SYNOPSIS
Osteoarthritis (OA) and type 2 diabetes mellitus (T2DM) often co-exist in older adults. There is increased susceptibility to develop arthritis in those with T2DM, which is supported by observations of higher prevalence of arthritis in those with T2DM (52%) compared to those without it (27%). The association between OA and T2DM has been traditionally attributed to underlying shared risk factors of age and obesity. Emerging evidence suggests that alterations in lipid metabolism and hyperglycemia might have a direct impact on cartilage health and subchondral bone that contribute to the development and/or progression of OA. Adequate management of older persons with both OA and T2DM benefits from a comprehensive understanding of the risk factors associated with these diseases. In this review, we discuss common risk factors and emerging underlying links between OA and T2DM, and emphasize the importance of physical activity to improve metabolism and decrease disability and pain in this population. Implications for safe and effective physical activity approaches in older patients with OA and T2DM are also discussed.
Keywords: Type 2 Diabetes, osteoarthritis, risk factors, glucose metabolism, arthritis, physical activity, exercise
INTRODUCTION
Osteoarthritis (OA) and Type 2 Diabetes Mellitus (T2DM) are two prevalent chronic diseases in the United States, Osteoarthritis (OA) affects 14% of adults aged 25 years and older and 34% of those above the age of 65.1 OA is a leading cause of disability and economic burden - around 40% of adults with OA report arthritis related limitations in daily activities and 30% report difficulties in work-related tasks.1 Diabetes affects 12% of adults 20 years and older and 26% of those above the age of 65.2 Diabetes is associated with mortality and serious complications such as heart disease and stroke, kidney failure, and lower-limb amputation.2
In the aging population, the co-existence of both OA and T2DM is frequent and can be a source of greater disability and economic burden.3,4 There seems to be an increased susceptibility to develop OA in those with T2DM.5,6 A recent report in adults ranging from 18–64 years showed that the prevalence of arthritis was 52% in those with T2DM compared to 27% in those without T2DM.7 The reason for the high prevalence of arthritis in those with T2DM is not entirely clear. OA and T2DM share common risk factors such as obesity and advanced aging, which may explain the higher prevalence of OA in the diabetic population.8 More recently, OA has been associated with systemic metabolic disturbances commonly seen in T2DM, suggesting that diabetes in and of itself influences the pathophysiology of OA independently of obesity or aging per se. These metabolic alterations have been proposed to serve as an underlying link between OA and T2DM.
With the growing prevalence of older persons diagnosed with both OA and T2DM, adequate prevention and management of these combined conditions becomes necessary. Optimal care of these patients depends upon understanding the risk factors leading to the development and progression of these commonly coexisting conditions. The purpose of this paper is to review the evidence on common risk factors and to discuss emerging underlying links between OA and T2DM. The review also discusses treatment considerations from a physical activity perspective when older individuals have both OA and T2DM.
SHARED RISK FACTORS FOR OA AND T2DM
Age and Obesity
Demographic and physiological risk factors for OA and T2DM are listed in Table 1. It is not surprising that OA and T2DM co-exist since they share several common risk factors, with age being a recognized link between them. The increased risk for OA and T2DM with aging is multifactorial. One factor is the decline in cell function with aging. For example, aging is associated with T2DM in part because pancreatic beta-cell function declines with aging.9 Aging also causes OA. In OA, senescent chondrocytes are more likely than young chondrocytes to secrete inflammatory mediators involved in cartilage degradation.10 Living longer also promotes cumulative joint loads and consequent cartilage wear and OA. Finally, aging has been attributed to a decline in mitochondrial health, and decreased mitochondrial health has been theorized to contribute to both diabetes and cartilage degradation.11–15
Table 1.
| Risk Factors for OA | Risk Factors for Diabetes |
|
|
| Risk Factors Common for OA and Diabetes | |
|
|
|
|
Risk factors that need further validation
Obesity is exceedingly prevalent among individuals with T2DM, and participates in the pathogenesis of T2DM. Obesity is also a risk factor for OA. Obesity contributes to the development of OA via biomechanical and systemic pathways. The biomechanical pathway is based on the direct effects of increased body weight. For example, increased body weight imposes greater loads on the weight-bearing joints, which has shown to affect cartilage wear.16,17 Excess body weight has also been associated with misalignment of weight-bearing joints (particularly the knee joint), which increases joint stress and promotes cartilage degradation that lead to OA.18 Moreover, obesity has been linked to decreased strength in muscles necessary for joint stabilization and therefore decreased ability to sustain mechanical joint stress.19,20
Although physical joint stress may be an important factor for obesity-mediated OA, it does not seem to be the only factor. An increased risk for OA in non-weight- bearing joints, such as those of the hands, has been previously reported in obesity,21 suggesting there is a systemic, non-mechanical influence on the risk for OA. This systemic link is not well understood, but there are theoretical precedents for supporting such notion. Obesity causes chronic low-grade inflammation in adipose tissue and enhanced expression and secretion of proinflammatory cytokines (e.g., IL-6, IL-1 and TNF-α) as well as adipokines (e.g., leptin, adiponectin, resistin, and visfatin).15,22 IL-1 and TNF-α have been shown to mediate the OA pathophysiology, possibly by modulating chondrocyte expression of proteases involved in matrix breakdown.
Adipokines are active in cartilage regulation and have been linked to the development of OA.23 Specifically, studies have shown that leptin is present in synovial fluid of joints with OA and high levels of this hormone in the synovial fluid are strongly associated with the radiographic severity of OA.24,25 High plasma levels of leptin are seen in obesity, but it is unclear whether the association between leptin and OA severity is merely a coincidence or reflects an underlying pathophysiologic link. Adiponectin is another adipokine, but its role in OA has been conflicting. One study reported higher levels of adiponectin in patients with erosive OA compared to those with non-erosive OA,26 while another study demonstrated that increased levels of this molecule protects against the progression of hand OA.27 For a comprehensive review on the role of adipokines to OA see review from Conde et al.28
Hypertension and Dyslipidemia
Hypertension and dyslipidemia, both widely recognized risk factors for T2DM,29,30 have been proposed to contribute to the development of OA.31–33 It has been theorized that hypertension might affect OA via narrowing of blood vessels and subchondral ischemia, which would initiate cartilage degradation.33,34 Although several studies demonstrated higher prevalence of OA in individuals with hypertension,35–38 adjustments for obesity were not conducted in those studies; therefore, the contribution of hypertension to OA needs further investigation.
Evidence for the contribution of dyslipidemia in OA is also not conclusive (for comprehensive review on this topic see papers from Zhuo and Velasquez).32,33 One study demonstrated a positive association between serum cholesterol levels and OA independent of obesity. Others have demonstrated that patients with OA have increased lipid deposits in the chondrocytes and deregulation of cellular lipid metabolism that might initiate OA development.31 There is also evidence that fatty acids are elevated in OA bone and that excessive intake of poly-unsaturated fatty acids is associated with increased risk of bone marrow lesions.39–44 However, it is important to make a distinction between circulating lipid levels and lipid metabolism within tissues, which should not be equated to each other and follow different metabolic regulation. These studies should be interpreted as hypothesis-generating as they do not definitely demonstrate that dyslipidemia is an etiological factor in OA.
Amongst the common risk factors of OA and T2DM, aging and obesity have a major impact on the functional ability of these individuals. Aging is characterized by physiologic changes such as altered endocrine function, loss of skeletal muscle function (decreased lean mass and muscle strength), and poor balance, as well as lifestyle changes such as decreased physical activity and nutritional deficiencies; all of these factors contribute to declines in functional ability and overall health.45 Studies have also shown that obese people demonstrate an increased incidence of pain in the weight-bearing joints and a higher pain perception during activity.46–48 The increased pain is likely mediated by heightened systemic inflammation and has been shown to be strongly related to the degree of disability in obese individuals.49 Thus, obesity-related factors such as increased pain, muscle weakness, and joint misalignment also limit mobility and contribute to functional limitations.
ASSOCIATION BETWEEN OA and T2DM
Does T2DM Independently Contribute to OA?
As OA and T2DM share common risk factors, the independent contribution of T2DM on OA is difficult to study. Several studies have tried to answer the question of whether T2DM predicts OA independently of age and obesity. Earlier studies failed to report significant associations.6,50,51 However, these studies had methodological limitations such as: a) the selection of fairly subjective criteria for the diagnosis of OA;50 b) having subjects with knee OA grouped by diabetes status but poorly matched for weight;51 c) assessment of generalized OA based only on radiographs of either hands and hips or hands and knees;6 or d) small sample sizes.6,51 Therefore, conclusions from those studies are far from definitive and should be approached with caution.
Nonetheless, recent studies have demonstrated an independent association between T2DM status and OA.5,52 Results of a 20-year longitudinal cohort study of 927 individuals suggest that T2DM predicts both joint failure and hip and knee arthroplasty surgery, independently of age, sex, and BMI.5 This study used both clinical measures (Knee Injury and Osteoarthritis Outcome Score, Western Ontario and McMaster Universities Osteoarthritis Index) and advanced imaging techniques (ultrasound) to assess severity of OA. In support of these findings, another study reported that individuals with T2DM had increased odds for clinical diagnosis of knee and hand OA (consistent with American College of Rheumatology criteria) after adjusting for age and BMI.52 Because these recent studies have used better methodological approaches and design, stronger evidence is accumulating in favor of an independent contribution of T2DM to the development and progression of OA.
Potential Underlying Links between OA and T2DM
Cartilage health is dependent upon a number of metabolic processes that regulate cartilage growth and nutrition, which if altered can lead to its degradation.53 Several investigators have postulated that altered glucose metabolism could be a direct link between OA and T2DM.10,21,32,54,55 Rosa et al investigated how glucose concentrations affect chondrocyte function in vitro using cartilage samples from healthy donors (posthumously) and from patients with OA undergoing a total knee arthroplasty.54 The cartilage samples were treated with varying concentrations of glucose in the medium and cartilage function measured. Findings revealed that chondrocytes of those with OA were unable to down-regulate transport of glucose into the chondrocyte in a hyperglycemic environment compared to a normoglycemic one. Higher levels of reactive oxygen species (ROS) were also detected in OA cartilage treated in a hyperglycemic-like environment, unlike the ROS levels in normal chondrocytes treated with high glucose medium. ROS are harmful to chondrocytes as they favor production of cytokines, such as IL-1β, and transcription factors, such as NF-κB, which give rise to catabolic processes implicated in cell degradation and cell apoptosis.56 The same group conducted a similar study to measure the effect of glucose concentrations on expression of proteolytic enzymes, mainly matrix metalloproteases (MMPs) that are responsible for cartilage degradation. Findings revealed a higher trend in the expression of MMPs in OA chondrocytes treated with hyperglycemic medium compared to normal chondrocytes in a hyperglycemic medium.57 Combined, those findings provide a plausible argument for the deleterious effects of hyperglycemia on articular cartilage.
An in vivo longitudinal cohort study investigated the relationship between fasting serum glucose levels and knee structural changes in 179 adults with no knee symptoms or diagnosis of T2DM. Knee cartilage volume and presence of bone marrow lesions were assessed by MRI. Results demonstrated that tibial cartilage volume loss and incidence of bone marrow lesions were positively associated with higher levels of fasting serum glucose levels in women, but not in men.58 The authors hypothesized that these gender differences might be due to greater cartilage loss in women as a result of decreased levels of estrogen (hormone that has a protective effect on cartilage) after menopause.58
Hyperglycemia is also known to favor the production of advanced glycation end products (AGEs) and their accumulation in articular cartilage, which contribute to a toxic environment that might facilitate OA pathogenesis.59 AGE accumulation has been reported with aging, and in vitro studies demonstrated that it contributes to cartilage stiffness60,61 and degradation.62 High intracellular glucose concentration in diabetes promotes formation of AGE; AGE compounds then interact with receptor of AGE (RAGE) to give rise to a cascade of events that promote release of pro-inflammatory factors such as TNF-α, and activate transcription factors such as Nf-kB, which cause inflammation and oxidative stress intracellularly and might promote cartilage degradation.59 AGEs may also contribute to the progression of OA through diabetic peripheral neuropathy; excess accumulation of AGEs may compromise proprioceptive and nociceptive receptors in joint structures.63 Impaired joint proprioception has been reported in OA and postulated to be a result of dysfunctional articular mechanoreceptors and reduced muscle spindle sensitivity in weak and atrophied muscles around the joints.64 Thus, impaired sensation in OA and diabetic neuropathy may conceal the perception of pain and further perpetuate joint damage by allowing constant harmful mechanical workloads.
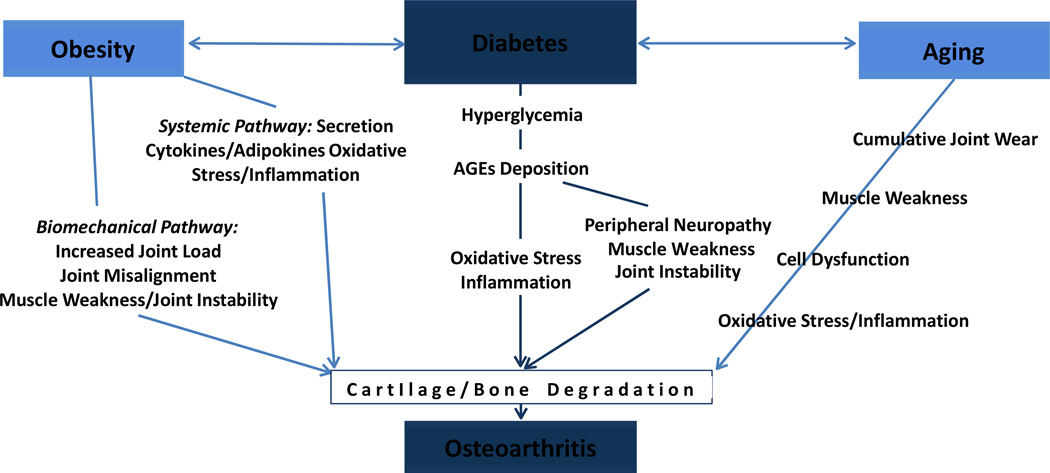
In summary, it is clear that aging, obesity, and T2DM interact to affect OA. This model is depicted in Figure 1. Shared components of aging, obesity, and T2DM, such as low-grade inflammation, oxidative stress, and dysregulation of cell function all lead to cell toxicity and consequent OA-related cartilage and bone abnormalities. Hyperglycemia also seems to have a direct effect on cartilage health. Although both lipid metabolism and vascularity play a role on joint health, the exact contribution of hypertension and dyslipidemia needs further validation.
Figure 1. Common Risk Factors of OA and T2DM.
Obesity and age are well-established shared risk factors for OA and T2DM. Obesity affects OA through biomechanical and systemic pathways. Age affects OA by factors such as cumulative joint load, muscle weakness, cell dysfunction (e.g., chondrocyte, mitochondria), and chronic inflammation. T2DM seems to have a direct impact on OA as hyperglycemia promotes deposition of advanced glycation end-products (AGEs) and affects cartilage health. Hyperglycemia also contributes to peripheral neuropathy, which can contribute to muscle weakness, joint instability, and consequent OA of weight-bearing joints.
Future Research on the Links between OA and T2DM
The metabolic processes linking OA and T2DM are not completely understood. We have identified several gaps in knowledge that should be addressed by future research. For example, large cross-sectional and longitudinal data should be used to clarify the associations between OA and T2DM prevalence and progression. These studies should apply rigorous methods to tease out the contributions of age, gender, and obesity. It is important to assess how different combinations of risk factors such as age and obesity interact with hyperglycemia to affect OA disease development, pain, and disability of patients. Moreover, although it is likely that the disruption of glucose metabolism promotes the development of OA, it could also be possible that in someone with OA, deregulated glucose metabolism just enhances the progression of OA, an issue that needs clarification. Studies should also discriminate the effects of T2DM on weight-bearing versus non weight-bearing joints, which has not been done. Such discrimination is needed to tease out the direct contributions of hyperglycemia versus complications from T2DM, such as diabetic neuropathy, on the development and progression of OA. Last, further research is needed to clarify if and how dyslipidemia and hypertension contribute to the development of OA.
Future investigations are equally needed to investigate the effects of altered glucose metabolism and AGE accumulation on cartilage health in patients with both OA and T2DM versus those with OA alone. While studies have implicated glucose and AGE accumulation with greater severity of OA, they have not included subjects with T2DM.65,66 Studies are necessary to confirm whether articular cartilage degradation in persons with T2DM is increased or accelerated compared to those without T2DM, which, if confirmed, could have pertinent implications for the management of these patients.
MANAGEMENT OF OLDER INDIVIDUALS WITH OA AND T2DM - A PHYSICAL ACTIVITY PERSPECTIVE
Approximately half of all adults with diabetes have arthritis and more than a quarter of these individuals report limitation in usual activities due to joint symptoms.3 The functional limitations observed in older individuals with both OA and T2DM is multifactorial and involve age and obesity-related factors such as pain, muscle dysfunction (weakness, atrophy and low quality of muscle), joint misalignment, deconditioning, poor balance and mobility, and inactivity. These limitations are exacerbated by the symptoms and complications of OA and T2DM, leading to complex interactions that further limit functional ability and compromise overall health.
A number of impairments associated with aging, OA and T2DM develop over many years as a consequence of sedentary lifestyle and poor eating habits. Physical inactivity is a contributor to these impairments and serves as a trigger for the vicious cycle of further impairments and additional physical inactivity. While inactivity is the trigger, physical activity is a potent intervention to break the vicious cycle. Physical activity is likely the only intervention capable of positively affecting all of the modifiable impairments listed above, including the underlying pathophysiology of all of the physical impairments and symptoms. To that end, the continuation of this review focuses specifically on the management of older patients with OA who have T2DM, emphasizing the physical activity component. For information about the management of individuals with T2DM only.
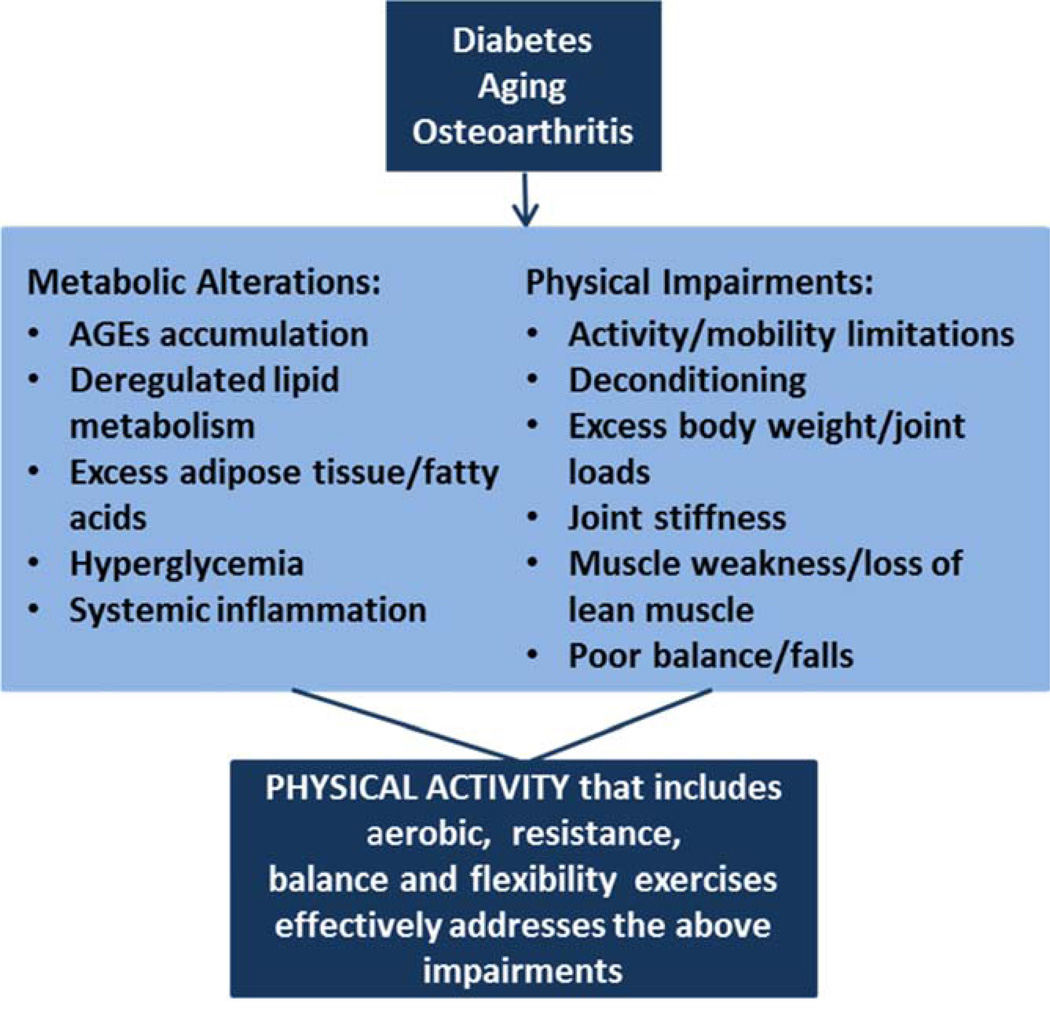
Physical activity is the first-line intervention to decrease pain and functional limitations in OA. Clinical practice guidelines from the American College of Rheumatology, the American Academy of Orthopaedic Surgeons, and the Osteoarthritis Research Society International; have all strongly recommended that patients with symptomatic OA of the lower extremities should participate in physical activity programs that include aerobic and resistance exercises.67–69 Regular physical activity is also one of the main treatment pillars in the management of T2DM and has been recommended by the American Diabetes Association (ADA) and the American College of Sports Medicine.70 In aging, physical activity has been recommended by the American Geriatrics Society/British Geriatrics Society and the US Department of Health and Human Services to prevent function decline.71–73 Thus, there is solid support for the benefits of physical activity in aging, T2DM, and OA. Additionally, physical activity affects metabolism by improving glucose tolerance,70,74–76 promoting healthy body weight, and decreasing inflammation.77–80 Physical activity also ameliorates physical impairments such as muscle weakness and atrophy,81,82 joint stiffness and pain,67–69 poor balance and mobility.71,73,83 Other benefits include decreased falls,71–73 improvements in cardiovascular health,84 mood and quality of life, and decreased mortality.71 Figure 2 depicts a model for the effectiveness of physical activity on metabolic and physical impairments related to aging, OA and T2DM.
Figure 2. Physical and Metabolic Impairments Resultant of T2DM, Aging and OA that Can Benefit from Physical Activity.
T2DM, aging and OA are associated with several physical impairments and metabolic alterations that can be addressed by physical activity. Physical impairments common in this population include: excess body weight and consequent increased adiposity and joint loads; poor balance and increased fall risk; muscle dysfunction such as muscle weakness, muscle atrophy, and decreased lean muscle mass; pain and joint stiffness; all of these impairments limit daily activities and mobility. Metabolic alterations include hyperglycemia, excess adipose tissue with deregulated lipid metabolism, accumulation of advanced glycation end products (AGEs), and systemic inflammation.
Physical Activity Prescription in Older Adults with OA and T2DM
The recommended modalities, dose, intensity, and benefits of exercise in aging, OA and T2DM are presented in Table 2. These recommendations have been compiled based on clinical guidelines for the management of these patients and align with the national recommendations for physical activity.67–71,73 It is recommended at least 150 minutes a week of aerobic activities and 2 days a week of resistive exercises. Specific to aging and OA, regular flexibility and balance exercises should also be performed.72,83,85
Table 2.
| Modality/Examples | Dose | Intensity Level | Benefits |
|---|---|---|---|
| Aerobic Exercise: Continuous movements of large muscle groups like walking, biking, swimming, gardening, and ballroom dancing. | 4 – 7 days per week, or every other day. Ideally, around 30 minutes every day. Do not stay more than 2 consecutive days without exercise. | For individuals with OA of weight-bearing joints use moderate intensity. It produces noticeable increases in heart and breathing rates while person is able to engage in a conversation. For individuals without OA of weight-bearing joints use moderate to vigorous intensity (consider overall health status). |
Improves cardiovascular health, maintains body weight, improves glucose and lipid metabolism, and improves physical function. |
| Resistive Exercise: Activities of brief duration with weights or elastic therapy bands such as calisthenics and weight training. | 2 – 3 days per week in non-consecutive days. Exercise the major muscle groups of the lower extremities and trunk. Progress from 1 to 3 sets of 8–10 repetitions at a weight that cannot be lifted more than 8–10 times | Moderate to high intensity - resistance should allow 10–15 repetitions of each exercise, representing a perceived effort from moderate to somewhat hard. | Strengthens muscles, increases lean muscle mass, decreases fat mass, improves glucose metabolism and physical function. |
| Flexibility Exercise: Exercise takes the joints to their full range of pain-free motion-stretching, range of motion. | 2 days per week for 10 minutes each day. Hold each stretch for approximately 30 seconds. | Light - these exercises demand low physical effort. | Maintains or increases flexibility and ameliorates joint stiffness. |
| Balance Exercise: should challenge the body’s dynamic stability - exercises are performed as modification of gait, such as advancing gait with changes in surfaces, rhythm, distance, load, attention, and postural transition (start, stop)- Yoga, Tai Chi, dance. | 2 days per week for 15–30 minutes. | Light -these exercises demand low physical effort. Balance exercises require more complex control of movement and elicit postural reactions of ankle and hip joints, along with step strategies. | Improves balance and mobility, reduces fall risk and disability. |
It is important to note that the optimal exercise volume may need to be individually tailored to maximize benefit and safety and minimize barriers. For example, to achieve weight loss in obesity the exercise volume ought to be larger than to achieve glycemic control and cardiovascular health. In obesity, current guidelines recommend from 250–300 minutes per week of aerobic exercise for weight loss and maintenance.86 In sarcopenia, if the aim is to promote muscle hypertrophy, the intensity level of resistive training should be kept towards the high- rather than moderate-range. Conversely, for individuals with severe disability the recommended volume of exercises may not be feasible and lower volume may be indicated. While the benefits of lower volume are less as compared to higher volume, emerging evidence demonstrates that low volume of exercise still promotes substantial health benefits.87
Although the national guidelines recommend aerobic exercises of moderate and vigorous intensities, Table 2 differentiates the intensity of exercise according with OA weight-bearing status. In those with OA of weight-bearing joints, vigorous intensity aerobics are discouraged because they generally involve high impact activities that could be deleterious to the joints. Moreover, many older adults with T2DM may not have sufficient aerobic capacity to undertake vigorous activities. It has also been shown that the cardiorespiratory benefits are comparable with aerobic exercises at moderate and vigorous intensities.88–90
When considering the type of aerobic exercise, walking has been the most widely used modality in this population. Yet, it is likely that walking is not as effective in obese patients with weight-bearing joint OA. In a recent literature review, we observed limited evidence for the effectiveness of walking programs on pain and physical function in patients with both obesity and OA, whereas prior studies on leaner individuals have reported beneficial effects.91 The increased joint load combined with the increased inflammation characteristic of obesity may preclude the effectiveness of walking programs. Therefore, alternative modes such as cycling, arm ergometers, underwater walking or water aerobics may be better choices.
Special considerations ought to be given for resistive exercises in individuals with joint pain. Resistive exercise should be performed at pain-free ranges and maximum resistance should be avoided. For example, if exercise for the knee extensor muscles cause pain at mid-range (e.g., 60 to 40 degrees of knee flexion), it is recommended to try to exercise at an alternative range of motion (e.g., 30 to 0 degrees). It is also recommended that a qualified exercise trainer provides initial supervision and periodic assessment to maximize benefits and minimize injury risk of resistive exercise.92
While the recommendations to exercise in Table 2 are generic to all older adults with both OA and T2DM, some patients may be unable to follow these recommendations due to specific impairments. For example, a patient with knee OA may present with moderate joint pain, poor balance, and weak thigh muscles; and these impairments may hinder the ability to reach the recommendations. In patients like this one, alternative provisions are needed to ameliorate the physical impairment before considering more generic recommendations. It may be necessary to refer the patient to physical therapy to learn resistive exercises that target the weak muscles using painfree ranges. Rehabilitation may also be needed to improve balance to allow the patient to safely perform exercise independently. Acquiring these new skills in rehabilitation will help the patient to engage in physical activity programs and achieve the more generic recommendations. Thus, patients who have functional limitations may need sporadic rehabilitation to resolve specific physical impairments or to adapt the exercise program to the existing physical impairments.
Special Considerations for Exercise in Older Adults with OA and T2DM
The combination of aging, OA and T2DM poses challenges that require particular precautions to exercise. For example, it is known that coronary artery disease is quite prevalent in this population and unstable disease should be brought to urgent medical attention before any exercise is instituted. Space limitations preclude us to discuss general precautions to exercise due to other related chronic diseases. For a complete review on the relative and absolute contraindications to exercise in the general population the reader is referred elsewhere.93,94
While challenges to exercise exist, the benefits of physical activity outweigh the risks. Inactivity leads to further complications and functional limitations. In general, it is safe to initiate exercise at light and moderate intensities (up to brisk walking) in this population without prior stress testing. Yet, it is recommended that those who are previously sedentary, have moderate to high risk of cardiovascular disease, and want to undertake more vigorous program of physical activity should be considered for stress testing prior to initiate an exercise program.95 Additionally, symptoms of exercise intolerance such as breathlessness, muscle pain and weakness during exercise, along with headache, nausea, dizziness or extreme fatigue after exercise warrant specialized medical assessment. Table 3 describes contraindications to exercise exclusively from the OA and T2DM standpoints. While in OA, joint pain and other signs of joint inflammation are the main criteria precluding exercise performance, in T2DM, glycemic control mainly dictates the contraindications to exercise. Contraindications related to aging are not depicted in Table 3 because age itself is an indication for exercise rather than a contraindication. However, aging accentuates several impairments common in OA and T2DM such as muscle weakness and poor balance and mobility; so, these age-related impairments should be considered to improve safety of exercise. In addition to contraindications, special considerations for exercise are also in order in this population to improve safety and comfort of exercise and those are described in Table 4.
Table 3.
| Osteoarthritis | ||
| Relative Contraindication | Mild to moderate joint pain | Most people can safely work through mild joint pain. If pain is worse two hours after exercise, stop exercise, for 1–2 days and:
|
| Absolute Contraindication | Moderate to severe joint pain | Continued pressure on inflamed joints can cause further damage
|
| Diabetes73,83,92,105 | ||
| Relative Contraindications Requiring Closer Monitoring- Based on Blood Glucose (BG) | B G 70–100 mg/dL | Have a snack - 15g carb every hour of moderately intense activity |
| BG 100–300 mg/dL | Proceed with exercise program | |
| BG> 300 mg/dL |
|
|
| Absolute Contraindications | Ingestion of alcohol 3 hours prior to exercise | |
| Hypoglycemia-BG< 70 mg/dL. Symptoms include shakiness, pale skin color, dizziness, behavior changes, sweating, clumsy/jerky movements, hunger, seizure, headache, tingling sensations around the mouth | ||
| Hyperglycemia-BG> 300 mg/dL with ketones and > 1 of the following require emergency treatment: shortness of breath, nausea and vomiting, breath that smells fruity, a very dry mouth | ||
Information in this table is not intended for use by patients
Table 4.
GENERAL CONSIDERATIONS
| |
EXERCISE PROGRESSION
| |
T2DM-SPECIFIC CONSIDERATIONS
|
In presence of Nephropathy:
|
In presence of Peripheral Neuropathy:
|
Weight Loss
The focus of this segment of the review is on physical activity intervention. Yet, a brief discussion on weight loss in this population is warranted. While strong evidence supports losing weight in obese individuals with OA67,69 and in those with T2DM;92 in older adults (above 70 years) weight loss seems to accelerate sarcopenia.96 Thus, we believe that for older adults with OA and T2DM, it is safe to recommend that weight loss be always accompanied by exercise to prevent loss of muscle mass. In support of this recommendation, it has been well established that the largest benefits for joint pain, metabolic impairments, and physical function have been observed in persons who exercise AND lose weight.97–100
Exercise Adherence
Benefits from exercise are best accrued by regular physical activity participation and are likely reversible if discontinued; so, improving adherence to exercise is key for promoting long-term benefits. Thus, the goal is to permanently increase physical activity level. Yet, long-term maintenance of physical activity programs is a challenge, especially given the combination of chronic diseases. The presence of multiple chronic diseases or poorer mobility in individuals with OA was shown to contribute to low exercise adherence.101
Although adherence to exercise is a complex issue beyond the scope of this review, several strategies may be helpful to promote sustainability of exercise. Efforts must be taken to engage participants and impress upon them the importance of physical activity while initiating an intervention program. Patients should be encouraged to set physical activity goals and discuss barriers to exercise and develop strategies to overcome the barriers. To that end, self-management programs have been shown effective to educate and engage patients, and improve exercise adherence.67–69 Exercises should also be easily instituted and individuals should engage in activities that they enjoy. Exercising at home instead of a fitness facility, and use simple exercise mode such as walking have shown to improve adherence.101 Additionally, physical activity programs need to be flexible, inexpensive, fun, and not greatly interfere with a person’s daily routine. Thus, perhaps the 30 minutes per day may need to be broken up into more feasible small bouts of 10 minutes walking or cycling several times a day, as this regimen is as effective as a single longer session of equivalent length and intensity.71 Monitoring activity through diaries and activity monitors or pedometers have also shown to increase exercise adherence.
We also believe an important hindrance to adherence to physical activity is the biases of healthcare professionals not to consider that OA and T2DM in older adults can be treated by physical activity. They tend to neglect the beneficial effects of exercise on cell function and on the common physiologic components of these conditions such as systemic inflammation, hyperglycemia and lipid metabolism. Physical activity is a powerful intervention that should be approached as a “pill” and taken as prescribed. A great advantage of this “pill” is its negligible side effects when special considerations and precautions to exercise are followed. If healthcare professionals prescribe the physical activity “pill” with the enthusiasm that it deserves, it would potentially improve exercise adherence.
Future Research on Physical Activity in Older Adult with both OA and T2DM
While there is strong evidence for the benefits of physical activity for older adults with T2DM or OA; the evidence for exercise in individuals with a combination of these conditions is limited. After an extensive search in MEDLINE we were unable to find studies on the effectiveness of exercise in those diagnosed with both OA and T2DM. So, it is unclear if the interaction of aging, OA and T2DM would modify the response or safety of exercise. To that end, future studies are warranted to clarify several important issues. For example, if research confirms the cartilage and subchondral bone of patients with T2DM are more prone to damage, it will likely prompt adaptations to exercise prescription in this population. Intervention studies should investigate the (1) dose-response of exercise, particularly the safety of weight-bearing exercises, (2) effects of exercise on cartilage and subchondral bone health, (3) consequences of increased joint loads in well controlled versus not well-controlled T2DM, (4) safety of weight loss interventions, (5) optimal combinations of aerobic and strength exercise to improve physical function and decrease pain.
SUMMARY
Emerging evidence supports that people with T2DM are more susceptible to develop arthritis. OA and T2DM in aging are linked by age and obesity factors such as cumulative joint loads, systemic inflammation, and abnormal lipid metabolism. Hyperglycemia may also directly impact OA. In this review we have discussed the common links between OA and T2DM in older adults along with the metabolic and physical impairments commonly present in this population. We emphasized the role of physical activity and the only intervention capable of addressing a large number of metabolic and physical impairments in older adults with both OA and T2DM.
KEY POINTS.
Individuals with T2DM are more susceptible to develop OA. This is partially explained by shared factors for these conditions such as aging and obesity.
Aging and obesity-related physical and physiological impairments contribute to the development of OA (e.g., muscle weakness and atrophy, poor balance, deconditioning, pain, excess joint loads, abnormal lipid metabolism, low-grade inflammation, and oxidative stress).
Hyperglycemia may also directly injure cartilage health in OA by mechanisms involving advanced glycation end-products (AGEs) and peripheral neuropathy.
Physical activity is the only available intervention capable of positively affecting the impairments that link aging, OA and T2DM.
Special considerations are necessary for safe and effective implementation of physical activity programs in older adults with OA and T2DM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2014. [Accessed on July 14, 2014];Estimates of Diabetes and Its Burden in the United States Fact Sheet. http://www.cdc.gov/diabetes/pubs/estimates14.htm.
- 3.Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation – United States, 2007–2009. MMWR Morb Mortal Wkly Rep. 2010;59(39):1261–1265. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2011 National Diabetes Fact Sheet. [Accessed February 10, 2014];2011 Available at http://www.cdc.gov/diabetes/pubs/factsheet11.htm.
- 5.Schett G, Kleyer A, Perricone C, et al. Diabetes is an independent predictor for severe osteoarthritis: results from a longitudinal cohort study. Diabetes care. 2013 Feb;36(2):403–409. doi: 10.2337/dc12-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sturmer TBH, Brenner RE, Gunther KP. Non-insulin dependent diabetesmellitus (NIDDM) and patterns of osteoarthritis. Scand J Rheumatol. 2001;30:169–171. doi: 10.1080/030097401300162969. [DOI] [PubMed] [Google Scholar]
- 7.Arthritis as a potential barrier to physical activity among adults with diabetes - United States, 2005 and 2007. MMWR Morb Mortal Wkly Rep. 2008;57(18):486–489. [PubMed] [Google Scholar]
- 8.Waine H, Nevinny D, Rosenthal J, Joffe IB. Association of osteoarthritis and diabetes mellitus. Tufts folia medica. 1961 Jan-Mar;7:13–19. [PubMed] [Google Scholar]
- 9.Cnop M, Igiollo-Esteve M, Hughes SJ, Walker JN, Cnop I, Clark A. Longevity of human islet α-and β-cells. Diabetes, Obesity, and Metabolism. 2001;13(Suppl 1):39–46. doi: 10.1111/j.1463-1326.2011.01443.x. [DOI] [PubMed] [Google Scholar]
- 10.Berenbaum F. Diabetes-induced osteoarthritis: from a new paradigm to a new phenotype. Annals of the rheumatic diseases. 2011 Aug;70(8):1354–1356. doi: 10.1136/ard.2010.146399. [DOI] [PubMed] [Google Scholar]
- 11.Harman D. Aging: a theory based on free radical and radiation chemistry. Journal of gerontology. 1956 Jul;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 12.Harman D. The biologic clock: the mitochondria? Journal of the American Geriatrics Society. 1972 Apr;20(4):145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 13.Trounce I, Byrne E, Marzuki S. Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet. 1989 Mar 25;1(8639):637–639. doi: 10.1016/s0140-6736(89)92143-0. [DOI] [PubMed] [Google Scholar]
- 14.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006 Dec 14;444(7121):840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 15.Goldring MB. Osteoarthritis and cartilage: the role of cytokines. Current rheumatology reports. 2000 Dec;2(6):459–465. doi: 10.1007/s11926-000-0021-y. [DOI] [PubMed] [Google Scholar]
- 16.Felson DT, Zhang Y, Hannan MT, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis and rheumatism. 1997 Apr;40(4):728–733. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- 17.Reijman M, Pols HA, Bergink AP, et al. Body mass index associated with onset and progression of osteoarthritis of the knee but not of the hip: the Rotterdam Study. Annals of the rheumatic diseases. 2007 Feb;66(2):158–162. doi: 10.1136/ard.2006.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma L, Lou C, Cahue S, Dunlop DD. The mechanism of the effect of obesity in knee osteoarthritis: the mediating role of malalignment. Arthritis and rheumatism. 2000 Mar;43(3):568–575. doi: 10.1002/1529-0131(200003)43:3<568::AID-ANR13>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 19.Syed IY, Davis BL. Obesity and osteoarthritis of the knee: hypotheses concerning the relationship between ground reaction forces and quadriceps fatigue in longduration walking. Medical hypotheses. 2000 Feb;54(2):182–185. doi: 10.1054/mehy.1999.0013. [DOI] [PubMed] [Google Scholar]
- 20.Slemenda C, Heilman DK, Brandt KD, et al. Reduced quadriceps strength relative to body weight: a risk factor for knee osteoarthritis in women? Arthritis and rheumatism. 1998 Nov;41(11):1951–1959. doi: 10.1002/1529-0131(199811)41:11<1951::AID-ART9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 21.Sellam J, Berenbaum F. Is osteoarthritis a metabolic disease? Joint, bone, spine : revue du rhumatisme. 2013 Dec;80(6):568–573. doi: 10.1016/j.jbspin.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39(1–2):237–246. [PubMed] [Google Scholar]
- 23.Toda Y, Toda T, Takemura S, Wada T, Morimoto T, Ogawa R. Change in body fat, but not body weight or metabolic correlates of obesity, is related to symptomatic relief of obese patients with knee osteoarthritis after a weight control program. J Rheumatol. 1998 Nov;25(11):2181–2186. [PubMed] [Google Scholar]
- 24.Dumond H, Presle N, Terlain B, et al. Evidence for a key role of leptin in osteoarthritis. Arthritis and rheumatism. 2003 Nov;48(11):3118–3129. doi: 10.1002/art.11303. [DOI] [PubMed] [Google Scholar]
- 25.Koskinen A, Vuolteenaho K, Nieminen R, Moilanen T, Moilanen E. Leptin enhances MMP-1, MMP-3 and MMP-13 production in human osteoarthritic cartilage and correlates with MMP-1 and MMP-3 in synovial fluid from OA patients. Clinical and experimental rheumatology. 2011 Jan-Feb;29(1):57–64. [PubMed] [Google Scholar]
- 26.Filkova M, Liskova M, Hulejova H, et al. Increased serum adiponectin levels in female patients with erosive compared with non-erosive osteoarthritis. Annals of the rheumatic diseases. 2009 Feb;68(2):295–296. doi: 10.1136/ard.2008.095737. [DOI] [PubMed] [Google Scholar]
- 27.Yusuf E, Ioan-Facsinay A, Bijsterbosch J, et al. Association between leptin, adiponectin and resistin and long-term progression of hand osteoarthritis. Annals of the rheumatic diseases. 2011 Jul;70(7):1282–1284. doi: 10.1136/ard.2010.146282. [DOI] [PubMed] [Google Scholar]
- 28.Conde J, Scotece M, Gomez R, Lopez V, Gomez-Reino JJ, Gualillo O. Adipokines and osteoarthritis: novel molecules involved in the pathogenesis and progression of disease. Arthritis. 2011;2011:203901. doi: 10.1155/2011/203901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National High Blood Pressure Education Program Working Group. National High Blood Pressure Education Program Working Group report on hypertension in diabetes. Hypertension. 1994;23:145–158. [PubMed] [Google Scholar]
- 30.Fukui M, Tanaka M, Toda H, et al. Risk factors for development of diabetes mellitus,hypertension and dyslipidemia. Diabetes research and clinical practice. 2011 Oct;94(1):e15–e18. doi: 10.1016/j.diabres.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Hart DJ, Doyle DV, Spector TD. Association between metabolic factors and knee osteoarthritis in women: the Chingford study. J Rheumatol. 1995;22:1118–1123. [PubMed] [Google Scholar]
- 32.Velasquez MT, Katz JD. Osteoarthritis: another component of metabolic syndrome? Metabolic syndrome and related disorders. 2010 Aug;8(4):295–305. doi: 10.1089/met.2009.0110. [DOI] [PubMed] [Google Scholar]
- 33.Zhuo Q, Yang W, Chen J, Wang Y. Metabolic syndrome meets osteoarthritis. Nature reviews. Rheumatology. 2012 Dec;8(12):729–737. doi: 10.1038/nrrheum.2012.135. [DOI] [PubMed] [Google Scholar]
- 34.Findlay DM. Vascular pathology and osteoarthritis. Rheumatology. 2007 Dec;46(12):1763–1768. doi: 10.1093/rheumatology/kem191. [DOI] [PubMed] [Google Scholar]
- 35.Puenpatom RA, Victor TW. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: an analysis of NHANES III data. Postgraduate medicine. 2009 Nov;121(6):9–20. doi: 10.3810/pgm.2009.11.2073. [DOI] [PubMed] [Google Scholar]
- 36.Engstrom G, Gerhardsson de Verdier M, Rollof J, Nilsson PM, Lohmander LS. Creactive protein, metabolic syndrome and incidence of severe hip and knee osteoarthritis. A population-based cohort study. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2009 Feb;17(2):168–173. doi: 10.1016/j.joca.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Marks R, Allegrante JP. Comorbid disease profiles of adults with end-stage hip osteoarthritis. Medical science monitor : international medical journal of experimental and clinical research. 2002 Apr;8(4):CR305–CR309. [PubMed] [Google Scholar]
- 38.Conaghan PG, Vanharanta H, Dieppe PA. Is progressive osteoarthritis an atheromatous vascular disease? Annals of the rheumatic diseases. 2005 Nov;64(11):1539–1541. doi: 10.1136/ard.2005.039263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lippiello L, Walsh T, Fienhold M. The association of lipid abnormalities with tissue pathology in human osteoarthritic articular cartilage. Metabolism: clinical and experimental. 1991 Jun;40(6):571–576. doi: 10.1016/0026-0495(91)90046-y. [DOI] [PubMed] [Google Scholar]
- 40.Gkretsi V, Simopoulou T, Tsezou A. Lipid metabolism and osteoarthritis: lessons from atherosclerosis. Progress in lipid research. 2011 Apr;50(2):133–140. doi: 10.1016/j.plipres.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Simopoulou T, Malizos KN, Tsezou A. Lectin-like oxidized low density lipoprotein receptor 1 (LOX-1) expression in human articular chondrocytes. Clinical and experimental rheumatology. 2007 Jul-Aug;25(4):605–612. [PubMed] [Google Scholar]
- 42.Wang Y, Wluka AE, Hodge AM, et al. Effect of fatty acids on bone marrow lesions and knee cartilage in healthy, middle-aged subjects without clinical knee osteoarthritis. Osteoarthritis and cartilage / OARSI, Osteoarthritis Research Society. 2008 May;16(5):579–583. doi: 10.1016/j.joca.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Plumb MS, Aspden RM. High levels of fat and (n-6) fatty acids in cancellous bone in osteoarthritis. Lipids in health and disease. 2004 Jun 18;3:12. doi: 10.1186/1476-511X-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Felson DT, McLaughlin S, Goggins J, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Annals of internal medicine. 2003 Sep 2;139(5 Pt 1):330–336. doi: 10.7326/0003-4819-139-5_part_1-200309020-00008. [DOI] [PubMed] [Google Scholar]
- 45.Clark BC, Manini TM. Functional consequences of sarcopenia and dynapenia in the elderly. Current opinion in clinical nutrition and metabolic care. 2010 May;13(3):271–276. doi: 10.1097/MCO.0b013e328337819e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hulens M, Vansant G, Claessens AL, Lysens R, Muls E. Predictors of 6-minute walk test results in lean, obese and morbidly obese women. Scandinavian journal of medicine & science in sports. 2003 Apr;13(2):98–105. doi: 10.1034/j.1600-0838.2003.10273.x. [DOI] [PubMed] [Google Scholar]
- 47.Melissas J, Kontakis G, Volakakis E, Tsepetis T, Alegakis A, Hadjipavlou A. The effect of surgical weight reduction on functional status in morbidly obese patients with low back pain. Obesity surgery. 2005 Mar;15(3):378–381. doi: 10.1381/0960892053576703. [DOI] [PubMed] [Google Scholar]
- 48.Hills AP, Hennig EM, McDonald M, Bar-Or O. Plantar pressure differences between obese and non-obese adults: a biomechanical analysis. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2001 Nov;25(11):1674–1679. doi: 10.1038/sj.ijo.0801785. [DOI] [PubMed] [Google Scholar]
- 49.Barofsky I, Fontaine KR, Cheskin LJ. Pain in the obese: impact on health-related quality-of-life. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 1997 Fall;19(4):408–410. doi: 10.1007/BF02895160. [DOI] [PubMed] [Google Scholar]
- 50.Frey MI, Barrett-Connor E, Sledge PA, Schneider DC, Weisman MH. The effect of noninsulin dependent diabetes mellitus on the prevalence of clinical osteoarthritis: a population based study. J Rheumatol. 1996;23(4):716–722. [PubMed] [Google Scholar]
- 51.Horn CA, Bradley JD, Brandt KD, Kreipke DL, Slowman SD, Kalasinski LA. Osteophyte formation in diabetic patients with OA. Arthritis and rheumatism. 1992;35(3):336–342. doi: 10.1002/art.1780350313. [DOI] [PubMed] [Google Scholar]
- 52.Nieves-Plaza M, Castro-Santana LE, Font YM, Mayor AM, Vila LM. Association of hand or knee osteoarthritis with diabetes mellitus in a population of Hispanics from Puerto Rico. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2013 Jan;19(1):1–6. doi: 10.1097/RHU.0b013e31827cd578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mobasheri A, Vannucci SJ, Bondy CA, et al. Glucose transport and metabolism in chondrocytes: a key to understanding chondrogenesis, skeletal development and cartilage degradation in osteoarthritis. Histology and histopathology. 2002 Oct;17(4):1239–1267. doi: 10.14670/HH-17.1239. [DOI] [PubMed] [Google Scholar]
- 54.Rosa SC, Goncalves J, Judas F, Mobasheri A, Lopes C, Mendes AF. Impaired glucose transporter-1 degradation and increased glucose transport and oxidative stress in response to high glucose in chondrocytes from osteoarthritic versus normal human cartilage. Arthritis research & therapy. 2009;11(3):R80. doi: 10.1186/ar2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan W, Li X. Impact of diabetes and its treatments on skeletal diseases. Frontiers of medicine. 2013 Mar;7(1):81–90. doi: 10.1007/s11684-013-0243-9. [DOI] [PubMed] [Google Scholar]
- 56.Goldring MB. Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best Pract Res Clin Rheumatol. 2006;20(5):1003–1025. doi: 10.1016/j.berh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 57.Rosa SC, Rufino AT, Judas FM, Tenreiro CM, Lopes MC, Mendes AF. Role of glucose as a modulator of anabolic and catabolic gene expression in normal and osteoarthritic human chondrocytes. J Cell Biochem. 2011 Oct;112(10):2813–2824. doi: 10.1002/jcb.23196. [DOI] [PubMed] [Google Scholar]
- 58.Davies-Tuck ML, Wang Y, Wluka AE, et al. Increased fasting serum glucose concentration is associated with adverse knee structural changes in adults with no knee symptoms and diabetes. Maturitas. 2012 Aug;72(4):373–378. doi: 10.1016/j.maturitas.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 59.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001 Dec 13;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 60.Verzijl N, DeGroot J, Ben ZC, et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002 Jan;46(1):114–123. doi: 10.1002/1529-0131(200201)46:1<114::AID-ART10025>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 61.Verzijl N, DeGroot J, Oldehinkel E, et al. Age-related accumulation of Maillard reaction products in human articular cartilage collagen. The Biochemical journal. 2000 Sep 1;350(Pt 2):381–387. [PMC free article] [PubMed] [Google Scholar]
- 62.Steenvoorden MM, Huizinga TW, Verzijl N, et al. Activation of receptor for advanced glycation end products in osteoarthritis leads to increased stimulation of chondrocytes and synoviocytes. Arthritis and rheumatism. 2006 Jan;54(1):253–263. doi: 10.1002/art.21523. [DOI] [PubMed] [Google Scholar]
- 63.Leaverton PE, Peregoy J, Fahlman L, Sangeorzan E, Barrett JP., Jr Does diabetes hide osteoarthritis pain? Medical hypotheses. 2012 Apr;78(4):471–474. doi: 10.1016/j.mehy.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 64.Knoop J, Steultjens MPM, Leeden M, Esch M, Thorstensson CA, Roorday LD, Lems WF. Proprioception in knee osteoarthritis: a narrative review. Osteoarthritis and Cartilage. 19(2011):381–388. doi: 10.1016/j.joca.2011.01.003. 2011. [DOI] [PubMed] [Google Scholar]
- 65.Nakamura H, Masuko K, Yudoh K, et al. Positron emission tomography with 18F-FDG in osteoarthritic knee. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2007 Jun;15(6):673–681. doi: 10.1016/j.joca.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 66.Hong YH, Kong EJ. (18F)Fluoro-deoxy-D-glucose uptake of knee joints in the aspect of age- related osteoarthritis: a case-control study. BMC Musculoskelet Disord. 2013;14:141. doi: 10.1186/1471-2474-14-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013 Sep;21(9):571–576. doi: 10.5435/JAAOS-21-09-571. [DOI] [PubMed] [Google Scholar]
- 68.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2008 Feb;16(2):137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 69.Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, knee. Arthritis care & research. 2012 Apr;64(4):465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 70.Colberg SR, Albright AL, Blissmer BJ, et al. Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Exercise and type 2 diabetes. Medicine and science in sports and exercise. 2010 Dec;42(12):2282–2303. doi: 10.1249/MSS.0b013e3181eeb61c. [DOI] [PubMed] [Google Scholar]
- 71.United States Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. [Accessed February 11, 2014]; Available at http://www.health.gov/paguidelines/
- 72.Agency for Healthcare Research and Quality. Physical activity and older Americans: benefits and strategies. [Accessed February 11, 2014]; Available at http://www.innovations.ahrq.gov/content.aspx?id=991.
- 73.Panel on Prevention of Falls in Older Persons AGS, British Geriatrics S. Summary of the Updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. Journal of the American Geriatrics Society. 2011 Jan;59(1):148–157. doi: 10.1111/j.1532-5415.2010.03234.x. [DOI] [PubMed] [Google Scholar]
- 74.Coker RH, Williams RH, Yeo SE, et al. The impact of exercise training compared to caloric restriction on hepatic and peripheral insulin resistance in obesity. The Journal of clinical endocrinology and metabolism. 2009 Nov;94(11):4258–4266. doi: 10.1210/jc.2008-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haus JM, Solomon TP, Marchetti CM, Edmison JM, Gonzalez F, Kirwan JP. Free fatty acid-induced hepatic insulin resistance is attenuated following lifestyle intervention in obese individuals with impaired glucose tolerance. The Journal of clinical endocrinology and metabolism. 2010 Jan;95(1):323–327. doi: 10.1210/jc.2009-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Solomon TP, Haus JM, Marchetti CM, Stanley WC, Kirwan JP. Effects of exercise training and diet on lipid kinetics during free fatty acid-induced insulin resistance in older obese humans with impaired glucose tolerance. American journal of physiology. Endocrinology and metabolism. 2009 Aug;297(2):E552–E559. doi: 10.1152/ajpendo.00220.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Balducci S, Zanuso S, Nicolucci A, et al. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2010 Oct;20(8):608–617. doi: 10.1016/j.numecd.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 78.Donges CE, Duffield R, Drinkwater EJ. Effects of resistance or aerobic exercise training on interleukin-6, C-reactive protein, and body composition. Medicine and science in sports and exercise. 2010 Feb;42(2):304–313. doi: 10.1249/MSS.0b013e3181b117ca. [DOI] [PubMed] [Google Scholar]
- 79.Smart NA, Larsen AI, Le Maitre JP, Ferraz AS. Effect of exercise training on interleukin-6, tumour necrosis factor alpha and functional capacity in heart failure. Cardiology research and practice. 2011;2011:532620. doi: 10.4061/2011/532620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Conraads VM, Beckers P, Bosmans J, et al. Combined endurance/resistance training reduces plasma TNF-alpha receptor levels in patients with chronic heart failure and coronary artery disease. European heart journal. 2002 Dec;23(23):1854–1860. doi: 10.1053/euhj.2002.3239. [DOI] [PubMed] [Google Scholar]
- 81.Hansen D, Dendale P, van Loon LJ, Meeusen R. The impact of training modalities on the clinical benefits of exercise intervention in patients with cardiovascular disease risk or type 2 diabetes mellitus. Sports medicine. 2010 Nov 1;40(11):921–940. doi: 10.2165/11535930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 82.Weinheimer EM, Sands LP, Campbell WW. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesity. Nutrition reviews. 2010 Jul;68(7):375–388. doi: 10.1111/j.1753-4887.2010.00298.x. [DOI] [PubMed] [Google Scholar]
- 83.McDermott AY, Mernitz H. Exercise and older patients: prescribing guidelines. American family physician. 2006 Aug 1;74(3):437–444. [PubMed] [Google Scholar]
- 84.Williams MA, Haskell WL, Ades PA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007 Jul 31;116(5):572–584. doi: 10.1161/CIRCULATIONAHA.107.185214. [DOI] [PubMed] [Google Scholar]
- 85.Elsawy B, Higgins KE. Physical activity guidelines for older adults. American family physician. 2010 Jan 1;81(1):55–59. [PubMed] [Google Scholar]
- 86.Donnelly JE, Blair SN, Jakicic JM, et al. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Medicine and science in sports and exercise. 2009 Feb;41(2):459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 87.Sattelmair J, Pertman J, Ding EL, Kohl HW, 3rd, Haskell W, Lee IM. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation. 2011 Aug 16;124(7):789–795. doi: 10.1161/CIRCULATIONAHA.110.010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kraus WE, Slentz CA. Exercise training, lipid regulation, and insulin action: a tangled web of cause and effect. Obesity. 2009 Dec;17(Suppl 3):S21–S26. doi: 10.1038/oby.2009.384. [DOI] [PubMed] [Google Scholar]
- 89.Hansen TM, Hansen G, Langgaard AM, Rasmussen JO. Longterm physical training in rheumatoid arthritis. A randomized trial with different training programs and blinded observers. Scand J Rheumatol. 1993;22(3):107–112. doi: 10.3109/03009749309099253. [DOI] [PubMed] [Google Scholar]
- 90.Slentz CA, Houmard JA, Kraus WE. Exercise, abdominal obesity, skeletal muscle, and metabolic risk: evidence for a dose response. Obesity. 2009 Dec;17(Suppl 3):S27–S33. doi: 10.1038/oby.2009.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khoja SS, Susko AM, Josbeno DA, Piva SR, Fitzgerald GK. Comparing physical activity programs for managing osteoarthritis in overweight or obese patients. Journal of Comparative Effectiveness Research. 2014 May 3;(3):283–299. doi: 10.2217/cer.14.15. [DOI] [PubMed] [Google Scholar]
- 92.American Diabetes Association. Standards of medical care in diabetes--2007. Diabetes care. 2007 Jan;30(Suppl 1):S4–S41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 93.Balady GJ, Arena R, Sietsema K, et al. Clinician's Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010 Jul 13;122(2):191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 94.Thompson WRGNF, Prescatello LS. ACSM's Guidelines for Exercise Testing and Prescription. Baltimore, M.D.: Lippincott Williams and Wilkins; 2010. [Google Scholar]
- 95.Hansen D, Peeters S, Zwaenepoel B, et al. Exercise assessment and prescription in patients with type 2 diabetes in the private and home care setting: clinical recommendations from AXXON (Belgian Physical Therapy Association) Physical Therapy. 2013 May;93(5):597–610. doi: 10.2522/ptj.20120400. [DOI] [PubMed] [Google Scholar]
- 96.Newman AB, Lee JS, Visser M, et al. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. The American journal of clinical nutrition. 2005 Oct;82(4):872–878. doi: 10.1093/ajcn/82.4.872. quiz 915–876. [DOI] [PubMed] [Google Scholar]
- 97.Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis and rheumatism. 2004 May;50(5):1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 98.Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA : the journal of the American Medical Association. 2013 Sep 25;310(12):1263–1273. doi: 10.1001/jama.2013.277669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miller GD, Nicklas BJ, Davis C, Loeser RF, Lenchik L, Messier SP. Intensive weight loss program improves physical function in older obese adults with knee osteoarthritis. Obesity. 2006 Jul;14(7):1219–1230. doi: 10.1038/oby.2006.139. [DOI] [PubMed] [Google Scholar]
- 100.Chomentowski P, Dube JJ, Amati F, et al. Moderate exercise attenuates the loss of skeletal muscle mass that occurs with intentional caloric restriction-induced weight loss in older, overweight to obese adults. The journals of gerontology. Series A, Biological sciences and medical sciences. 2009 May;64(5):575–580. doi: 10.1093/gerona/glp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van Gool CH, Penninx BW, Kempen GI, et al. Determinants of high and low attendance to diet and exercise interventions among overweight and obese older adults. Results from the arthritis, diet, and activity promotion trial. Contemporary clinical trials. 2006 Jun;27(3):227–237. doi: 10.1016/j.cct.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 102.Herrero-Beaumont G, Roman-Blas JA, Castaneda S, Jimenez SA. Primary osteoarthritis no longer primary: three subsets with distinct etiological, clinical, and therapeutic characteristics. Seminars in arthritis and rheumatism. 2009 Oct;39(2):71–80. doi: 10.1016/j.semarthrit.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 103.Mayo Clinic. Risk Factors for Diabetes. 2013. [Accessed January 2, 2014]; Available at http://www.mayoclinic.org/diseases-conditions/diabetes/basics/risk-factors/con-20033091. [Google Scholar]
- 104.Centers for Disease Control and Prevention. Osteoarthritis Risk Factors. 2014. [Accessed January 2, 2014]; Available at http://www.cdc.gov/arthritis/basics/risk-factors.htm.
- 105.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes care. 2006 Jun;29(6):1433–1438. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]