Abstract
Accumulation of Aβ in the brains of Alzheimer disease (AD) patients reflects an imbalance between Aβ production and clearance from their brains. Alternative cleavage of amyloid precursor protein (APP) by processing proteases generates soluble APP fragments including the neurotoxic amyloid Aβ40 and Aβ42 peptides that assemble into fibrils and form plaques. Plaque-buildup occurs over an extended time-frame, and the early detection and modulation of plaque formation are areas of active research. Radiolabeled probes for the detection of amyloid plaques and fibrils in living subjects are important for noninvasive evaluation of AD diagnosis, progression, and differentiation of AD from other neurodegenerative diseases and age-related cognitive decline. Tritium-labeled (E,E)-1-[3H]-2,5-bis(4’-hydroxy-3’-carbomethoxystyryl)benzene possesses an improved level of chemical stability relative to a previously reported radioiodinated analog for radiometric quantification of Aβ plaque and tau pathology in brain tissue and in vitro studies with synthetic Aβ and tau fibrils.
Accumulation of extracellular Aβ senile plaques in brain tissue and tangles of hyperphosphorylated tau protein inside brain neurons are the classical histopathological signs of Alzheimer’s disease (AD).1,2 According to the AD β-amyloid hypothesis,3,4 the imbalance between the production and clearance of Aβ from brains of AD patients results from the alternative processing of the amyloid precursor protein5,6 (APP) by β- and γ-secretases resulting in the generation of soluble APP fragments:7 amyloid Aβ38, Aβ40, and Aβ42, which assemble into plaques.8 Tau protein hyperphosphorylation is induced by Aβ leading to neurofibrillary tangle formation.9,10 The soluble oligomers of Aβ42 that result from the assembly of monomer produced by the sequential proteolytic cleavage of APP by β- and γ-secretase are neurotoxic,11 cause neuroinflammation and neuronal death, and ultimately result in cognitive impairment, irreversible memory loss, and disorientation in AD.
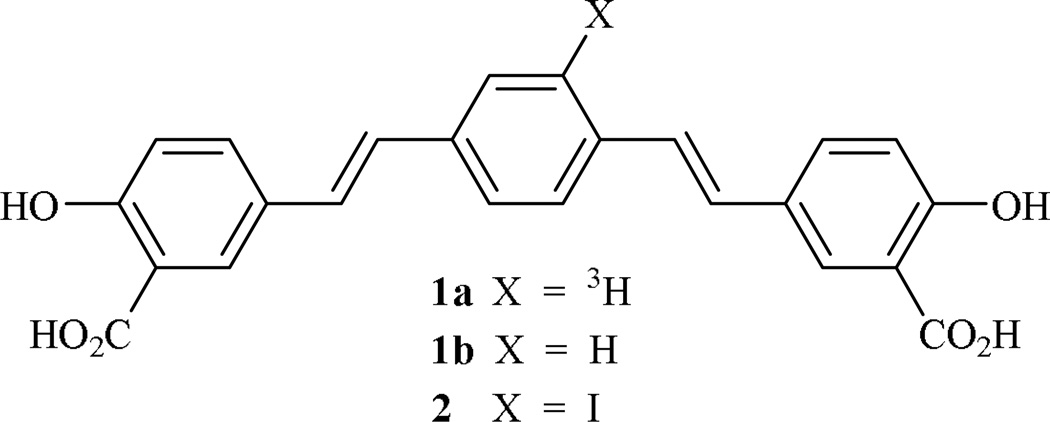
Because Aβ plaque formation occurs over a lengthy time period, methods for the early and conclusive detection12, monitoring, and prevention13–16 of Aβ deposition are of considerable importance. The development of probes for amyloid plaques and tangles17,18 provides one avenue for the noninvasive diagnosis and evaluation of AD progression in living subjects and its differentiation from age-related cognitive decline in AD patients.19,20 We report the synthesis of a radiolabeled Congo Red analog, (E,E)-1-[3H]-2,5-bis(4’-hydroxy-3’-carboxystyryl)benzene (1a), which has not been reported previously, and its non-radiolabeled counterpart21 1b, which is commonly known as X-34 (Fig. 1). Both ligands bind to fibrillar forms of both Aβ and tau, and we demonstrate the utility of 1a in studies of synthetic Aβ40 and Aβ42 fibrils. We also report a cautionary note regarding the oxidative sensitivity of an intermediate in this route, namely 2,5-bis(4’-hydroxy-3’-carbomethoxystyryl)-1-iodobenzene (2) (Fig. 1) and by analogy, a radioiodinated version of 2, which has also been reported22 as a probe for Aβ deposition.
Figure 1. Iodinated and tritiated (E,E)-bis-1,4-styrylbenzenes required as imaging agents.
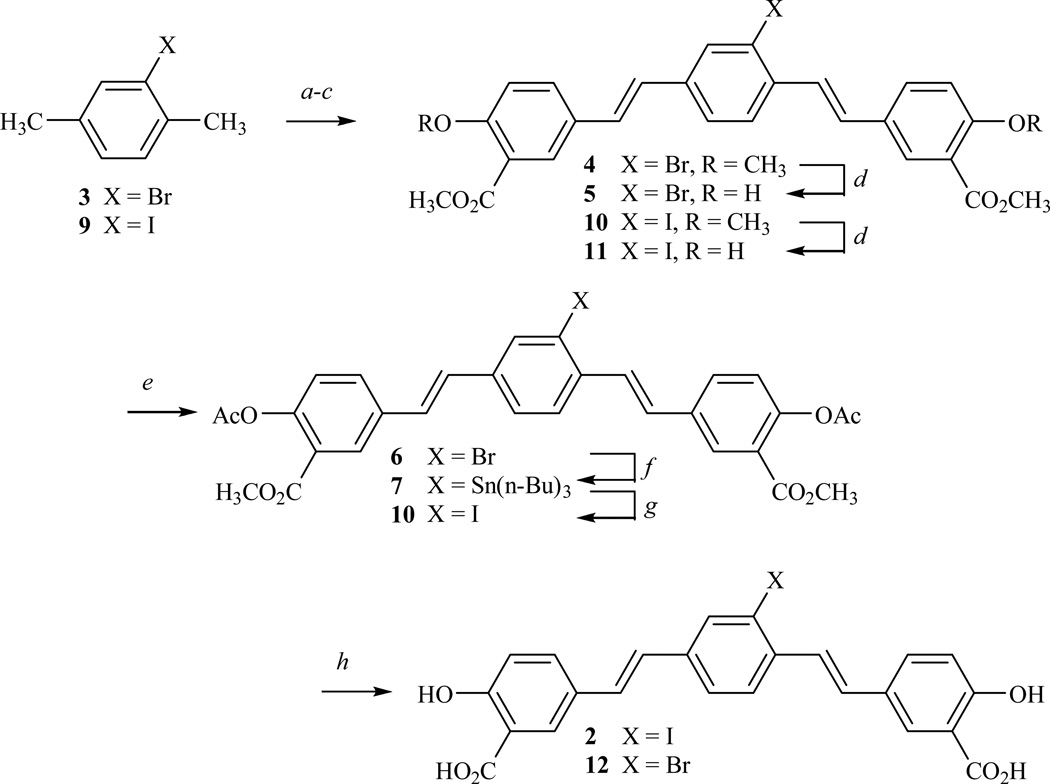
Plans for the synthesis of 1a or 1b focused on the regioselective, catalytic hydrogenolysis of a suitable iodinated 4-styrylstilbene, namely (E,E)-2,5-bis(4’-hydroxy-3’-carbomethoxystyryl)-1-iodobenzene (2) (Fig. 2), using tritium gas and a palladium catalyst or sodium borohydride and a palladium catalyst, respectively. Initial efforts, as a consequence, focused on the synthesis of 2. Following the procedure of Zhuang,22 the benzylic bromination of 2-bromo-para-xylene (3) and Arbusov reaction with triethyl phosphite provided tetraethyl (2-bromo-1,4-phenylene)bis(methylene)-diphosphonate. Wadsworth-Emmons condensation of this phosphonate with 3-carbomethoxy-4-methoxybenzaldehyde and demethylation of the intermediate bisanisole 4 secured the bisphenol 5 (Fig. 2). Efforts, however, to effect the tri-nbutylstannylation of 5 were unsuccessful, contrary to a report22 claiming a 25% yield. Suspecting that this failure was a consequence of the phenolic hydroxyl groups in 5, we converted 5 to the bisacetate 6 and were successful in converting 6 to the arylstannane 7, albeit only in 18–23% yields. Iodination of 7 using sodium iodide and hydrogen peroxide, and saponification of 8 furnished the iodinated 4-styrylstilbene 2 in very poor yield. The low yield in the iodination reaction was unexpected until subsequent work, as discussed below, brought to our attention the oxidative sensitivity of 2.
Figure 2. Synthesis of (E,E)-2,5-bis(4’-hydroxy-3’-carboxystyryl)-1-iodobenzene (2).
a, NBS (recrystallized), AIBN (cat), CCl4; b, P(OCH2CH3)3; c, NaOCH3, 3-carbomethoxy-4-methoxybenzaldehyde, CH3OH; d, BBr3, CH2Cl2, −78°C; e, Ac2O, Py; f, Pd(PPh3)4, (n-Bu)3SnSn(n-Bu)3; g, NaI, H2O2; h, NaOH, aq CH3OH.
The poor yields in the stannylation and iodination reactions led us to explore an alternate route to the iodinated 4-styrylstilbene 2. Elaboration of 2-iodo-p-xylene (9) to the iodinated bisphenol 11 followed a similar sequence to that described earlier (Fig. 2). The acetylation of 11 afforded the bisacetate 8 that was identical to material prepared from the route originating with 2-bromo-p-xylene (3). The saponification of 8 and subsequent acidification gave a precipitate that was unambiguously identified as the iodinated 4-styrylstilbene 2 according to NMR and mass spectral data. Efforts, however, to purify 2 by recrystallization or chromatography were complicated by the apparent instability of 2 on exposure to air. For example, the 1H NMR of a chromatographed sample of the iodinated 4-styrylstilbene 2 did not display the sharp signals seen in either the non-chromatographed sample of 2 or in the brominated analog, 2,5-bis(4’-hydroxy-3’-carbomethoxystyryl)-1-bromobenzene (12). Oxidative cyclization23–25 of other 1,4-bis(styryl)benzenes to 3-styrylphenanthrenes suggested at least one avenue for the oxidation of 2, and indeed, the treatment of non-chromatographed, well characterized 2 with hydrogen peroxide alone led to a plethora of products. In summary, we developed an unambiguous route to 2,5-bis(4’-hydroxy-3’-carbomethoxystyryl)-2-iodobenzene (2) that did not involve an oxidative process, and we utilized 2, without further purification, in the synthesis of the desired 1a and 1b, as discussed below. In our opinion, investigators opting to use the radioiodinated [125I]-version22 of 2 as a probe for Aβ deposition should be aware of this chemical instability, avoid chromatographic purification on silica gel, and consider the tritiated ligand 1a (Fig. 1) as a preferable alternative. It should also be noted that the “cold” ligand 1b is sufficiently fluorescent to find application in its own right as a probe for Aβ deposition.26
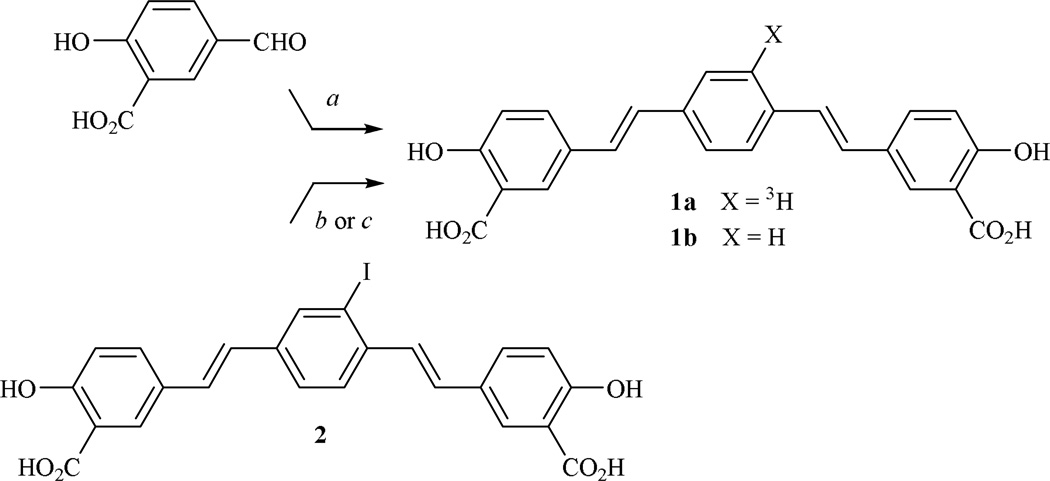
Catalytic hydrogenolysis27 of iodinated 4-styrylstilbene 2 using sodium borohydride and a catalytic amount of tetrakis(triphenylphosphine)palladium(0) furnished 2,5-bis(4’-hydroxy-3’-carbomethoxystyryl)benzene (1b) or X-34,26,28 as it is commonly known in the literature (Fig. 3). The “cold” 4-styrylstilbene (1b) was also synthesized independently using the Horner-Emmons condensation of 5-formylsalicylic acid with p-xylenediphosphonic acid tetraethyl ester.28 In order to achieve high specific activity, hydrogenolysis of 2 was performed using tritium gas and a palladium catalyst to provide radiolabeled (E,E)-1-[3H]-2,5-bis(4’-hydroxy-3’-carbomethoxystyryl)benzene (1a) at 23 Ci/mmole. Tritiated sodium borohydride and the palladium catalyst could be used to secure 1a, albeit at lower specific activities than those reported here.
Figure 3. Synthesis of (E,E)-1-[3H]-2,5-bis(4’-methoxy-3’-carboxystyryl)benzene (1a) and its “cold” analog (1b or X-34).
a, NaOCH3, p-xylenediphosphonic acid tetraethyl ester; b, tritium gas, proprietary palladium catalyst (ViTrax, Placentia, CA) or [3H]-NaBH4, Pd(PPh3)4 to give 1a; c, NaBH4, Pd(PPh3)4 to give 1b.
The binding of tritiated 1a was selective for the Congo Red-binding site on Aβ40 and Aβ42 fibrils and was displaced only by those unlabeled ligands, including the “cold” 1b that mimicked Congo Red (Table 1). Non-specific retention of 1a on the GF/B filter material was very low, and as expected, ligands with structures similar to Congo Red (i.e., possess extended π systems) vied for binding against 1a with efficacy values (EC50, Table 1) for either Aβ40 or Aβ42 fibrils (i.e., displayed varied EC50 values) that varied with substitution patterns. In contrast, benzothiazole ligands, such as Thioflavine T, Pittsburgh Compound B (PIB), or 2-(4´-methylaminophenyl)benzothiazole (BTA-1), were ineffective in displacing 1a as were several other molecules reported to bind to Aβ fibrils but with structures unlike the Congo Red structure. From the Scatchard analysis, the binding stoichiometry of tritiated 1a approached one ligand per two peptides. The similarity of the Scatchard Kd to the EC50 for “cold” 1b competition against tritiated 1a suggested that the EC50’s were equivalent to Kd values, namely for Aβ40, Kd = 0.60 µM and the ratio of molecules of 1b/molecules of Aβ40 (as monomers) = 0.43 and for Aβ42, Kd = 0.25 µM and the ratio of molecules of 1b/ molecules of Aβ42 (as monomers) = 0.63.
Table 1. Displacement of 5 nM of (E,E)-1-[3H]-2,5-bis(4’-hydroxy-3’-carbomethoxystyryl)benzene (1a) from synthetic Aβ fibrils by unlabeled compounds.
[3H]-X-34 is another name for (E,E)-1-[3H]-2,5-bis(4’-hydroxy-3’-carbomethoxystyryl)benzene (1a); X-34 is another name for (E,E)-2,5-bis(4’-hydroxy-3’-carbomethoxystyryl)benzene (1b); BSB, (E,E)-1-bromo-2,5-bis(4’-hydroxy-3’-carbomethoxystyryl)benzene; ISB, (E,E)-1-iodo-2,5-bis(4’-hydroxy-3’-carbomethoxystyryl)benzene; K114, (E,E)-1-bromo-2,5-bis(4’-hydroxystyryl)benzene; BMB, (E,E)-1-methoxy-2,5-bis(4’-aminostyryl)benzene or as it also known 1,4-bis(4-aminophenylethenyl)-2-methoxybenzene; PIB, Pittsburgh Compound B; BTA-1, 2-(4´-methylaminophenyl)benzothiazole; IMPY, 2-(4'-dimethylaminophenyl)-6-iodoimidazo[1,2-a]pyridine; ThT, Thioflavine T; ThS, Thioflavin S.
| Compound | Aβ(1–40) EC50, µM |
Aβ(1–42) EC50, µM |
|---|---|---|
| Chrysamine G | 9 | 0.9 |
| Congo Red | 2.3 | 0.7 |
| 1b or X-34 | 0.53 | 0.2 |
| BSB | 0.23 | 0.12 |
| ISB | 0.2 | 0.15 |
| K114 | >10 | 3 |
| BMB | >10 | 3 |
| PIB | >10 | >10 |
| BTA-1 | >10 | >10 |
| IMPY | >10 | >10 |
| ThT | >10 | >10 |
| ThS | >10 | >10 |
| primulin | >10 | >10 |
| curcumin | >10 | 4 |
| resveratrol | >10 | >10 |
| resorufin | >10 | >10 |
| naproxen | >10 | >10 |
| ibuprofen | >10 | >10 |
The structure and conformation of amyloid fibrils reflects the conditions under which they are assembled. This polymorphism is most evident in comparing the Aβ deposits from animal models of AD and human AD brain. A large amount of high affinity PIB binding is found in AD brain, but binding of this ligand is virtually undetectable in brain tissue of transgenic mouse models29 or non-human primate brains30 with similar amounts of Aβ deposition, as judged by immunohistochemistry or Congo Red binding. Since (E,E)-1-[3H]-2,5-bis(4’-hydroxy-3’-carbomethoxystyryl)benzene possesses an extended π system like the panfibrillar amyloid, Congo Red ligand, tritiated 1a will be useful for standardizing the quantitative assessment of relationships between chemical structures of existing and future amyloid probes with the ligand binding site types present on amyloid deposits.31,32 Ligands, like the one reported here, will be important for understanding the molecular mechanisms of deposition linked to AD and other neurodegenerative diseases with misfolded protein pathology.
Supplementary Material
Acknowledgments
HL was supported by the Coins for Alzheimer’s Research Trust (C.A.R.T.) of the Rotary Clubs of North Carolina, South Carolina, and Georgia; the National Institute of Neurological Disorders and Stroke (R21 NS080576); an unrestricted “Grants4Targets” Grant from Bayer Healthcare; and a University of Kentucky Research Support Grant. DSW was supported by the Office of the Dean of the College of Medicine and by NIH Grant Number P20 RR020171 from the National Institute of General Medical Sciences to L. Hersh, PI. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, the NINDS, or the NIGMS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Marcell E, Epis R, Di Luca M. Eur. J. Pharmacol. 2008;558:109. doi: 10.1016/j.ejphar.2007.11.083. [DOI] [PubMed] [Google Scholar]
- 2.Ikonomovic MD, Abrahamson EE, Isanski BA, Debnath ML, Mathis CA, DeKosky ST, Klunk WE. Methods Enzymol. 2006;412:123. doi: 10.1016/S0076-6879(06)12009-1. [DOI] [PubMed] [Google Scholar]
- 3.Selkoe DJ. Physiol. Rev. 2001;81:741. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong RA. Int. J. Alzheimers Dis. 2011:630865. doi: 10.4061/2011/630865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim T, Hinton DJ, Choi D-S. Int. J. Alzheimers Dis. 2011:857368. doi: 10.4061/2011/857368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mawneuyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman R. Science. 2010 Dec 24;330(6012):1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Strooper B. Physiol. Rev. 2010;90:465. doi: 10.1152/physrev.00023.2009. [DOI] [PubMed] [Google Scholar]
- 8.Small GW, Kepe V, Ercoli LM, Siddarth P, Bookheimer SY, Miller KJ, Lawretsky H, Burgreen AC, Cole GM, Vinters HV, Thompson PM, Huang S-C, Satyamurthy N, Phelps ME, Barrio JR. New Eng. J. Med. 2006;355:2652. doi: 10.1056/NEJMoa054625. [DOI] [PubMed] [Google Scholar]
- 9.Oddo S, Caccamo A, Cheng D, Jouleh B, Torp R, Laferla FM. J. Neurochem. 2007;102:1053. doi: 10.1111/j.1471-4159.2007.04607.x. [DOI] [PubMed] [Google Scholar]
- 10.Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Neurobiol. Aging. 2003;24:1063. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Abramowski D, Rabe S, Upadhaya AR, Raichwald J, Simone D, Staab D, Capetillo-Zarate E, Yamagushi H, Saido TC, Wiederholt K-H, Tahl DR, Staufenbiel MJ. Neurosci. 2012;32:1273. doi: 10.1523/JNEUROSCI.4586-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kepe V, Moghbel MC, Långström B, Zaidi H, Vinterts HV, Huang S-Ch, Satyamyrthy N, Doudet D, Mishani E, Cohen RM, Høilund-Carlsen PF, Alavi A, Barrio JR. Int. J. Alzheimers Dis. 2013;36:613. doi: 10.3233/JAD-130485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nugroschl J, Sano M. Curr. Neurol. Neurosci. Rep. 2009;9:368. doi: 10.1007/s11910-009-0054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Styczyńska M, Strosznajder JB, Chodakowska-Żebrowska M, Pfeffer A, Gabrylewicz T, Czapski GA, Kobryś M, Karciauskas G, Barcikowska M. Folia Neuropathol. 2008;46:249. [PubMed] [Google Scholar]
- 15.Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, Yan SD. FASEB J. 2005;19:2040. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 16.Anand R, Gill KD, Mahdi AA. Neuropharmacol. 2014 Jan; doi: 10.1016/j.neuropharm.2013.07.004. 76 Pt A:27. [DOI] [PubMed] [Google Scholar]
- 17.Reineke AA, Gestwicki JE. Chem. Biol. Drug Des. 2011;77:399. doi: 10.1111/j.1747-0285.2011.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribeiro Morais G, Paulo A, Santos IA. Eur. J. Org. Chem. 2012;7:1279. [Google Scholar]
- 19.Sutharsan J, Dakanali N, Capule ChC, Haidekker MA, Yang J, Theodorakis EA. Chem. Med. Chem. 2010;5:56. doi: 10.1002/cmdc.200900440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckroad TJ, Mayhoub AS, Garneau-Tsodikova S, Beilstein J. Org. Chem. 213:9. doi: 10.3762/bjoc.9.116. 1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shrestha S, Bhattarai BR, Kafle B, Lee K-H, Cho H. Bioorg. Med. Chem. 2008;16:8643. doi: 10.1016/j.bmc.2008.07.090. [DOI] [PubMed] [Google Scholar]
- 22.Zhuang Z-P, Kung M-P, Hou C, Skovronsky DM, Gur TL, Plössl K, Trojanowski JQ, Lee VMY, Kung HF. J. Med. Chem. 2001;44:1905. doi: 10.1021/jm010045q. [DOI] [PubMed] [Google Scholar]
- 23.Wang K-L, Lu M-Y, Wang Q-M, Huang R-Q. Tetrahedron. 2008;64:7504. [Google Scholar]
- 24.Wang K, Hu Y, Li Z, Wu M, Liu Z, Su B, Yu A, Wang Q. Synthesis. 2010;7:1083. [Google Scholar]
- 25.Ding D-J, Cao X-Y, Dai F, Li X-Z, Liu G-Y, Lin D, Fu X, Jin X-L, Zhou B. Food Chem. 2012;135:1011. doi: 10.1016/j.foodchem.2012.05.074. [DOI] [PubMed] [Google Scholar]
- 26.Ikonomovic MD, Abrahamson EE, Isanski BA, Debnath ML, Mathis CA, DeKosky ST, Klunk WE. Methods Enzymol. 2006;412:123. doi: 10.1016/S0076-6879(06)12009-1. [DOI] [PubMed] [Google Scholar]
- 27.Nagasaki T, Sakai K, Segawa M, Katsuyama Y, Haga N, Koike M, Kawada K, Takechi SJ. Labelled Compd. Radiopharm. 2001;44:993. [Google Scholar]
- 28.Styren SD, Hamilton RL, Styren GC, Klunk WE. J. Histochem. Cytochem. 2000;48:1223. doi: 10.1177/002215540004800906. [DOI] [PubMed] [Google Scholar]
- 29.Klunk WE, Lopresti BJ, Ikonomovic MD, Lefterov IM, Koldamova RP, Abrahamson EE, Debnath ML, Holt DP, Huang GF, Shao L, DeKosky ST, Price JC, Mathis CA. J. Neurosci. 2005;25:10598. doi: 10.1523/JNEUROSCI.2990-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosen RF, Walker LC, LeVine H., III Neurobiol. Aging. 2011;32:223. doi: 10.1016/j.neurobiolaging.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lockhart A, Ye L, Judd DB, Merritt AT, Lowe PN, Morgenstern JL, Hong G, Gee AD, Brown JJ. Biol. Chem. 2005;280:7677. doi: 10.1074/jbc.M412056200. [DOI] [PubMed] [Google Scholar]
- 32.Ye L, Morgenstern JL, Gee AD, Hong G, Brown J, Lockhart AJ. Biol. Chem. 2005;280:23599. doi: 10.1074/jbc.M501285200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.