to possess pro-atherogenic properties (10,11,17), which might partially contribute to the adverse prognosis associated with higher TMAO levels, the equivalent adverse prognostic value of elevated TMAO levels in both ischemic and nonischemic HF suggests the possibility that other potential mechanisms contribute to the increased mortality risks observed in HF subjects with elevated TMAO levels. Our recent identification of the host-microbiome relationship in formation of TMAO (10,11,17) has generated novel insights into potential contributions of environmental factors within the traditional definition of “self.” While the “gut hypothesis” in HF has prevailed over the years with evidence implying bacterial translocation and heightened inflammatory responses and oxidative stress, few studies have definitively linked such associations with outcomes or directly demonstrated such processes at the bedside. Our current observations therefore provided direct evidence that accumulation in the systemic circulation of TMAO, an obligatory down-stream metabolite of gut microbiota, heralds increased long-term adverse clinical outcomes in subjects with HF independent of multiple cardiorenal indices. It remains to be determined whether elevated TMAO levels are associated with enhanced gut edema and bacterial translocation in HF, nor have we identified an alteration in the microbial composition within HF subjects at increased adverse prognosis as the source of elevated TMAO among those at increased mortality risk. In the setting of HF, where ischemic or atherogenic processes appear less important in long-term prognosis and progression, the underlying mechanisms for the association between TMAO and adverse outcomes are not entirely understood.
Clearly, other factors such as individual variations in gut microbiota composition and impaired renal clearance may influence systemic TMAO levels. The notion that alterations in microbial composition and gut microbial functional physiology may be part of the HF syndrome is attractive (2). Changes in bacterial composition have been shown to serve as a primary driver of TMAO levels in other studies (10,15). Whether microbial composition changes in HF directly or indirectly contribute to enhanced oxidative stress and inflammation is unclear, but we do note that plasma TMAO levels were modestly yet positively correlated with serum uric acid levels and inversely correlated with PON1 activity as monitored by serum arylesterase activity. Importantly, the association between TMAO levels and eGFR was strong within our study cohort. However, we also observed that TMAO levels confer mortality risk prediction beyond traditional risk factors, BNP, and eGFR, though recognize that the kidneys may be physiologically impaired in eliminating TMAO beyond estimates of glomerular filtration rates.
The present study is the first demonstration of an association between elevated TMAO levels and poor prognosis in patients with HF. Our findings have several important implications that should be discussed. First, by demonstrating the association between TMAO and mortality risks in patients with HF, our findings suggest that gut microbiota composition and functional consequence may contribute to disease progression during HF. With the mechanistic link between TMAO and atherogenesis in animal models (10,17), it is conceivable that progressive vascular remodeling and coronary atherogenesis may occur in the setting of high TMAO. Even in those with no known ischemic heart disease, subclinical concomitant coronary artery disease may occur(18). In fact, previous autopsy findings showed that progressive coronary vasculopathy is not uncommon in patients with HF, nor is the occurrence of sudden cardiac death (19). On the other hand, others have speculated there exists a potential link between progressive wasting in advanced HF and other chronic disease that can be associated with the gut microbiota axis (20).
Second, the present studies suggest a potential clinical utility for clearance of gut microbiome-generated uremic toxins as a possible adjunct therapy for HF. Of note, recent oral potassium binders have shown some promise in clinical testing in patients with HF and cardiorenal compromise (21,22). The ability of these interventions to eliminate harmful compounds via the alimentary track remains unknown. In the setting of advanced renal diseases, direct binders have been reported to reduce indoxyl sulfate, a tryptophan-based gut microbiome metabolite that in animal models shows antiatherosclerotic effects (23). Hence it is conceivable that targeted interventions can directly impact microbiome-host relationships and further investigations are warranted into direct effects of TMAO and other gut microbiome derived metabolites on cardiac remodeling.
STUDY LIMITATIONS
Several limitations of this study are important to point out. First, this is a single-center study that recruited patients at the point of cardiac evaluation for coronary angiography; hence selection bias may ensue to identify a higher proportion of patients with ischemic cardiomyopathy. Also, this is a cohort of patients who underwent elective coronary angiography, thus right heart catheterization was not performed in a large majority of subjects and was not readily available. Also, because of this catchment, the large majority of patients have relatively preserved renal function, and few have advanced cardiorenal diseases or advanced HF (annualized mortality of 5.8%). We also did not have complete information regarding New York Heart Association functional class, presence of cachexia, or atrial fibrillation in our study population, even though we believe that the inclusion of BNP can integrate many of these risk profiles. Another potential limitation is that only a single time-point blood draw after overnight fast was available, and we do not have dietary history to confirm or refute the impact of diet on TMAO levels or their cardiorenal consequences. Specifically, we do not have any information regarding gastrointestinal symptoms and pathology or prior antibiotic use or knowledge of prior supplements other than those taken the day of enrollment. As in most outcome studies, interim events and treatment also may influence the primary outcome of all-cause mortality.
Despite these limitations, our findings point to novel insights that provide mechanistic links between gut microbiota-associated metabolism involved in TMAO formation and cardiorenal pathophysiology. Further investigations to test the hypothesis that targeted interventions are warranted to alter the gut microbiota composition to lower TMAO production or enhancement of TMAO clearance to determine whether it is possible to alter the natural history of HF disease progression.
CONCLUSIONS
Patients with HF have elevated TMAO levels compared to those without HF. Elevated fasting TMAO levels portend higher long-term mortality risk independent of traditional risk factors, BNP, and renal function.
FIGURE 3. Mortality Rates Stratified by Tertiles of BNP and TMAO.
Five-year mortality rates according to BNP tertiles and TMAO tertiles. All cohorts were significantly different compared to the reference cohort of BNP <160 pg/ml and TMAO <3.6 μM (all p < 0.01). BNP = B-type natriuretic peptide; other abbreviations as in Figure 1.
TABLE 2.
Hazard Ratio of Fasting Plasma TMAO Levels For 5-Year All-Cause Mortality
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
|---|---|---|---|---|
| Range (μM) | <3.03 | 3.03–5 | 5–8.51 | ≥8.51 |
| Unadjusted | 1 | 1.39 (0.87–2.24) | 2.19 (1.39–3.43)* | 3.42 (2.24–5.23)* |
| Adjusted model 1 | 1 | 1.17 (0.72–1.90) | 1.44 (0.90–2.30) | 2.20 (1.42–3.43)* |
| Adjusted model 2 | 1 | 1.14 (0.70–1.86) | 1.33 (0.83–2.13) | 1.75 (1.07–2.86)† |
| Adjusted model 3 | 1 | 1.18 (0.73–1.91) | 1.34 (0.84–2.15) | 1.85 (1.14–3.00)† |
Model 1: adjusted for traditional risk factors include age, sex, systolic blood pressure, LDL cholesterol, HDL cholesterol, smoking, diabetes mellitus, BNP (log-transformed).
Model 2: adjusted for Model 1 plus eGFR (log-transformed).
Model 3: adjusted for Model 2 plus hsCRP (log-transformed).
p<0.01;
p<0.05
Abbreviations as in Table 1.
PERSPECTIVES.
Competency in Medical Knowledge
The intestinal microbe-dependent metabolite, trimethylamine-N-oxide (TMAO) is related to the pathogenesis of atherosclerosis and may play a role in the development and progression of heart failure.
Translational Outlook
Clinical studies should address the effects of dietary modifications and other interventions that alter microbe-generated intestinal TMAO on the development and progression of heart failure.
Acknowledgments
FUNDING
This research was supported by grants from the National Institutes of Health and the Office of Dietary Supplements (R01HL103866, P20HL113452). The GeneBank study has been supported by NIH grants P01HL076491, P01HL098055, R01HL103931, and the Cleveland Clinic Clinical Research Unit of the Case Western Reserve University CTSA (UL1TR 000439). Dr. Wang was partially supported by an American Heart Association Scientist Development Grant 12SDG12050473. Dr. Hazen is also partially supported by a gift from the Leonard Krieger endowment and by the Foundation LeDucq.
ABBREVIATIONS
- BNP
B-type natriuretic peptidel
- eGFR
estimated glomerular filtration rate
- HF
heart failure
- HR
hazard ratio
- TMAO
trimethylamine N-oxide
Footnotes
DISCLOSURE
Dr. Tang has previously received investigator-initiated research grant support from Abbott Laboratories, Inc. with no personal financial payments. Dr. Wang and Dr. Hazen are named as co-inventor on pending patents held by the Cleveland Clinic relating to cardiovascular diagnostics. Dr. Wang reports that he has the right to receive royalty payment for inventions or discoveries related to cardiovascular diagnostics from Liposciences, Inc. Dr. Hazen reports having been paid as a consultant for the following companies: Abbott Diagnostics, Cleveland Heart Lab, Esperion, Lilly, Liposcience Inc., Merck & Co., Inc., P&G, and Pfizer Inc. Dr. Hazen reports receiving research funds from Abbott, Cleveland Heart Lab, Liposcience Inc., P&G, Pfizer Inc., and Takeda. Dr. Hazen reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from the companies shown here: Abbott Laboratories, Inc., Cleveland Heart Lab., Esperion, Frantz Biomarkers, LLC, and Liposcience Inc. All other authors have no relationships to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krack A, Sharma R, Figulla HR, Anker SD. The importance of the gastrointestinal system in the pathogenesis of heart failure. Eur Heart J. 2005;26:2368–74. doi: 10.1093/eurheartj/ehi389. [DOI] [PubMed] [Google Scholar]
- 2.Verbrugge FH, Dupont M, Steels P, et al. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol. 2013;62:485–95. doi: 10.1016/j.jacc.2013.04.070. [DOI] [PubMed] [Google Scholar]
- 3.Rauchhaus M, Anker SD. Plasma concentrations of bacterial lipopolysaccharide: a marker of infection or inflammation? J Am Coll Cardiol. 2000;36:656–7. doi: 10.1016/s0735-1097(00)00750-6. [DOI] [PubMed] [Google Scholar]
- 4.Sandek A, Bauditz J, Swidsinski A, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1561–9. doi: 10.1016/j.jacc.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Vasko MR, Cartwright DB, Knochel JP, Nixon JV, Brater DC. Furosemide absorption altered in decompensated congestive heart failure. Ann Intern Med. 1985;102:314–8. doi: 10.7326/0003-4819-102-3-314. [DOI] [PubMed] [Google Scholar]
- 6.Carlton LD, Pollack GM, Brouwer KL. Physiologic pharmacokinetic modeling of gastrointestinal blood flow as a rate-limiting step in the oral absorption of digoxin: implications for patients with congestive heart failure receiving epoprostenol. J Pharm Sci. 1996;85:473–7. doi: 10.1021/js9503993. [DOI] [PubMed] [Google Scholar]
- 7.Mullens W, Abrahams Z, Skouri HN, et al. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol. 2008;51:300–6. doi: 10.1016/j.jacc.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 8.Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–96. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–7. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–84. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang WH, Wu Y, Mann S, et al. Diminished antioxidant activity of high-density lipoprotein-associated proteins in systolic heart failure. Circ Heart Fail. 2011;4:59–64. doi: 10.1161/CIRCHEARTFAILURE.110.958348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 14.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–43. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 15.Kanbay M, Segal M, Afsar B, Kang DH, Rodriguez-Iturbe B, Johnson RJ. The role of uric acid in the pathogenesis of human cardiovascular disease. Heart. 2013;99:759–66. doi: 10.1136/heartjnl-2012-302535. [DOI] [PubMed] [Google Scholar]
- 16.Anker SD, Doehner W, Rauchhaus M, et al. Uric acid and survival in chronic heart failure: validation and application in metabolic, functional, and hemodynamic staging. Circulation. 2003;107:1991–7. doi: 10.1161/01.CIR.0000065637.10517.A0. [DOI] [PubMed] [Google Scholar]
- 17.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frankenstein L, Hees H, Taeger T, et al. Clinical characteristics, morbidity, and prognostic value of concomitant coronary artery disease in idiopathic dilated cardiomyopathy. Clin Res Cardiol. 2013;102:771–80. doi: 10.1007/s00392-013-0589-7. [DOI] [PubMed] [Google Scholar]
- 19.Uretsky BF, Thygesen K, Armstrong PW, et al. Acute coronary findings at autopsy in heart failure patients with sudden death: results from the assessment of treatment with lisinopril and survival (ATLAS) trial. Circulation. 2000;102:611–6. doi: 10.1161/01.cir.102.6.611. [DOI] [PubMed] [Google Scholar]
- 20.Bindels LB, Delzenne NM. Muscle wasting: the gut microbiota as a new therapeutic target? Int J Biochem Cell Biol. 2013;45:2186–90. doi: 10.1016/j.biocel.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 21.Pitt B, Anker SD, Bushinsky DA, Kitzman DW, Zannad F, Huang IZ. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011;32:820–8. doi: 10.1093/eurheartj/ehq502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costanzo MR, Heywood JT, Massie BM, et al. A double-blind, randomized, parallel, placebo-controlled study examining the effect of cross-linked polyelectrolyte in heart failure patients with chronic kidney disease. Eur J Heart Fail. 2012;14:922–30. doi: 10.1093/eurjhf/hfs074. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto S, Zuo Y, Ma J, et al. Oral activated charcoal adsorbent (AST-120) ameliorates extent and instability of atherosclerosis accelerated by kidney disease in apolipoprotein E-deficient mice. Nephrol Dial Transplant. 2011;26:2491–7. doi: 10.1093/ndt/gfq759. [DOI] [PMC free article] [PubMed] [Google Scholar]