Abstract
Given the high incidence and mortality of ARDS in critically ill patients, every practitioner needs a bedside approach both for early identification of patients at risk for ARDS and for the appropriate evaluation of patients who meet the diagnostic criteria of ARDS. Recent advances such as the Lung Injury Prediction Score, the Early Acute Lung Injury score, and validation of the SpO2/FiO2 ratio for assessing the degree of hypoxemia are all practical tools to aid the practitioner in caring for patients at risk of ARDS and will likely become more important in the future as more preventative therapies for ARDS are investigated. For patients who meet the diagnostic criteria for ARDS, the practitioner should focus on a thorough search for an underlying cause as well as the concurrent possibility of an underlying disease process that mimics the clinical syndrome of ARDS.
Keywords: Diagnosis, Evaluation, Acute Respiratory Distress Syndrome, Risk
Introduction
The acute respiratory distress syndrome (ARDS) is a common complication of a variety of illnesses and is associated with significant morbidity and mortality 1,2. Early recognition of the patient at-risk for or with ARDS and identification of the underlying cause allows more timely application of potentially life-saving therapies 3–5. However, in a study by Ferguson et al 6, over 50% of patients with ARDS went unrecognized by their physician and ARDS was only recognized at the time of autopsy. This underrecognition of ARDS can partly be related to the lack of sensitivity in the clinical definitions of this syndrome 6,7; however regardless of the reasons, the underrecognition of ARDS likely leads to an underutilization of ARDS-specific therapies. For example, the benefit of lower tidal volume protective ventilator strategy has been established for over ten years 3, but approximately 25% of patients worldwide with ARDS still do not receive this therapy 8.
Therapies for ARDS have been reported to improve ARDS-related mortality by 8–16% 3–5. Use of these therapies is dependent on the practitioner applying the definition of ARDS 7 at the bedside to make the diagnosis. However, the clinical definition of ARDS does not identify patients at risk for later development of ARDS, consider other conditions that can mimic ARDS, nor does it take into account the respiratory dysfunction that exists prior to meeting ARDS criteria that might benefit from early implementation of therapy. Illustrated with case-based presentations, this review aims to describe a bedside approach to the early identification of critically ill patients at risk of developing ARDS as well as a practical approach to diagnosis and evaluation for the underlying cause in patients with ARDS.
Identifying patients at-risk of developing ARDS
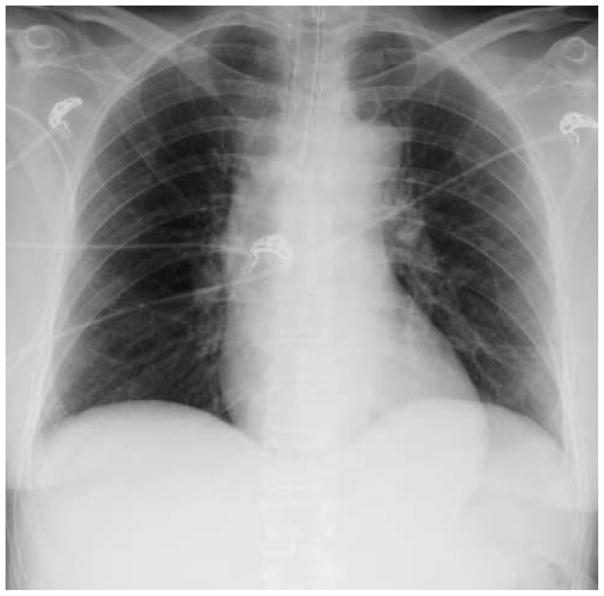
A 65-year-old woman with a history of diabetes mellitus presents with an acute abdomen, fever, tachycardia, leukocytosis and hypotension. She is found to have a perforated diverticulum with an intra-abdominal abscess that is effectively drained in the operating room. Post-operatively and despite fluid resuscitation and broad-spectrum antibiotics, she arrives in the ICU hypotensive and mechanically ventilated. Vasoactive medications are initiated for blood pressure support. Her chest radiograph shows no pulmonary infiltrates (Figure 1) and she has an arterial oxygen saturation of 95% on mechanical ventilation with a fraction of inspired oxygen of 0.40. What is the subsequent risk of this patient developing ARDS during his course in the ICU?
Figure 1. Chest Radiograph.
No infiltrates were seen on the chest radiograph of the intubated and mechanically ventilated patient in this case presentation.
Recently, increased attention has been given to the critically ill patient at risk of developing ARDS. With numerous studies showing a lack of benefit of pharmacologic interventions aimed at treating patients with established ARDS 9–12, focus has shifted to identifying patients at risk of developing ARDS in order to provide earlier preventative therapies. As an example, early application of low tidal volume ventilation may prevent the development of ARDS in at risk patients 13,14. For this reason, clinical recognition of patients who are at risk of development of ARDS is critically important. A number of strategies have been used to identify patient factors associated with the development of ARDS, including data that is collected both non-invasively and invasively.
Traditional Clinical-Risk Factors and Risk Modifiers for ARDS
ARDS usually develops in the setting of an appropriate clinical risk factor. Awareness of these risk factors and other clinical factors that may increase or decrease risk can facilitate early diagnosis. The acutely injured lung is the end result of a pathologic process characterized by diffuse alveolar damage with influx of inflammatory cells and protein-rich pulmonary edema fluid into the alveolus 15. Although the pathologic findings are similar regardless of the underlying cause, there are many different underlying diagnoses that put patients at-risk for diffuse alveolar damage. Risk factors for the development of ARDS can be divided into diagnoses that induce direct injury to the lung and diagnoses that have an extra-pulmonary origin, with ensuing systemic inflammation causing indirect lung injury. Among diagnoses that directly injure the alveolus, pneumonia (46%) and aspiration of gastric contents (11%) are the most common causes; severe sepsis of a non-pulmonary origin (33%) and trauma (7%) are the most common causes of indirect lung injury 1. Distinct from risk factors, risk modifiers are patient characteristics that are not thought to cause ARDS but may make a risk factor, for example sepsis, more or less likely to cause ARDS. Risk modifiers thought to decrease the risk of ARDS include diabetes mellitus 16; whereas smoking 17, alcohol use 17, hypoalbuminemia 18, oxygen therapy 19, and chemotherapy 20 have all been reported to increase the risk of ARDS in the setting of an appropriate risk factor such as sepsis or severe trauma 21 (Table 1).
Table 1.
Lung Injury Prediction Score
| LIPS Points | Score | Positive-Predictive Value (Risk of the future Development of ARDS) | |
|---|---|---|---|
| Predisposing Conditions | >3 | 14% | |
| Shock | 2 | >4 | 18% |
| Aspiration | 2 | >5 | 23% |
| Sepsis | 1 | ||
| Pneumonia | 1.5 | ||
| High-risk Surgery | |||
| Orthopedic Spine | 1 | ||
| Acute Abdomen | 2 | ||
| Cardiac | 2.5 | ||
| Aortic Vascular | 3.5 | ||
| High-risk Trauma | |||
| Traumatic Brain Injury | 2 | ||
| Smoke Inhalation | 2 | ||
| Near Drowning | 2 | ||
| Lung Contusion | 1.5 | ||
| Multiple Fractures | 1.5 | ||
| Risk Modifiers | |||
| Alcohol Abuse | 1 | ||
| Obesity (BMI >30) | 1 | ||
| Hypoalbuminemia | 1 | ||
| Chemotherapy | 1 | ||
| FiO2 >0.35 (> 4 L/min) | 2 | ||
| Tachypnea (RR > 30) | 1.5 | ||
| SpO2 < 95% | 1 | ||
| Acidosis (pH < 7.35) | 1.5 | ||
| Diabetes Mellitus | -1 | ||
BMI = body mass index
FiO2 = fraction of inspired oxygen
RR = respiratory rate
SpO2 = arterial oxygen saturation
Adapted from Gajic O, Dabbagh O, Park PK, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183(4):462–470. doi:10.1164/rccm.201004-0549OC; with permission.
The Lung Injury Prediction Score
In addition to recognition of broad categories of clinical risk factors, bedside calculation of a risk prediction score may aid in identifying patients at highest risk of ARDS. Early in the study of ARDS it was recognized that certain clinical variables, such as ventilator settings, blood transfusions, and pre-disposing diagnoses were associated with the subsequent development of ARDS 1,22–26. Recent emphasis 27 has been placed on the use of these and other easily obtained clinical variables to predict the development of ARDS in critically ill patients in order to design clinical trials of interventions aimed at the prevention of ARDS. The Lung Injury Prediction Score (LIPS), first described in 2011 21 attempts to account for risk factors for the subsequent development of ARDS, such as sepsis, along with accounting for potential risk modifiers.
The LIPS was developed through an initial single-center, retrospective and prospective observational cohort study 21 and a subsequent larger, multi-center validation study involving over 5,000 at-risk patients 28. In the larger, multi-center study, clinical variables that included both known ARDS risk factors and risk modifiers were collected during the first 6 hours after presentation to an emergency department. Of 5,584 at-risk patients enrolled, 277 (6.8%) subsequently developed ARDS, a median of 2 days after admission. After analyzing the association of both risk factors and risk modifiers with the future development of ARDS, points were assigned to each factor and modifier based on the strength of association in a regression model (Table 1). Calculation of the LIPS allows a percentage describing the risk of future development of ARDS to be assigned to each at-risk patient. For example, in the initial case presentation of a patient with a history of diabetes mellitus, sepsis, shock, FiO2 greater than 0.35, and high-risk surgery, the calculated LIPS score is 6, corresponding to an approximate 23% risk of future development of ARDS.
In the original study, a LIPS of > 4 was found to have good discriminatory power, in that 97% of patients with a score of ≤ 4 did not go on to develop ARDS, while 18% of patients with a score > 4 went on to develop ARDS. As such, clinical trials 29 aimed at preventing the development of ARDS in high risk patients have used a LIPS ≥ 4 to enrich for patients to target for preventative interventions. Although the low positive predictive value of the LIPS 28 (Table 1) is discouraging for the bedside practitioner in predicting which patient will develop ARDS, the LIPS remains the only validated scoring system available and involves almost no invasive testing.
While we await the results of preventative trials to guide the management of the at-risk patient, the bedside clinician can still use the LIPS and other tools 30 to identify patients at risk of ARDS and perform interventions that may decrease such risk. For example, a patient determined to be at high risk for development of ARDS may benefit from the earlier initiation of resuscitation and antibiotics for severe sepsis 20, a more conservative fluid strategy early in their ICU course 31, and lung-protective ventilation even in the absence of ARDS, an intervention that has been associated with improved clinical outcomes 13,14,32. In the case presented above where the patient is in shock, has been volume resuscitated, treated with early antibiotics, and has a LIPS of 6, the addition of lung-protective ventilation may reduce the risk of pulmonary and extra-pulmonary complications and shorten the patient’s hospital stay 14.
Plasma Biomarkers for the Risk Prediction of ARDS
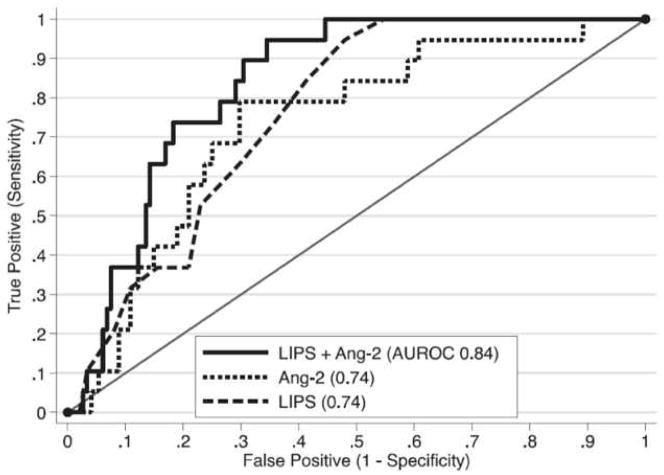
ARDS is the culmination of multiple inflammatory and coagulopathic processes involving both the lung endothelium and epithelium that can produce measurable biomarkers prior to the development of bilateral pulmonary infiltrates on chest imaging 33. As a biomarker of injury to the lung endothelium, plasma angiopoietin-2 (Ang-2) has received the most attention for prediction of the development of ARDS in at-risk patients 34–36. In a recent study by Agrawal et al 36, plasma Ang-2 was measured in a heterogeneous group of 230 patients in the emergency department determined to be at risk for the development of ARDS based on planned admission to an ICU. Not only was Ang-2 significantly higher in patients who went on to develop ARDS, but Ang-2 was at least as predictive of the development of ARDS as the Lung Injury Prediction Score (LIPS) (area under the receiver operating characteristic curve (AUC) of 0.74 and 0.74, respectively). Adding Ang-2 levels to the LIPS further improved discriminatory power (AUC 0.84)(Figure 2). Although less studied, other plasma biomarkers such as club cell protein (CC-16) 37, IL-8 36, and tissue factor 38, have also shown promise in identifying at risk patients.
Figure 2. Receiver Operating Characteristic Curves for the Prediction of the Development of ARDS.
When measured in the emergency department, Angiopoietin-2 (Ang-2) was as predictive as the LIPS in determining which critically ill patients would go on to develop ARDS. The predictive power increased when Ang-2 was added to the LIPS.
From Agrawal A, Matthay MA, Kangelaris KN, et al. Plasma angiopoietin-2 predicts the onset of acute lung injury in critically ill patients. Am J Respir Crit Care Med. 2013;187(7):736–742. doi:10.1164/rccm.201208-1460OC; with permission.
The obvious limitations of using plasma biomarkers in risk prediction are cost, speed, and timing of measurement. None of the above tests are currently available for clinical use nor is it clear what the optimal time is for measurement. The Ang-2 study 36 was performed in the emergency department. However, it is unclear whether Ang-2 measurements would be useful in the already hospitalized patient, post-operative patients, or critically ill patients transferred from other ICU settings. As further data are collected on the predictive value of plasma biomarkers and the cost and time of measurement decreases, perhaps plasma biomarkers will be incorporated to improve the predictive power of other tools such as the LIPS.
Diagnosing ARDS
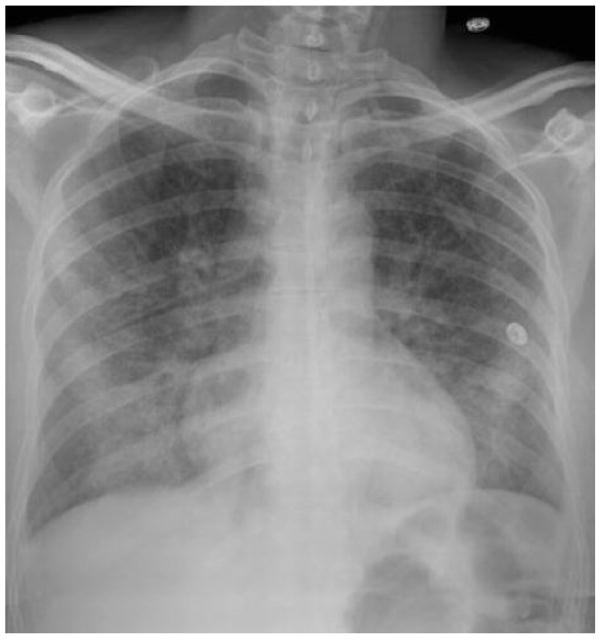
A 40 year old woman who has been admitted to the intensive care unit with community acquired pneumonia is currently requiring 4 liters of oxygen per minute to maintain an arterial oxygen saturation by pulse oximetry (SpO2) of 88%, has a respiratory rate of 32, and has bilateral alveolar infiltrates on her chest radiograph (Figure 3). Does this patient have ARDS and what is her risk of progressing to requiring mechanical ventilation?
Figure 3. Chest Radiograph.
This patient had bilateral alveolar infiltrates on chest radiography; however was not currently requiring mechanical ventilation.
ARDS is a clinical syndrome that is diagnosed by application of clinical definitions that have been developed by expert consensus. The clinical definition of ARDS has undergone recent revision 7 (Table 2) from its original form 39. The current definition’s requirement of positive pressure ventilation, measurement of arterial blood gases, and evaluation of left ventricular function reduce the sensitivity to detect ARDS at the bedside in patients who may not fit these criteria (as in the case presentation), but are still at risk of progressive respiratory failure. A number of strategies have been recently reported to address these shortcomings of the current definitions 7 in diagnosing ARDS at the bedside.
Table 2.
The Berlin Definition of ARDS
| Criteria | Notes | |
|---|---|---|
| Timing | Within 1 week of a known clinical risk factor | If no ARDS risk factor present, echocardiography or PAOP measurement to rule out cardiac causes |
| Chest Imaging | Bilateral opacities (excluding effusions, atelectasis, and nodules) | |
| Cause of Edema | Respiratory failure not purely of cardiac origin | |
| Oxygenation | ||
| Mild ARDS | PaO2/FiO2 = 201–300 mm Hg and PEEP or CPAP ≥ 5 cm H2O | Mild ARDS can be diagnosed even if the patient is receiving non-invasive ventilation |
| Moderate ARDS | PaO2/FiO2 = 101–200 mm Hg and PEEP ≥ 5 cm H2O | |
| Severe ARDS | PaO2/FiO2 ≤ 100 mm Hg and PEEP ≥ 5 cm H2O | |
Adapted from ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. In: Vol 307. 2012:2526–2533. doi:10.1001/jama.2012.5669.
Early Acute Lung Injury
In autopsy studies, the sensitivity of the current definition of ARDS 7 is approximately 89% 40 when practitioners suspect the diagnosis and apply the criteria at the bedside. However the sensitivity of this definition may be reduced by the requirement for either invasive or non-invasive positive pressure ventilation and measurement of arterial blood gases, along with dichotomizing ARDS as either being present or absent rather than recognizing that this syndrome encompasses a spectrum of severity of lung injury.
To address these concerns, Levitt et al 41 recently performed a prospective cohort study to develop a definition of early acute lung injury (EALI) in order to alert the practitioner to patients who have acute lung injury that do not yet meet criteria for diagnosis of ARDS but who have high risk of progression to ARDS and need for invasive mechanical ventilation. Patients enrolled in this study had bilateral infiltrates on chest radiograph, were not mechanically ventilated, did not have a clinical suspicion of isolated left atrial hypertension, and did not have arterial blood gases available. In this group, a requirement of > 2 liters/minute of supplemental oxygen, a respiratory rate ≥ 30 breaths per minute, and immune suppression were found to be independent risk factors for subsequent progression to ARDS with the need for mechanical ventilation. Patients with any two of these factors, termed an EALI score ≥2 (as in the case presentation) had a 53% subsequent risk of progression to ARDS with mechanical ventilation, a higher risk than calculated from concomitant LIPS scoring (33%). The median time from meeting criteria for EALI to need for positive pressure ventilation was 20 hours. The creation of this definition of EALI recognizes ARDS as a spectrum of illness and provides the bedside practitioner with a diagnostic tool to identify ARDS early in its progression which may result in the early application of therapeutic interventions. For example, intensive care unit admission for patients presenting to the emergency department with bilateral infiltrates on chest radiograph and an EALI score ≥2 should strongly be considered given the high risk of rapid progression of respiratory failure requiring mechanical ventilation.
The Diagnosis of ARDS
Recent modifications in the definition of ARDS are worth noting, since they may affect application of the definitions at the bedside. The original American-European Consensus Conference (AECC) definition of ARDS 39 did not specify a timeframe as to what represented an “acute” onset of ARDS. The new Berlin definition 7 requires that the development of ARDS, including bilateral infiltrates on chest radiograph, occur within 1 week of a known precipitant. Secondly, patients receiving ≥ 5 cmH20 of continuous positive airway pressure via non-invasive positive pressure ventilation may now be diagnosed with mild ARDS without the need for invasive mechanical ventilation. The AECC definition of ARDS required that patients have pulmonary arterial occlusion pressures (PAOP), if measured, ≤ 18 mm Hg, whereas the current Berlin definition recognizes that elevated PAOP and ARDS can coexist 42,43 in patients receiving volume resuscitation with pre-existing cardiac disease and elevated end-expiratory intrathoracic pressures. Currently, if a patient’s respiratory failure cannot be explained fully by an ARDS risk factor, objective cardiac testing is needed, such as echocardiography or pulmonary artery catheterization. Finally, the term “acute lung injury (ALI)” has been eliminated from the Berlin definition and mild, moderate and severe categories of ARDS have been created based on ratio of inspired to arterial oxygen (PaO2/FiO2). This clinical definition of ARDS is currently the primary diagnostic tool available at the bedside for practitioners to identify patients with ARDS.
Although both the AECC and Berlin definitions of ARDS require measurement of arterial PaO2 in order to calculate the PaO2/FiO2 ratio, less invasive strategies for measurement of the oxygenation defect that utilize the arterial oxygen saturation measured by pulse oximetry (SpO2) may be useful in diagnosing ARDS and are more continuously available. In a derivation and validation study 44 of approximately 1,000 patients and over 4,000 simultaneous measurements of PaO2, SpO2, and FiO2, use of a SpO2/FiO2 ratio performed very well in comparison to a PaO2/FiO2 ratio in the diagnosis of ARDS. Specifically, SpO2/FiO2 ratios of 315 and 235 corresponded to PaO2/FiO2 ratios of 300 and 200, respectively. In pediatric patients where arterial blood sampling is more difficult than adults, the SpO2/FiO2 ratio may be useful in the diagnosis and prediction of respiratory failure requiring invasive mechanical ventilation in patients with ARDS 45. Although use of a SpO2/FiO2 ratio is not accurate in the extremes of SpO2 and PaO2 given a non-linear relationship at these levels (i.e. when SpO2 is ≥97%), the SpO2/FiO2 ratio represents a simple, noninvasive alternative to arterial blood gas measurement in diagnosing patients with ARDS.
Another challenge in diagnosing ARDS is the differentiation between ARDS and cardiogenic pulmonary edema 46. Several indices on the chest radiograph may aid in this differentiation. Radiographic features of cardiogenic pulmonary edema include increased heart size, widened vascular pedicle width (> 70mm measured from the origin of the left subclavian artery from the aorta to the intersection of the right mainstem bronchus and superior vena cava) 47, centrally located edema, and pleural effusions 48. Transthoracic echocardiography is also useful in this differentiation and has 86% agreement with pulmonary-artery catheters when diagnosing cardiac dysfunction 49. Finally, if the diagnosis of ARDS or cardiogenic pulmonary edema is still in question after chest radiography and echocardiography, pulmonary-artery catheterization may be necessary, while being mindful that the complication rate with this procedure in the critically-ill has been reported as high as 9.5% 50–52. Furthermore, a PAOP of > 18 mm Hg with a normal cardiac index was reported in 29% of patients with known ARDS 43.
Taking into account the EALI definition 41 and the Berlin definition of ARDS 7, the case patient presented above would be considered to have EALI but would not yet meet diagnostic criteria for ARDS. Evaluation of cardiac function would not be necessary to meet diagnostic criteria for ARDS as the patient’s respiratory failure can be explained by the presence of pneumonia. Although the patient is receiving only a modest amount of oxygen, the patient has a 53% risk of the future development of ARDS and need for mechanical ventilation. Therefore ICU-level monitoring is an appropriate disposition for this patient.
Evaluation of the Patient with ARDS
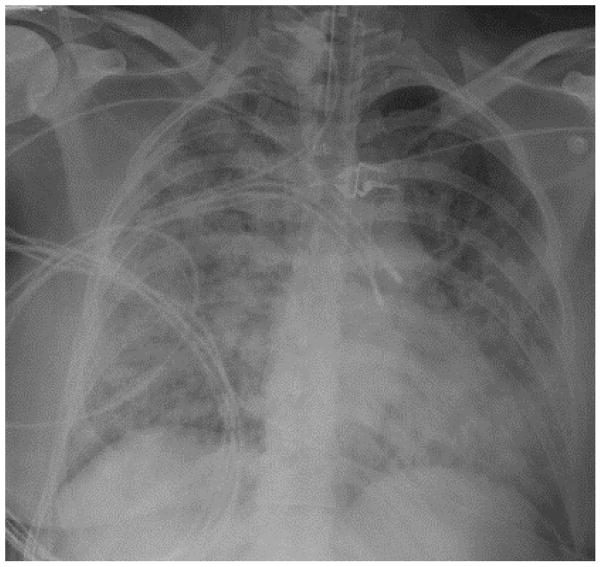
A 24-year-old woman with no past medical history but who recently started smoking tobacco is admitted to the ICU with fever, cough, dyspnea, and rapidly progressive hypoxemic respiratory failure. She requires invasive mechanical ventilation with an initial PaO2/FiO2 of 80 mmHg. Chest radiography shows bilateral alveolar infiltrates (Figure 4), and there are no signs of left ventricular dysfunction. Despite treatment with broad-spectrum antibiotics for community-acquired pneumonia she fails to improve over the subsequent three days. Bacterial cultures of the sputum on presentation are negative. Would any additional testing be beneficial in the evaluation of this patient with ARDS?
Figure 4. Chest Radiograph.
Bilateral alveolar infiltrates were seen on this patient’s chest radiograph during her critical illness.
It cannot be overemphasized that ARDS is a syndrome indicative of an underlying diagnosis. Without recognition and treatment of the underlying diagnosis, ARDS is unlikely to improve. Potential underlying diagnoses may not be readily apparent in the critically ill, sedated or comatose patient who is unable to provide a complete history. For example, intra-abdominal processes such as pancreatitis, cholecystitis or viscous perforation may be occult unless clinical suspicion leads to appropriate testing. Atypical infectious processes such as fungal pneumonias, psittacosis, or tick borne illness will not respond to usual antibiotic therapy for community acquired pneumonia and may be missed if an appropriate history and testing are not obtained. Drug overdose can also lead to ARDS either directly or due to consequent aspiration and may be missed unless appropriate toxicology tests are ordered. For this reason, the diagnosis of ARDS should be viewed as a starting point in the diagnostic evaluation rather than the endpoint, and the underlying diagnosis leading to ARDS 15 should always be thoroughly investigated, including the consideration of more unusual causes of ARDS 53,54. The importance of a thorough history cannot be overstated and if the patient is unable to provide a history (as is often the case), every effort should be made to contact family or friends to obtain a complete description of the antecedent illness and any exposures.
Mimickers of ARDS
The approach to the differential diagnosis in patients with ARDS should also include mimickers of ARDS. Although the definitions of ARDS 7,39 include parameters that should reduce the possibility of misclassifying pure cardiogenic pulmonary edema and chronic lung diseases as ARDS, there are other conditions that can present acutely with hypoxemia, bilateral alveolar infiltrates, and no evidence of left ventricular dysfunction. Diagnoses such as diffuse alveolar hemorrhage, pulmonary alveolar proteinosis, acute interstitial pneumonia, cryptogenic organizing pneumonia, acute eosinophilic pneumonia, and acute exacerbations of idiopathic pulmonary fibrosis may meet the diagnostic criteria for ARDS (Table 3); however, these syndromes are not a result of the same inflammatory mechanisms that underlie the direct and indirect causes of ARDS and treatment may vary widely based on the diagnosis. Careful attention should be paid to the possibility of an alternative diagnosis in patients with ARDS, particularly when no apparent underlying cause for ARDS is readily identified.
Table 3.
Mimickers of ARDS
| Chest Imaging Characteristics | Diagnostic Tests | Potential Changes in Therapy | |
|---|---|---|---|
| Diffuse Alveolar Hemorrhage | Bilateral alveolar and ground glass infiltrates | Bronchoscopy with bronchoalveolar lavage | Glucocorticoids Transfusion Immunosuppressive therapy |
| Pulmonary Alveolar Proteinosis | Central and lower lung zone alveolar infiltrates, “bat wing” appearance, “crazy paving” on CT | High-resolution Computed Tomography, bronchoscopy with bronchoalveolar lavage | Whole lung lavage, Granulocyte macrophage-colony stimulating factor |
| Acute Interstitial Pneumonia | Bilateral alveolar and ground glass infiltrates, septal thickening, traction bronchiectasis | No alternative cause of ARDS identified, open or thoracoscopic lung biopsy | Glucocorticoids |
| Cryptogenic Organizing Pneumonia | Peripheral distribution of alveolar infiltrates, migratory infiltrates | Bronchoscopy with Transbronchial lung biopsy | Glucocorticoids |
| Acute Exacerbation of Idiopathic Pulmonary Fibrosis | Ground glass opacities superimposed on peripheral, basilar fibrotic changes | Computed Tomography | Glucocorticoids |
| Acute Eosinophilic Pneumonia | Bilateral alveolar and ground glass infiltrates | Bronchoscopy with bronchoalveolar lavage | Glucocorticoids |
Invasive Evaluation of ARDS
Invasive sampling of the lung, in the absence of a diagnosis after non-invasive testing, may aid in determining the cause of ARDS. Flexible bronchoscopy with bronchoalveolar lavage may play a role in determining the cause of ARDS and evaluating for mimickers of ARDS. In the setting of pneumonia as the cause of ARDS, bronchoalveolar lavage may have a sensitivity as high as 60% for identification of a specific pathogen 55. Bronchoscopy may also be helpful in the patient with persistent ARDS given that new, superimposed ventilator-associated pneumonia diagnosed by bronchoalveolar lavage occurs in approximately 36% of patients with ARDS 56 and may prolong the patient’s recovery from the initial diagnosis. In the case presented above, the patient underwent flexible bronchoscopy and was found to have a differential cell count of 48% eosinophils on bronchoalveolar lavage, and was diagnosed with acute eosinophilic pneumonia. Once this diagnosis was made, she was treated with glucocorticoids leading to extubation three days later 57. This case of acute eosinophilic pneumonia mimicking ARDS emphasizes the point that if an underlying cause of ARDS is not identified and the patient is not improving with empiric therapy for common causes of ARDS, invasive testing may be useful for specific diagnosis and treatment.
Open lung biopsy may also play a similar role in patients with undifferentiated ARDS and has produced an alternative diagnosis and change in therapy in up to 60% of patients, with few major complications 58,59. In one study, even in the setting of marked hypoxemic respiratory failure (mean PaO2/FiO2 = 145 mmHg, SD ± 61), major complications occurred in only 7% of patients with no procedure-related deaths 59. In fact, a study by Papazian et al 58 showed that there was no significant change in arterial blood gas values pre- and post-procedure, while there was an increase in the PaO2/FiO2 ratio after the procedure. From the total of 93 cases of open lung biopsy described in these two studies, we can conclude that in a selected patient population with ARDS and no identified underlying cause, the risks of open lung biopsy may be acceptable given that this procedure may provide additional information that may change therapy.
Conclusion
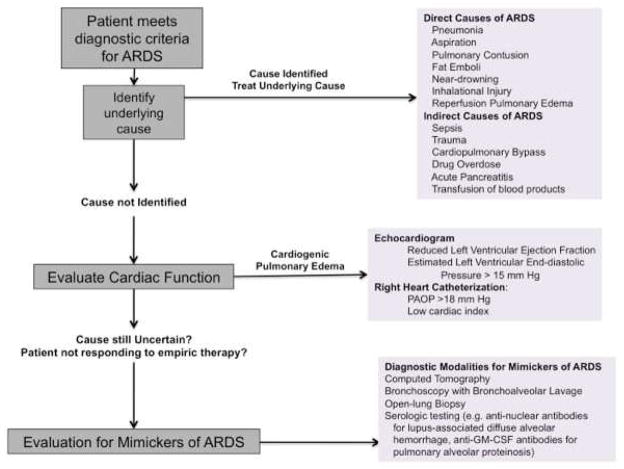
Given the high incidence and mortality of ARDS in critically ill patients, every practitioner needs a bedside approach (Figure 5) both for early identification of patients at risk for ARDS and for the appropriate evaluation of patients who meet the diagnostic criteria of ARDS. Recent advances such as the Lung Injury Prediction Score, the Early Acute Lung Injury score, and validation of the SpO2/FiO2 ratio for assessing the degree of hypoxemia are all practical tools to aid the practitioner in caring for patients at risk of ARDS and will likely become more important in the future as more preventative therapies for ARDS are investigated. For patients who meet the diagnostic criteria for ARDS, the practitioner should focus on a thorough search for an underlying cause as well as the concurrent possibility of an underlying disease process that mimics the clinical syndrome of ARDS.
Figure 5.
Algorithm for the Bedside Approach to the Patient with ARDS
Key Points.
Prior to meeting the formal criteria for the diagnosis of ARDS, patients may exhibit signs that can be used to inform the bedside practitioner as to the risk of future development of ARDS and respiratory failure.
Early implementation of therapies, such as lung protective ventilation, in the at-risk patient may prevent the development of ARDS.
Non-invasive testing, such as the arterial oxygen saturation to fraction of inspired oxygen ratio and echocardiography, can be valuable in the bedside evaluation of a patient with respiratory failure and bilateral infiltrates.
Once the diagnosis of ARDS is made, the bedside practitioner should begin a thorough search for the underlying cause of ARDS.
In the absence of a direct or indirect risk factor for ARDS, the practitioner should also consider cardiac dysfunction and mimickers of ARDS in their differential diagnosis of respiratory failure and bilateral infiltrates on chest imaging.
Acknowledgments
Sources of funding: Supported in part by the NIH T32 HL087738 and UL1 RR024975-01
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Herridge MS, Tansey CM, Matté A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 3.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 4.Guérin C, Reignier J, Richard J-C, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 5.Papazian L, Forel J-M, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson ND, Frutos-Vivar F, Esteban A, et al. Acute respiratory distress syndrome: underrecognition by clinicians and diagnostic accuracy of three clinical definitions. Crit Care Med. 2005;33(10):2228–2234. doi: 10.1097/01.ccm.0000181529.08630.49. [DOI] [PubMed] [Google Scholar]
- 7.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 8.Esteban A, Frutos-Vivar F, Muriel A, et al. Evolution of Mortality over Time in Patients Receiving Mechanical Ventilation. Am J Respir Crit Care Med. 2013;188(2):220–230. doi: 10.1164/rccm.201212-2169OC. [DOI] [PubMed] [Google Scholar]
- 9.Ketoconazole for early treatment of acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. The ARDS Network. JAMA. 2000;283(15):1995–2002. doi: 10.1001/jama.283.15.1995. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354(16):1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 11.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Matthay MA, Brower RG, et al. Randomized, placebo-controlled clinical trial of an aerosolized β2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184(5):561–568. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice TW, Wheeler AP, Thompson BT, et al. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306(14):1574–1581. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Determann RM, Royakkers A, Wolthuis EK, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care. 2010;14(1):R1. doi: 10.1186/cc8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Futier E, Constantin J-M, Paugam-Burtz C, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369(5):428–437. doi: 10.1056/NEJMoa1301082. [DOI] [PubMed] [Google Scholar]
- 15.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 16.Moss M, Guidot DM, Steinberg KP, et al. Diabetic patients have a decreased incidence of acute respiratory distress syndrome. Crit Care Med. 2000;28(7):2187–2192. doi: 10.1097/00003246-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Iribarren C, Jacobs DR, Sidney S, Gross MD, Eisner MD. Cigarette smoking, alcohol consumption, and risk of ARDS: a 15-year cohort study in a managed care setting. Chest. 2000;117(1):163–168. doi: 10.1378/chest.117.1.163. [DOI] [PubMed] [Google Scholar]
- 18.Mangialardi RJ, Martin GS, Bernard GR, et al. Hypoproteinemia predicts acute respiratory distress syndrome development, weight gain, and death in patients with sepsis. Ibuprofen in Sepsis Study Group. Crit Care Med. 2000;28(9):3137–3145. doi: 10.1097/00003246-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Levitt JE, Bedi H, Calfee CS, Gould MK, Matthay MA. Identification of early acute lung injury at initial evaluation in an acute care setting prior to the onset of respiratory failure. Chest. 2009;135(4):936–943. doi: 10.1378/chest.08-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iscimen R, Cartin-Ceba R, Yilmaz M, et al. Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Crit Care Med. 2008;36(5):1518–1522. doi: 10.1097/CCM.0b013e31816fc2c0. [DOI] [PubMed] [Google Scholar]
- 21.Trillo-Alvarez C, Cartin-Ceba R, Kor DJ, et al. Acute lung injury prediction score: derivation and validation in a population-based sample. Eur Respir J. 2011;37(3):604–609. doi: 10.1183/09031936.00036810. [DOI] [PubMed] [Google Scholar]
- 22.Matthay MA, Zimmerman GA, Esmon C, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med. 2003;167(7):1027–1035. doi: 10.1164/rccm.200208-966WS. [DOI] [PubMed] [Google Scholar]
- 23.Gong MN. Genetic epidemiology of acute respiratory distress syndrome: implications for future prevention and treatment. Clin Chest Med. 2006;27(4):705–24. doi: 10.1016/j.ccm.2006.07.001. abstract x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gajic O, Frutos-Vivar F, Esteban A, Hubmayr RD, Anzueto A. Ventilator settings as a risk factor for acute respiratory distress syndrome in mechanically ventilated patients. Intensive Care Med. 2005;31(7):922–926. doi: 10.1007/s00134-005-2625-1. [DOI] [PubMed] [Google Scholar]
- 25.Khan H, Belsher J, Yilmaz M, et al. Fresh-frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest. 2007;131(5):1308–1314. doi: 10.1378/chest.06-3048. [DOI] [PubMed] [Google Scholar]
- 26.Janz DR, Zhao Z, Koyama T, et al. Longer storage duration of red blood cells is associated with an increased risk of acute lung injury in patients with sepsis. Ann Intensive Care. 2013;3(1):33. doi: 10.1186/2110-5820-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spragg RG, Bernard GR, Checkley W, et al. Beyond mortality: future clinical research in acute lung injury. 2010;181:1121–1127. doi: 10.1164/rccm.201001-0024WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gajic O, Dabbagh O, Park PK, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183(4):462–470. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kor DJ, Talmor DS, Banner-Goodspeed VM, et al. Lung Injury Prevention with Aspirin (LIPS-A): a protocol for a multicentre randomised clinical trial in medical patients at high risk of acute lung injury. BMJ Open. 2012;2(5) doi: 10.1136/bmjopen-2012-001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmickl CNC, Shahjehan KK, Li GG, et al. Decision support tool for early differential diagnosis of acute lung injury and cardiogenic pulmonary edema in medical critically ill patients. Chest. 2012;141(1):43–50. doi: 10.1378/chest.11-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park PK, Birkmeyer NO, Gentile NT, Chang SY, Dabbagh O, Gajic O. Early cumulative fluid balance and development of acute lung injury. Am J Respir Crit Care Med. 2011:A5592. [Google Scholar]
- 32.Serpa Neto A, Cardoso SO, Manetta JA, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA. 2012;308(16):1651–1659. doi: 10.1001/jama.2012.13730. [DOI] [PubMed] [Google Scholar]
- 33.Janz DR, Ware LB. Biomarkers of ALI/ARDS: Pathogenesis, Discovery, and Relevance to Clinical Trials. Semin Respir Crit Care Med. 2013;34(4):537–548. doi: 10.1055/s-0033-1351124. [DOI] [PubMed] [Google Scholar]
- 34.van der Heijden M, van Nieuw Amerongen GP, Koolwijk P, van Hinsbergh VWM, Groeneveld ABJ. Angiopoietin-2, permeability oedema, occurrence and severity of ALI/ARDS in septic and non-septic critically ill patients. Thorax. 2008;63(10):903–909. doi: 10.1136/thx.2007.087387. [DOI] [PubMed] [Google Scholar]
- 35.Gallagher DC, Parikh SM, Balonov K, et al. Circulating angiopoietin 2 correlates with mortality in a surgical population with acute lung injury/adult respiratory distress syndrome. Shock. 2008;29(6):656–661. doi: 10.1097/shk.0b013e31815dd92f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agrawal A, Matthay MA, Kangelaris KN, et al. Plasma angiopoietin-2 predicts the onset of acute lung injury in critically ill patients. Am J Respir Crit Care Med. 2013;187(7):736–742. doi: 10.1164/rccm.201208-1460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Determann RM, Millo JL, Waddy S, Lutter R, Garrard CS, Schultz MJ. Plasma CC16 levels are associated with development of ALI/ARDS in patients with ventilator-associated pneumonia: a retrospective observational study. BMC Pulm Med. 2009;9:49. doi: 10.1186/1471-2466-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuchs-Buder T, de Moerloose P, Ricou B, et al. Time course of procoagulant activity and D dimer in bronchoalveolar fluid of patients at risk for or with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;153(1):163–167. doi: 10.1164/ajrccm.153.1.8542111. [DOI] [PubMed] [Google Scholar]
- 39.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 40.Thille AW, Esteban A, Fernández-Segoviano P, et al. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am J Respir Crit Care Med. 2013;187(7):761–767. doi: 10.1164/rccm.201211-1981OC. [DOI] [PubMed] [Google Scholar]
- 41.Levitt JE, Calfee CS, Goldstein BA, Vojnik R, Matthay MA. Early Acute Lung Injury: Criteria for Identifying Lung Injury Prior to the Need for Positive Pressure Ventilation. Crit Care Med. 2013 doi: 10.1097/CCM.0b013e31828a3d99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferguson ND, Meade MO, Hallett DC, Stewart TE. High values of the pulmonary artery wedge pressure in patients with acute lung injury and acute respiratory distress syndrome. Intensive Care Med. 2002;28(8):1073–1077. doi: 10.1007/s00134-002-1354-y. [DOI] [PubMed] [Google Scholar]
- 43.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Wheeler AP, Bernard GR, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354(21):2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 44.Rice TW, Wheeler AP, Bernard GR, et al. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132(2):410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 45.Mayordomo-Colunga J, Pons M, López Y, et al. Predicting non-invasive ventilation failure in children from the SpO2/FiO2 (SF) ratio. Intensive Care Med. 2013;39(6):1095–1103. doi: 10.1007/s00134-013-2880-5. [DOI] [PubMed] [Google Scholar]
- 46.Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med. 2005;353(26):2788–2796. doi: 10.1056/NEJMcp052699. [DOI] [PubMed] [Google Scholar]
- 47.Ely EW, Haponik EF. Using the chest radiograph to determine intravascular volume status: the role of vascular pedicle width. Chest. 2002;121(3):942–950. doi: 10.1378/chest.121.3.942. [DOI] [PubMed] [Google Scholar]
- 48.Aberle DR, Wiener-Kronish JP, Webb WR, Matthay MA. Hydrostatic versus increased permeability pulmonary edema: diagnosis based on radiographic criteria in critically ill patients. Radiology. 1988;168(1):73–79. doi: 10.1148/radiology.168.1.3380985. [DOI] [PubMed] [Google Scholar]
- 49.Kaul S, Stratienko AA, Pollock SG, Marieb MA, Keller MW, Sabia PJ. Value of two-dimensional echocardiography for determining the basis of hemodynamic compromise in critically ill patients: a prospective study. J Am Soc Echocardiogr. 1994;7(6):598–606. doi: 10.1016/s0894-7317(14)80082-5. [DOI] [PubMed] [Google Scholar]
- 50.Harvey S, Harrison DA, Singer M, et al. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. Lancet. 2005;366(9484):472–477. doi: 10.1016/S0140-6736(05)67061-4. [DOI] [PubMed] [Google Scholar]
- 51.Binanay C, Califf RM, Hasselblad V, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294(13):1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 52.Shah MR, Hasselblad V, Stevenson LW, et al. Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA. 2005;294(13):1664–1670. doi: 10.1001/jama.294.13.1664. [DOI] [PubMed] [Google Scholar]
- 53.Morgan WK. “Zamboni disease.” Pulmonary edema in an ice hockey player. Arch Intern Med. 1995;155(22):2479–2480. doi: 10.1001/archinte.155.22.2479. [DOI] [PubMed] [Google Scholar]
- 54.Abdulla KA, Davidson NM. A woman who collapsed after painting her soles. Lancet. 1996;348(9028):658. doi: 10.1016/S0140-6736(96)04506-0. [DOI] [PubMed] [Google Scholar]
- 55.Meduri GU, Reddy RC, Stanley T, El-Zeky F. Pneumonia in acute respiratory distress syndrome. A prospective evaluation of bilateral bronchoscopic sampling. Am J Respir Crit Care Med. 1998;158(3):870–875. doi: 10.1164/ajrccm.158.3.9706112. [DOI] [PubMed] [Google Scholar]
- 56.Markowicz P, Wolff M, Djedaïni K, et al. Multicenter prospective study of ventilator-associated pneumonia during acute respiratory distress syndrome. Incidence, prognosis, and risk factors. ARDS Study Group. Am J Respir Crit Care Med. 2000;161(6):1942–1948. doi: 10.1164/ajrccm.161.6.9909122. [DOI] [PubMed] [Google Scholar]
- 57.Janz DR, O'Neal HR, Ely EW. Acute eosinophilic pneumonia: A case report and review of the literature. Crit Care Med. 2009;37(4):1470–1474. doi: 10.1097/CCM.0b013e31819cc502. [DOI] [PubMed] [Google Scholar]
- 58.Papazian L, Thomas P, Bregeon F, et al. Open-lung biopsy in patients with acute respiratory distress syndrome. Anesthesiology. 1998;88(4):935–944. doi: 10.1097/00000542-199804000-00013. [DOI] [PubMed] [Google Scholar]
- 59.Patel SR, Karmpaliotis D, Ayas NT, et al. The role of open-lung biopsy in ARDS. Chest. 2004;125(1):197–202. doi: 10.1378/chest.125.1.197. [DOI] [PubMed] [Google Scholar]