SUMMARY
Background
In most cells, the cilium is formed within a compartment separated from the cytoplasm. Entry into the ciliary compartment is regulated by a specialized gate located at the base of the cilium in a region known as the transition zone. The transition zone is closely associated with multiple structures of the ciliary base including the centriole, axoneme, and ciliary membrane. However, the contribution of these structures to the ciliary gate remains unclear.
Results
Here, we report that in Drosophila spermatids, a conserved module of transition zone proteins mutated in Meckel-Gruber Syndrome (MKS) including Cep290, Mks1, B9d1, and B9d2 comprise a ciliary gate that continuously migrates away from the centriole to compartmentalize the growing axoneme tip. We show that Cep290 is essential for transition zone composition, compartmentalization of the axoneme tip, and axoneme integrity, and find that MKS proteins also delimit a centriole-independent compartment in mouse spermatids.
Conclusion
Our findings demonstrate that the ciliary gate can migrate away from the base of the cilium, thereby functioning independently of the centriole and of a static interaction with the axoneme to compartmentalize the site of axoneme assembly.
INTRODUCTION
Compartmentalization of cellular processes is a critical aspect of cell biology. In many cases—such as in mitochondria and the endoplasmic reticulum—a semipermeable membrane surrounds an organelle to create a compositionally distinct compartment. In other cases, such as in the nucleus and cilium, the lumen of the organelle is continuous with the cytoplasm and compartmentalization is achieved by a specialized gate at its cytoplasmic interface [1]. The ciliary gate is found in a region known as the transition zone at the base of the cilium. Within the transition zone, axonemal microtubules are crosslinked to the surrounding membrane and components of the ciliary gate mediate attachments between the axoneme, ciliary membrane, and centriole, giving the impression that the transition zone is a static and rigid structure [2].
Aside from housing the ciliary gate, the transition zone is the site of multiple ciliary activities [3]. As such, the transition zone is compositionally diverse and it is unclear which of its components comprise the ciliary gate. However, recent studies have suggested that genes mutated in the ciliopathies Nephronophthisis (NPHP) and Meckel-Gruber syndrome (MKS) encode proteins that comprise two distinct modules essential for ciliary gate function [4-7].
Centrioles and basal bodies, as well as cilia and flagella, are synonymous terms in different cellular contexts [8]. For simplicity, we maintain the terms centriole and cilium throughout the text. Cilia can be divided into two types based on the location of the axoneme. In compartmentalized cilia, such as Drosophila sensory cilia, the entire axoneme is assembled and maintained within a membrane-bound compartment projecting from the transition zone at the distal end of a centriole (Figure 1A) [1, 9, 10]. Cilia formed by compartmentalized ciliogenesis require intraflagellar transport (IFT) to move cargo through the transition zone [11], and IFT machinery directly interacts with transition zone proteins [12]. Thus, the centriole, IFT, and the transition zone are generally essential for compartmentalized ciliogenesis.
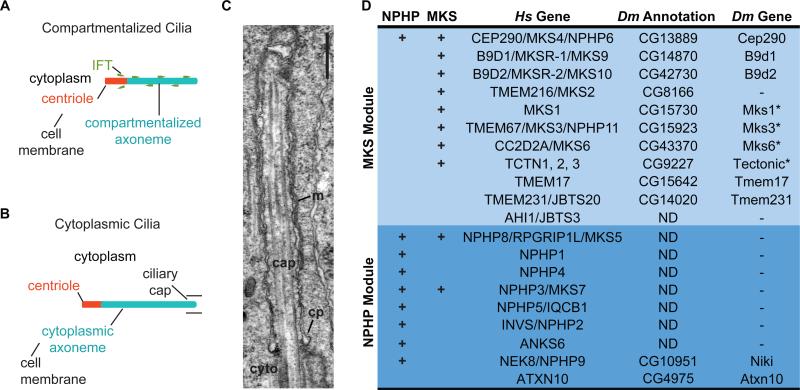
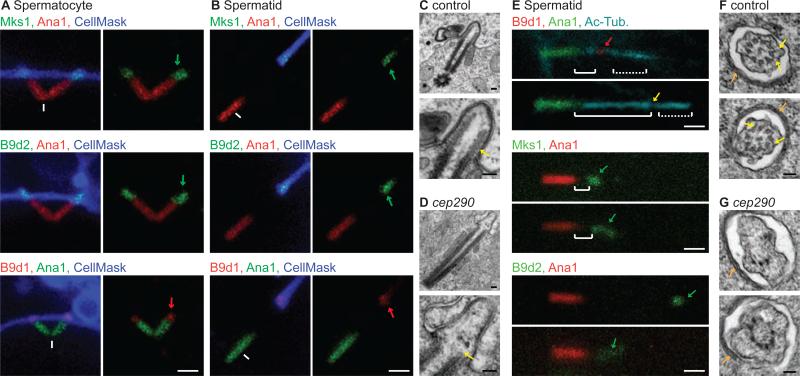
Figure 1. NPHP- and MKS-associated Genes in Drosophila.
(A-B) Illustration of compartmentalized and cytoplasmic cilia. (C) Electron micrograph of the Drosophila ciliary cap (cap); ciliary pocket (cp), cytoplasm (cyto), juxtaposed ciliary and cell membranes (m). Scale bar, 500 nm. (D) MKS module genes are conserved in Drosophila. Hs, human; Dm, Drosophila. ND, ortholog not detected; *, previously identified [25, 26].
In cytoplasmic cilia, such as in mammalian and Drosophila spermatids and microgametes of the malarial parasite Plasmodium, the axoneme is found in the cytoplasm and the centriole is not attached to the cell or ciliary membrane (Figure 1B) [13-15]. In Drosophila spermatids, the axoneme is initially composed of bare microtubules and later acquires its mature composition by incorporating proteins in the cytoplasm [13, 16]. Furthermore, IFT is not essential for cytoplasmic axoneme formation in Plasmodium microgametes and Drosophila spermatids [17-19]. Thus, the separation of the centriole from a membrane-bound compartment and dispensability of IFT appear contradictory for a role of compartmentalization in cytoplasmic ciliogenesis.
Interestingly, electron microscopy of spermatids in various insects including Drosophila identifies a membranous cap-like structure associated with the growing axoneme tip [16, 20]. This, structure, which we refer to as the ciliary cap, is morphologically similar to a typical compartmentalized cilium. (Figure 1C). Furthermore, mutations in candidate Drosophila transition zone proteins result in defects in spermatogenesis [21-23], and many MKS module genes are highly expressed in Drosophila testes [24]. Together, these observations suggest that transition zone components—which are typically implicated in cilium compartmentalization—may also be involved in the formation of cytoplasmic cilia.
Here, we provide insight into the mechanism by which transition zone proteins function in cytoplasmic ciliogenesis. We show that MKS module proteins comprise a ciliary gate that migrates to compartmentalize the growing axoneme tip in Drosophila spermatids. Our findings provide an example whereby the ciliary gate is dynamically associated with the surrounding ciliary architecture to sustain a compartment housing the site of axoneme assembly.
RESULTS
In vertebrates and C. elegans, NPHP and MKS module genes are essential for transition zone function [4-6], but few of these genes have been identified in Drosophila [25, 26]. Using BLAST reciprocal best-hit, we identified orthologs of all but one gene of the MKS module in the Drosophila genome. However, we were only able to identify two peripheral NPHP module components (Figure 1D). Interestingly, these modules appear to be functionally redundant in organisms containing both modules. In C. elegans, for example, dramatic ciliary defect is only observed in mutants containing a mutation in each module [4, 27]. Our inability to identify core NPHP genes such as NPHP1, NPHP4, and NPHP8 implies that, in Drosophila, there is reduced functional redundancy at the transition zone.
Cep290 is a Transition Zone Protein Essential for Compartmentalized Sensory Cilium Formation
In Drosophila. the mechanosensory transduction machinery that mediates proprioception is housed within a sensory cilium (Figure 2A). Consequently, ciliary mutants exhibit a distinctive adult proprioception deficit [17, 28]. In a collection of EMS mutagenized lines [29], we identified cep290mecH, a recessive loss-of-function mutant of cep290 that exhibits severe proprioception deficit (Figure 2B-C) . This proprioception deficit was rescued by transgenic expression of wildtype Cep290 and Cep390-GFP under control of the cep290 promoter (Figure 2C and Movie S1).
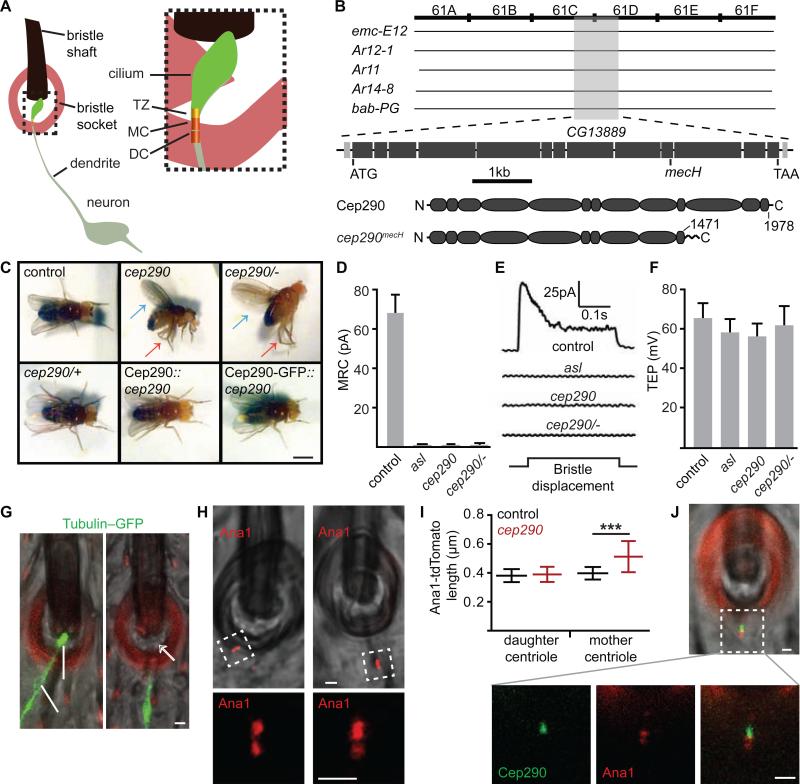
Figure 2. Cep290 is Required for Drosophila Sensory Cilium Formation and Localizes to the Transition Zone.
(A) Illustration of the Drosophila mechanosensory organ. TZ, transition zone; MC, mother centriole; DC, daughter centriole. (B) Positional cloning of mecH found a mutation in cep290 (CG13889). cep290mecH does not complement deficiencies emc-E12, Ar12-1, Ar11, Ar14-8 but complements bab-PG. cep290mecH has a deletion from bases 5040-5170, resulting in a frameshift and premature stop. Cep290mecH includes normal residues 1-1,471. (C) cep290mecH homozygotes (cep290) and hemizygotes (cep290/−) cannot stand, hold their wings erect (blue arrow), and cross their legs (red arrow). cep290mecH heterozygotes (cep290/+) and rescue (Cep290, Cep290-GFP) are normal. Scale bar, 1 mm. Also, see Movie S1. (D-F) cep290mecH has an abnormal MRC but robust TEP; the MRC defect is similar to aslmecD, a mutant lacking sensory cilia [45]. N≥5. (G) cep290mecH sensory dendrites lack ciliary GFP-tubulin (white arrow). Red, cuticle autofluorescence. (H) Both sensory dendrite centrioles marked by Ana1-tdTomato are present in cep290mecH. (I) cep290mecH sensory dendrite mother centrioles are long as labeled by Ana1-tdtomato. N=20 (J) Cep290-GFP labels the transition zone distal to the mother centriole. (G-H, J) Scale bar, 1 μm.
To analyze cep290mecH sensory cilium functionality, we recorded the mechanoreceptor current (MRC) and transepithelial potential (TEP) from thoracic mechanosensory bristles. Mutations that affect the neuronal sensory cilium show a defective MRC [17, 18]. In contrast, mutations that affect accessory cells of the mechanosensory organ also abolish the TEP [30]. We found that cep290mecH bristles transduce no MRC (Figure 2D-E) but possess robust TEPs (Figure 2F), suggesting that cep290mecH has a severe and specific ciliary defect. To test this, we labeled sensory neurons and their cilia by expressing GFP-α1-tubulin84B and the centriolar marker Ana1-tdTomato via a pan-neuronal promoter and found that cep290mecH mechanosensory neurons have dendrites, but these dendrites lack tubulin labeling of their cilia (Figure 2G).
Mechanosensory neurons have two centrioles (mother and daughter) at the tip of their dendrites. Of these, only the mother centriole nucleates a cilium. Interestingly, although the length distribution of Ana1-tdTomato in the daughter centriole was normal in cep290mecH, its distribution in the mother centriole was abnormally long (Figure 2H-I).
Cep290 localizes to the transition zone in vertebrates and Chlamydomonas [31, 32]. Similarly, we found that Cep290-GFP localizes to the transition zone at the tip of the mother centriole in Drosophila sensory cilia(Figure 2J). Together, our findings in sensory neurons demonstrate that Drosophila Cep290 localizes to the transition zone and is essential for compartmentalized ciliogenesis.
Cep290 is Important for Sperm Cytoplasmic Ciliogenesis
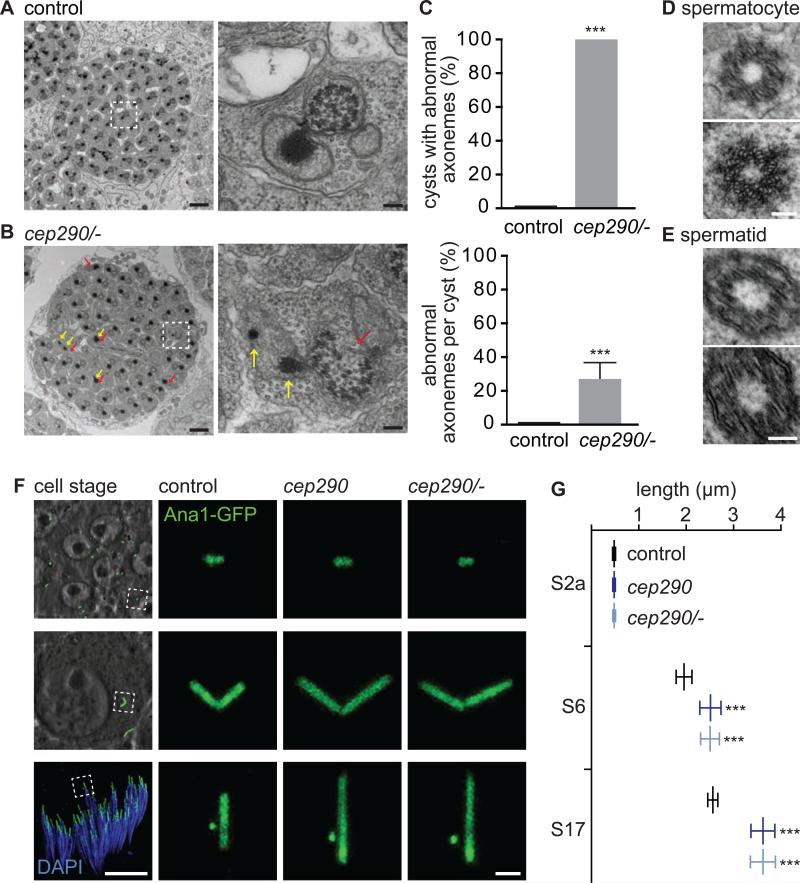
Electron microscopy of cep290mecH revealed that developing spermatid axonemes are frequently absent or structurally defective (Figure 3A-C and Figure S1A). Such structural defects are not observed in centrioles of cep290mecH spermatocytes or spermatids (Figure 3D-E), suggesting that the axoneme defect does not originate in the centriole. Further, similar to sensory cilia, the length distribution of centriolar proteins is increased in cep290mecH sperm (Figure 3F-G and Figure S1B-E). This increase is not observed in young spermatocytes that have not yet given rise to a cilium (Figure 3F-G, stage 2a). Despite this, testis and mature spermatid length are normal in cep290mecH (Figure S1F-H). Together, these findings demonstrate that Cep290 is important in sperm cytoplasmic ciliogenesis.
Figure 3. Cep290 is Essential for Drosophila Sperm Ciliogenesis.
(A-C) Cep290 is essential for spermatid axoneme formation. (A) Cross-section through a spermatid cyst in control, and (B) cep290mecH/- shows missing or defective axonemes (red arrows) and abnormal mitochondria (yellow arrows). Scale bars, 1 μm and 100 nm. Quantified in (C). N=10. (D-E) Centrioles of cep290mecH spermatocytes (C) and spermatids (D) are intact. Scale bar, 100 nm. (F-G) Cep290 is essential for centriole length. (F) Mature spermatocyte (S6) and spermatid (S17) centrioles are long in cep290mecH. Centriole length is normal before the onset of ciliogenesis (S2a). Stages described in [46]. Scale bars, 10 μm and 1 μm. (G) Quantification of Ana1-GFP length. N≥40. Also, see Figure S1.
Cep290 is a Component of the Spermatocyte Primary Cilium and Spermatid Ciliary Cap
Cilium assembly in Drosophila sperm (Figure 4A) begins when spermatocyte centrioles dock to the cell membrane to give rise to primary cilia, triggering the elongation of both the centriole and the primary cilium. During meiosis, the centrioles retract from the cell membrane into the cytoplasm, forming an invagination of the cell membrane that remains associated with the primary cilium at the distal end of the centriole. As the spermatid differentiates, the axoneme elongates and matures within the cytoplasm by incorporating structural proteins. Although most of the axoneme is not surrounded by the cell membrane, its distal growing tip always remains associated with a membranous cap derived from the spermatocyte primary cilium. This membranous cap, which we refer to as the ciliary cap, resembles a typical compartmentalized cilium [13, 16, 33] (Figure 1C).
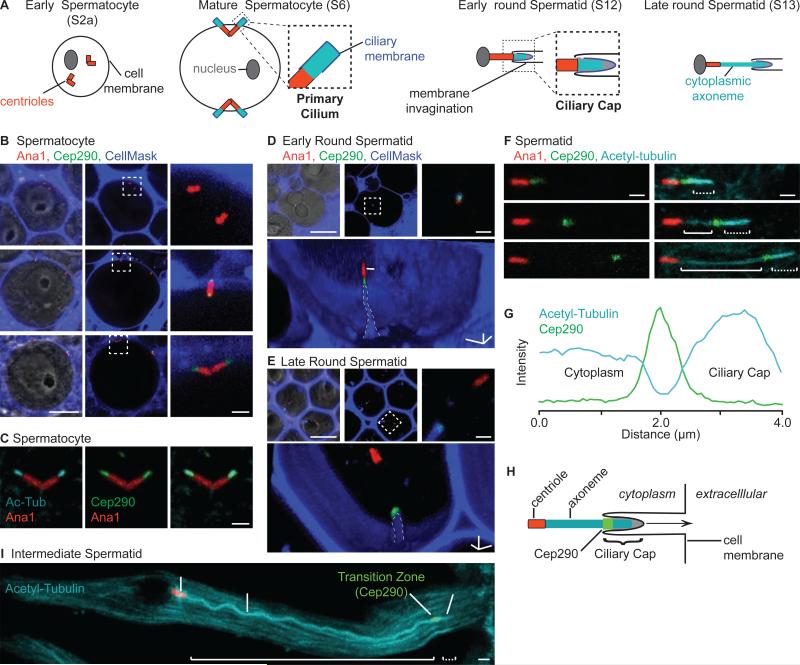
Figure 4. Cep290 Labels the Spermatocyte Primary Cilium and Base of the Spermatid Ciliary Cap.
(A) Illustration of Drosophila spermatogenesis. (B-C) Cep290 labels the spermatocyte primary cilium. (B) Cep290-GFP localizes to the growing primary cilium during spermatocyte differentiation, and (C) labels the entire primary cilium marked by anti-acetyl-tubulin in mature spermatocytes. Scale bars, 10 μm and 1 μm. (D G) Cep290-GFP labels the base of the ciliary cap. (D-E) Cep290-GFP localizes to base of the cell membrane invagination (outlined) in early (D) and late (E) round spermatids (S12 and S13). Bottom panel, 3D projection; Scale bar, 1 μm in each dimension. Note, invagination projects into the page in (D). (F) Cep290-GFP migrates away from the centriole and localizes to the base of the ciliary cap during axoneme elongation. (G) Anti-acetyltubulin is reduced at the position of Cep290-GFP. N=30. (H) Illustration of the ciliary cap. (I) Intermediate spermatid (S15). N, nucleus. (F, I) Solid brackets, cytoplasmic axoneme segment; dotted brackets, ciliary cap axoneme segment. Scale bar, 1 μm.
We analyzed Cep290-GFP localization during spermatogenesis. In early spermatocytes, centrioles are not attached to the cell membrane and Cep290-GFP is not detectable (Figure 4B, stage 2a). As spermatocytes differentiate, ciliogenesis begins with the centrioles migrating to the cell membrane, where Cep290-GFP appears as a focus at the membrane-associated tip of each centriole (Figure 4B, stages 2b and 3/4). Subsequently during primary cilium formation, both the centriole and the region defined by Cep290-GFP elongate until centriole length reaches ~2 μm and Cep290-GFP length reaches ~1 μm just prior to meiosis (Figure 4C, stage 6). Staining with anti-acetyl-tubulin, a marker of the primary cilium [33], colocalizes precisely with Cep290-GFP, suggesting that the spermatocyte primary cilium is homologous to a transition zone.
In early spermatids, the ciliary cap and associated cell membrane invagination are just distal to the centriole and Cep290-GFP (Figure 4D). As the membrane invagination moves away from the centriole, Cep290-GFP remains associated with the invagination and migrates away from the centriole (Figure 4E). Staining with anti-acetyl-tubulin demonstrates that Cep290-GFP is consistently located ~2 μm away from the axoneme's distal tip (Figure 4F-G). Interestingly, anti-acetyl-tubulin staining distinctly marked three axoneme segments; the ciliary cap segment at the distal ~2 μm of the axoneme stained most intensely, the segment marked by Cep290-GFP stained minimally, and the cytoplasmic axoneme segment stained with intermediate intensity (Figure 4F-I).
MKS Module Proteins Label the Spermatocyte Primary Cilium and Base of the Spermatid Ciliary Cap
Altogether, the spatial and temporal dynamics of Cep290 localization suggest that assembly of a transition zone at the distal end of spermatocyte centrioles initiates axoneme assembly. After meiosis, this transition zone migrates away from the centriole along the growing axoneme to remain localized at the base of the ciliary cap. Therefore, we hypothesize that this migrating transition zone functions to maintain the ciliary cap as a distinct compartment housing the growing axoneme tip. To test this, we engineered flies expressing Mks1-GFP, B9d1-mRFP, and B9d2-GFP and found that these proteins also label the spermatocyte transition zone (Figure 5A), and the migrating base of the spermatid ciliary cap (Figure 5B), suggesting that the ciliary cap contains a true transition zone.
Figure 5. Cep290 is Required for MKS Module Localization and Spermatid Ciliary Cap Formation.
(A-B) MKS proteins label the spermatocyte primary cilium (A) and base of the spermatid ciliary cap (B) . White arrowheads, cell membrane invagination; green or red arrows, Mks1-GFP, B9d2-GFP, and B9d1-mRFP. (C-D) Axonemal microtubules (yellow arrow) are not observed in cep290mecH spermatocyte primary cilia. (E) B9d1-mRFP, MKS1-GFP and B9d2-GFP localization is abnormal in cep290mecH. (F-G) Ciliary cap cross-sections in control (F) and cep290mecH (G); two examples are provided of each. Vague links (yellow arrows) are observed between the axoneme and membrane. Ciliary cap-associated ER (orange arrows) indicates the position of the section at ~500 μm distal to the ciliary cap base [16]. Also, see Figure S2. (A-B, E) Scale bar, 1 μm. (C-D, F-G) Scale bar, 100 nm.
Cep290 is Required for Transition Zone Assembly and Ciliary Cap Structure
We next examined the spermatocyte primary cilium in cep290mecH. We found that although spermatocyte centrioles remain associated with the cell membrane (Figure S2A), the size of the spermatocyte primary cilium was significantly reduced as labeled by anti-acetyl-tubulin and B9d1-mRFP (Figure S2B-C). Furthermore, cep290mecH spermatocyte primary cilia lacked organized axonemal microtubules (Figure 5C-D). Thus, Cep290 is required for the proper formation of the spermatocyte primary cilium/transition zone.
We then studied the spermatid ciliary cap in cep290mecH. We found that cep290mecH spermatids faithfully formed the membrane invagination of the ciliary cap (Figure S2D) and anti-acetyl-tubulin staining resulted in a similar profile to control spermatids (Figure 5E and S2E), suggesting that the cep290mecH spermatids form a ciliary cap. Ultrastructural analysis of the ciliary cap near the transition zone in late elongating spermatids(Figure 5F) found that the axoneme was normal in 91% (N=22) of control, and vague links connect the axonemal microtubules to the ciliary membrane. In contrast, 8% (N=12) of ciliary cap axonemes exhibited normal structure in cep290mecH (Figure 5G). We also found that B9d1-mRFP, Mks1-GFP, and B9d2-GFP localization at the ciliary cap was abnormal in cep290mecH (Figure 5E). While these markers were focused at the base in control spermatids, they were diffuse along the ciliary cap in cep290mecH. Nevertheless, sum Mks1-GFP and B9d2-GFP fluorescence was not altered (Figure S2F-H) in cep290mecH, suggesting that Cep290 is required to concentrate Mks1 and B9d2 at the base. Together, these data suggest that although Cep290 is dispensable for the association of centrioles or the axoneme with the ciliary membrane and for ciliary cap formation, Cep290 is required for proper transition zone assembly.
Cep290 is Essential for Compartmentalization of the Ciliary Cap
Next, we investigated whether the ciliary cap is a distinct cellular compartment. While the nonionic surfactant Triton X-100 permeabilizes both the cell membrane and the ciliary membrane, low concentrations of the glycoside Digitonin permeabilize the cell membrane without disrupting other membrane-bound organelles [34]. Thus, we developed an assay to differentially permeabilize the cell and ciliary cap membranes and analyzed compartmentalization by staining the axoneme with anti-acetyl-tubulin. We found that treatment of spermatids with Triton X-100 permeabilized both the cell membrane and ciliary cap membrane, allowing for anti-acetyltubulin staining of the entire axoneme (Figure 6A and Figure S3A). On the other hand, Digitonin permeabilization permitted staining of only the cytoplasmic axoneme segment and not of the ciliary cap (Figure 6B and Figure S3B). The inability of anti-acetyl-tubulin to label the ciliary cap axoneme segment after treatment with Digitonin demonstrates that Digitonin preferentially permeabilizes the cell membrane without disrupting the ciliary cap membrane and suggests that diffusion into the ciliary cap is restricted at its base.
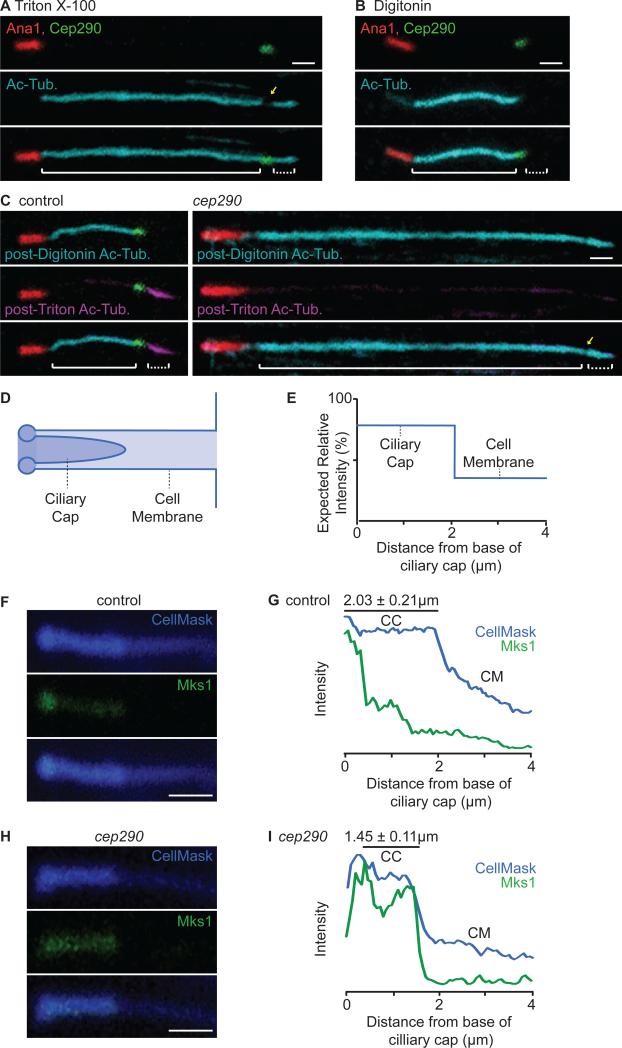
Figure 6. The Ciliary Cap is a Distinct Compartment that is Compromised in cep290mecH.
(A-B) Triton X-100 and Digitonin differentially permeabilize the cell and ciliary cap membranes. (A) Triton X-100 permeabilizes both the cell and ciliary cap membranes, allowing for anti-acetyl-tubulin staining of the entire axoneme. (B) Digitonin preferentially permeabilizes the cell membrane, allowing for anti-acetyl-tubulin staining of only the cytoplasmic axoneme. (C) Ciliary cap compartmentalization is compromised in cep290mecH. (C, left) In control spermatids, post-Digitonin staining specifically labels the cytoplasmic axoneme segment (cyan) while post-Triton X-100 staining specifically labels the ciliary cap axoneme (magenta) (N>70). (C, right) In cep290mecH, post-Digitonin staining labels both the cytoplasmic and ciliary cap axoneme segments (N≥60). (A, C) Yellow arrow, reduction in anti-acetyl-tubulin at the transition zone. Solid brackets, cytoplasmic axoneme; dotted brackets, ciliary cap axoneme. Note that all spermatids analyzed were at the same developmental stage; any difference in length displayed is within normal variation. (D) Illustration depicting the cell membrane invagination containing the ciliary cap and (E) expected CellMaskTM signal. (F-I) Ciliary cap length and Mks1-GFP distribution are abnormal in cep290mecH. (F-G) In control spermatids, CellMaskTM and Mks1-GFP mark the ciliary cap membrane and transition zone. (H-I) In cep290mecH, CellMaskTM marks a shorter ciliary cap and Mks1-GFP labeling is not focused at the base. (G, I) Mean signal was quantified along a 4 μm region distal to the ciliary cap base (N=10). CM, cell membrane invagination; CC, ciliary cap membrane. (A-C, F, H) Scale bar, 1 μm. Also, see Figure S3.
Next, we examined the role of Cep290 in ciliary cap compartmentalization. For this, we developed a two-step permeabilization protocol and labeled anti-acetyl-tubulin with two different conjugates to visualize the cytoplasmic and ciliary cap axoneme segments distinctly. First, we used Digitonin to preferentially permeabilize the cell membrane and labeled the cytoplasmic axoneme segment with anti-acetyl-tubulin and AlexaFluor®647 to saturation. Subsequently, we permeabilized the ciliary cap membrane with Triton X-100 and labeled the freshly exposed ciliary cap axoneme segment with anti-acetyl-tubulin and DyLight™405 (Figure S3C). As a proof of principle, in control spermatids, post-Digitonin staining specifically labeled the cytoplasmic axoneme segment whereas post-Triton X-100 staining specifically labeled the ciliary cap (Figure 6C, left). We applied the same protocol to cep290mecH and found that post-Digitonin anti-acetyl-tubulin stained both the cytoplasmic and ciliary cap axoneme segments, suggesting that the integrity of ciliary cap compartmentalization is compromised in cep290mecH (Figure 6C, right).
MKS module transition zone proteins exhibit a dual function in cilium compartmentalization. Besides creating a barrier to cytoplasmic entry into the ciliary compartment (cytosolic diffusion barrier), some MKS module proteins are also required to restrict the exchange of the cell and ciliary membrane (membrane diffusion barrier) [5]. Thus, the entry of soluble antibody into the ciliary cap in cep290mecH (Figure 6C, right) may either be due to a defect in the cytoplasmic diffusion barrier at the base of the ciliary cap, or to a compromise in the membrane diffusion barrier that allows the mixing of cell and ciliary cap membranes, thereby rending the membrane of the ciliary cap sensitive to Digitonin.
We analyzed ciliary cap membrane integrity in cep290mecH by examining the distribution of membrane markers along the cell membrane invagination containing the ciliary cap. This invagination is composed of both a cell membrane domain and a ciliary cap membrane domain (Figure 6D-E). Importantly, the ciliary cap domain is enveloped by the cell membrane invagination and is therefore composed of two layers of external membrane. We visualized and quantified the amounts of membrane in each domain by observing the differential signal intensities achieved by staining with the general membrane stain CellMaskTM (Figure 6F-G) [35]. We analyzed the distribution of CellMaskTM along both domains in cep290mecH and found that CellMaskTM staining resulted in similar signal distribution to control spermatids. Interestingly, however, CellMaskTM marked a distinctly shorter ciliary cap in cep290mecH. As expected (Fig 5C), Mks1-GFP was delocalized from its focus at the ciliary cap transition zone and was instead distributed along the entirety of the ciliary cap (Figure 6H-I). These experiments establish CellMaskTM as a reliable and quantitative marker for the ciliary cap membrane.
Considering this, we analyzed the integrity of the ciliary cap membrane diffusion barrier by measuring the distribution of peripheral membrane proteins including Sktl-YFP [36], Src-GFP, and Ack-mCherry [37] (Figure S3D). We found that all three markers were distributed evenly along the cell membrane and cell membrane invagination in both control and cep290mecH spermatids. Importantly, these markers were not enriched in the ciliary cap membrane (Figure S3E-I), indicating that the ciliary cap membrane diffusion barrier is not compromised in cep290mecH. Together, our findings suggest that Cep290 functions at the ciliary cap transition zone to create a cytoplasmic compartment housing the growing axoneme tip in Drosophila sperm.
Cep290 and MKS1 Mark a Cytoplasmic Axoneme Segment in Mouse Spermatids
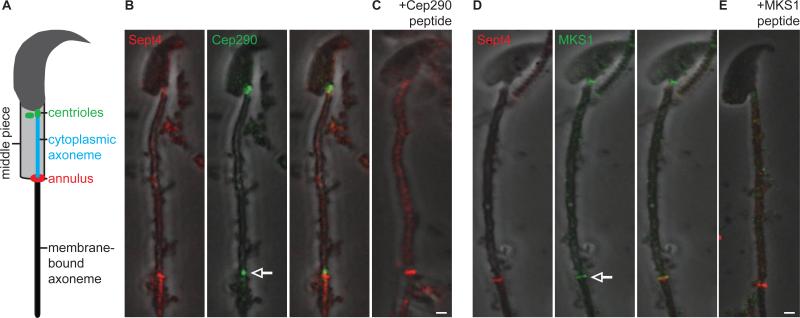
Mammalian spermatids also contain both a cytoplasmic axoneme segment and a distal segment that is closely associated with a membrane (Figure 7A) [15]. The interface of the cytoplasmic and membrane-bound axoneme segments is marked by a structure known as the annulus, which has been implicated in separating adjacent membrane domains [38]. However, it is unknown whether a transition zone compartmentalizes the membrane-bound axoneme segment in mammalian spermatids. Considering our findings in Drosophila, we hypothesized that MKS module transition zone proteins also delimit the membrane-bound segment of mammalian spermatid axonemes. To test this, we stained mouse spermatids with antibodies against CEP290, MKS1, and TCTN1 as well as Sept4, a marker of the annulus [39]. We found that anti-CEP290, anti-MKS1, and anti-TCTN1 stained the spermatid centrioles near the nucleus, demonstrating that all three markers recognized their expected epitopes under our experimental conditions (Figure 7 and Figure S4). In addition, we found that both anti-CEP290 and anti-MKS1 stained a structure at the base of the membrane-bound axoneme segment near the annulus (Figure 7B-D). As a specificity control, staining of anti-CEP290 and anti-MKS1 was blocked by competition with epitope-specific peptides (Figure 7C-E and Figure S4E-F). These observations imply that the distal end of mammalian spermatid axonemes is also compartmentalized in an MKS module-dependent manner.
Figure 7. Cep290 and MKS1 Mark the Base of a Membrane-Bound Axoneme Segment in Mouse Spermatids.
(A) Mouse spermatid illustration. Anti-Cep290 (B-C) and anti-MKS1 (D-E) label the centriole near the nucleus (arrowhead), and at a focus near the annulus (anti-Sept4, arrow); staining is blocked by anti-Cep290 (C) and anti-MKS1 (E) peptide competition. Scale bar, 1 μm. Also, see Figure S4.
DISCUSSION
In most species, ciliary gate function is dependent on both the NPHP and MKS modules, which exhibit some redundancy at the transition zone [4-6]. However, mutation of MKS module genes in humans is associated with more severe ciliary disease than is mutation of the NPHP module, suggesting that the MKS module plays a more basic role in cilium formation or function. Consistent with this, we were unable to detect core genes of the NPHP module in Drosophila, implying that the MKS module is sufficient for cilium compartmentalization and that compartmentalization can be achieved by a relatively small subset of proteins.
Classically, the transition zone has been regarded as a characteristic of the ciliary base that is restricted to the distal end of the centriole. Indeed, a mechanism involved in anchoring transition zone proteins to the centriole has been recently described [10]. However, our work demonstrates that the transition zone can function independently of the centriole and suggests that the transition zone, and not the centriole, is the determining factor in positioning the ciliary compartment along the axoneme. The assembly of the transition zone upon the distal ends of centrioles prior to cytoplasmic axoneme elongation in Drosophila spermatids suggests that initial transition zone assembly requires the centriole. However, as the axoneme elongates, this transition zone becomes spatially separated from the centriole and continuously migrates to compartmentalize the growing axoneme tip. The spatial separation between the centriole and the ciliary cap demonstrates that the transition zone can function free of centriolar association and while constantly migrating relative to the growing axoneme to create a centriole-independent ciliary compartment.
Transition zone migration may also underlie centriole elongation. In normal spermatocytes, the centriole continues to elongate after the assembly of the transition zone upon its distal end. Since centrioles are not known to grow at their proximal ends, centriole elongation likely occurs by transition zone migration along the axoneme, followed by the expansion of centriolar proteins over freshly exposed axonemal microtubules. The excessive length distribution of centriolar proteins observed in cep290mecH spermatocytes may result from an impairment in the transition zone barrier, allowing centriolar proteins to aberrantly expand along the axoneme at the expense of the transition zone. This interpretation is consistent with our observation that transition zone size is decreased in cep290mecH.
The base of the ciliary cap may contain additional proteins besides those of the MKS module. Previously, it was shown that two proteins, Uncoordinated and Chibby, are found simultaneously in both the centriole and at a distal focus, presumably the ciliary cap [22, 40-42]. Although the role of these proteins at the ciliary cap remains unclear, it is possible that they cooperate with the MKS module in ciliary cap compartmentalization. Furthermore, another structure associated with the base of the ciliary cap, referred to as the ring centriole, has been described in insects by electron microscopy. It was proposed that the ring centriole is homologous to a structure known as the annulus of mammalian sperm [20]. However, unlike the transition zone, the ring centriole/annulus seems to be localized outside of the ciliary cap on the cytoplasmic side of its base and does not appear to contact the axoneme [20, 43]. Considering this, we speculate that the ring centriole is a distinct structure from the transition zone, and that MKS proteins are not part of the ring centriole but are instead components of the ciliary cap transition zone as in compartmentalized cilia. In support of this, we observed that CEP290 and MKS1 do not precisely colocalize with Sept4 in mouse spermatids.
In compartmentalized cilia of C. elegans, Chlamydomonas, and vertebrates, transition zone ultrastructure is characterized by features known as “Y-links,” which are thought to mediate attachments between the axoneme and ciliary membrane [31]. These Y-links are also thought to be involved in regulating entry into the ciliary compartment, and Y-link formation seems to be dependent on MKS protein function [4, 31]. Although Y-links have been observed in the transition zone of some insect sensory cilia [44] they have not been identified in Drosophila, suggesting that NPHP module proteins—which are absent from the Drosophila genome—are involved in Y-link formation. Still, electron microscopy of Drosophila spermatids identifies vague links between the axoneme and the ciliary cap membrane [16], implying that structural connections between the axoneme and ciliary cap membrane are also present in migrating transition zones. Indeed, we observed similar vague links in our analysis of both the Drosophila ciliary cap base and sensory cilium transition zone (Figure S5).
Similar to Drosophila, mammalian spermatid axonemes also contain a segment that is exposed to the cytoplasm. Distal to this segment, the axoneme resembles a compartmentalized cilium and is closely associated with a membrane of distinct composition [15, 38]. However, it was unknown whether a transition zone defines these distinct axoneme segments in mammalian spermatids. Our finding that CEP290 and MKS1 localize to the boundary of cytoplasmic and membrane-bound axoneme segments in mouse spermatids suggests that, similar to Drosophila, the distal, growing region of mammalian spermatid axonemes is also compartmentalized.
Although axoneme compartmentalization is a defining characteristic of cilia, it has remained unclear if compartmentalization is essential for cilium formation or if it is instead required to satisfy ciliary functional requirements. Our observations suggest that compartmentalization is a conserved feature of ciliogenesis that is required for the integrity of axoneme assembly. Although the axoneme may be exposed to the cytoplasm after its initial formation, the process of axoneme assembly must occur within a distinct cellular environment. The transition zone may also play a direct role in axoneme assembly and it has previously been proposed that the base of the ciliary cap confers 9-fold symmetry to the axoneme by organizing growing axonemal microtubules [16]. This idea is supported by the observation that ciliary cap axonemes of cep290mecH are structurally destabilized.
In summary, our findings demonstrate that the transition zone can migrate relative to the axoneme to sustain a compartment housing the site of axoneme assembly. We propose that axoneme compartmentalization is conserved as a prerequisite for proper cilium assembly and that the ciliary gate possesses a high degree of independence from the surrounding ciliary architecture to specify the location of the ciliary compartment along the axoneme.
EXPERIMENTAL PROCEDURES
Statistical Methods
Statistical analyses were done with GraphPad Prism 5. A two-tailed, unpaired Student's T-test (with samples of unequal variance) was used. Data shown as mean ± SEM. ***, P<0.001.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs. D. Leaman, B. Lin, F. Dong, P. Du, C.A. Johnson, J.A. Brill, and H.C. Chang for materials, Drs. S. Blachon and. J. Malicki for discussions, T. Janowicz, M. Buczek, and the EM facilities at UNC Chapel Hill and Harvard Medical School for technical assistance. This work was supported by R01GM098394 from the National Institute of General Medical Sciences. NIGMS Diversity Supplement supported M. Basiri. RFBR 10-04-01027 supported A. Polyanovsky.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Detailed experimental procedures can be found in the Supplemental Information.
REFERENCES
- 1.Reiter JF, Blacque OE, Leroux MR. The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep. 2012;13:608–618. doi: 10.1038/embor.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malicki J, Avidor-Reiss T. From the cytoplasm into the cilium: bon voyage. Organogenesis. 2014;10:138–157. doi: 10.4161/org.29055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobayashi T, Dynlacht BD. Regulating the transition from centriole to basal body. J Cell Biol. 2011;193:435–444. doi: 10.1083/jcb.201101005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams CL, Li C, Kida K, Inglis PN, Mohan S, Semenec L, Bialas NJ, Stupay RM, Chen N, Blacque OE, et al. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J Cell Biol. 2011;192:1023–1041. doi: 10.1083/jcb.201012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chih B, Liu P, Chinn Y, Chalouni C, Komuves LG, Hass PE, Sandoval W, Peterson AS. A ciliopathy complex at the transition zone protects the cilia as a privileged membrane domain. Nat Cell Biol. 2012;14:61–72. doi: 10.1038/ncb2410. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Gonzalo FR, Corbit KC, Sirerol-Piquer MS, Ramaswami G, Otto EA, Noriega TR, Seol AD, Robinson JF, Bennett CL, Josifova DJ, et al. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat Genet. 2011;43:776–784. doi: 10.1038/ng.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sang L, Miller JJ, Corbit KC, Giles RH, Brauer MJ, Otto EA, Baye LM, Wen X, Scales SJ, Kwong M, et al. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell. 2011;145:513–528. doi: 10.1016/j.cell.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avidor-Reiss T, Gopalakrishnan J, Blachon S, Polyanovsky A. Centriole duplication and inheritance in Drosophila melanogaster. In: Schatten H, editor. The Centrosome: Cell and Molecular Mechanisms of Functions and Dysfunctions in Disease. Humana Press; 2012. [Google Scholar]
- 9.Szymanska K, Johnson CA. The transition zone: an essential functional compartment of cilia. Cilia. 2012;1:10. doi: 10.1186/2046-2530-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang WJ, Tay HG, Soni R, Perumal GS, Goll MG, Macaluso FP, Asara JM, Amack JD, Bryan Tsou MF. CEP162 is an axoneme-recognition protein promoting ciliary transition zone assembly at the cilia base. Nat Cell Biol. 2013;15:591–601. doi: 10.1038/ncb2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenbaum J. Intraflagellar transport. Curr Biol. 2002;12:R125. doi: 10.1016/s0960-9822(02)00703-0. [DOI] [PubMed] [Google Scholar]
- 12.Zhao C, Malicki J. Nephrocystins and MKS proteins interact with IFT particle and facilitate transport of selected ciliary cargos. EMBO J. 2011;30:2532–2544. doi: 10.1038/emboj.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tates AD. Cytodifferentiation during Spermatogenesis in Drosophila melanogaster: An Electron Microscope Study. Rijksuniversiteit de Leiden; Leiden, Netherlands: 1971. [Google Scholar]
- 14.Sinden RE, Canning EU, Spain B. Gametogenesis and fertilization in Plasmodium yoelii nigeriensis: a transmission electron microscope study. Proc R Soc Lond B Biol Sci. 1976;193:55–76. doi: 10.1098/rspb.1976.0031. [DOI] [PubMed] [Google Scholar]
- 15.Fawcett DW, Eddy EM, Phillips DM. Observations on the fine structure and relationships of the chromatoid body in mammalian spermatogenesis. Biology of reproduction. 1970;2:129–153. doi: 10.1095/biolreprod2.1.129. [DOI] [PubMed] [Google Scholar]
- 16.Tokuyasu KT. Dynamics of spermiogenesis in Drosophila melanogaster. VI. Significance of “onion” nebenkern formation. J Ultrastruct Res. 1975;53:93–112. doi: 10.1016/s0022-5320(75)80089-x. [DOI] [PubMed] [Google Scholar]
- 17.Avidor-Reiss T, Maer AM, Koundakjian E, Polyanovsky A, Keil T, Subramaniam S, Zuker CS. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell. 2004;117:527–539. doi: 10.1016/s0092-8674(04)00412-x. [DOI] [PubMed] [Google Scholar]
- 18.Han YG, Kwok BH, Kernan MJ. Intraflagellar transport is required in Drosophila to differentiate sensory cilia but not sperm. Curr Biol. 2003;13:1679–1686. doi: 10.1016/j.cub.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 19.Sarpal R, Todi SV, Sivan-Loukianova E, Shirolikar S, Subramanian N, Raff EC, Erickson JW, Ray K, Eberl DF. Drosophila KAP interacts with the kinesin II motor subunit KLP64D to assemble chordotonal sensory cilia, but not sperm tails. Curr Biol. 2003;13:1687–1696. doi: 10.1016/j.cub.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 20.Phillips DM. Insect sperm: their structure and morphogenesis. J Cell Biol. 1970;44:243–277. doi: 10.1083/jcb.44.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma L, Jarman AP. Dilatory is a Drosophila protein related to AZI1 (CEP131) that is located at the ciliary base and required for cilium formation. J Cell Sci. 2011;124:2622–2630. doi: 10.1242/jcs.084798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enjolras C, Thomas J, Chhin B, Cortier E, Duteyrat JL, Soulavie F, Kernan MJ, Laurencon A, Durand B. Drosophila chibby is required for basal body formation and ciliogenesis but not for Wg signaling. J Cell Biol. 2012;197:313–325. doi: 10.1083/jcb.201109148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker JD, Adhikarakunnathu S, Kernan MJ. Mechanosensory-defective, male-sterile unc mutants identify a novel basal body protein required for ciliogenesis in Drosophila. Development. 2004;131:3411–3422. doi: 10.1242/dev.01229. [DOI] [PubMed] [Google Scholar]
- 24.Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39:715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- 25.Reiter JF, Skarnes WC. Tectonic, a novel regulator of the Hedgehog pathway required for both activation and inhibition. Genes Dev. 2006;20:22–27. doi: 10.1101/gad.1363606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodges ME, Scheumann N, Wickstead B, Langdale JA, Gull K. Reconstructing the evolutionary history of the centriole from protein components. J Cell Sci. 2010;123:1407–1413. doi: 10.1242/jcs.064873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams CL, Winkelbauer ME, Schafer JC, Michaud EJ, Yoder BK. Functional redundancy of the B9 proteins and nephrocystins in Caenorhabditis elegans ciliogenesis. Mol Biol Cell. 2008;19:2154–2168. doi: 10.1091/mbc.E07-10-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kernan M, Cowan D, Zuker C. Genetic dissection of mechanosensory transduction: mechanoreception-defective mutations of Drosophila. Neuron. 1994;12:1195–1206. doi: 10.1016/0896-6273(94)90437-5. [DOI] [PubMed] [Google Scholar]
- 29.Koundakjian EJ, Cowan DM, Hardy RW, Becker AH. The Zuker collection: a resource for the analysis of autosomal gene function in Drosophila melanogaster. Genetics. 2004;167:203–206. doi: 10.1534/genetics.167.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willingham AT, Keil T. A tissue specific cytochrome P450 required for the structure and function of Drosophila sensory organs. Mech Dev. 2004;121:1289–1297. doi: 10.1016/j.mod.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 31.Craige B, Tsao CC, Diener DR, Hou Y, Lechtreck KF, Rosenbaum JL, Witman GB. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J Cell Biol. 2010;190:927–940. doi: 10.1083/jcb.201006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsang WY, Bossard C, Khanna H, Peranen J, Swaroop A, Malhotra V, Dynlacht BD. CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease. Dev Cell. 2008;15:187–197. doi: 10.1016/j.devcel.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riparbelli MG, Callaini G, Megraw TL. Assembly and persistence of primary cilia in dividing Drosophila spermatocytes. Dev Cell. 2012;23:425–432. doi: 10.1016/j.devcel.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breslow DK, Koslover EF, Seydel F, Spakowitz AJ, Nachury MV. An in vitro assay for entry into cilia reveals unique properties of the soluble diffusion barrier. J Cell Biol. 2013;203:129–147. doi: 10.1083/jcb.201212024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang X, Dausend J, Hafner M, Musyanovych A, Rocker C, Landfester K, Mailander V, Nienhaus GU. Specific effects of surface amines on polystyrene nanoparticles in their interactions with mesenchymal stem cells. Biomacromolecules. 2010;11:748–753. doi: 10.1021/bm901348z. [DOI] [PubMed] [Google Scholar]
- 36.Fabian L, Wei HC, Rollins J, Noguchi T, Blankenship JT, Bellamkonda K, Polevoy G, Gervais L, Guichet A, Fuller MT, et al. Phosphatidylinositol 4,5-bisphosphate directs spermatid cell polarity and exocyst localization in Drosophila. Mol Biol Cell. 2010;21:1546–1555. doi: 10.1091/mbc.E09-07-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdallah AM, Zhou X, Kim C, Shah KK, Hogden C, Schoenherr JA, Clemens JC, Chang HC. Activated Cdc42 kinase regulates Dock localization in male germ cells during Drosophila spermatogenesis. Dev Biol. 2013;378:141–153. doi: 10.1016/j.ydbio.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 38.Kwitny S, Klaus AV, Hunnicutt GR. The annulus of the mouse sperm tail is required to establish a membrane diffusion barrier that is engaged during the late steps of spermiogenesis. Biology of reproduction. 2010;82:669–678. doi: 10.1095/biolreprod.109.079566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ihara M, Kinoshita A, Yamada S, Tanaka H, Tanigaki A, Kitano A, Goto M, Okubo K, Nishiyama H, Ogawa O, et al. Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev Cell. 2005;8:343–352. doi: 10.1016/j.devcel.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Wei HC, Rollins J, Fabian L, Hayes M, Polevoy G, Bazinet C, Brill JA. Depletion of plasma membrane PtdIns(4,5)P2 reveals essential roles for phosphoinositides in flagellar biogenesis. J Cell Sci. 2008;121:1076–1084. doi: 10.1242/jcs.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riparbelli MG, Cabrera OA, Callaini G, Megraw TL. Unique properties of Drosophila spermatocyte primary cilia. Biology open. 2013;2:1137–1147. doi: 10.1242/bio.20135355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gottardo M, Callaini G, Riparbelli MG. The cilium-like region of the Drosophila spermatocyte: an emerging flagellum? J Cell Sci. 2013;126:5441–5452. doi: 10.1242/jcs.136523. [DOI] [PubMed] [Google Scholar]
- 43.Colley SM, Wintle L, Searles R, Russell V, Firman RC, Smith S, Deboer K, Merriner DJ, Genevieve B, Bentel JM, et al. Loss of the nuclear receptor corepressor SLIRP compromises male fertility. PLoS One. 2013;8:e70700. doi: 10.1371/journal.pone.0070700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thurm U. An insect mechanoreceptor. I. Fine structure and adequate stimulus. Cold Spring Harb Symp Quant Biol. 1965;30:75–82. doi: 10.1101/sqb.1965.030.01.011. [DOI] [PubMed] [Google Scholar]
- 45.Blachon S, Gopalakrishnan J, Omori Y, Polyanovsky A, Church A, Nicastro D, Malicki J, Avidor-Reiss T. Drosophila asterless and vertebrate Cep152 Are orthologs essential for centriole duplication. Genetics. 2008;180:2081–2094. doi: 10.1534/genetics.108.095141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cenci G, Bonaccorsi S, Pisano C, Verni F, Gatti M. Chromatin and microtubule organization during premeiotic, meiotic and early postmeiotic stages of Drosophila melanogaster spermatogenesis. J Cell Sci. 1994;107(Pt 12):3521–3534. doi: 10.1242/jcs.107.12.3521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.