Abstract
Background
Patients with alcoholic liver disease have been reported to have a significantly lower percentage of body fat (%BF) than controls. The mechanism for the reduction in %BF in heavy alcohol users has not been elucidated. In adipose tissue, Pref-1 is specifically expressed in pre-adipocytes but not in adipocytes. Pref-1 inhibits adipogenesis and elevated levels are associated with reduced adipose tissue mass. We investigated the association between serum Pref-1 and %BF, alcohol consumption, and serum free fatty acids (FFA) in a well-characterized cohort of heavy alcohol users compared to controls.
Methods
One hundred forty-eight subjects were prospectively recruited. The Time Line Follow-Back (TLFB) questionnaire was used to quantify the amount of alcohol consumed over the 30-day period before their enrollment. Anthropometric measurements were performed to calculate %BF. Serum Pref-1 and FFA were measured.
Results
Fifty-one subjects (mean age 32 ± 9 years, 88% men) were non-excessive drinkers whereas 97 were excessive drinkers (mean age 41 ± 18 years, 69% men). Compared to non-excessive drinkers, individuals with excessive drinking had significantly higher levels of Pref-1 (p < 0.01), FFA (p < 0.001), and lower %BF (p = 0.03). Serum levels of Pref-1 were associated with the amount of alcohol consumed during the previous 30 days. Serum Pref-1 was negatively correlated with %BF, but positively associated with serum FFA.
Conclusions
Our data suggest that elevated Pref-1 levels in excessive drinkers might inhibit the expansion of adipose tissue, decreasing %BF in alcoholics. Further work is needed to validate these findings and to better understand the role of Pref-1 and its clinical significance in subjects with heavy alcohol use.
Keywords: Pref-1, free fatty acid, body fat, alcohol
Introduction
Adipose tissue is a metabolically active tissue that plays important roles in the risk and pathogenesis of chronic liver diseases, in particular those related to insulin resistance and the metabolic syndrome (Lumeng, 2010; Vuppalanchi & Chalasani, 2009). There are interesting and unexplained interactions between alcohol intake and fat metabolism. Studies of body composition of alcoholics vs. weight-matched controls (Addolorato et al., 2000) showed that heavy drinkers had significantly lower body fat mass, suggesting that they were less capable of storing ethanol-derived or other calories as fat compared with controls. These changes were reversible with 3 months of abstinence, coincident with restoration of a normal body fat mass (Addolorato, Capristo, Greco, Caputo, et al., 1998; Addolorato, Capristo, Greco, Stefanini, & Gasbarrini, 1997, 1998). On the other hand, moderate alcohol use does not change metabolic rate, post-prandial free fatty acids, or insulin levels (Contaldo et al., 1989). Thus, the interaction between drinking and adipose tissue biology depends on the level of alcohol consumption. Using the large population-based data of the third National Health and Nutrition Examination Survey (NHANES), we have found that there is a decrease in percentage of body fat (%BF) as alcohol use increases (Liangpunsakul, Crabb, & Qi, 2010).
The findings of reduced fat mass in heavy drinkers might have clinical consequences. Recent studies showed that the interaction between liver and adipose tissue plays an important role in whole body lipid homeostasis. In physiological states, white adipose tissue is the major organ to store excess energy (i.e., under positive energy balance conditions) in the form of triglycerides (TG), and it provides the major source of energy for other tissues by releasing fatty acids under negative energy balance conditions. Decreasing body fat and its storage in adipose tissue may cause excess fatty acid influx into the liver, leading to hepatic steatosis (Cusi, 2010; Lafontan & Girard, 2008; Wree, Kahraman, Gerken, & Canbay, 2011). In fact, a few clinical studies have demonstrated that lower fat mass is associated with higher liver fat in alcoholics (Addolorato et al., 1997; Addolorato, Capristo, Greco, Stefanini, et al., 1998).
Reduced fat mass in heavy drinkers suggests either loss of stored lipid in the form of TG or reduced differentiation of pre-adipocytes into mature adipocytes. Reduction of adipocyte size due to loss of stored lipid might be related to alcohol-induced hyperlipolysis (Zhong et al., 2012).
Adipocytes arise from pre-adipocytes that undergo commitment and differentiation to mature adipocytes (Sul, 2009). Pre-adipocyte factor-1 (Pref-1) is a 385-amino acid protein and a member of the epidermal growth factor-like family of proteins. It is highly expressed in pre-adipocytes but decreases during the process of differentiation and is absent in mature adipocytes. In fact, high levels of Pref-1 inhibit adipogenesis (Sul, 2009). Previously, our group showed that alcohol inhibits the differentiation of pre-adipocytes into adipocytes (Crabb, Zeng, Liangpunsakul, Jones, & Considine, 2011). This process might involve the activation of Pref-1, reflected by increased serum concentrations. The role of Pref-1 and body fat in human subjects with heavy alcohol use has not been studied. We hypothesized that excessive drinkers would have higher levels of Pref-1 than non-excessive drinkers and that higher Pref-1 would be associated with lower %BF. To test this hypothesis, we conducted a clinical study to investigate the association between serum Pref-1 and %BF, amount of alcohol consumption, and free fatty acid in a well-characterized cohort of excessive alcohol use compared to controls.
Materials and methods
Subjects
Ninety-seven subjects with a history of alcohol use disorder who were admitted for alcohol rehabilitation at Fairbanks Drug and Alcohol Treatment Center in Indianapolis, Indiana were recruited. All subjects reported the last alcohol drinking to be from 0–4 days before the enrollment. They all met the criteria for ‘excessive drinking’ according to the NIH/NIAAA, which are defined as men who drink more than 4 standard drinks in a day (or more than 14 per week) and women who drink more than 3 standard drinks in a day (or more than 7 per week). Fifty-one non-excessive drinkers were recruited from Richard L. Roudebush Veterans Administration Medical Center in Indianapolis, Indiana. The inclusion criteria required subjects to be at least 21 years of age or older and to be able to provide informed consent. Subjects were excluded if they had active and serious medical diseases at the time of screening (such as congestive heart failure, chronic obstructive pulmonary disease, cancer, uncontrolled diabetes, and chronic renal failure), had a history of any systemic infection within 4 weeks prior to the study, and had a history of recent major surgeries within the past 3 months. The study design and protocol were approved by the Institutional Review Board at the Indiana University Purdue University Indianapolis (IUPUI), Richard L. Roudebush VAMC Research and Development Program, and at Fairbanks Alcohol Rehabilitation Center. Written informed consent was obtained from each participant.
Data collection
Participants completed a self-administered questionnaire. Demographic data, smoking history, past medical history, and the AUDIT (Alcohol use disorders identification test) alcohol consumption questions (AUDIT-C) were collected. In this study, the Time Line Follow-Back questionnaire was used to determine the amount of alcohol consumption over the 30-day period before the study date. A timeline follow back (TLFB) questionnaire was administered in person by trained study coordinators who reviewed the instructions with the subjects prior to administering the questionnaire, and it was completed by each subject during the interview. The TLFB offers a daily retrospective report of alcohol consumption over the previous 30 days, from which quantity, drinks per drinking occasion, and pattern of drinking can be obtained (Sobell et al., 1988, 2003; Vakili, Sobell, Sobell, Simco, & Agrawal, 2008). Memory aids (weekends and special occasions) were used to enhance recall of alcohol consumption amounts. TLFB consisted of subjects' retrospective recall of the number of “standard” drinks (i.e., defined as the alcohol equivalent of 12 oz. of domestic (U.S.) beer, 5 oz. of 12%-alcohol wine, or 1.5 oz. of 80-proof distilled spirits) consumed on each of the previous 30 days. Participants completed a log based on a monthly calendar to indicate daily alcohol drinking to provide the total number of alcoholic drinks consumed in the previous 30 days prior to enrollment. This quantity was then divided by the total number of drinking days, also within the previous 30 days, to calculate drinks per drinking day. TLFB is established as an effective measure of drinking patterns (Collins, Kashdan, Koutsky, Morsheimer, & Vetter, 2008; Sobell et al., 1988). It also demonstrates a test–retest reliability correlation ranging from 0.86 to 0.90 for normal drinkers within the previous 30 drinking days (Sobell et al., 1988).
Anthropometric measures
Anthropometric measures were obtained with subjects wearing hospital gowns. Height and weight were measured and BMI was calculated as weight (kg)/height2 (m2). Waist circumference was measured by trained study coordinators using a tape measure at the midpoint between the lower margin of the last palpable rib and the top of the iliac crest. The percentage of whole body fat was calculated using the waist to height ratio and age stratified by gender as previously described (Kagawa, Byrne, & Hills, 2008).
Laboratory measures and biochemical analyses
Laboratory evaluation included complete blood counts and comprehensive metabolic panels. Fasting serum from all participants was used to measure serum Pref-1 and serum free fatty acid. Serum Pref-1 concentrations were measured by a sandwich ELISA assay (R&D Systems, Inc., Minneapolis, MN; Catalog number DPRF10, sensitivity 0.034 ng/mL and assay range at 0.156–10 ng/mL). Serum free fatty acid concentrations were determined by an in vitro enzymatic calorimetric method (Wako HR series NEFA-HR[2], Richmond, VA).
Statistical analysis
The basic characteristics of the study participants are presented as mean ± S.D. for continuous variables and as numbers with percentages for categorical variables. Chi-square tests and t tests were used to compare values of categorical variables and continuous variables, respectively. The level and patterns of alcohol consumption during the previous 30 days were modeled categorically into non-excessive and excessive drinking based on the NIAAA definition, as described above. The serum Pref-1, serum free fatty acid (FFA), and percentage of body fat (%BF) between these 2 groups were compared. Alcohol intake was also modeled as a continuous variable by calculating the total number of drinks during the previous 30 days from TLFB. We used linear regression models to examine the association between the total amount of alcohol consumed in the past month with serum Pref-1 concentrations, FFA, and %BF. To determine the relationship of these variables and different levels of alcohol consumption, separate linear regression analyses were performed to explore the relationship between Pref-1, FFA, %BF, and the amount of alcohol consumption during the previous 30 days stratified by quartiles (Q1: < 26 drinks, Q2: 26–116 drinks, Q3: 117–273 drinks, and Q4: ≥ 273 drinks). A 2-tailed p value < 0.05 was considered statistically significant.
Results
One hundred forty-eight subjects were enrolled. Subjects classified as excessive drinkers (n = 91) were older compared to non-excessive drinkers (n = 57; mean age 41 vs. 32 years, p < 0.01). There were no significant differences in height, weight, and body mass index as well as waist circumference between both groups. Excessive drinkers, as expected, had significantly higher AUDIT-C scores (26.7 vs. 4.2, p < 0.001). The average total drinks over the previous month were 241 for excessive drinkers, and 15 for non-excessive drinkers (p < 0.001). Excessive drinkers had higher levels of MCV, AST, ALT, GGT, and %CDT. The detailed demographic and clinical characteristics of subjects in these 2 groups are shown in Table 1.
Table 1.
Selected demographic, clinical and laboratory characteristics of participants with non-excessive and excessive drinking
| Non-excessive drinkers (n = 51) | Excessive drinkers (n = 97) | p value | |
|---|---|---|---|
| Age (years) | 31.8 ± 9.3 | 40.9 ± 12.2 | < 0.01 |
| Males (n, %) | 45 (88) | 67 (70) | 0.01 |
| Race (Caucasian, n, %) | 43 (84) | 78 (80) | 0.12 |
| Smoking (n, %) | 29 (56%) | 74 (76%) | 0.01 |
| Height (cm) | 176.7 ± 7.1 | 175.1 ± 8.9 | 0.27 |
| Weight (kg) | 89.9 ± 15 | 85.5 ± 19.5 | 0.16 |
| BMI (kg/m2) | 28.7 ± 4.2 | 27.9 ± 6.2 | 0.38 |
| Waist circumference (cm) | 98.1 ± 11.6 | 95.8 ± 12.9 | 0.30 |
| AUDIT-C scores | 4.2 ± 4.0 | 26.7 ± 7.3 | < 0.001 |
| Total standard drinks previous 30 days | 15.2 ± 15.2 | 241.2 ± 146.8 | < 0.001 |
| Average drinks per drinking day | 2.4 ± 2.2 | 12.2 ± 6.4 | < 0.001 |
| Creatinine (mg/dL) | 1.0 ± 0.2 | 0.9 ± 0.2 | 0.11 |
| Albumin (g/dL) | 4.2 ± 0.44 | 3.8 ± 0.4 | 0.001 |
| MCV (Fl) | 90.0 ± 3.9 | 92.6 ± 6.5 | 0.01 |
| AST (U/L) | 23.2 ± 10.2 | 29.8 ± 17.9 | 0.02 |
| ALT (U/L) | 29.7 ± 19.6 | 44.7 ± 17.7 | < 0.001 |
| GGT (U/L) | 35.2 ± 27.6 | 89.8 ± 9.2 | < 0.001 |
| %Carbohydrate deficient transferrin | 1.4 ± 0.8 | 2.6 ± 1.3 | < 0.01 |
| Percentage of body fat | 31.7 ± 5.7 | 29.7 ± 4.7 | 0.03 |
| Serum Pref-1 (ng/mL) | 0.13 ± 0.06 | 0.32 ± 0.13 | < 0.01 |
| Free fatty acid (mmol/L) | 0.42 ± 0.28 | 2.31 ± 0.78 | < 0.001 |
| Free fatty acid/serum albumin ratio | 0.1 ± 0.07 | 0.62 ± 0.23 | 0.0001 |
The levels of alcohol consumption were dichotomized into non-excessive and excessive drinking using the NIAAA definition.
Serum levels of Pref-1 were significantly elevated in excessive drinkers and correlated with the amount of alcohol consumption during the previous 30 days
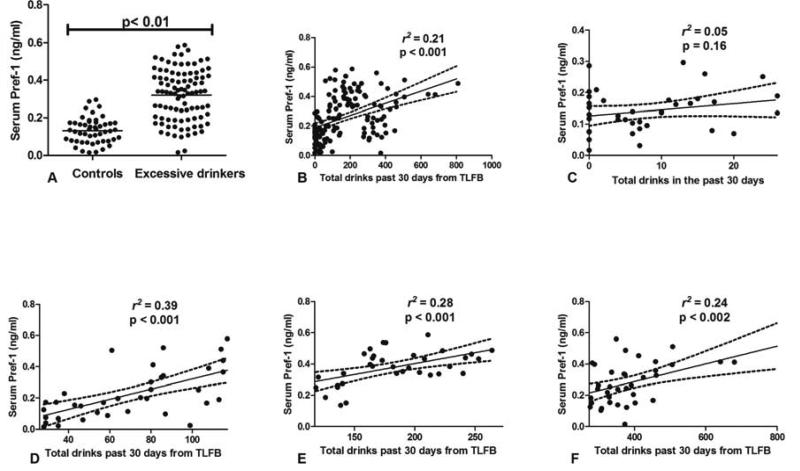
We found that serum concentrations of Pref-1 were significantly higher in excessive drinkers compared to non-excessive drinkers (0.32 ± 0.13 vs. 0.13 ± 0.06 ng/mL, p < 0.01; Table 1 and Fig. 1A). Additionally, serum levels of Pref-1 were significantly correlated with the amount of alcohol consumption as quantified by the TLFB (r2 = 0.21, p < 0.001; Fig. 1B). In a sub-analysis, we explored the relationship between serum Pref-1 and different levels of alcohol consumption by quartiles. We found no association between the levels of serum Pref-1 and the levels of alcohol consumption in the first quartile range (Fig. 1C). We observed a significant association when the levels of alcohol consumption were in the 2nd–4th quartiles range (Fig. 1D–F). Our data suggested that increasing serum Pref-1 levels were observed only when alcohol drinking became excessive.
Figure 1.
(A) Dot plot demonstrating the levels of serum Pref-1 in excessive drinkers and non-excessive drinkers (B) The linear regression analysis between serum Pref-1 and alcohol consumption in the previous 30 days (C–F) The linear regression analysis between serum Pref-1 and the levels of alcohol consumption stratified by quartiles (C for Q1, D for Q2, E for Q3, and F for Q4)
The levels of alcohol consumption were negatively associated with percentage of body fat (%BF)
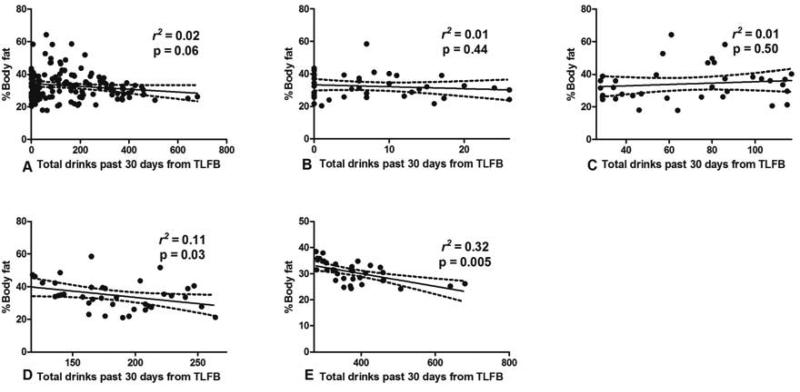
Excessive alcohol drinkers had significantly lower %BF than non-excessive drinkers (29.7% and 31.7%, p = 0.03). We next performed the linear regression analysis to determine the relationship between level of alcohol drinking and %BF. When considering the data from all participants, we observed a trend in the paradoxical relationship between the percentage of body fat and the amount of alcohol consumed (r2 = 0.02, p = 0.06; Fig. 2A). To determine the effect of different levels of drinking and %BF, we next performed separate analyses by dividing the patients into quartiles based on the levels of alcohol consumption. We found that there was no association between alcohol consumption and %BF for participants in the 1st and 2nd quartiles (Fig. 2B and C). However, the %BF was significantly decreased with increased alcohol consumption, specifically, drinking in the 3rd (r2 = 0.11, p = 0.03; Fig. 2D) and 4th quartiles (r2 = 0.32, p = 0.005; Fig. 2E).
Figure 2.
The relationship between %BF and the levels of alcohol consumption in the previous 30 days (A) The linear regression analysis between %BF and the levels of alcohol consumption in the previous 30 days (B–E) The linear regression analysis between %BF and the levels of alcohol consumption stratified by quartiles (B for Q1, C for Q2, D for Q3, and E for Q4)
Percentage of body fat was negatively correlated with serum Pref-1
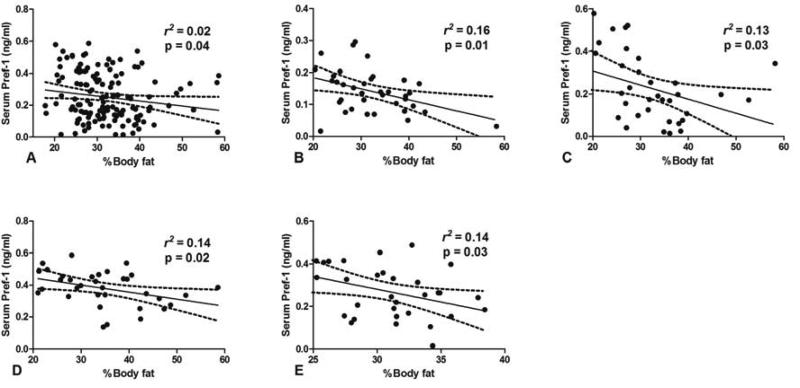
To test the hypothesis that the increased levels of Pref-1 observed in excessive drinkers inhibited adipogenesis, and thus reduced the %BF, we next determined the association between %BF and the levels of serum Pref-1. We observed an inverse correlation between the serum Pref-1 and the %BF (r2 = 0.02, p = 0.04; Fig. 3A). We also performed an additional analysis to further determine the relationship of serum Pref-1 and percentage of body fat, when stratified by different quartiles of alcohol consumption. We found that higher levels of Pref-1 were correlated with lower %BF at all levels of alcohol consumption (Fig. 3B–E).
Figure 3.
(A) The relationship between serum Pref-1 and %BF in all participants (B–E) The linear regression analysis between serum Pref-1 and the levels of %BF of subjects with different degrees of alcohol consumption stratified by quartiles (B for Q1, C for Q2, D for Q3, and E for Q4)
The levels of serum free fatty acids were increased in excessive drinkers and correlated with serum Pref-1 levels
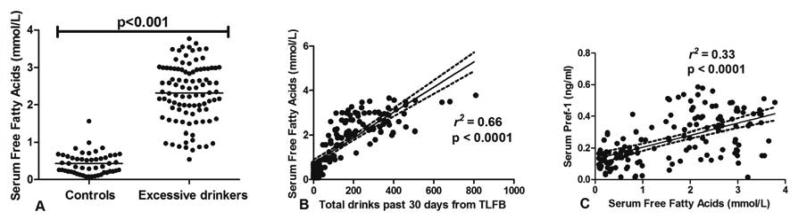
Since excessive alcohol drinking led to a decrease in %BF, we hypothesized that this would reduce the ability of adipose tissues to store TG, as reflected by increasing serum FFA. We found that the levels of serum free fatty acids were significantly higher in excessive drinkers (2.3 vs. 0.42 mmol/L, p < 0.001; Table 1 and Fig. 4A). Since serum albumin levels may affect the levels of free fatty acid, we also did an analysis of FFA corrected with serum albumin (Table 1). We again found that the FFA:albumin ratio was significantly higher in excessive drinkers (0.62 ± 0.23 vs. 0.1 ± 0.07, p = 0.0001). Their levels are positively associated with total alcohol consumption during the previous 30 days (r2 = 0.66, p < 0.0001; Fig. 4B). Interestingly, when we analyzed the relationship between serum Pref-1 and serum FFA to test the hypothesis that decreasing %BF by Pref-1 is associated with an increase in serum FFA, we found that serum Pref-1 is strongly associated with serum free fatty acids (r2 = 0.33, p < 0.0001; Fig. 4C).
Figure 4.
(A) Dot plot demonstrating the levels of serum free fatty acids (FFA) in excessive drinkers and non-excessive drinkers (B) The relationship between serum FFA and the levels of alcohol consumption in the previous 30 days (C) The linear regression analysis between serum Pref-1 and FFA %BF and the levels of alcohol consumption in the previous 30 days
No gender differences found in the serum Pref-1 and the levels of alcohol consumption
Women generally have a higher %BF than men. To determine the influence of gender and alcohol consumption on the levels of serum Pref-1, we analyzed our data stratified by gender. The serum Pref-1 was significantly higher in males with excessive drinking (n = 44) compared to control males (n = 66, 0.13 ± 0.32 vs. 0.03 ± 0.06 ng/mL, p < 0.001). Likewise, females with excessive drinking (n = 29) had higher levels of serum Pref-1 when compared to female controls (n = 6, 0.33 ± 0.13 vs. 0.08 ± 0.03, p < 0.001). We also analyzed the levels of Pref-1 in those with heavy alcohol consumption (n = 97). We found no gender differences in the levels of Pref-1 in this group (male and female: 0.32 ± 0.13 and 0.32 ± 0.14, respectively, p = 0.87).
Discussion
This is the first study to demonstrate an association between Pref-1 and %BF as well as the levels of alcohol consumptions in human subjects. We show here that i) excessive alcohol use is associated with increased levels of serum Pref-1 regardless of gender, ii) Pref-1 is negatively associated with %BF, and iii) Pref-1 concentration is positively correlated with the levels of serum FFA.
Pref-1, a trans-membrane protein highly expressed in the pre-adipocytes, is a member of the EGF-like family of proteins. It is an important negative regulator of adipocyte differentiation (Sul, 2009). In murine models, over-expression of Pref-1 results in mice with significantly reduced fat mass (Sul, 2009). It has been observed that subjects with chronic and heavy alcohol use had significantly lower %BF when compared to controls (Addolorato et al., 1997), although the exact mechanism has not yet been explained. In this study, we demonstrated that serum Pref-1 was higher in excessive drinkers and its levels were significantly associated with the amount of alcohol consumption. Further, we showed that the higher levels of Pref-1 were in fact negatively correlated with the %BF. Our results suggest that Pref-1 could be a mechanistic link between alcohol use and the adipose tissue biology.
Heavy alcohol drinking impairs lipolysis and increases free fatty acid fluxes from adipose tissue to the liver, which eventually can contribute to hepatic steatosis (Zhong et al., 2012). This liver-adipose tissue axis is being recognized as an important pathway to maintain body lipid homeostasis. Our study showed that serum FFA was strongly correlated with the amount of alcohol consumption, suggesting an increase in lipolysis in those with excessive alcohol use. Of importance, we suggest that this might be related to high serum Pref-1 and a decrease in %BF. In general, adipose tissue mass is determined by the storage and removal of triglycerides in the adipocytes. It is known that the rate of triglyceride removal from adipose tissue is increased due to lipolysis in chronic alcohol drinkers (Zhong et al., 2012). However, our data suggest that alcohol might also interfere with the differentiation of pre-adipocytes into adipocytes by increasing levels of Pref-1. Estimates of adipocyte turnover rate and adipocyte death in humans vary greatly, from a low of 10% per year determined by analyzing the integration of 14C derived from nuclear bomb tests into genomic DNA (Spalding et al., 2008) to a high of greater than 60% per year (0.16–0.29% per day) by 2H2O long-term labeling with 2H2O (Strawford, Antelo, Christiansen, & Hellerstein, 2004). The turnover rates are believed to be substantially higher for pre-adipocytes than adipocytes, 4.5% and 0.16– 0.29% per day, respectively (Strawford et al., 2004). It is plausible that alcohol reduced the %BF by reducing the replacement of adipocytes secondary to high Pref-1.
This study has a few limitations. One would argue that the effects of alcohol on adipose tissue and alcoholic steatosis depend not only on the amount of alcohol consumption but also the duration of use. Alcohol consumption during the previous month does not provide a measure of life-time alcohol use. In our study, all excessive drinkers have an alcohol use disorder and the duration of heavy alcohol use ranged from 5–15 years. It is difficult to estimate the amount of lifetime alcohol consumption; however, a previous report has shown correlations between daily reports of drinking pattern/amount and TLFB (Searles, Helzer, & Walter, 2000). Further, TLFB is established as an effective measure of the amount of alcohol consumption (Collins et al., 2008; Sobell et al., 1988). Thus, we believed that it is justified to use the amount of alcohol consumption over the previous month as the independent variable in our study. We also do not know what duration of drinking is required to affect Pref-1 levels. Next, the %BF in our study was calculated based on the anthropometric measurement, not from a body composition analyzer (such as BOD POD). However, the formula that we used to estimate the %BF showed a high correlation with direct body fat measurement in other studies (Kagawa et al., 2008).
In summary, we have shown that Pref-1 is increased in heavy drinkers, and propose that this may reflect an important role of alcohol in adipose tissue biology. Further studies are needed to delineate the role of Pref-1 and its clinical significance in subjects with heavy alcohol use.
Acknowledgment
This study is supported by K08 AA016570 from the NIH/NIAAA, 1I01CX000361-01 from the Veterans Affairs Research and Administration, and W81XWH-12-1-0497 from the U.S. Department of Defense (SL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addolorato G, Capristo E, Greco AV, Caputo F, Stefanini GF, Gasbarrini G. Three months of abstinence from alcohol normalizes energy expenditure and substrate oxidation in alcoholics: a longitudinal study. The American Journal of Gastroenterology. 1998;93:2476–2481. doi: 10.1111/j.1572-0241.1998.00707.x. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Capristo E, Greco AV, Stefanini GF, Gasbarrini G. Energy expenditure, substrate oxidation, and body composition in subjects with chronic alcoholism: new findings from metabolic assessment. Alcoholism: Clinical and Experimental Research. 1997;21:962–967. [PubMed] [Google Scholar]
- Addolorato G, Capristo E, Greco AV, Stefanini GF, Gasbarrini G. Influence of chronic alcohol abuse on body weight and energy metabolism: is excess ethanol consumption a risk factor for obesity or malnutrition? Journal of Internal Medicine. 1998;244:387–395. doi: 10.1046/j.1365-2796.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Capristo E, Marini M, Santini P, Scognamiglio U, Attilia ML, et al. Body composition changes induced by chronic ethanol abuse: evaluation by dual energy X-ray absorptiometry. The American Journal of Gastroenterology. 2000;95:2323–2327. doi: 10.1111/j.1572-0241.2000.02320.x. [DOI] [PubMed] [Google Scholar]
- Collins RL, Kashdan TB, Koutsky JR, Morsheimer ET, Vetter CJ. A self-administered Timeline Followback to measure variations in underage drinkers' alcohol intake and binge drinking. Addictive Behaviors. 2008;33:196–200. doi: 10.1016/j.addbeh.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contaldo F, D'Arrigo E, Carandente V, Cortese C, Coltorti A, Mancini M, et al. Short-term effects of moderate alcohol consumption on lipid metabolism and energy balance in normal men. Metabolism. 1989;38:166–171. doi: 10.1016/0026-0495(89)90257-6. [DOI] [PubMed] [Google Scholar]
- Crabb DW, Zeng Y, Liangpunsakul S, Jones R, Considine R. Ethanol impairs differentiation of human adipocyte stromal cells in culture. Alcoholism: Clinical and Experimental Research. 2011;35:1584–1592. doi: 10.1111/j.1530-0277.2011.01504.x. [DOI] [PubMed] [Google Scholar]
- Cusi K. The role of adipose tissue and lipotoxicity in the pathogenesis of type 2 diabetes. Current Diabetes Reports. 2010;10:306–315. doi: 10.1007/s11892-010-0122-6. [DOI] [PubMed] [Google Scholar]
- Kagawa M, Byrne NM, Hills AP. Comparison of body fat estimation using waist:height ratio using different ‘waist’ measurements in Australian adults. The British Journal of Nutrition. 2008;100:1135–1141. doi: 10.1017/S0007114508966095. [DOI] [PubMed] [Google Scholar]
- Lafontan M, Girard J. Impact of visceral adipose tissue on liver metabolism. Part I: heterogeneity of adipose tissue and functional properties of visceral adipose tissue. Diabetes & Metabolism. 2008;34:317–327. doi: 10.1016/j.diabet.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Liangpunsakul S, Crabb DW, Qi R. Relationship among alcohol intake, body fat, and physical activity: a population-based study. Annals of Epidemiology. 2010;20:670–675. doi: 10.1016/j.annepidem.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN. Adipose tissue macrophages: a piece of the PAI of metabolic syndrome. Science Translational Medicine. 2010;2:20ps7. doi: 10.1126/scitranslmed.3000850. [DOI] [PubMed] [Google Scholar]
- Searles JS, Helzer JE, Walter DE. Comparison of drinking patterns measured by daily reports and timeline follow back. Psychology of Addictive Behaviors. 2000;14:277–286. doi: 10.1037//0893-164x.14.3.277. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Agrawal S, Sobell MB, Leo GI, Young LJ, Cunningham JA, et al. Comparison of a quick drinking screen with the timeline followback for individuals with alcohol problems. Journal of Studies on Alcohol. 2003;64:858–861. doi: 10.15288/jsa.2003.64.858. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, et al. The reliability of alcohol abusers’ self-reports of drinking and life events that occurred in the distant past. Journal of Studies on Alcohol. 1988;49:225–232. doi: 10.15288/jsa.1988.49.225. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- Strawford A, Antelo F, Christiansen M, Hellerstein MK. Adipose tissue triglyceride turnover, de novo lipogenesis, and cell proliferation in humans measured with 2H2O. American Journal of Physiology. Endocrinology and Metabolism. 2004;286:E577–E588. doi: 10.1152/ajpendo.00093.2003. [DOI] [PubMed] [Google Scholar]
- Sul HS. Minireview: Pref-1: role in adipogenesis and mesenchymal cell fate. Molecular Endocrinology. 2009;23:1717–1725. doi: 10.1210/me.2009-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakili S, Sobell LC, Sobell MB, Simco ER, Agrawal S. Using the Timeline Followback to determine time windows representative of annual alcohol consumption with problem drinkers. Addictive Behaviors. 2008;33:1123–1130. doi: 10.1016/j.addbeh.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology. 2009;49:306–317. doi: 10.1002/hep.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wree A, Kahraman A, Gerken G, Canbay A. Obesity affects the liver –the link between adipocytes and hepatocytes. Digestion. 2011;83:124–133. doi: 10.1159/000318741. [DOI] [PubMed] [Google Scholar]
- Zhong W, Zhao Y, Tang Y, Wei X, Shi X, Sun W, et al. Chronic alcohol exposure stimulates adipose tissue lipolysis in mice: role of reverse triglyceride transport in the pathogenesis of alcoholic steatosis. The American Journal of Pathology. 2012;180:998–1007. doi: 10.1016/j.ajpath.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]