Abstract
Gene therapy for the muscular dystrophies has evolved as a promising treatment for this progressive group of disorders. While corticosteroids and/or supportive treatments remain standard of care for Duchenne muscular dystrophy (DMD), loss of ambulation, respiratory failure and compromised cardiac function is the inevitable outcome. Recent developments in genetically mediated therapies have allowed for personalized treatments that strategically target individual muscular dystrophy subtypes based on disease pathomechanism and phenotype. In this review, we highlight therapeutic progress with emphasis on evolving pre-clinical data and our own experience in completed clinical trials, and others currently underway. We also discuss the lessons we have learned along the way and the strategies developed to overcome limitations and obstacles in this field.
Introduction
The revolution in genetics, witnessed over the past few decades, has transformed the practice of medicine. Gene therapy exemplifies the move towards personalized medicine, where molecular-based therapies are tailored to the individual’s genetic disorder. Approaches to gene therapy include: direct gene replacement or gene repair or indirectly through a surrogate gene that enhances cellular performance. The promising findings from pre-clinical, proof-of-concept studies in Duchenne muscular dystrophy (DMD) and the limb-girdle muscular dystrophies (LGMDs) have moved the field forward for treatment of neuromuscular disorders.
In this review, we highlight the progress and lessons learned with an emphasis on our own experience in clinical trials that are underway or have been completed.
Gene Repair Through Mutation-specific Therapies in DMD (non-viral vectors)
Exon skipping using antisense oligonucleotides (AONs)
Mutations in the DMD gene disrupt the open reading frame and result in incomplete translation of the dystrophin protein. Exon skipping is an approach to gene repair that targets the pre-mRNA transcript, introducing alternative splice sites that result in skipping one or more targeted exons with resultant restoration of the dystrophin reading frame. Antisense oligonucleotides (AONs) are synthetically modified strands of nucleic acids, typically 20–30 nucleotides in length, composed of complementary sequences to dystrophin pre-mRNA. This treatment approach was inspired by evidence of restoration of the open reading frame of the dystrophin gene expressed as “revertant fibers” by intrinsic alternative splicing appearing in a high percentage of DMD boys [1]. The result is small clusters of dystrophin positive fibers; too few to provide clinical efficacy. Synthetically designed AONS were designed to simulate naturally occurring alternative splicing, with the intention of achieving this in a higher percentage of muscle fibers that could attain a clinically meaningful result. It is an approach that could apply to approximately 83% of all DMD patients [2]. The early emphasis for clinical trials has been to target the hotspot region for deletions between exon 42 and 55, where mutations are generally amenable to exon skipping. Exon 51 skipping is applicable to the largest group of all DMD patients (13%) inclusive of deletions: 45–50, 47–50, 48–50, 49–50, 50; 52, or 52–63 [2]. Pre-clinical efficacy of AONs has been demonstrated in the mdx, dystrophin/utrophin knockout mouse, and the dystrophin-deficient dog using 2’O-methyl-ribo-oligonucleoside-phosphorothioate (2’OMePS) and phosphorodiamidate morpholino oligomers (PMOs) [3–5]. These two oligomers share a common mechanism of action but differ in their biochemical structure, stability against endonucleases and toxicity profiles.
Two Phase I proof-of principle clinical trials using a 2’OMePS oligomer (PRO051/GSK2402968, ProsensaTherapeutics) or a PMO (AVI-4658/Eteplirsen®; Sarepta Therapeutics) targeting exon 51 were completed by direct muscle injection, both demonstrating expression of dystrophin fibers [6, 7]. Safety profiles were comparable, neither showing adverse events. These studies led to subsequent Phase IIa, open-label trials in which higher doses of systemically delivered AONs were given to study participants. In the PRO051 trial, 12 patients participated in a dose escalation, 5-week trial of product given by subcutaneous injection [8]. Dystrophin expression was seen in approximately 60–100% of muscle fibers reaching 15.6% of normal. A 12-week extension study improved the 6-minute walk distance by about 35 meters. Complications included injection site reactions and proteinuria, without loss of renal function. In the phase II 12-week, open-label, dose-escalation PMO study (dose range 0.5 to 20.0 mg/kg), dystrophin expression at the sarcolemma was modestly increased in 7 of 19 patients to a mean of 8.9% to 16.4% [9]. One patient reached 55% dystrophin positive fibers following treatment with 20 mg/kg. No drug-related adverse events were encountered.
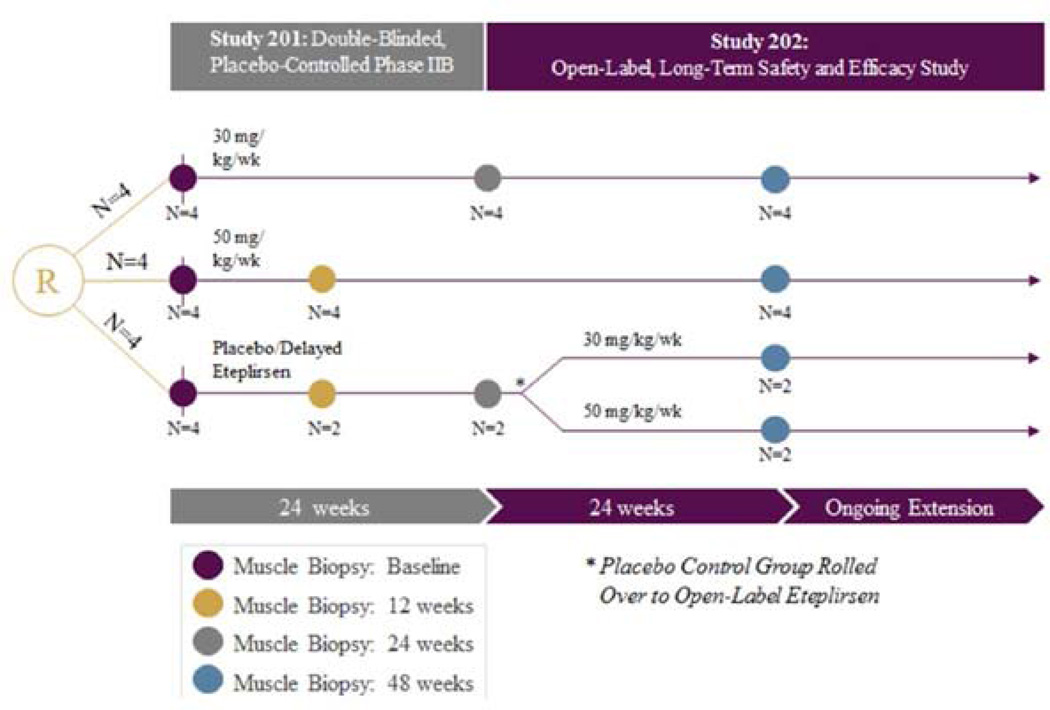
The first double blind randomized controlled trial of exon skipping using a PMO (eteplirsen Sarepta Therapeutics) took place at Nationwide Children’s Hospital in Columbus, OH [10]. Enrollment included 12 DMD boys, ages 7 to 13 with confirmed out of frame DMD gene deletions potentially correctable by skipping exon 51 that were randomized to 1 of 3 eteplirsen-treated cohorts: Cohort 1, received 30 mg/kg/wk; Cohort 2, 50 mg/kg/wk; and Cohort 3, placebo-treated. At week 25, Cohort 3, switched to open-label treatment, either 30 or 50 mg/kg/wk; thereafter referred to as “placebo-delayed” (Fig. 1). All were on a stable dose of corticosteroids for six months minimum prior to enrollment. Outcome measures included dystrophin production, both percent of muscle fibers and intensity at the sarcolemma. Muscle biopsies were done at baseline, at 12 weeks for the 50 mg/kg cohort and 24 weeks for the 30 mg/kg cohort. Two blinded placebo-treated subjects were biopsied at 12 weeks and two at week 24. At the week 25, the placebo-treated subjects (n=4) received weekly eteplirsen dosing; two received 30mg/kg and two received 50 mg/kg. All subjects had a third biopsy at week 48. The 6MWT was the primary functional outcome, performed pre- and post-treatment through week 48.
Figure 1.
Eteplirsen exon skipping study design. Twelve subjects with DMD were randomized (R) to 1 of 3 eteplirsen-treated cohorts in study 201: Cohort 1, received 30 mg/kg/wk; Cohort 2, 50 mg/kg/wk; and Cohort 3, placebo-treated. At week 25, Cohort 3, switched to open-label treatment, either 30 or 50 mg/kg/wk; thereafter referred to as “placebo-delayed”. Patients were maintained on the same starting dose of eteplirsen under the open label extension Study 202. Biceps biopsies were obtained on all patients at baseline and deltoid biopsies at week 48 for analysis of dystrophin expression. At week 12 biceps biopsies were obtained from patients in Cohort 2 (50 mg/kg/wk) and 2 Placebo-treated patients in Cohort 3. At week 24 biceps biopsies were obtained from patients in Cohort 1 (30 mg/kg/wk) and 2 placebo-treated patients in Cohort 3.
The results of dystrophin production at all time points are shown in (Fig. 2). At 12 weeks post treatment, no significant dystrophin was produced (50 mg/kg cohort plus 2 placebo-treated). Dystrophin production could be seen after 24 weeks of eteplirsen except for the two placebo-treated subjects (as expected). At week 48 all patients, including the placebo-delayed cohort, produced significant dystrophin. These findings are a clear indication that dystrophin production started sometime after week 12 and was more influenced by duration of treatment than dose. We also found restoration of the sarcoglycans and nNOS binding site accompanying dystrophin production.
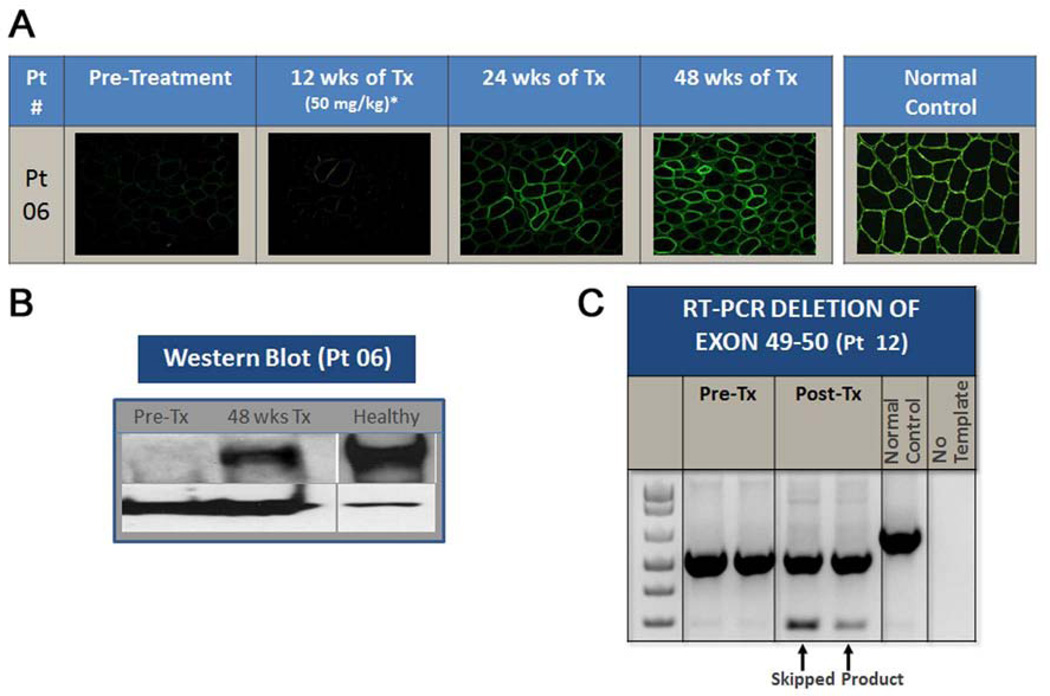
Figure 2.
Effects of eteplirsen on dystrophin expression. (A) Representative example of time dependent increases in dystrophin-positive fibers throughout the study can be seen. Dystrophin expression begins between week 12 and week 24 of eteplirsen treatment, and continues to increase after that time point. (B) Western blot from a representative patient demonstrates measureable dystrophin at week 48 compared to baseline. (C) RT-PCR demonstrates presence of skipped mRNA product (289 bp) following eteplirsen treatment in the muscle of a representative patient with exons 49–50 deletion.
The 6MWT results for the four patients in the placebo cohort were consistent with natural history studies [11, 12] resulting in decline of almost 70 meters from baseline by week 48. In the treated cohorts from study initiation, analysis was complicated by twin boys in the 30 mg/kg cohort who showed rapid disease progression. By week 24 when consistent increases in dystrophin positive fibers were observed, one of the twins lost ambulation and the other was teetering on loss. In retrospect their baseline profile predicted imminent loss of ambulation (the tallest and among the oldest of the 12 patients enrolled and had the lowest baseline 6MWT values) [11, 13]. The severe deviation from normality due to their extreme values placed them in an “outliers” category justifying exclusion from the early treatment group. Thus, we compared the 6 ambulation-evaluable subjects in the 30 mg/kg (n=2) and 50 mg/kg (n=4) cohorts who received eteplirsen for 48 weeks and demonstrated stable performance on the 6MWT from baseline to Week 48. This was significantly different (67.3 meter difference; p≤0.001) compared to the placebo-delayed cohort (Fig. 3).
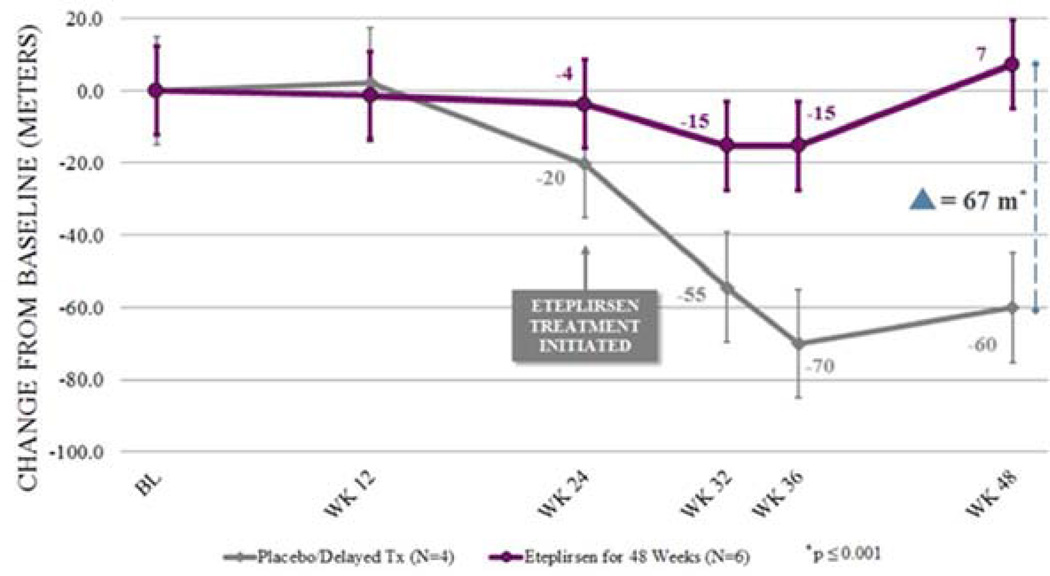
Figure 3.
Functional efficacy of eteplirsen. The purple line illustrates the change from baseline (BL) in distance walked on the 6-minute walk test (6MWT) over time for the 6 evaluable patients who received eteplirsen from the start of Study 201. The dark gray line represents change from baseline in distance walked on the 6MWT for the 4 patients who received placebo for the first 24 weeks and eteplirsen for the last 24 weeks. It is estimated from dystrophin expression studies and multiple biopsies that significant dystrophin was produced after 12 weeks. This is clearly indicated in the placebo/delayed cohort starting on eteplirsen at week 25 and stabilizing in function at week 36. Eteplirsen-treated cohorts beginning at study initiation maintained a plateau without decline through week 48, diverging from placebo/delayed patients at week 12. Two of the treated patients starting at week 1 lost ambulation before they produced significant dystrophin and are not represented on this graph.
The findings from this study show a clear relationship between systemic treatment with eteplirsen, an exon-skipping drug, and demonstration of dystrophin production in DMD muscle, followed by a statistically significant difference in walking ability in treated versus placebo/delayed patients. Eteplirsen’s clinical benefit mirrored the time required to produce consistent increases in dystrophin. This observed delay between initiation of eteplirsen and detectable dystrophin production and stabilization in clinical function likely results from an accumulation of novel dystrophin over time. It also has to be underscored that the safety profile of eteplirsen could not be any better as not a single significant adverse event has been attributable to this agent. The study has now extended for more than 1.5 years and stability and safety continue without deviation.
This very promising method of treatment is now spreading to other exons. The effect of skipping different exons would be expected to vary given the binding sites and folding of smaller dystrophin proteins. Nevertheless, this is a promising mode of therapy that is moving forward.
Suppression of Nonsense Mutations
Aminoglycosides (Gentamicin)
Premature termination codons (PTC) due to nonsense mutations account for approximately 13–15% of all DMD mutations [14, 15]. As with exon skipping, stop codon readthrough is also mutation-specific. The end product is potentially a full-length dystrophin protein. Biochemical and molecular studies of readthrough suggest that aminoglycosides bind a specific site in ribosomal RNA and impair codon-anticodon recognition at the aminoacyl-tRNA acceptor site. This introduces a missense mutation permitting translation by ignoring the premature stop codon. An important question is whether this finding could be harnessed for translational benefit to patients. Proof-of principle studies were first demonstrated using aminoglycosides to treat the cystic fibrosis mouse model restoring the full-length cystic fibrosis transmembrane conductance regulator (CFTR) protein [16, 17]. Studies were extended to the mdx mouse, where the aminoglycoside, gentamicin, successfully reduced creatine kinase levels by 70% and increased dystrophin expression to about 20% [18]. Gentamicin-treated mice showed improved histology and protection against contraction-induced injury. The encouraging pre-clinical studies served as a template for a similar approach in DMD patients with confirmed nonsense mutations in the dystrophin gene. The initial clinical trial reported in 2001 involved two DMD and two BMD patients who received 7.5 mg/kg intravenous gentamicin for 2 weeks [19] with a drop in serum CK but restoration of dystrophin was not seen at this early time-point. A subsequent trial conducted in a small cohort of four DMD patients reported more favorable results with gentamicin given over two cycles, each for 6 days with a 7-week interval between dosing [20]. Dystrophin expression was observed in three out of four patients’ muscle biopsies but no clinical meaningful outcomes were obtained in any of the patients.
A third clinical trial conducted by our group at the Nationwide Children’s Hospital consisted of a two-phase protocol [21]. The first phase was intended to establish biopotency in a short-term, 14-day dosing study, simulating what had been done in mdx mice and ensuring adequate translation to the clinic prior to an extended 6-month trial. Ten DMD boys with proven stop codon mutations were recruited for daily gentamicin administration using a standard dosing regimen (7.5 mg/kg) for treatment of gram-negative infections. Serum CK served as the primary outcome measure and both a biological effect and a 50% reduction of CK at day 14 were established. Having demonstrated biopotency in the short-term trial, a six-month study was designed using muscle strength and function as outcomes for clinical assessment. The dosing regimen for gentamicin was dictated based on the known prolonged half-life of dystrophin, with estimates of as long as 6–8 weeks [22, 23] justifying weekly and twice-weekly dosing regimens. Enrollment was contingent on normal hearing and kidney function, and negative testing for the known A1555G mitochondrial DNA mutation in the 12S rRNA gene predisposing to aminoglycoside-induced hearing loss [24]. Renal function was monitored using cystatin C to avoid false negative findings related to decreased serum creatinine and creatinine clearance levels in this population [25].
Sixteen subjects with documented stop codon mutations received weekly (n=12) or twice weekly (n=4) gentamicin. No patient demonstrated a decline in renal or hearing function except one patient given a miscalculated dose (125% of recommended) for the first four administrations resulting in a transient high frequency hearing loss that returned to normal within 3 months. In nine patients, pretreatment dystrophin levels ranged from 0.8% to 4.5%; six of which showed increased levels following six months of gentamicin treatment. In three subjects dystrophin protein increased to a potentially therapeutic range with independent concordance by immunofluorescence (Bioquant image analysis ®) and Western blot analyses. The most significant readthrough was seen in three patients increasing dystrophin levels to 13.0%, 15.4%, and 15.4% of wild-type levels (Fig. 4). No conclusion could be reached regarding preferential stop codon predicting readthrough or relation to the fourth base surrounding the stop codon as suggested by others [26].
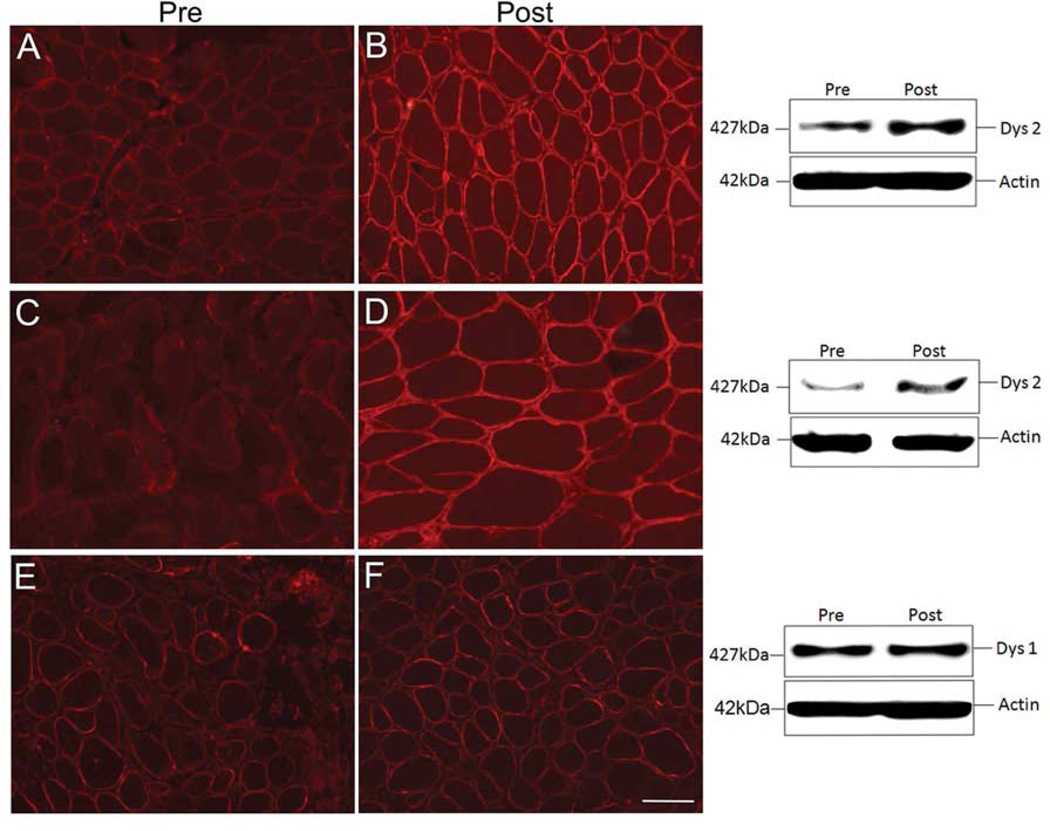
Figure 4.
Dystrophin expression following gentamicin treatment. (A, B) Pre- and post-gentamicin treatment muscle biopsies from Patient 6 demonstrating dystrophin expression by immunofluorescence (IF) using DYS 2 Novacastra; adjacent Western blot (WB) shows full-length dystrophin at 427kDa. Gentamicin-induced dystrophin expression increased to 15.44%. (C, D) Pre- and post-treatment muscle biopsy from Patient 10; IF sections stained with DYS2 antibody; adjacent WB shows full-length dystrophin at 427 kDa. Gentamicin-induced dystrophin increased to 13.0%. (E, F) Pre- and post-treatment muscle biopsy sections from Patient 5 without response to gentamicin showing negligible increase after treatment by IF and WB. Scale bar = 100 µm.
In addition to dystrophin expression in muscle tissue, serum CK was mildly reduced after 6 months of gentamicin treatment (9851± 2109 U/l to 5316± 850 U/l, p= 0.04). However, functional outcomes failed to improve including time to walk 30 feet, or climb four standard steps, and stand from supine position on the floor.
Overall the clinical trial validates pre-clinical observations in mdx mice showing that mutation suppression or readthrough can be translated to patients. However, only three patients in this clinical trial reached dystrophin levels that could potentially slow the progression of the disease. Results could theoretically be improved by increased dosing but the risk-benefit considerations of aminoglycoside therapy must be weighed in favor of agents that have potentially fewer side effects and can be administered orally, as discussed in the next section.
PTC124 (Ataluren®)
Ataluren is a small molecule developed by PTC Therapeutic in an effort to advance an orally bioavailable product that selectively bypasses disease-causing nonsense mutations without an effect on stop codons and avoids the potential renal and ototoxicity of aminoglycosides. It was originally developed by means of an optimized high throughput screening campaign. Dose-dependent readthrough of all three nonsense codons was observed with the highest readthrough at UGA, followed by UAG and then UAA. When compared to gentamicin, PTC124 demonstrated higher potency of nonsense suppression at lower concentrations [27]. Treatment of mdx mice restored dystrophin production in all skeletal muscles examined, including the diaphragm, and cardiac muscle within 2–8 weeks of exposure. A significant reduction in serum CK levels was observed after two weeks of treatment. The dystrophin levels were found to be 20–25% of control mouse muscles, and partially restored force generation and resistance against eccentric exercise.
In two Phase 1 dose-escalating safety and tolerability studies, PTC124 was well-tolerated with the exception of mild headaches, dizziness and gastrointestinal discomfort at higher doses [28]. A Phase 2b double blind, placebo-controlled, dose-ranging, efficacy and safety study failed to demonstrate improvement in the 6MWT [29]. Further detailed analysis of the Phase 2b data, however, suggested the possibility of a bell-shaped dose-response with clinical benefit to DMD boys receiving low dose, and in high dose patients in whom serum levels of PTC124 were low. In view of these tentative findings, PTC124 has moved ahead with a Phase 3, multicenter randomized double-blind, placebo-controlled study to determine efficacy and safety of Ataluren in DMD boys 7 to 16 years of age with known stop codons. The study is currently underway and results are not yet available (ClinicalTrials.gov identifier: NCT01826487).
Gene Replacement Through Viral Vector-mediated Therapies in Muscular Dystrophy
Duchenne muscular dystrophy (DMD)
DMD, the most common childhood muscular dystrophy, is an X-linked recessive disorder due to mutations in the dystrophin gene. Currently, the only treatment with proven efficacy is corticosteroids. Although a detailed review of corticosteroid trials is beyond the scope of this review, it is pertinent to emphasize the unequivocal evidence of functional benefit from this class of drugs. Prolonged ambulation was well documented in a randomized double blind controlled trial of daily Prednisone versus placebo [30]. Treatment with corticosteroids has become standard of care and a requirement for enrollment in most clinical trials. Several regimens for initiating steroid treatment are used worldwide with some variability in the adverse effect profile of each regimen [31].
Dystrophin, the largest gene in the human genome, is composed of 79 exons encoding a ∼427 kD subsarcolemmal protein that is widely expressed in both skeletal and smooth muscle. Spontaneous mutations lead to a high incidence of disease in newborn males estimated at approximately1:5000 [32, 33]. Using a gene replacement approach potentially circumvents restricted applicability of mutation-specific gene repair therapies. However, the large size of the dystrophin gene impedes packaging into viral vectors considered to be the most suitable for gene delivery to skeletal muscle and cardiac muscle. In an effort to overcome these hurdles, two promising strategies emerged in the field: functional miniaturization of dystrophin that can fit into recombinant adeno-associated virus (rAAV) and vascular delivery that allows for a wider distribution of therapeutic gene.
The concept of miniature forms of the dystrophin gene that could translate into a functional protein came from the clinical observation of a BMD patient who remained ambulatory until the 7th decade despite a deletion in 46% of his DMD gene [34]. Starting with this as a template, mini- and micro-dystrophin constructs were engineered to encode integral domains of the dystrophin protein sufficient to produce a functional protein. Pre-clinical studies in the mdx mouse using a mini-dystrophin gene (Δ 3990 kb) under control of a cytomegalovirus (CMV) set the stage for translation [35, 36]. Robust and sustained gene expression was observed for six months, with protection of muscle fibers from uptake of Evans blue dye, and an increase isometric force and resistance to contraction-induced injury.
In a first clinical gene therapy trial of its kind in DMD, six boys were enrolled in a double blind randomized controlled dose escalation study evaluating safety and efficacy of dystrophin gene transfer [37]. All six subjects had confirmed frame-shifting deletions of the dystrophin gene; four were on corticosteroid treatment that continued throughout the study. The vector used in this trial was a modified AAV serotype 2 capsid with an insertion of 5 amino acids from AAV1 encapsulating a functional mini-dystrophin transgene, representing approximately 40% of the 11-kb coding sequence of the human dystrophin gene (rAAV2.5.CMV.Δ3990). This was a dose escalation trial; the biceps muscle of one side received the therapeutic gene while the contralateral side received either saline (Cohort 1, n=3) or empty capsids (Cohort 2, n=3).
Gene transfer efficiency was evaluated by examining biopsy specimens of vector-injected versus untreated biceps muscles on day 42 (in Patients 1, 3, 4, and 6) and day 90 (in Patients 2 and 5). Expression of the mini-dystrophin was only detectable in a few myofibers from two of the four treated muscle samples evaluated at day 42 post-injection (Patients 3 and 6). Neither the treated samples taken at day 90 post-injection nor the untreated side showed any evidence of transgene expression. An important aspect of gene therapy trials is safety and in this trial no adverse events were encountered.
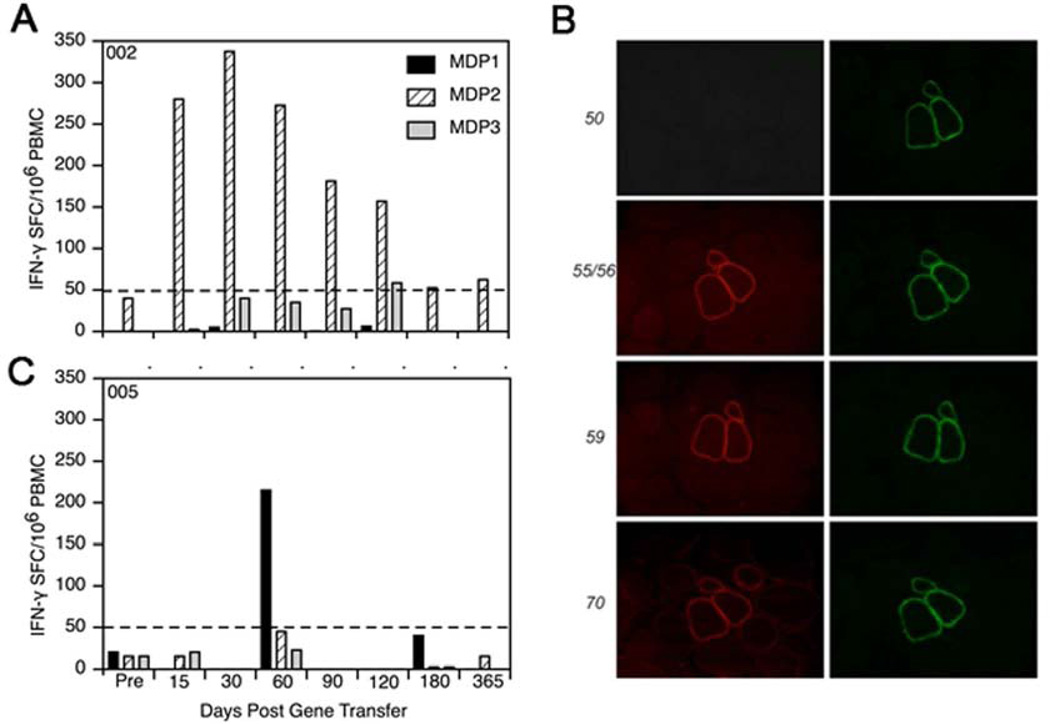
Despite failure to achieve successful transgene expression, this trial proved to be informative with regards to the impact of cellular immune responses on the outcome of gene replacement therapy. Peripheral blood mononuclear cells (PBMCs) were tested in an interferon-γ enzyme-linked immunosorbent spot (ELISpot) assay for reactivity to mini-dystrophin. Three synthetic peptide pools spanning the entire mini-dystrophin sequence (designated MDP1, MDP2, and MDP3) were used to stimulate PBMCs in the ELISpot assay. Patient 2 showed a robust T-cell response against MDP2 beginning on the 15th day post gene transfer (Fig. 5). The response peaked on day 30, slowly declining during the next 3 months, and was intermittently positive after day 120. In Cohort 2, Patients 4, and 5 had positive ELISpots to MDP3. Additional studies revealed that at least two different pathways provoked an immune response to mini-dystrophin. In Patient 5 the delayed, transient T-cell response detected discrete dystrophin epitopes, all in the region of the patient’s deletion. Because exons 3 through 17 were deleted from the DMD gene in this patient, it was clear that CD4+ and CD8+ T cells were primed by mini-dystrophin that expressed exons 3 through 12 in the region of the patient’s deletion (Fig. 5). In Patient 2, a different and unexpected mechanism of immunity was elicited. An MDP2-specific interferon-γ T cell response was triggered by amino acids 2809 to 2829 of exon 57, downstream from the patient’s exon-50 deletion. The patient’s mutation predicted a frame-shift, thereby impeding dystrophin expression. However, in the muscle biopsy from Patient 2, revertant fibers were visualized with the use of exon specific antibodies against dystrophin (Fig. 5). Thus, T cells were primed from exon 57, and we were able to demonstrate a robust interferon-γ response in PBMCs not only after gene transfer, but prior to treatment as well. A similar situation was seen in Patient 4 who had a deletion at exons 49 through 54, in whom we found revertant fibers with novel epitopes encoding exon 59, downstream of the mutation.
Figure 5.
Dystrophin-specific T-cell response. Peripheral blood mononuclear cells (PBMCs) were tested in an interferon-γ enzyme-linked immunosorbent spot (ELISpot) assay for reactivity to mini-dystrophin in two patients in the clinical DMD gene therapy trial. Three synthetic peptide pools spanning the entire mini-dystrophin sequence (designated MDP1, MDP2, and MDP3) were used to stimulate PBMCs interferon-γ T cells in the ELISpot assay. (A) Patient with exon 50 deletion showed a showed a robust T-cell response against MDP2 beginning on the 15th day post gene transfer. The response peaked on day 30 and slowly declined during the next 3 months. (B) An unexpected mechanism of immunity was found for this Patient with exon 50 frame-shifting deletion. The immune response was triggered from amino acids 2809 to 2829 of exon 57, downstream from the patient’s deletion. Exon specific antibodies against dystrophin showed that exon 57 was expressed in the revertant cluster (antibody stains showed expression of exons 55–56 and 59 implying that exon 57 was also expressed). (C) In a different patient with exons 49 through 54 deleted, T cells were found directed against exon 59, downstream of the frame-shifting mutation. These patients illustrate that misfolded protein expressed on revertant fibers can trigger a T cell immune response.
The findings from this study are important as we move forward with gene therapy for DMD. First we learned that we could not express mini-dystrophin in a deleted region of the patient’s DMD gene. In retrospect, this seems intuitive but it was underappreciated as inclusion/exclusion criteria for this study. The second important lesson learned is that revertant fibers, expressing dystrophin by restoring the reading frame in the DMD gene through alternative splicing or a second-site mutation, can express an immunogenic novel epitope never before encountered by the patient, most likely related to misfolding of the newly expressed dystrophin. This finding was very much unexpected given that most clinicians and scientists predicted that revertant fibers would induce immune tolerance. Validation of this principle was demonstrated in a follow up study to determine the prevalence of such immunity within a DMD cohort from our Muscular Dystrophy Association clinic [38]. Dystrophin-specific T cell immunity was evaluated in subjects with DMD who were either receiving corticosteroids in the form of prednisone (n = 24) or deflazacort (n = 29), or who were not receiving steroids (n = 17), as well as from normal age-matched control subjects (n = 21). Anti-dystrophin T cell responses were detected in none of the normal control patients, and responses were found in 9 of 17 (52.9%) untreated patients with DMD. In contrast, they were found in only 11 of 53 (20.8%) treated patients with DMD. These findings confirm the presence of preexisting circulating T cell immunity to dystrophin in a sizable proportion of patients with DMD, and emphasize the need to screen patients for dystrophin immunity prior to clinical gene therapy trials.
Limb-girdle muscular dystrophy type 2D (LGMD2D)
The alpha-sarcoglycan (α-SG) deficient subtype of LGMD (LGMD2D) is the most common of the four sarcoglycanopathies [39]. This disease shares many phenotypic similarities to DMD with the exception of its autosomal recessive inheritance pattern. Cognitive function is not affected and the heart is most often unaffected. Clinically the spectrum of disease is similar to dystrophin deficiency including a severe DMD-like phenotype and a milder form simulating Becker muscular dystrophy (BMD) with preserved ambulation into adulthood. Currently, there is no effective treatment for LGMD2D. In preparation for a clinical trial, proof of principle studies were conducted at our center in the α-SG knockout (KO) mouse using a muscle-specific creatine kinase promoter and rAAV1 serotype for intramuscular delivery [40]. This choice of vector construct allowed for robust and long-term gene expression in the tibialis anterior muscle without signs of inflammation or toxicity paving the path for clinical trial.
The LGMD2D clinical trial of α-SG gene transfer carried the responsibility to address the lessons learned from the prior DMD gene transfer trial as well as the concerns of safety. In a randomized, double blind control study; rAAV1.tMCK.hSGCA was injected into the extensor digitorum brevis (EDB) of six subjects with genetically confirmed LGMD2D [41]. The contralateral EDB served as a control receiving saline injections. The investigators performing the injections were blinded to the side of gene transfer. The EDB injections provide a source for valuable tissue assessment post-gene transfer and removal of the muscle causes no loss of function to the patient. Coincident with gene transfer, to suppress an immune response, patients received a three-day course of methylprednisone starting 4 hours prior to gene injections with repeat at 24 and 48 hours post-injection.
In this trial the subjects were initially divided into low and high dose cohorts to allow for dose-escalation. However, sufficient evidence of successful gene transfer at 6 weeks (patients 1 and 2) and 12 weeks (patient 3) at low dose (3.25 X 1011 vg) precluded the need for a higher dose regimen in the remaining three participants (4, 5 and 6). This second cohort of patients received the same dose of vector but had gene expression evaluated at a six month post-injection time point [42]. In all six participants the question of safety was answered with no adverse events recorded throughout the study. Unequivocal muscle fiber transduction of α-SG was consistently observed in both cohorts with the exception of Patient 6, whose biopsy showed no differences pre- and post-injection (Fig 6). Accounting for this was an early rise in neutralizing antibodies at day 2 post-gene delivery and early T-cell responses to AAV1 capsid measured by IFN-γ ELISpot assay (Fig 7). These early immune responses favored an amnestic response related to preexisting immunity to AAV.
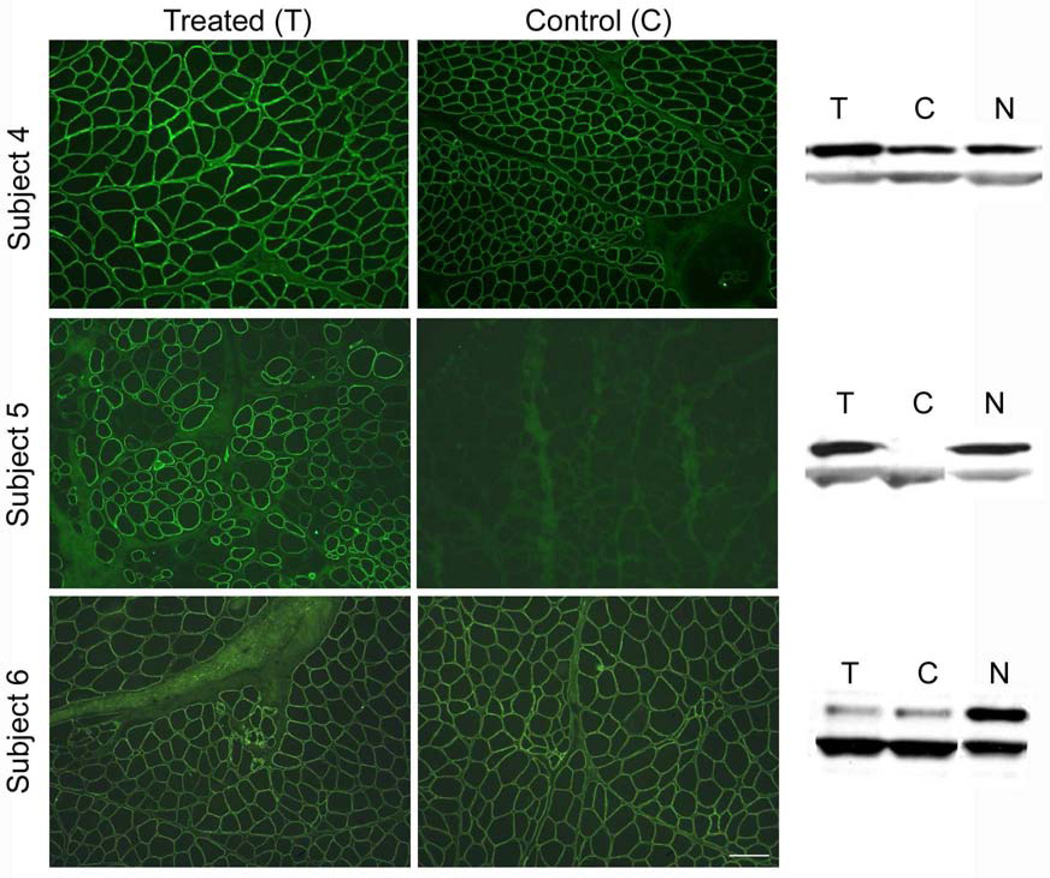
Figure 6.
α-Sarcoglycan (SG) expression following gene transfer in LGMD2D patients. (A) Post gene transfer tissue sections from EDB muscles of subjects 4–6 were stained with antibody to α-SG. Subjects 4 and 5 expressed increased staining on treated side (T) compared to control side (C). Subject 6 showed no difference in α-SG staining intensity pre- and post gene transfer (findings verified by Bioquant Image Analysis®) (scale bar =150 µm). (B) Western blots of subject 4–6 show increased α-SG gene expression on the side of gene transfer compared to the contralateral side (treated on left, control on right) for Subjects 4 and 5; residual gene expression from mutant protein prominently exhibited in Subject 4.
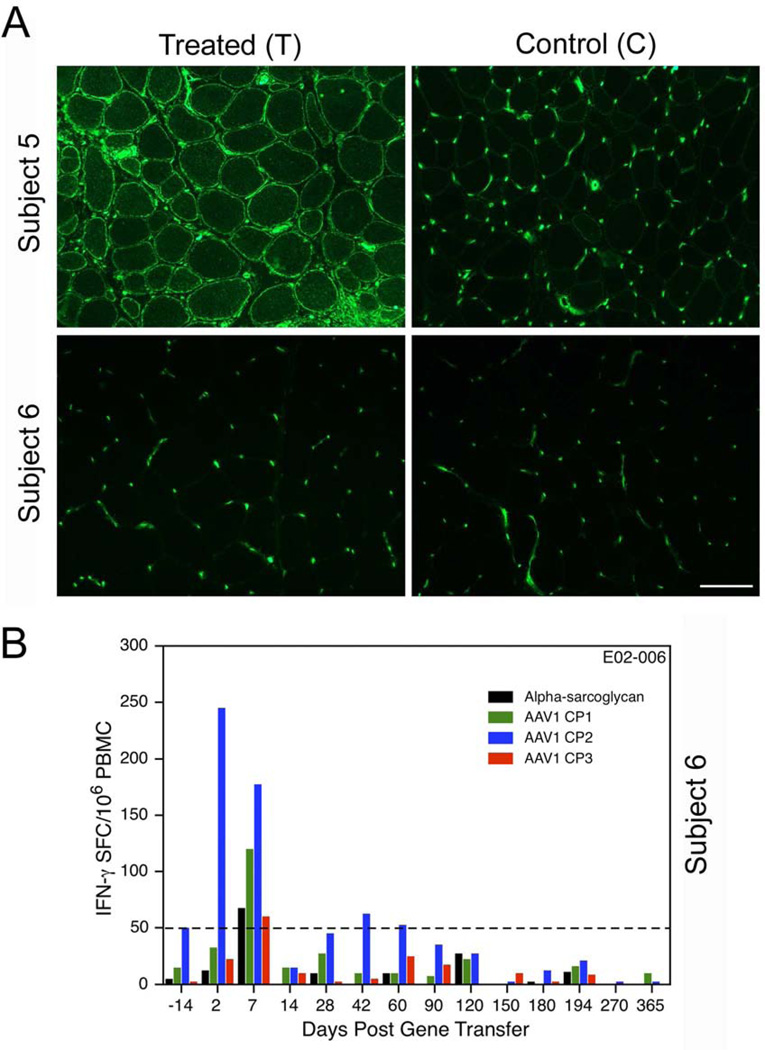
Figure 7.
MHC class I staining of sarcolemmal membrane of muscle sections on treated (T) and control (C) sides. (A) Gene transfer leads to antigen presentation at the sarcolemma. The absence of MHC I in Subject 6 of the LGMD2D study was in direct contrast to the findings of all other participants in the clinical trial, illustrated by prominent MHC I expression shown in Subject 5 on the treated side. Blood vessels will show non-specific positive staining for MHC Igene . (B) Subject 6 of the LGMD2D gene transfer study also had an immune response demonstrated in the IFN-γ ELISpot assay to AAV1 capsid pool 2 (AAV1 CP2). This was seen as early as day 2 (Blue), and again on day 7. Responses were negative for other capsid pools (AAV1 CP1 and AAV1 CP3). The positive IFN-γ ELISpot assay occurring shortly after gene transfer was indicative of an amnestic response suggesting pre-existing immunity to AAV1.
This clinical trial helps provide the foundation for moving forward with gene delivery of the α-SG transgene dispelling any concerns regarding toxicity. The safety profile is further enhanced with the demonstration of gene transfer using a muscle-specific (tMCK) promoter by avoiding concerns of off target gene expression. The trial also revealed the importance of testing all patients for pre-existing immunity to AAV; both neutralizing antibody levels and T cell immunity.
Genetic Enhancement of Alternate Genes to Improve Cellular Performance
Follistatin gene therapy
Blocking the myostatin pathway shows dramatic muscle building properties that are highly conserved across species, including mice, sheep, cattle, canines, and humans [62–64]. Myostatin is a member of the transforming growth factor (TGF)-β superfamily and numerous studies have demonstrated that it is a negative regulator of skeletal muscle growth. Follistatin is a powerful inhibitor of myostatin plus having the advantage of controlling muscle mass through pathways independent of the myostatin signaling cascade [65]. In studies of the myostatin knockout mice crossed to mice carrying a follistatin transgene there was a quadrupling of muscle mass compared with the doubling of muscle mass that is observed from lack of myostatin alone.
In preparation for taking this to clinical trial, we identified an alternatively spliced isoform of follistatin (FS344) that can be packaged in AAV1. In the mdx mouse, intramuscular injections of AAV1 carrying the FS344 gene increased muscle mass (histological measure), hind- and forelimb grip strength, and quantitative measures force generation and protection against eccentric contractions [65]. The benefits persisted for more than two years and there were no adverse effects. In non-human primates, direct muscle injections of AAV1.CMV.FS344 into the quadriceps muscle increased size and strength for as long as 15 months [66]. Necropsies showed no abnormalities in any organ, and clinical chemistries and hormone levels related to gonadal function including follicle stimulating hormone, luteinizing hormone, testosterone, and estradiol were normal.
We have now taken this to clinical trial in two patient groups who could specifically benefit by increasing size and strength of the quadriceps muscle. In Becker muscular dystrophy (BMD) and in sporadic inclusion body myositis (sIBM) the quadriceps muscle is targeted by the disease leading to frequent falls and loss of ambulation. In the gene therapy trial, the first three patients (sIBM) were injected with AAV1.CMV.FS344 at low dose vector (2 X 1011 vg/kg) in a single limb required by the FDA. No adverse events were encountered and patients transiently improved the distance walked on the 6-minute walk test (in spite of unilateral injections). In the second group, three BMD patients received an intermediate dose (6 X 1011 vg/kg). The distance walked on the 6-minute walk test improved for six months in two of three subjects. Treatment has now extended to the high dose BMD group (n =3); bilateral intramuscular injections of 1.2 X 1012 vg/kg have been given. Results are still under evaluation. The FDA has given approval for six sIBM patients to receive this same high dose in both legs. The distance walked on the 6-MWT is the primary outcome measure with secondary endpoints including muscle biopsy analysis and magnetic resonance imaging of quadriceps muscles.
Lessons Learned and Strategies to Enhance Gene Delivery
LGMD2D vascular delivery
In most gene replacement trials for the muscular dystrophies, proof-of-principle studies have utilized intramuscular (IM) injections to deliver the therapeutic gene. In an effort to achieve wider distribution of gene expression, vascular delivery can be used to target single limbs or can be administered to provide systemic distribution. Beyond the benefit of widespread distribution, vascular delivery holds several advantages. Most importantly vascular delivery is less immunogenic compared to IM delivery [43, 44]. Immunogenicity results from increased vector genome copy number per nucleus, approximately 5–10 times greater following direct IM muscle injections versus vascular delivery. The goal of a more equal distribution of uniform protein expression across the length of the muscle [45] accompanies vascular delivery.
Different strategies have been used in large animals to deliver the gene to isolated limbs through either the venous or arterial circulation [44, 46–49]. Our group has preferred to use an arterial approach that avoids the high volume, high pressure method of gene delivery that is required for delivery through the venous circulation [50]. We have utilized fluoroscopy-guided catheterization to target individual muscle groups, such as the gastrocnemius and quadriceps, or to deliver vector to the entire lower limb. To optimize arterial gene delivery and distribution, we have developed a method utilizing recirculation that improves vector transduction. This method employs a closed circuit with a tourniquet placed at the level of the groin accompanied by catheterization of both the femoral artery and vein. Venous blood is first removed from the circuit and stored in a reservoir followed by infusion of a saline-based solution to remove platelets and other cells from the extremity that could bind to vector. The vector is then infused and recirculated using a mechanical pump, thereby offering transgene to many muscle groups in the extremity through multiple passes. The promising pre-clinical results of this recirculation approach provided a rationale for translation to a clinical trial in LGMD2D patients. We have permission from the FDA to move ahead with a limb recirculation approach using AAV to deliver the α-SG transgene. This will be the first vascular gene therapy trial for muscular dystrophy and be adapted to for treatment of DMD, LGMD2B, and other dystrophies.
LGMD2B homologous recombination
Limb girdle muscular dystrophy type 2B (LGMD2B) is an autosomal recessive form of muscular dystrophy due to dysferlin deficiency. It represents one of four allelic phenotypes that also include: Miyoshi myopathy (MM) with calf muscle weakness and atrophy, a distal anterior compartment myopathy (DACM) with tibialis muscle atrophy, and a less common subtype with rigid spine syndrome [51–53]. This spectrum of myopathies is due to mutations in the dysferlin gene. The major challenge for gene therapy in dysferlinopathies is that the 6.5 kb dysferlin cDNA exceeds the packaging capacity of AAV. Compared to dystrophin, the modest size of dysferlin implores the development of novel ways to bypass packaging restraints with the benefit of restoring a full-length protein. Three different approaches have been explored for generating full-length dysferlin (DYSF) in vivo with varying degrees of efficacy [54–56]. Utilizing a trans-splicing strategy, Lostal and colleagues engineered a two vector system comprised of one vector that contained the first half of DYSF and a splice donor site and a second vector containing a splice acceptor site and the 3’ portion of DYSF [56]. In dysferlin deficient mice, they showed this approach was efficient and improved membrane repair by intramuscular injection. However, when given systemically expression was limited to <5% of muscle fibers.
We designed an alternative strategy taking advantage of the unique plasticity demonstrated by the AAV5 serotype [54]. Using AAV5 we discovered that we could spontaneously package two halves of DYSF yielding virions with regions of homology that result in a full-length transgene following delivery to muscle. Implementation of this approach leads to robust gene expression following direct intramuscular or vascular delivery using AAV5 to transfer DYSF to muscle (Fig 8). In dysferlin deficient muscle, the resulting expression improves histology, protects the muscle membrane against laser-induced injury and restores force and resistance to fatigue in the diaphragm. Long-term studies demonstrate sustained dysferlin expression for at least one year with no measurable decrease in protein levels. While only a clinical trial will be able to define how these findings translate to treatment in LGMD2B patients, this pre-clinical data implies that delivery of full-length dysferlin by homologous recombination could correct both the defects in membrane repair and the underlying histopathology of dysferlinopathy.
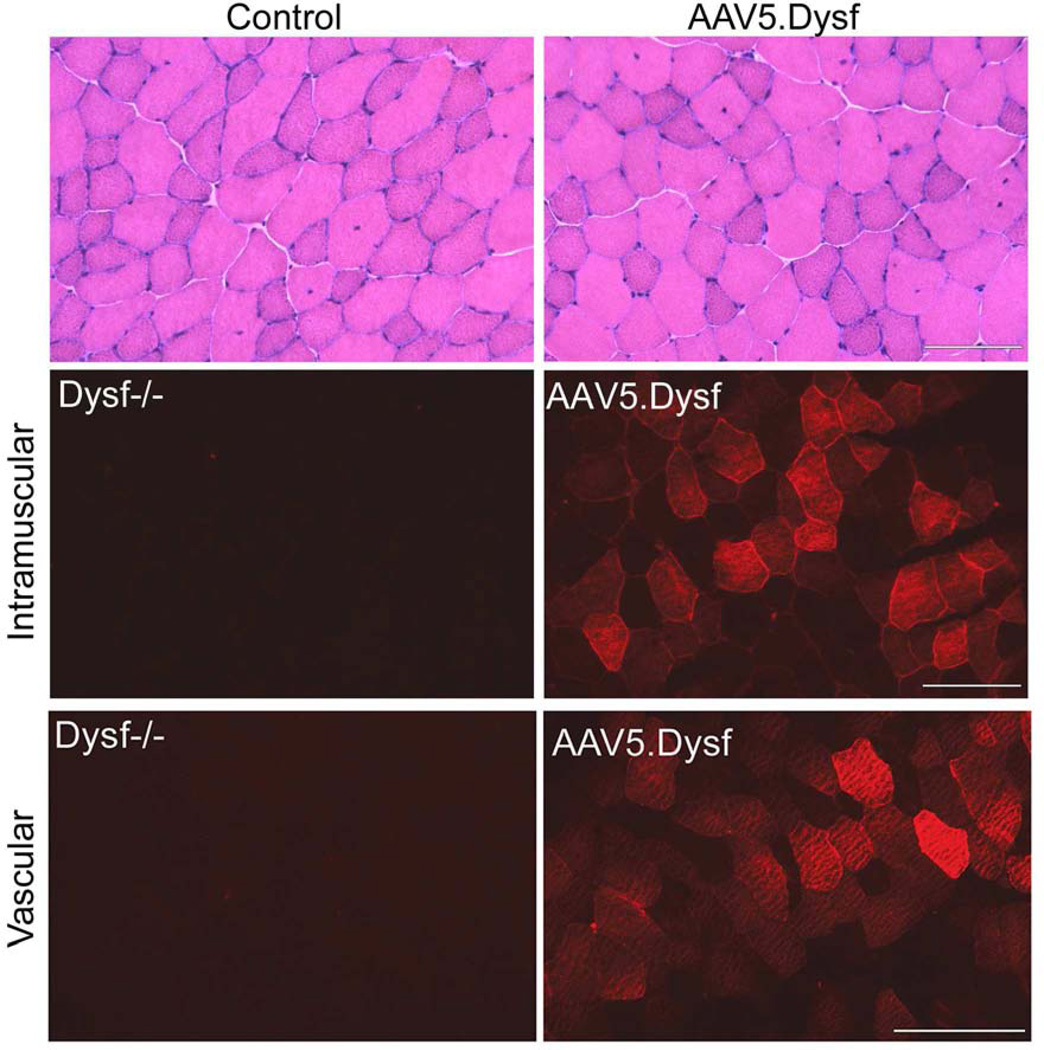
Figure 8.
AAV5.Dysf gene delivery. Dysferlin deficient mice (Dysf−/−) mice were treated by intramuscular or vascular delivery via the femoral artery with AAV5.Dysf. Dysferlin expression was restored one month after treatment (right panel) compared to untreated mice (left panel). The overexpression of dysferlin in the muscle fibers is typical of this method of gene transfer and is non-toxic to the cell.
Summary
In summary, remarkable progress has been made in gene therapy approaches for muscular dystrophies with potential for translation in clinical trials. This is the result of stepwise developments providing a foundation upon which to build. Success in exon skipping provides proof of concept for non-viral approach to gene repair. Gene delivery using AAV demonstrates both safety and efficacy in local administration and will be further promoted through vascular delivery. Lessons learned include careful screening of patients for preexisting immunity to either transgene or viral vector that will be used for delivery. Alternate strategies that employ disruption of the myostatin pathway also show potential merit. An approach to deliver genes through homologous recombination as demonstrated for dysferlin may pay unexpected dividends in clinical trials. All in all the future looks bright for multiple molecular therapeutic strategies for the muscular dystrophies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klein CJ, et al. Somatic Reversion Suppression in Duchenne Muscular-Dystrophy (Dmd) - Evidence Supporting a Frame-Restoring Mechanism in Rare Dystrophin-Positive Fibers. American Journal of Human Genetics. 1992;50(5):950–959. [PMC free article] [PubMed] [Google Scholar]
- 2.Aartsma-Rus A, et al. Theoretic applicability of antisense-mediated exon skipping for Duchenne muscular dystrophy mutations. Hum Mutat. 2009;30(3):293–299. doi: 10.1002/humu.20918. [DOI] [PubMed] [Google Scholar]
- 3.Mann CJ, et al. Antisense-induced exon skipping and synthesis of dystrophin in the mdx mouse. Proc Natl Acad Sci U S A. 2001;98(1):42–47. doi: 10.1073/pnas.011408598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goyenvalle A, et al. Prevention of dystrophic pathology in severely affected dystrophin/utrophin-deficient mice by morpholino-oligomer-mediated exon-skipping. Mol Ther. 2010;18(1):198–205. doi: 10.1038/mt.2009.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yokota T, et al. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann Neurol. 2009;65(6):667–676. doi: 10.1002/ana.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Deutekom JC, et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med. 2007;357(26):2677–2686. doi: 10.1056/NEJMoa073108. [DOI] [PubMed] [Google Scholar]
- 7.Kinali M, et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8(10):918–928. doi: 10.1016/S1474-4422(09)70211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goemans NM, et al. Systemic administration of PRO051 in Duchenne's muscular dystrophy. N Engl J Med. 2011;364(16):1513–1522. doi: 10.1056/NEJMoa1011367. [DOI] [PubMed] [Google Scholar]
- 9.Cirak S, et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet. 2011;378(9791):595–605. doi: 10.1016/S0140-6736(11)60756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.JR M, et al. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann Neurol. 2013 doi: 10.1002/ana.23982. [DOI] [PubMed] [Google Scholar]
- 11.McDonald CM, et al. The 6-minute walk test and other clinical endpoints in duchenne muscular dystrophy: Reliability, concurrent validity, and minimal clinically important differences from a multicenter study. Muscle & Nerve. 2013;48(3):357–368. doi: 10.1002/mus.23905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonald CM, et al. The 6-minute walk test in Duchenne/Becker muscular dystrophy: longitudinal observations. Muscle & Nerve. 2010;42(6):966–974. doi: 10.1002/mus.21808. [DOI] [PubMed] [Google Scholar]
- 13.Strober JB. Therapeutics in duchenne muscular dystrophy. NeuroRx. 2006;3(2):225–234. doi: 10.1016/j.nurx.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendell JR, et al. Diagnosis of Duchenne dystrophy by enhanced detection of small mutations. Neurology. 2001;57(4):645–650. doi: 10.1212/wnl.57.4.645. [DOI] [PubMed] [Google Scholar]
- 15.Flanigan KM, et al. Rapid direct sequence analysis of the dystrophin gene. American Journal of Human Genetics. 2003;72(4):931–939. doi: 10.1086/374176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du L, et al. Nonaminoglycoside compounds induce readthrough of nonsense mutations. J Exp Med. 2009;206(10):2285–2297. doi: 10.1084/jem.20081940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du M, et al. Aminoglycoside suppression of a premature stop mutation in a Cftr−/− mouse carrying a human CFTR-G542X transgene. J Mol Med (Berl) 2002;80(9):595–604. doi: 10.1007/s00109-002-0363-1. [DOI] [PubMed] [Google Scholar]
- 18.Barton-Davis ER, et al. Aminoglycoside antibiotics restore dystrophin function to skeletal muscles of mdx mice. Journal of Clinical Investigation. 1999;104(4):375–381. doi: 10.1172/JCI7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner KR, et al. Gentamicin treatment of Duchenne and Becker muscular dystrophy due to nonsense mutations. Ann Neurol. 2001;49(6):706–711. [PubMed] [Google Scholar]
- 20.Politano L, et al. Gentamicin administration in Duchenne patients with premature stop codon. Preliminary results. Acta Myol. 2003;22(1):15–21. [PubMed] [Google Scholar]
- 21.Malik V, et al. Gentamicin-induced readthrough of stop codons in Duchenne muscular dystrophy. Ann Neurol. 2010;67(6):771–780. doi: 10.1002/ana.22024. [DOI] [PubMed] [Google Scholar]
- 22.Ahmad A, et al. Mdx mice inducibly expressing dystrophin provide insights into the potential of gene therapy for duchenne muscular dystrophy. Human Molecular Genetics. 2000;9(17):2507–2515. doi: 10.1093/hmg/9.17.2507. [DOI] [PubMed] [Google Scholar]
- 23.Ghahramani Seno MM, et al. RNAi-mediated knockdown of dystrophin expression in adult mice does not lead to overt muscular dystrophy pathology. Human Molecular Genetics. 2008;17(17):2622–2632. doi: 10.1093/hmg/ddn162. [DOI] [PubMed] [Google Scholar]
- 24.Tang HY, et al. Genetic susceptibility to aminoglycoside ototoxicity: how many are at risk? Genet Med. 2002;4(5):336–345. doi: 10.1097/00125817-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Viollet L, et al. Utility of Cystatin C to Monitor Renal Function in Duchenne Muscular Dystrophy. Muscle & Nerve. 2009;40(3):438–442. doi: 10.1002/mus.21420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howard MT, et al. Sequence specificity of aminoglycoside-induced stop condon readthrough: potential implications for treatment of Duchenne muscular dystrophy. Ann Neurol. 2000;48(2):164–169. [PubMed] [Google Scholar]
- 27.Welch EM, et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447(7140):87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- 28.Hirawat S, et al. Safety, tolerability, and pharmacokinetics of PTC124, a nonaminoglycoside nonsense mutation suppressor, following single- and multiple-dose administration to healthy male and female adult volunteers. J Clin Pharmacol. 2007;47(4):430–444. doi: 10.1177/0091270006297140. [DOI] [PubMed] [Google Scholar]
- 29.Quinlivan R KJ, Comi G, Bushby K the Ataluren DBMD Study Group. Safety and efficacy of ataluren 10,10,20 mg/kg TID in patients with nonsense mutation dystrophinopathy. European Paediatric Neurology Society Congress, 9th EPNS Congress [online] 2010 [Google Scholar]
- 30.Mendell JR, et al. Randomized, double-blind six-month trial of prednisone in Duchenne's muscular dystrophy. N Engl J Med. 1989;320(24):1592–1597. doi: 10.1056/NEJM198906153202405. [DOI] [PubMed] [Google Scholar]
- 31.Griggs RC, et al. Corticosteroids in Duchenne muscular dystrophy: major variations in practice. Muscle & Nerve. 2013;48(1):27–31. doi: 10.1002/mus.23831. [DOI] [PubMed] [Google Scholar]
- 32.Mendell JR, et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol. 2012;71(3):304–313. doi: 10.1002/ana.23528. [DOI] [PubMed] [Google Scholar]
- 33.Mendell JR, Lloyd-Puryear M. Report of MDA muscle disease symposium on newborn screening for Duchenne muscular dystrophy. Muscle & Nerve. 2013;48(1):21–26. doi: 10.1002/mus.23810. [DOI] [PubMed] [Google Scholar]
- 34.England SB, et al. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature. 1990;343(6254):180–182. doi: 10.1038/343180a0. [DOI] [PubMed] [Google Scholar]
- 35.Wang B, Li J, Xiao X. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc Natl Acad Sci U S A. 2000;97(25):13714–13719. doi: 10.1073/pnas.240335297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watchko J, et al. Adeno-associated virus vector-mediated minidystrophin gene therapy improves dystrophic muscle contractile function in mdx mice. Hum Gene Ther. 2002;13(12):1451–1460. doi: 10.1089/10430340260185085. [DOI] [PubMed] [Google Scholar]
- 37.Mendell JR, et al. Dystrophin immunity in Duchenne's muscular dystrophy. N Engl J Med. 2010;363(15):1429–1437. doi: 10.1056/NEJMoa1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flanigan KM, et al. Anti-Dystrophin T Cell Responses in Duchenne Muscular Dystrophy: Prevalence and a Glucocorticoid Treatment Effect. Hum Gene Ther Methods. 2013 doi: 10.1089/hum.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore S, Shilling C, Mendell J. Limb-Girdle Muscular Dystrophy in the United States. J Neuropath Exp Neurol. 2006;65(10):995–1003. doi: 10.1097/01.jnen.0000235854.77716.6c. [DOI] [PubMed] [Google Scholar]
- 40.Rodino-Klapac LR, et al. Lack of toxicity of alpha-sarcoglycan overexpression supports clinical gene transfer trial in LGMD2D. Neurology. 2008;71(4):240–247. doi: 10.1212/01.wnl.0000306309.85301.e2. [DOI] [PubMed] [Google Scholar]
- 41.Mendell JR, et al. Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann Neurol. 2009;66(3):290–297. doi: 10.1002/ana.21732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mendell JR, et al. Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D. Ann Neurol. 2010;68(5):629–638. doi: 10.1002/ana.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toromanoff A, et al. Safety and efficacy of regional intravenous (r.i.) versus intramuscular (i.m.) delivery of rAAV1 and rAAV8 to nonhuman primate skeletal muscle. Mol Ther. 2008;16(7):1291–1299. doi: 10.1038/mt.2008.87. [DOI] [PubMed] [Google Scholar]
- 44.Toromanoff A, et al. Lack of immunotoxicity after regional intravenous (RI) delivery of rAAV to nonhuman primate skeletal muscle. Mol Ther. 2010;18(1):151–160. doi: 10.1038/mt.2009.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodino-Klapac LR, et al. Persistent expression of FLAG-tagged micro dystrophin in nonhuman primates following intramuscular and vascular delivery. Mol Ther. 2010;18(1):109–117. doi: 10.1038/mt.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hegge JO, et al. Evaluation of hydrodynamic limb vein injections in nonhuman primates. Hum Gene Ther. 2010;21(7):829–842. doi: 10.1089/hum.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arruda VR, et al. Regional intravascular delivery of AAV-2-F.IX to skeletal muscle achieves long-term correction of hemophilia B in a large animal model. Blood. 2005;105(9):3458–3464. doi: 10.1182/blood-2004-07-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katwal AB, et al. Adeno-associated virus serotype 9 efficiently targets ischemic skeletal muscle following systemic delivery. Gene Ther. 2013;20(9):930–938. doi: 10.1038/gt.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodino-Klapac LR, et al. AAV-mediated gene therapy to the isolated limb in rhesus macaques. Methods Mol Biol. 2011;709:287–298. doi: 10.1007/978-1-61737-982-6_19. [DOI] [PubMed] [Google Scholar]
- 50.Rodino-Klapac LR, et al. A translational approach for limb vascular delivery of the micro-dystrophin gene without high volume or high pressure for treatment of Duchenne muscular dystrophy. J Transl Med. 2007;5:45. doi: 10.1186/1479-5876-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu J, et al. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nature Genetics. 1998;20(1):31–36. doi: 10.1038/1682. [DOI] [PubMed] [Google Scholar]
- 52.Illa I, et al. Distal anterior compartment myopathy: a dysferlin mutation causing a new muscular dystrophy phenotype. Ann Neurol. 2001;49(1):130–134. [PubMed] [Google Scholar]
- 53.Nagashima T, et al. Dysferlinopathy associated with rigid spine syndrome. Neuropathology. 2004;24(4):341–346. doi: 10.1111/j.1440-1789.2004.00573.x. [DOI] [PubMed] [Google Scholar]
- 54.Grose WE, et al. Homologous Recombination Mediates Functional Recovery of Dysferlin Deficiency following AAV5 Gene Transfer. Plos One. 2012;7(6) doi: 10.1371/journal.pone.0039233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krahn M, et al. A naturally occurring human minidysferlin protein repairs sarcolemmal lesions in a mouse model of dysferlinopathy. Sci Transl Med. 2010;2(50):50ra69. doi: 10.1126/scitranslmed.3000951. [DOI] [PubMed] [Google Scholar]
- 56.Lostal W, et al. Efficient recovery of dysferlin deficiency by dual adeno-associated vector-mediated gene transfer. Human Molecular Genetics. 2010;19(10):1897–1907. doi: 10.1093/hmg/ddq065. [DOI] [PubMed] [Google Scholar]