Abstract
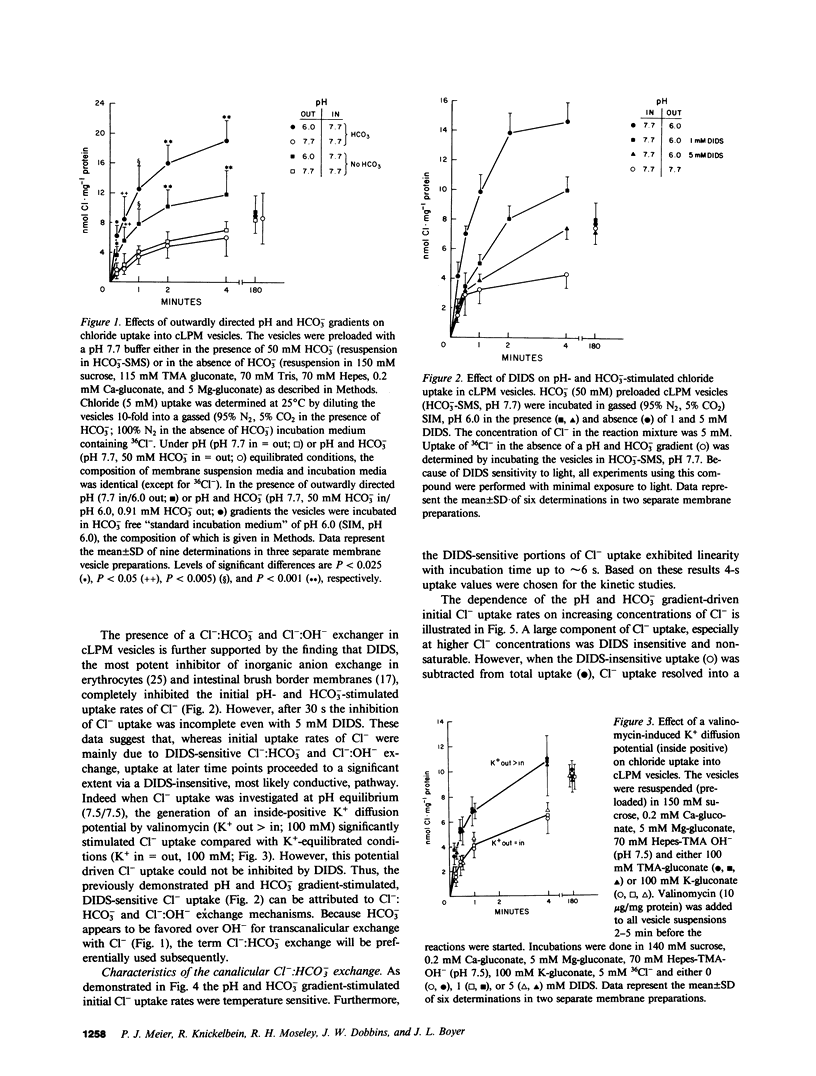
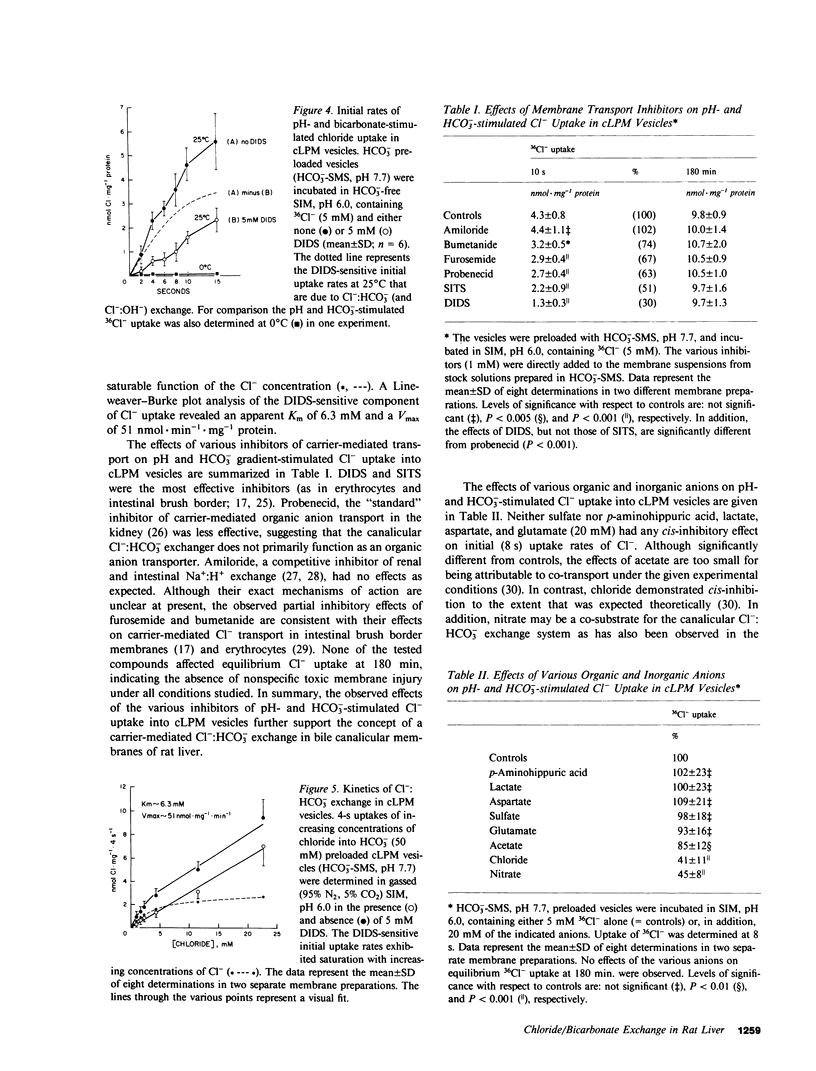
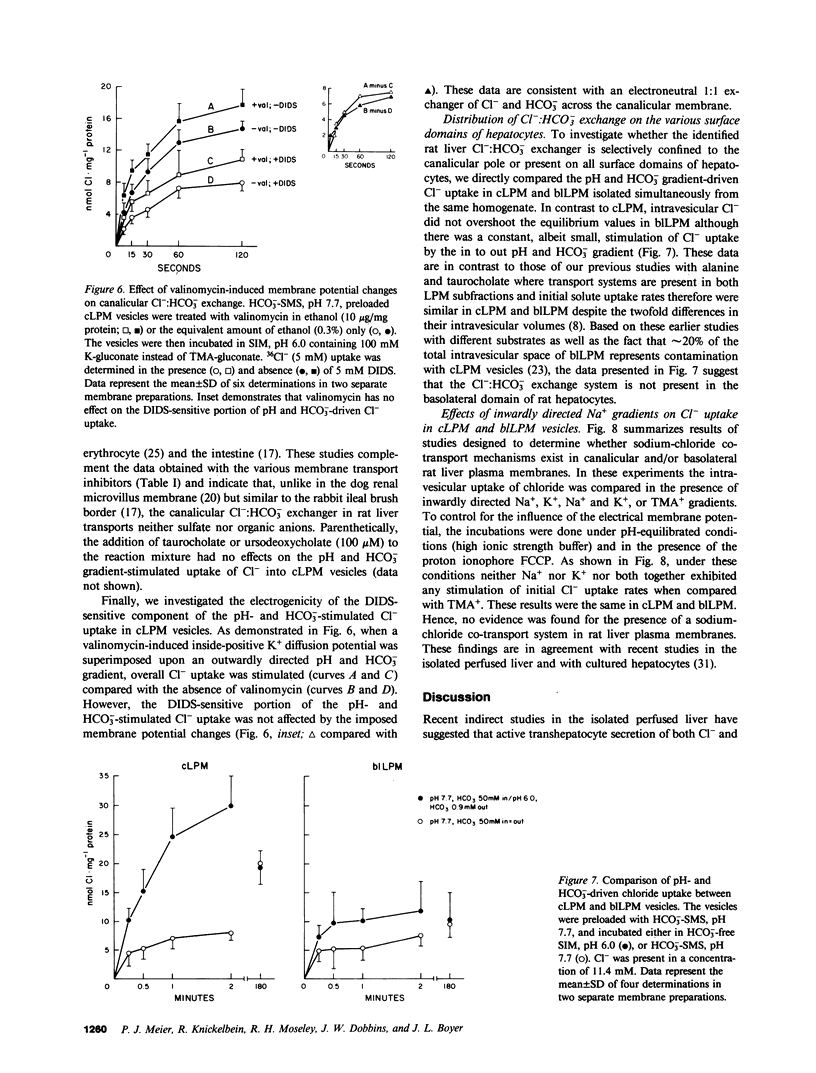
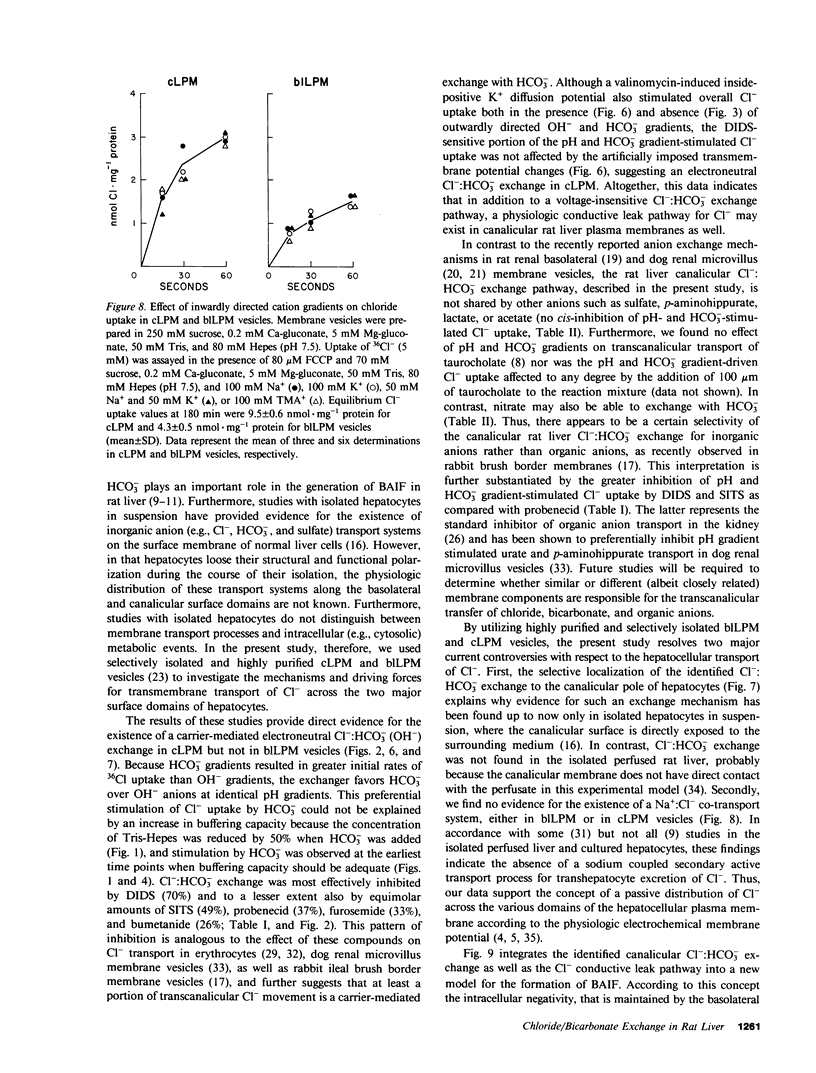
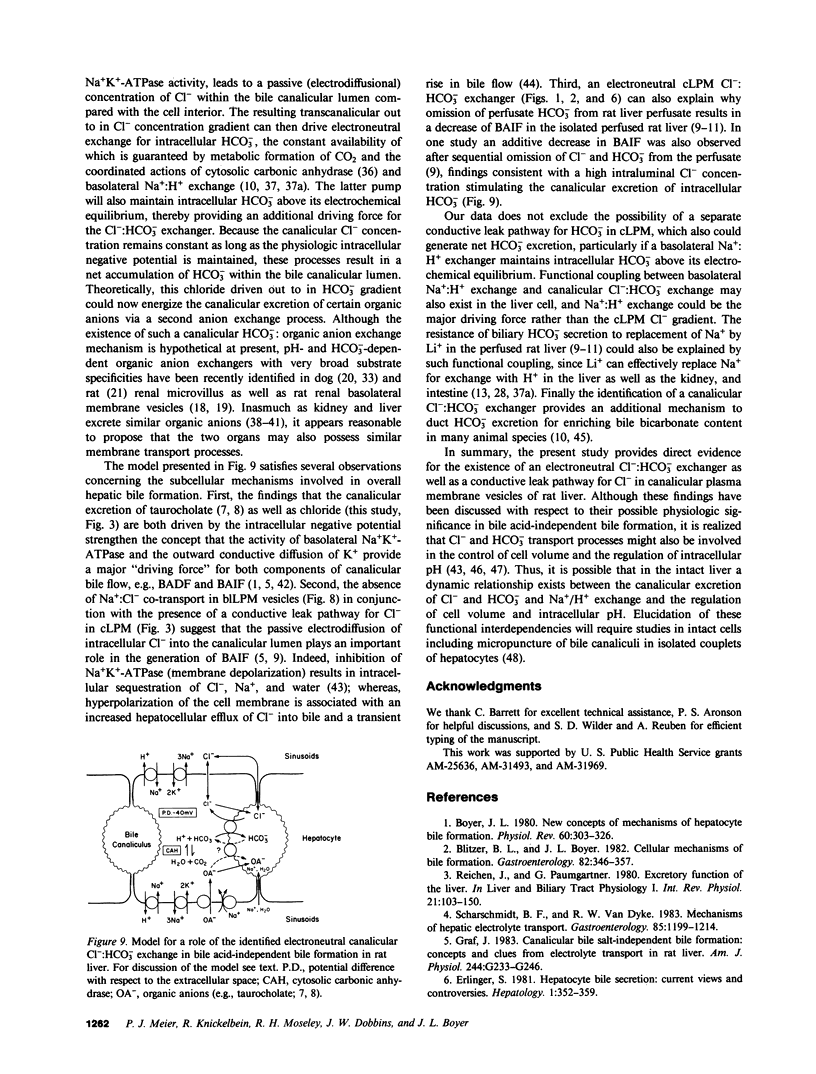
To determine whether anion exchangers might play a role in hepatic bile formation, we looked for the presence of Cl-:OH- and Cl-:HCO3- exchange in highly purified canalicular (c) and basolateral (bl) rat liver plasma membrane (LPM) vesicles. In cLPM vesicles, a pH gradient (7.7 in/6.0 out) stimulated 36Cl- uptake twofold above values obtained during pH-equilibrated conditions (7.7 in = out). When 50 mM HCO3- was also present inside the vesicles, the same pH gradient (7.7 in/6.0 out) resulted in Cl- uptake to levels fourfold above pH- and HCO3--equilibrated controls and two- to threefold above Cl- equilibrium (overshoot). Initial rates of both pH and HCO3- gradient-stimulated Cl- uptake were completely inhibited by 4,4'-diisothiocyano-2,2'-disulfonic acid stilbene (DIDS). A valinomycin-induced K+ diffusion potential (inside positive) also stimulated Cl- uptake in cLPM, but this conductive Cl- pathway was insensitive to DIDS. The DIDS-sensitive, pH and HCO3- gradient-stimulated Cl- uptake demonstrated: saturation with Cl- (Km approximately 6.3 mM; Vmax approximately 51 nmol X mg-1 X min-1); partial inhibition by bumetanide (26%), furosemide (33%), probenecid (37%), and 4-acetamido-4'-isothiocyano-2,2'-disulfonic acid stilbene (49%); cis-inhibition by chloride and nitrate but not by sulfate and various organic anions, and independence from the membrane potential. These data demonstrate the presence of an electroneutral Cl-:OH- and Cl-:HCO3- exchanger in rat liver canalicular membranes that favors Cl-:HCO3- exchange. In contrast, no evidence was found for the presence of a Cl-:HCO3- (OH-) exchange system in blLPM vesicles. Furthermore, neither blLPM nor cLPM vesicles exhibited Na+-stimulatable Cl- uptake, indicating the absence of a NaCl co-transport system in either LPM subfraction. These findings are consistent with a functional role for a Cl-:HCO3- (OH-) exchanger in canalicular bile formation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwer M. S., Hegner D. Role of inorganic electrolytes in bile acid-independent canalicular bile formation. Am J Physiol. 1983 Feb;244(2):G116–G124. doi: 10.1152/ajpgi.1983.244.2.G116. [DOI] [PubMed] [Google Scholar]
- Arias I. M., Forgac M. The sinusoidal domain of the plasma membrane of rat hepatocytes contains an amiloride-sensitive Na+/H+ antiport. J Biol Chem. 1984 May 10;259(9):5406–5408. [PubMed] [Google Scholar]
- Aronson P. S. Mechanisms of active H+ secretion in the proximal tubule. Am J Physiol. 1983 Dec;245(6):F647–F659. doi: 10.1152/ajprenal.1983.245.6.F647. [DOI] [PubMed] [Google Scholar]
- Blitzer B. L., Boyer J. L. Cellular mechanisms of bile formation. Gastroenterology. 1982 Feb;82(2):346–357. [PubMed] [Google Scholar]
- Boyer J. L. New concepts of mechanisms of hepatocyte bile formation. Physiol Rev. 1980 Apr;60(2):303–326. doi: 10.1152/physrev.1980.60.2.303. [DOI] [PubMed] [Google Scholar]
- Bracht A., Bracht A. K., Schwab A. J., Scholz R. Transport of inorganic anions in perfused rat liver. Eur J Biochem. 1981 Mar;114(3):471–479. doi: 10.1111/j.1432-1033.1981.tb05169.x. [DOI] [PubMed] [Google Scholar]
- Brazy P. C., Gunn R. B. Furosemide inhibition of chloride transport in human red blood cells. J Gen Physiol. 1976 Dec;68(6):583–599. doi: 10.1085/jgp.68.6.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik Z. I., Knauf P. A., Rothstein A. The anion transport system of the red blood cell. The role of membrane protein evaluated by the use of 'probes'. Biochim Biophys Acta. 1978 Sep 29;515(3):239–302. doi: 10.1016/0304-4157(78)90016-3. [DOI] [PubMed] [Google Scholar]
- Claret M., Mazet J. L. Ionic fluxes and permeabilities of cell membranes in rat liver. J Physiol. 1972 Jun;223(2):279–295. doi: 10.1113/jphysiol.1972.sp009847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despopoulos A. Congruence of excretory functions in liver and kidney: hippurates. Am J Physiol. 1966 Apr;210(4):760–764. doi: 10.1152/ajplegacy.1966.210.4.760. [DOI] [PubMed] [Google Scholar]
- Despopoulos A. Congruence of renal and hepatic excretory functions: sulfonic acid dyes. Am J Physiol. 1971 Jun;220(6):1755–1758. doi: 10.1152/ajplegacy.1971.220.6.1755. [DOI] [PubMed] [Google Scholar]
- Erlinger S. Does Na+-K+-atpase have any role in bile secretion? Am J Physiol. 1982 Oct;243(4):G243–G247. doi: 10.1152/ajpgi.1982.243.4.G243. [DOI] [PubMed] [Google Scholar]
- Erlinger S. Hepatocyte bile secretion: current views and controversies. Hepatology. 1981 Jul-Aug;1(4):352–359. doi: 10.1002/hep.1840010413. [DOI] [PubMed] [Google Scholar]
- Graf J. Canalicular bile salt-independent bile formation: concepts and clues from electrolyte transport in rat liver. Am J Physiol. 1983 Mar;244(3):G233–G246. doi: 10.1152/ajpgi.1983.244.3.G233. [DOI] [PubMed] [Google Scholar]
- Graf J., Gautam A., Boyer J. L. Isolated rat hepatocyte couplets: a primary secretory unit for electrophysiologic studies of bile secretory function. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6516–6520. doi: 10.1073/pnas.81.20.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggino S. E., Martin G. J., Aronson P. S. Specificity and modes of the anion exchanger in dog renal microvillus membranes. Am J Physiol. 1983 Jun;244(6):F612–F621. doi: 10.1152/ajprenal.1983.244.6.F612. [DOI] [PubMed] [Google Scholar]
- Hardison W. G., Wood C. A. Importance of bicarbonate in bile salt independent fraction of bile flow. Am J Physiol. 1978 Aug;235(2):E158–E164. doi: 10.1152/ajpendo.1978.235.2.E158. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kinne R., Tran T., Arias I. M. Taurocholate transport by rat liver canalicular membrane vesicles. Evidence for the presence of an Na+-independent transport system. J Clin Invest. 1984 Mar;73(3):659–663. doi: 10.1172/JCI111257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives H. E., Rector F. C., Jr Proton transport and cell function. J Clin Invest. 1984 Feb;73(2):285–290. doi: 10.1172/JCI111212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn A. M., Aronson P. S. Urate transport via anion exchange in dog renal microvillus membrane vesicles. Am J Physiol. 1983 Jan;244(1):F56–F63. doi: 10.1152/ajprenal.1983.244.1.F56. [DOI] [PubMed] [Google Scholar]
- Kahn A. M., Branham S., Weinman E. J. Mechanism of urate and p-aminohippurate transport in rat renal microvillus membrane vesicles. Am J Physiol. 1983 Aug;245(2):F151–F158. doi: 10.1152/ajprenal.1983.245.2.F151. [DOI] [PubMed] [Google Scholar]
- Kinsella J. L., Aronson P. S. Amiloride inhibition of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am J Physiol. 1981 Oct;241(4):F374–F379. doi: 10.1152/ajprenal.1981.241.4.F374. [DOI] [PubMed] [Google Scholar]
- Klos C., Paumgartner G., Reichen J. Cation-anion gap and choleretic properties of rat bile. Am J Physiol. 1979 Apr;236(4):E434–E440. doi: 10.1152/ajpendo.1979.236.4.E434. [DOI] [PubMed] [Google Scholar]
- Knickelbein R., Aronson P. S., Atherton W., Dobbins J. W. Sodium and chloride transport across rabbit ileal brush border. I. Evidence for Na-H exchange. Am J Physiol. 1983 Oct;245(4):G504–G510. doi: 10.1152/ajpgi.1983.245.4.G504. [DOI] [PubMed] [Google Scholar]
- Löw I., Friedrich T., Burckhardt G. Properties of an anion exchanger in rat renal basolateral membrane vesicles. Am J Physiol. 1984 Mar;246(3 Pt 2):F334–F342. doi: 10.1152/ajprenal.1984.246.3.F334. [DOI] [PubMed] [Google Scholar]
- Maren T. H., Sanyal G. The activity of sulfonamides and anions against the carbonic anhydrases of animals, plants, and bacteria. Annu Rev Pharmacol Toxicol. 1983;23:439–459. doi: 10.1146/annurev.pa.23.040183.002255. [DOI] [PubMed] [Google Scholar]
- Meier P. J., St Meier-Abt A., Barrett C., Boyer J. L. Mechanisms of taurocholate transport in canalicular and basolateral rat liver plasma membrane vesicles. Evidence for an electrogenic canalicular organic anion carrier. J Biol Chem. 1984 Aug 25;259(16):10614–10622. [PubMed] [Google Scholar]
- Meier P. J., Sztul E. S., Reuben A., Boyer J. L. Structural and functional polarity of canalicular and basolateral plasma membrane vesicles isolated in high yield from rat liver. J Cell Biol. 1984 Mar;98(3):991–1000. doi: 10.1083/jcb.98.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motais R., Cousin J. L. The inhibitor effect of probencid and structural analogues on organic anions and chloride permeabilities in ox erythrocytes. Biochim Biophys Acta. 1976 Jan 21;419(2):309–313. doi: 10.1016/0005-2736(76)90356-4. [DOI] [PubMed] [Google Scholar]
- Murer H., Ahearn G., Biber J., Cassano G., Gmaj P., Stieger B. Co- and counter-transport mechanisms in brush border membranes and basal-lateral membranes of intestine and kidney. J Exp Biol. 1983 Sep;106:163–180. doi: 10.1242/jeb.106.1.163. [DOI] [PubMed] [Google Scholar]
- Murer H., Burckhardt G. Membrane transport of anions across epithelia of mammalian small intestine and kidney proximal tubule. Rev Physiol Biochem Pharmacol. 1983;96:1–51. doi: 10.1007/BFb0031006. [DOI] [PubMed] [Google Scholar]
- Møller J. V., Sheikh M. I. Renal organic anion transport system: pharmacological, physiological, and biochemical aspects. Pharmacol Rev. 1982 Dec;34(4):315–358. [PubMed] [Google Scholar]
- Pritchard J. B., Renfro J. L. Renal sulfate transport at the basolateral membrane is mediated by anion exchange. Proc Natl Acad Sci U S A. 1983 May;80(9):2603–2607. doi: 10.1073/pnas.80.9.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichen J., Paumgartner G. Excretory function of the liver. Int Rev Physiol. 1980;21:103–150. [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- SPERBER I. Secretion of organic anions in the formation of urine and bile. Pharmacol Rev. 1959 Mar;11(1):109–134. [PubMed] [Google Scholar]
- Scharschmidt B. F., Van Dyke R. W. Mechanisms of hepatic electrolyte transport. Gastroenterology. 1983 Nov;85(5):1199–1214. [PubMed] [Google Scholar]
- Scharschmidt B. F., Van Dyke R. W., Stephens J. E. Chloride transport by intact rat liver and cultured rat hepatocytes. Am J Physiol. 1982 Jun;242(6):G628–G633. doi: 10.1152/ajpgi.1982.242.6.G628. [DOI] [PubMed] [Google Scholar]
- Seifter J. L., Aronson P. S. Cl- transport via anion exchange in Necturus renal microvillus membranes. Am J Physiol. 1984 Dec;247(6 Pt 2):F888–F895. doi: 10.1152/ajprenal.1984.247.6.F888. [DOI] [PubMed] [Google Scholar]
- Van Dyke R. W., Stephens J. E., Scharschmidt B. F. Effects of ion substitution on bile acid-dependent and -independent bile formation by rat liver. J Clin Invest. 1982 Sep;70(3):505–517. doi: 10.1172/JCI110642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOO T. H., HONG S. K. Renal and hepatic excretions of phenol red and bromsulphalein in the dog. Am J Physiol. 1963 May;204:776–780. doi: 10.1152/ajplegacy.1963.204.5.776. [DOI] [PubMed] [Google Scholar]
- van Rossum G. D., Russo M. A. Requirement of Cl- and Na+ for the ouabain-resistant control of cell volume in slices of rat liver. J Membr Biol. 1984;77(1):63–76. doi: 10.1007/BF01871101. [DOI] [PubMed] [Google Scholar]
- von Dippe P., Levy D. Analysis of the transport system for inorganic anions in normal and transformed hepatocytes. J Biol Chem. 1982 Apr 25;257(8):4381–4385. [PubMed] [Google Scholar]



