Abstract
The autonomic nervous system is known to play a significant role in the genesis and persistence of arrhythmias. Neuromodulation has become a new therapeutic strategy for the treatment of ventricular arrhythmias. Catheter-based renal denervation (RDN) is being studied as a treatment option for drug-refractory hypertension. Ablation within the renal arteries, by altering efferent and afferent signaling, has the potential to improve blood pressure, as well as heart failure, atrial, and ventricular tachyarrhythmias. We present a brief review of the anatomic and pathophysiological rationale for RDN as an adjunctive treatment for ventricular tachyarrhythmias.
Introduction
The understanding of the mechanisms and treatment strategies for refractory ventricular tachyarrhythmias (VT) continues to evolve. Pharmacologic therapy for VT has had limited clinical efficacy. The advent of catheter ablation for VT has been of significant benefit to patients with recurrent VT that is resistant to medical therapy. However, intermediate and long-term freedom from VT is limited, particularly in nonischemic cardiomyopathy (NICM) patients. This may be due to the more important role of functional mechanisms of VT in NICM and likely progression of underlying cardiac disease. Furthermore, this could also reflect the limitations of available catheter ablation technology. Specific substrates common to NICM are difficult to successfully ablate, including septal substrates, as well as arrhythmias originating from the mid-myocardium and left ventricular summit. Further, NICM patients have a lower scar burden and a propensity for epicardial scars, with less available substrate for modification during an ablation procedure. In addition, some arrhythmias in NICM may not be scar-related macro-reentrant tachycardias, but focal or microreentrant.
Modulation of regulatory systems (the autonomic nervous system) has been a subject of intense research in the past several years, especially given the current limitations of therapies. There is clear evidence that the autonomic nervous system is a driver of VT [1]. Medications, such as beta-blockers and angiotensin-converting enzyme inhibitors that target the autonomic nervous system, have been shown to reduce incidence of sudden cardiac death. Autonomic modulation provides an adjunctive, and in some cases, alternative treatment modality for the treatment of VT. Autonomic modulation utilizing cardiac sympathetic denervation for the management of VT is not a new concept [2]. Left-sided [3–5] and bilateral [6] cervicothoracic sympathectomy have demonstrated benefit in treatment of patients with refractory VT in the setting of structural heart disease and channelopathies resistant to medical therapy and ablation. Data for spinal cord stimulation are also evolving [7–9]. Spinal cord stimulation, initially thought to mediate its effects via parasympathetic stimulation, may in fact work by increasing parasympathetic and decreasing sympathetic activation [7]. Therefore, neuromodulation as an adjunctive or alternative treatment option is of substantial current interest.
RDN in the context of the symplicity HTN-3 trial
Catheter-based renal denervation (RDN) has gained interest for the treatment of drug-resistant hypertension (HTN) and has been shown in pre-clinical and clinical trials [10] to decrease ambulatory blood pressure in patients with medication-refractory HTN. However, the recently published prospective symplicity HTN-3 trial did not meet its expected pre-specified end points [11], raising questions about the future direction of RDN for the treatment of HTN.
In this trial, Bhatt and colleagues randomized 535 patients in a 2:1 ratio to undergo RDN or a sham procedure with a primary efficacy end point of office systolic blood pressure at 6 months. Systolic blood pressure in the RDN arm decreased by 14.13 ± 23.93 mmHg vs. 11.74 ± 25.94 mmHg in the sham arm, which did not meet statistical significance. While a placebo effect in the sham arm, inadequate ablation, and regression to the mean may have contributed to the results, these findings demonstrate the need for further mechanistic studies.
The mixed results for the treatment of HTN do not necessarily decrease the enthusiasm for RDN as a treatment option in other disorders, such as VT. Our understanding of neurocardiology and the role of autonomic modulation as a treatment strategy for cardiac disorders continue to evolve. In this article, we describe the potential role of RDN as an adjunctive treatment for refractory VT.
Autonomic control of arrhythmias
The autonomic interplay between organ systems is complex, and its study in humans is constrained by the limited ability to measure neuronal firing within the sympathetic ganglia and nerves. To date, much of the human work in this area has involved assessment of neuronal firing rates in skeletal muscle and skin, and the measurement of plasma levels of norepinephrine, as surrogates. Therefore, much of our understanding of interorgan direct neurologic connections and reflexes comes from animal models.
Anatomy and physiology
Renal efferent signaling controls renin secretion [12], intra-renal vascular distribution, and water and sodium retention [13]. Sympathetic stimulation leads to increased renin secretion with increased sodium uptake, vasoconstriction, and activation of the renin–angiotensin–aldosterone (RAAS) system with associated decreased renal blood flow. These effects occur at different frequencies of nerve stimulation [14].
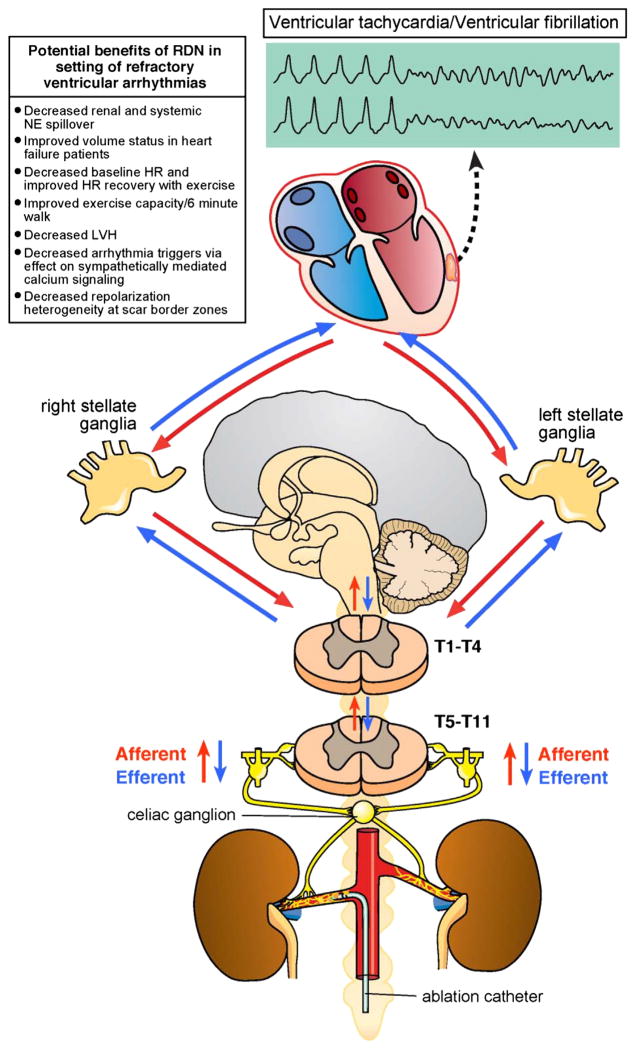
Sensory afferent signals originate from multiple organs and centrifugally reach the central nervous system. These afferent signals sensed by chemo- and mechanoreceptors travel along the sympathetic chain and reach the central nervous system via the dorsal roots ganglia. Renal afferent signals are processed in the central nervous system in the hypothalamus as well as nucleus of the solitary tract, insular cortex, anterior cingulate cortex, and infralimbic cortex based on functional MRI studies [15]. Efferent signals can then activate the sympathetic fibers in the sympathetic chain, inhibit parasympathetic cardio-motor efferents through the vagal nerve, and cause release of catecholamines from the adrenal gland. Cardiac efferent preganglionic neurons exit the T1–T4 ventral rami where they synapse in the sympathetic chain within the cervicothoracic (stellate) and T2–T4 ganglia. Many of the cardiac neuron cell bodies, however, also reside in the middle cervical and superior cervical ganglion. Post-ganglionic fibers arise from the left and right stellate ganglia and form the cardiac nerves that enter the heart at the base, projecting to the atrial and ventricular myocardium in addition to the epicardial ganglia of the intrinsic cardiac nervous system (Fig. 1).
Fig. 1.
Cardio-renal neuraxial pathways of autonomic nerve signaling. Major elements of the cardio-renal neural axis with known and unknown directions of nerve signaling are shown. HR= heart rate; LVH = left ventricular hypertrophy; NE = norepinephrine.
Cardiac benefits of renal autonomic modulation for VT
Cardiac afferent and efferent neural signaling can be altered in pathologic states. Myocardial infarction not only causes denervation of the scar [16–18], supersensitivity to circulating catecholamines [19], and nerve sprouting along the border zones of infarcts [20], but also has global remodeling effects on viable regions of the myocardium distal from the infarct. The heterogenous denervation and up-regulation of beta-adrenergic receptors can lead to an increase in dispersion of repolarization in humans with cardiomyopathy as compared to normal hearts [17], allowing for persistence of VT. Release of catecholamines via activation of renal afferent fibers, therefore, can increase risk of arrhythmias. The benefit of RDN in reducing VT in post-infarct models may in part occur via inhibition of sympathetically mediated repolarization heterogeneity at scar border zones.
Sympathetic nerve activity tends to precede VT [21]. Blockade of cardiac sympathetic stimulation with beta blockers, thoracic epidural anesthesia, and cervicothoracic sympathectomy reduces the burden of VT and the risk of sudden death. By reducing circulating catecholamines, RDN has the potential to reduce the electrical heterogeneity between regions of scarred myocardium that demonstrate denervation supersensitivity to catecholamines and border zone regions with “hyperinnervation” due to nerve sprouts. Further, changes may not be relegated to cardiac structures, with the recent evidence that the stellate ganglia shows remodeling in patients with cardiomyopathy, suggesting the possibility of pathologic neural remodeling due to cardiac disease from the heart [22].
Surgical RDN has been shown to reduce VT during acute ischemia in a porcine model [23]. Linz et al. demonstrated a significant reduction in VF after acute ischemia was induced in animals undergoing RDN versus a sham procedure. Ventricular fibrillation (VF) occurred in 5 of 6 (83%) of the sham but only in 1 of 7 (14%) of the RDN animals during left anterior descending coronary artery occlusion. The reduction in inducible VF was also associated with a reduction in spontaneous PVCs. Monophasic action potential duration during reperfusion was not affected by RDN.
Human data specific to treatment of VT predominantly consist of small case series of patients treated with RDN. Ukena et al. [24] were the first to describe the use of RDN for the treatment of life-threatening VT, reporting their experience in two patients. In this report, one patient had hypertrophic cardiomyopathy with an intramural source of VT refractory to multiple attempts at ablation from both an endocardial and epicardial approach. The second patient had NICM and refused ablation. Both procedures resulted in significant reduction in VT burden. The patient with hypertrophic cardiomyopathy demonstrated a decrease from 594 episodes pre-RDN to 57 episodes in the first week after RDN and finally only a single episode in the 3 weeks that followed. The NICM patient had 28 episodes pre-RDN, which decreased to 12 episodes one-day post-RDN and no further episodes up to 24 weeks post-RDN.
Hoffmann et al. [25] presented a case report of a 63-year-old patient who presented with an ST-elevation myocardial infarction and recurrent monomorphic VT and VF. The patient underwent VT ablation but continued to have recurrent arrhythmias despite medical therapy. The authors utilized the symplicity RDN system (Medtronic Inc., Minneapolis, MN). VT episodes decreased from 1.8 to 0.5 episodes/day after RDN, and the patient had no further VT after day 23 and up to 6 months of follow-up.
Tsioufis et al. [26] reported in abstract form a beneficial effect on atrial and ventricular arrhythmias seen in a series of 14 patients. Ventricular extra-systole burden seen on 24-h Holter monitoring was significantly reduced after RDN. Decreased sympathetic activity may cause a decrease in intracellular calcium transit, and therefore, a decrease in PVC burden, which may be a trigger for sustained VT. Therefore, decreasing PVC burden is the postulated mechanism for the benefit of RDN in this case.
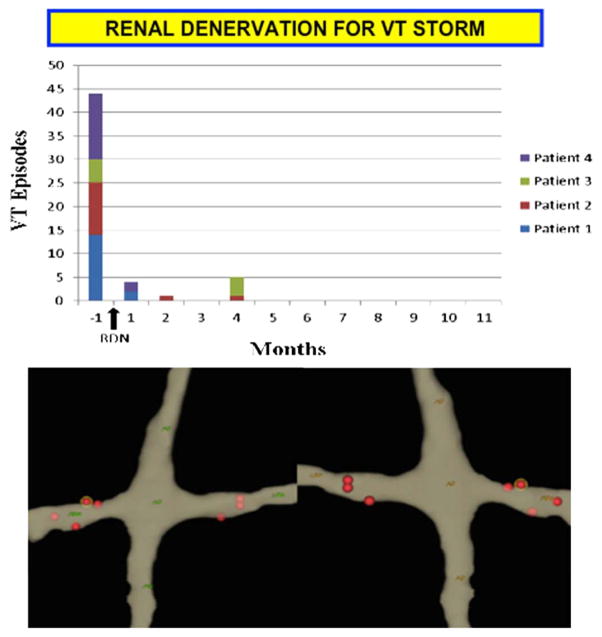
The largest series to date by Remo et al. [27] was a multi-center series of four patients (2 ICM and 2 NICM) that underwent RDN for refractory VT. All patients in this series had failed antiarrhythmic therapy and had undergone endocardial or combined endocardial/epicardial ablation. Both standard open-irrigated (10–12 W for 30–60 s) and non-irrigated (6 W at 50° and 60 s) ablation catheters were used at the discretion of the operator. After a median follow-up of 8.8 ± 2.6 months, all patients in the series had a significant reduction in VT burden. VT burden decreased from 11.0 ± 4.2 episodes per month before RDN to 0.3 ± 0.1 after RDN. There was no difference in effect for the ICM and NICM patients. Recurrent VTs were seen only in the first 4 months after RDN (Fig. 2).
Fig. 2.
Human data demonstrating decreased VT burden after RDN. The upper panel demonstrates the number of VT episodes prior to and after RDN. Time of RDN is denoted by the black arrow. The lower panel demonstrates a 3-D electroanatomic map (NavX, St. Jude, Minneapolis, MN, USA) of the spiral lesions delivered with an open-irrigated ablation catheter. (Reprinted with permission from Remo et al. [27])
Early clinical evidence suggests a possible benefit of RDN as an adjunct treatment option not just for VT but for atrial fibrillation (AFIB) as well [28,29]. Pukushalov et al. randomized 27 patients with AFIB and drug-refractory HTN to pulmonary vein isolation (n = 14) vs. pulmonary vein isolation plus RDN (n = 13). The patients that received the combined pulmonary vein isolation and RDN had a significantly higher freedom from AFIB than those patients that underwent pulmonary vein isolation alone (69% vs. 29%, p = 0.033). This data builds on previous translational studies showing that RDN suppresses AFIB in a sleep apnea model [30] and also provides improved heart rate control while in AFIB [29]. RDN has also shown promise for improvements in glucose metabolism, reduction in left ventricular hypertrophy (LVH), and sleep apnea. These benefits, while not directly antiarrhythmic, may indirectly improve AFIB by improving blood pressure, diabetes, and decreasing LVH, factors that may potentially then decrease left atrial stress and AFIB episodes.
Additional cardio-protective benefits of RDN that may secondarily benefit VT management include decreased baseline heart rate, improved exercise capacity, and heart rate recovery after exercise, as well as potential benefits in heart failure. Ukena et al. [31] demonstrated that in 136 patients undergoing RDN, heart rate decreased both at rest and with exertion and the decrease in heart rate was more pronounced if the patients started with a higher heart rate at baseline. Patients with a baseline heart rate of 60–71 beats per minute (bpm) had a mean decrease of 2.9 bpm, while the patients with a baseline heart rate of ≥71 bpm had a mean decrease of 9.0 bpm. The authors also noted a slight prolongation in PR interval on the resting ECG, possibly suggesting increased vagal control of AV nodal conduction.
Further, there are data showing that exercise capacity as well as heart rate recovery after exercise may improve after RDN [32]. Resting heart rate was modestly decreased as noted in the previous study and peak heart rate did not change, however, the heart rate recovery after exercise improved. A mean improvement of 4 ± 7 bpm was seen in the RDN group.
Increased sympathetic nervous system activity is known to have detrimental effects in patients with heart failure [33]. Overactivation of the sympathetic nervous system in heart failure patients can lead to fluid overload due to increased salt and water absorption, increased arterial constriction, and inotropic/chronotropic stimulation, all of which can worsen VT. RDN has been shown to improve LV function in a rat model after induced myocardial infarction [34]. Hasking et al. [35] described increased norepinephrine spillover due to increased cardio-renal sympathetic nerve activity in heart failure patients, while Davies et al. [36] were the first to demonstrate a possible symptomatic benefit for humans with heart failure undergoing RDN. The majority of the 7 New York Heart Association class III/IV patients enrolled had a decrease in diuretic requirements after RDN with additional improvements in 6-min walk distance. Given the possible role of renal afferents in reducing the effects of atrial natriuretic peptide [37], the decrease in diuretic use and improved functional capacity may have merit.
RDN also has the potential to help patients with diastolic heart failure, often due to poorly controlled HTN. Data suggests a benefit in improving LVH [38] that could be in part related to the associated improvement in blood pressure control. If RDN provides improved volume status, decreased LVH, and improved LV function, it can then indirectly reduce VT.
RDN procedure
Much of the early work on RDN involved a surgical approach. However, as the sympathetic neural fibers of the kidney are within a 2–3-mm distance from the renal artery lumen and surround the renal arteries, a catheter-based denervation approach under fluoroscopic and/or electroanatomic mapping guidance is feasible. Although RDN for resistant hypertension is currently performed predominantly by using specific multi-electrode ablation catheters designed for this purpose, there is evidence that similar end points can be achieved utilizing standard ablation catheters for both hypertension and arrhythmia management (Fig. 3).
Fig. 3.
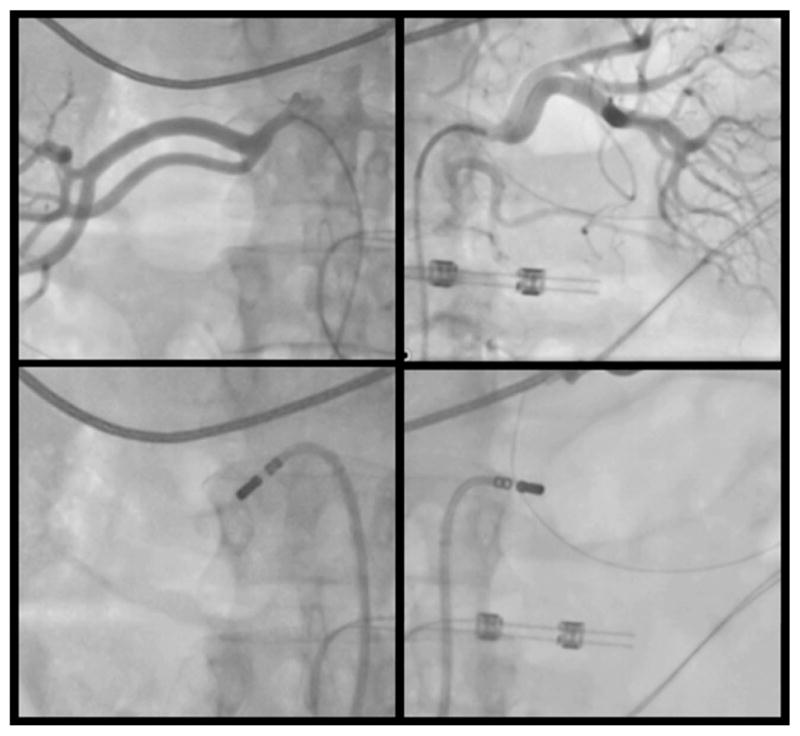
Image of RDN in a patient with refractory VT resistant to catheter ablation. Image of the renal angiogram (upper panel) and an associated position of the ablation catheter at the inferior aspect of the renal artery bifurcation (lower panel) is shown. The ablation catheter in the lower right panel has been directed inferiorly for this lesion and subsequently positioned at a superior location. This was a 63-year-old patient with an ischemic cardiomyopathy following left ventricular assist device and previous ablation who underwent RDN and was VT free for 5 months post-RDN after presenting with VT storm.
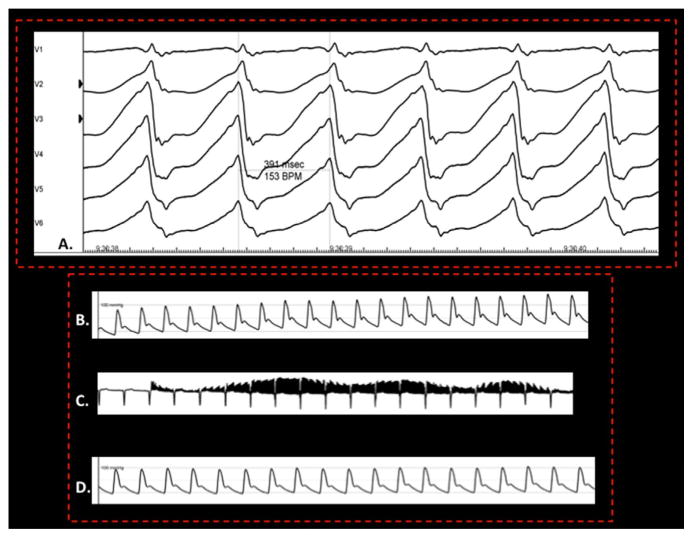
Acute procedural end points for this procedure need further evaluation. High-frequency stimulation (Fig. 4) to assess pre- and post-ablation blood pressure response is a technique that has been used. While this technique has primarily been utilized in assessing sites of ganglionated plexi during mapping and ablation of atrial fibrillation [39], it has also been utilized during RDN [28]. Effective RDN is thought to be associated with a lack of blood pressure rise with high-frequency stimulation. However, correlation of this acute end point with intermediate and long-term success requires further study.
Fig. 4.
Monomorphic ventricular tachycardia (A) resistant to conventional therapy. High-frequency stimulation at 20 Hz/15 V/10 ms (C) was used to demonstrate an initial increase in blood pressure (B) from 88/45 to 125/68 mmHg and a subsequent lack of blood pressure response (D) as a surrogate for successful denervation after RDN.
Potential complications of RDN procedure
Risks of RDN include vascular access complications, such as femoral artery pseudoaneurysm and dissection and direct renal artery damage, including dissection and post-procedure stenosis with the potential for associated renal failure. To date, renal failure as a complication has been exceedingly uncommon. In the symplicity HTN-3 trial, major complications were rare. In this study, one patient (of 352, 0.3%) required intervention for a vascular complication, one patient had documented renal artery stenosis after ablation, and no patients developed new onset end-stage renal disease. These findings were similar to the sham treatment group. A meta-analysis by Davis et al. [40] further supports the low complication rate with an overall procedure complication rate of <1% (included one renal artery dissection and 4 pseudoaneurysms).
Conclusion
Autonomic control of cardiac function and its role in the genesis and persistence of VT is complex. Pathological neural remodeling that alters the sympathetic and parasympathetic input to the heart, and increased circulating catecholamines increase the risk of VT. Neural modulation, by potentially restoring the baseline balance, is an evolving therapeutic option for refractory VT. Interruption of afferent and efferent sympathetic stimuli to the heart has clear benefit in pathologic conditions such as refractory VT. The extent that adjunctive autonomic interventions such as RDN will benefit the management of arrhythmias warrants further investigation, though early studies are promising.
The recent negative results from the symplicity HTN-3 trial have raised a number of issues with regard to the delivery of the therapy itself. There is no good way to “dose” the therapy, and no agreement on technology or end points to titrate therapy. These issues can be sorted out by careful mechanistic studies to define the exact role of RDN in cardiovascular and arrhythmia therapeutics.
Footnotes
The authors have indicated there are no conflicts of interest.
References
- 1.Zipes DP, Rubart M. Neural modulation of cardiac arrhythmias and sudden cardiac death. Heart Rhythm. 2006;3:108–13. doi: 10.1016/j.hrthm.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zipes DP, Festoff B, Schaal SF, Cox C, Sealy WC, Wallace AG. Treatment of ventricular arrhythmia by permanent atrial pacemaker and cardiac sympathectomy. Ann Intern Med. 1968;68:591–7. doi: 10.7326/0003-4819-68-3-591. [DOI] [PubMed] [Google Scholar]
- 3.Bourke T, Vaseghi M, Michowitz Y, Sankhla V, Shah M, Swapna N, et al. Neuraxial modulation for refractory ventricular arrhythmias: value of thoracic epidural anesthesia and surgical left cardiac sympathetic denervation. Circulation. 2010;121:2255–62. doi: 10.1161/CIRCULATIONAHA.109.929703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz PJ, Locati EH, Moss AJ, Crampton RS, Trazzi R, Ruberti U. Left cardiac sympathetic denervation in the therapy of congenital long qt syndrome. A worldwide report. Circulation. 1991;84:503–11. doi: 10.1161/01.cir.84.2.503. [DOI] [PubMed] [Google Scholar]
- 5.Collura CA, Johnson JN, Moir C, Ackerman MJ. Left cardiac sympathetic denervation for the treatment of long qt syndrome and catecholaminergic polymorphic ventricular tachycardia using video-assisted thoracic surgery. Heart Rhythm. 2009;6:752–9. doi: 10.1016/j.hrthm.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 6.Vaseghi M, Gima J, Kanaan C, Ajijola OA, Marmureanu A, Mahajan A, et al. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and long-term follow-up. Heart Rhythm. 2014;11:360–6. doi: 10.1016/j.hrthm.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Issa ZF, Zhou X, Ujhelyi MR, Rosenberger J, Bhakta D, Groh WJ, et al. Thoracic spinal cord stimulation reduces the risk of ischemic ventricular arrhythmias in a postinfarction heart failure canine model. Circulation. 2005;111:3217–20. doi: 10.1161/CIRCULATIONAHA.104.507897. [DOI] [PubMed] [Google Scholar]
- 8.Lopshire JC, Zhou X, Dusa C, Ueyama T, Rosenberger J, Courtney N, et al. Spinal cord stimulation improves ventricular function and reduces ventricular arrhythmias in a canine postinfarction heart failure model. Circulation. 2009;120:286–94. doi: 10.1161/CIRCULATIONAHA.108.812412. [DOI] [PubMed] [Google Scholar]
- 9.Odenstedt J, Linderoth B, Bergfeldt L, Ekre O, Grip L, Mannheimer C, et al. Spinal cord stimulation effects on myocardial ischemia, infarct size, ventricular arrhythmia, and noninvasive electrophysiology in a porcine ischemia-reperfusion model. Heart Rhythm. 2011;8:892–8. doi: 10.1016/j.hrthm.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 10.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the symplicity htn-2 trial): a randomised controlled trial. Lancet. 2010;376:1903–9. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393–401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 12.Zanchetti AS. Neural regulation of renin release: experimental evidence and clinical implications in arterial hypertension. Circulation. 1977;56:691–8. doi: 10.1161/01.cir.56.5.691. [DOI] [PubMed] [Google Scholar]
- 13.Bell-Reuss E, Trevino DL, Gottschalk CW. Effect of renal sympathetic nerve stimulation on proximal water and sodium reabsorption. J Clin Investig. 1976;57:1104–7. doi: 10.1172/JCI108355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiBona GF. Dynamic analysis of patterns of renal sympathetic nerve activity: implications for renal function. Exp Physiol. 2005;90:159–61. doi: 10.1113/expphysiol.2004.029215. [DOI] [PubMed] [Google Scholar]
- 15.Cechetto DF. Cortical control of the autonomic nervous system. Exp Physiol. 2014;99:326–31. doi: 10.1113/expphysiol.2013.075192. [DOI] [PubMed] [Google Scholar]
- 16.Barber MJ, Mueller TM, Henry DP, Felten SY, Zipes DP. Transmural myocardial infarction in the dog produces sympathectomy in noninfarcted myocardium. Circulation. 1983;67:787–96. doi: 10.1161/01.cir.67.4.787. [DOI] [PubMed] [Google Scholar]
- 17.Vaseghi M, Lux RL, Mahajan A, Shivkumar K. Sympathetic stimulation increases dispersion of repolarization in humans with myocardial infarction. Am J Physiol Heart Circ Physiol. 2012;302:H1838–46. doi: 10.1152/ajpheart.01106.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fallavollita JA, Heavey BM, Luisi, Michalek SM, Baldwa S, Mashtare, et al. Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J Am Coll Cardiol. 2014;63:141–9. doi: 10.1016/j.jacc.2013.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kammerling JJ, Green FJ, Watanabe AM, Inoue H, Barber MJ, Henry DP, et al. Denervation supersensitivity of refractoriness in noninfarcted areas apical to transmural myocardial infarction. Circulation. 1987;76:383–93. doi: 10.1161/01.cir.76.2.383. [DOI] [PubMed] [Google Scholar]
- 20.Cao JM, Chen LS, KenKnight BH, Ohara T, Lee MH, Tsai J, et al. Nerve sprouting and sudden cardiac death. Circ Res. 2000;86:816–21. doi: 10.1161/01.res.86.7.816. [DOI] [PubMed] [Google Scholar]
- 21.Zhou S, Jung BC, Tan AY, Trang VQ, Gholmieh G, Han SW, et al. Spontaneous stellate ganglion nerve activity and ventricular arrhythmia in a canine model of sudden death. Heart Rhythm. 2008;5:131–9. doi: 10.1016/j.hrthm.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Ajijola OA, Wisco JJ, Lambert HW, Mahajan A, Stark E, Fishbein MC, et al. Extracardiac neural remodeling in humans with cardiomyopathy. Circulation. 2012;5:1010–116. doi: 10.1161/CIRCEP.112.972836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linz D, Wirth K, Ukena C, Mahfoud F, Poss J, Linz B, et al. Renal denervation suppresses ventricular arrhythmias during acute ventricular ischemia in pigs. Heart Rhythm. 2013;10:1525–30. doi: 10.1016/j.hrthm.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Ukena C, Bauer A, Mahfoud F, Schreieck J, Neuberger HR, Eick C, et al. Renal sympathetic denervation for treatment of electrical storm: first-in-man experience. Clin Res Cardiol. 2012;101:63–7. doi: 10.1007/s00392-011-0365-5. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann BA, Steven D, Willems S, Sydow K. Renal sympathetic denervation as an adjunct to catheter ablation for the treatment of ventricular electrical storm in the setting of acute myocardial infarction. J Cardiovasc Electrophysiol. 2013;24:E21. doi: 10.1111/jce.12282. [DOI] [PubMed] [Google Scholar]
- 26.Tsioufis KP, Tsiachris V, Dimitriadis D, Thomopoulos K, Kefala C, Antonakis A, et al. Renal sympathetic denervation significantly reduces mean heart rate and exerts a favorable effect on atrial and ventricular arrhythmias in resistant hypertesives. J Am Coll Cardiol. 2013;61:E1376. [Google Scholar]
- 27.Remo BF, Preminger M, Bradfield J, Mittal S, Boyle N, Gupta A, et al. Safety and efficacy of renal denervation as a novel treatment of ventricular tachycardia storm in patients with cardiomyopathy. Heart Rhythm. 2014;11(4):541–6. doi: 10.1016/j.hrthm.2013.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Baranova V, Turov A, et al. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol. 2012;60:1163–70. doi: 10.1016/j.jacc.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 29.Linz D, Mahfoud F, Schotten U, Ukena C, Hohl M, Neuberger HR, et al. Renal sympathetic denervation provides ventricular rate control but does not prevent atrial electrical remodeling during atrial fibrillation. Hypertension. 2013;61:225–31. doi: 10.1161/HYPERTENSIONAHA.111.00182. [DOI] [PubMed] [Google Scholar]
- 30.Linz D, Mahfoud F, Schotten U, Ukena C, Neuberger HR, Wirth K, et al. Renal sympathetic denervation suppresses postapneic blood pressure rises and atrial fibrillation in a model for sleep apnea. Hypertension. 2012;60:172–8. doi: 10.1161/HYPERTENSIONAHA.112.191965. [DOI] [PubMed] [Google Scholar]
- 31.Ukena C, Mahfoud F, Spies A, Kindermann I, Linz D, Cremers B, et al. Effects of renal sympathetic denervation on heart rate and atrioventricular conduction in patients with resistant hypertension. Int J Cardiol. 2013;167:2846–51. doi: 10.1016/j.ijcard.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 32.Ukena C, Mahfoud F, Kindermann I, Barth C, Lenski M, Kindermann M, et al. Cardiorespiratory response to exercise after renal sympathetic denervation in patients with resistant hypertension. J Am Coll Cardiol. 2011;58:1176–82. doi: 10.1016/j.jacc.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 33.Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol. 1995;26:1257–63. doi: 10.1016/0735-1097(95)00332-0. [DOI] [PubMed] [Google Scholar]
- 34.Nozawa T, Igawa A, Fujii N, Kato B, Yoshida N, Asanoi H, et al. Effects of long-term renal sympathetic denervation on heart failure after myocardial infarction in rats. Heart vessels. 2002;16:51–6. doi: 10.1007/s380-002-8317-8. [DOI] [PubMed] [Google Scholar]
- 35.Hasking GJ, Esler MD, Jennings GL, Burton D, Johns JA, Korner PI. Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation. 1986;73:615–21. doi: 10.1161/01.cir.73.4.615. [DOI] [PubMed] [Google Scholar]
- 36.Davies JE, Manisty CH, Petraco R, Barron AJ, Unsworth B, Mayet J, et al. First-in-man safety evaluation of renal denervation for chronic systolic heart failure: primary outcome from reach-pilot study. Int J Cardiol. 2013;162:189–92. doi: 10.1016/j.ijcard.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 37.Pettersson A, Hedner J, Hedner T. Renal interaction between sympathetic activity and anp in rats with chronic ischaemic heart failure. Acta Physiol Scand. 1989;135:487–92. doi: 10.1111/j.1748-1716.1989.tb08607.x. [DOI] [PubMed] [Google Scholar]
- 38.Brandt MC, Mahfoud F, Reda S, Schirmer SH, Erdmann E, Bohm M, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012;59:901–9. doi: 10.1016/j.jacc.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 39.Po SS, Nakagawa H, Jackman WM. Localization of left atrial ganglionated plexi in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:1186–9. doi: 10.1111/j.1540-8167.2009.01515.x. [DOI] [PubMed] [Google Scholar]
- 40.Davis MI, Filion KB, Zhang D, Eisenberg MJ, Afilalo J, Schiffrin EL, et al. Effectiveness of renal denervation therapy for resistant hypertension: a systematic review and meta-analysis. J Am Coll Cardiol. 2013;62:231–41. doi: 10.1016/j.jacc.2013.04.010. [DOI] [PubMed] [Google Scholar]