Abstract
Human studies have shown that adolescents who repeatedly use alcohol are more likely to be dependent on alcohol and are more likely to suffer from psychological problems later in life. There has been limited research examining how ethanol exposure in adolescence might contribute to later abuse or addiction in adulthood. The present experiment examined effects of intermittent ethanol exposure during adolescence on sign-tracking behavior in adulthood, indexed by a Pavlovian conditioned approach (PCA) task wherein an 8-s lever presentation served as a cue predicting subsequent delivery of a flavored food pellet. Although no response was required for food delivery, after multiple pairings, 1 of 2 different responses often emerged during the lever presentation: goal tracking (head entries into the food trough) or sign tracking (engagement with the lever when presented). Sign tracking is thought to reflect the attribution of incentive salience to reward-paired cues and has been previously correlated with addiction-like behaviors. Following the last PCA session, blood samples were collected for analysis of post-session corticosterone levels. Sixty-two rats (n = 10–12/group) were pseudo-randomly assigned to 1 of 2 intragastric (i.g.) exposure groups (water or 4 g/kg ethanol) or a non-manipulated (NM) control group. Animals were intubated with ethanol or water every other session from postnatal session (PND) 28–48 or PND 70–90. Rats were then tested in adulthood (PND 71–79 or PND 113–122) on the PCA task. Animals exposed chronically to ethanol during adolescence exhibited significantly higher levels of sign-tracking behavior in adulthood than NM and water-treated animals, and showed higher corticosterone than NM control animals. These effects were not seen after comparable ethanol exposure in adulthood. These results suggest that adolescent alcohol exposure has long-term consequences on the expression of potential addiction-relevant behaviors in adulthood.
Keywords: adolescence, ethanol, rat, sign tracking, goal tracking
Introduction
Adolescence is a developmental transition characterized by increases in social interactions with peers, elevated levels of risk taking, impulsivity, and frequent initiation of alcohol and other drug use, along with other behavioral and physiological changes (Doremus-Fitzwater, Barreto, & Spear, 2012; Johnston, O’Malley, Bachman, & Schulenberg, 2012). Alcohol is the most widely used drug during this time, with approximately 42% of 12th graders reporting use within the past 30 days (Johnston, O’Malley, Bachman, & Schulenberg, 2013). Further, human adolescents show higher levels of binge drinking than their adult counterparts, with levels per occasion of ethanol use in adolescence approximately 2 times higher than in adulthood (SAMHSA, 2013). Studies with animal models likewise typically report similar increases of ethanol use in adolescence (Spear, 2013; Vetter, Doremus-Fitzwater, & Spear, 2007; Vetter-O’Hagen, Varlinskaya, & Spear, 2009). Elevations in adolescent drinking may have long-term consequences. Adolescents diagnosed with alcohol disorders show cognitive impairments (Brown, Tapert, Tate, & Abrantes, 2000), and adolescent drinkers are more likely to develop alcohol dependence (Grant & Dawson, 1997). From these kinds of studies alone, however, it is not possible to attribute causality of effects to alcohol exposure per se, and as such, animal studies have proved useful for investigating whether repeated exposure to alcohol during adolescence induces long-lasting consequences that are evident in adulthood.
An increasing body of literature to date has investigated the impact of adolescent intermittent alcohol exposure (AIE) on adult behaviors, with some evidence emerging that AIE may have long-term repercussions. For example, cognitive deficits (Coleman, He, Lee, Styner, & Crews, 2011; Pascual, Blanco, Cauli, Miñarro, & Guerri, 2007) and increased alcohol intake under certain circumstances (Broadwater & Spear, 2013; Matthews, Tinsley, Diaz-Granados, Tokunaga, & Silvers, 2008) have been seen in adulthood after AIE. Yet, the data are mixed with, for instance, reports of increased ethanol consumption, contrasting with other findings of decreased ethanol consumption in adulthood after AIE (Gilpin, Karanikas, & Richardson, 2012). Few of the studies to date in this emerging literature have included adult exposure groups to determine whether or not observed consequences are specific to ethanol exposure during adolescence. Those studies that have included adults generally suggest age-specificity of effects (Fleming, Acheson, Moore, Wilson, & Swartzwelder, 2012; Pascual, Boix, Felipo, & Guerri, 2009).
One little-investigated area to date is the impact of AIE on future addiction vulnerabilities. Among the theories of drug addiction that may provide insight into predispositions for elevated drinking is the Incentive Sensitization Theory, which postulates a dichotomy among potential processes contributing to addiction between “wanting” and “liking”, with each having different underlying neural substrates (Robinson & Berridge, 1993). “Liking” can be thought of as the hedonic value of a reward (Robinson & Berridge, 2003), and is thought to require opioid activation in the nucleus accumbens (NAc) (Kelley & Berridge, 2002; Peciña & Berridge, 2000). In contrast, “wanting” reflects motivation for a drug reward, and is more closely associated with dopamine release within the NAc (Robinson & Berridge, 2003). It is thought that during the development of addiction, “liking” of a drug may not change, whereas “wanting” of a drug may increase with the development of incentive sensitization. Further, a cue for a reward, such as an addictive substance, can be imbued with incentive salience, thereby contributing to cue-related relapse (Berridge & Robinson, 2003).
“Wanting”/incentive salience vs. hedonic “liking” has been explored in rats using Pavlovian-conditioned approach (PCA). PCA is an auto-shaping procedure in which a conditioned stimulus (CS; such as a lever) is repeatedly paired with an unconditioned stimulus (US; such as a food reward). Immediate engagement with the CS when it is presented is termed sign tracking (ST), and is thought to reflect the incentive salience of the reward cue. While some animals develop ST during PCA, others will preferentially attend to the US delivery area (i.e., food trough) during CS presentation, a behavior termed goal tracking (GT). Emergence of ST is thought to reflect the motivational property of the reward, and has the potential to develop into “pathological” incentive salience (Flagel, Akil, & Robinson, 2009; Robinson & Berridge, 2003). Sign tracking has also been linked to addiction-like behaviors (Tomie, Grimes, & Pohorecky, 2008) and can be induced by drugs of abuse (Doremus-Fitzwater & Spear, 2011; Uslaner, Acerbo, Jones, & Robinson, 2006). Our lab has previously shown that sign tracking in the PCA task seen in adulthood is sensitive to adolescent stress exposure (Anderson & Spear, 2011).
The purpose of experiment (Exp.) 1 was to determine the effect of adolescent ethanol exposure on later sign-tracking behavior in adulthood. The purpose of Exp. 2 was to investigate whether effects observed in Exp. 1 were age-dependent by exposing adults to comparable amounts of ethanol and testing them later in adulthood. Our hypothesis was that repeated alcohol exposure in adolescence, but not adulthood, would increase later propensity for sign tracking, perhaps reflecting an increase in later addiction vulnerability via exacerbation of the incentive salience animals assign to cues predicting rewards.
Methods
Subjects
Male Sprague-Dawley rats reared and raised in our colony in Binghamton University were used in the present experiments. On the day after birth, postnatal day (P)1, litters were culled to 8–10 pups, with a ratio of 6 males to 4 females preferred. Pups remained with their mothers until P21, at which point animals were pair-housed with same-sex littermates. Animals were maintained in a temperature-controlled vivarium with a 12:12 h light-dark cycle (lights on at 0700), and food (Purina rat chow, Lowell, MA) and water available ad libitum. All animals were maintained and treated in accordance with the Guide for the Care and Use of Laboratory Animals established by the National Institutes of Health, using protocols approved by the Binghamton University Institutional Animal Care and Use Committee.
Exposure (n = 62, 10–12/group)
Animals were exposed to ethanol as adolescents (Exp. 1) or as adults (Exp. 2). Animals were pair-housed with non-littermates on P25 (Exp. 1) or P65 (Exp. 2) to allow assignment of housing partners to the same experimental group while avoiding potential litter effects. Animals were pseudo-randomly assigned to 1 of 3 exposure groups: ethanol, water, or non-manipulated (NM). Aside from routine cage maintenance, non-manipulated animals were only disturbed on P28 and P48 (Exp. 1) or P70 and P90 (Exp. 2) for weighing. From P28–48 (Exp. 1) or P70–90 (Exp. 2), ethanol- and water-treated animals were weighed and given their assigned exposure of either 4 g/kg (25% v/v) ethanol, or an equivalent volume of water intragastrically between 1100 and 1300 h every other day for a total of 11 intubations. This dose has been found to produce initial intoxication ratings in the slight (adolescents) to moderate (adult rats) range (Broadwater & Spear, 2013) and peak blood ethanol levels (BECs) of approximately 200 mg/dL (unpublished observations). Although above the threshold for binge drinking (i.e., > 80 mg/dL), these ethanol levels are well within the range of those reported in underage drinkers and in young drinkers of legal age in recent field studies (Day, Celio, Lisman, Johansen, & Spear, 2013). Operant training began 22 days after the end of the exposure period (i.e., P70 in Exp. 1; P113 in Exp. 2).
Apparatus
Eight operant chambers (30.5 × 24.1 × 21 cm, Med Associates, # ENV-016M) housed within sound-attenuating boxes (55.9 × 38 × 35.6 cm) were used. The chambers consisted of 3 metal-paneled walls with removable inserts. On one wall, 2 retractable, illuminated levers (4.8 cm wide; # ENV-112CML) flanked a food trough, all of which were mounted 3 cm above the floor. Levers were illuminated only while being presented during the task. Photosensors in the food trough counted head entries into the trough. Lever presses and trough entries were measured during the 8 s between conditioned stimulus (CS) presentation (the lever), and reward delivery. Each reward consisted of delivery of a single 45 mg dustless precision banana-flavored pellet (Bio-Serv, Frenchtown, NJ).
Procedure
Pellet exposure (P69; P112)
On either P69 (Exp. 1) or P112 (Exp. 2), animals were given approximately 7 g of banana-flavored pellets in their home cages in order to reduce potential neophobia.
Pre-training (P70–71; P113–114)
For 2 days, animals were placed into the operant box with levers retracted during the entire session. Banana pellets were delivered on a variable interval (VI) 90-s schedule during their pre-training period. Each session lasted approximately 35 min.
Pavlovian conditioned approach (P72–79; P115–122)
Following pre-training, animals were tested on Pavlovian conditioned approach (PCA). Each of the 8 daily sessions consisted of 25 trials. Each trial consisted of an 8-s presentation of a single illuminated lever (counter-balanced over animals between a left and right lever). At the start of each session the house light was illuminated. After a VI of 90 s had passed, the lever was presented and illuminated. Eight seconds later, the lever was retracted and the lever light extinguished. Immediately thereafter, the food trough was illuminated and a food pellet was delivered into the food trough. The VI was then begun anew for a total of 25 lever presentations per session. Following the last trial, the house light was extinguished and the lever remained retracted, and each animal was removed from the operant chamber and returned to its home cage.
Stress hormone collection
Immediately following the last session of PCA, animals were decapitated, and trunk blood was collected for assessments of corticosterone (CORT) and progesterone (PROG). All blood samples were collected in heparinized tubes. Following collection, blood samples were centrifuged at 2 °C for 20 min a t 3000 rpm. Plasma samples were then stored in a −80 °C freezer until analysis. COR T levels were determined using radioimmunoassay (RIA) kits using competitive binding tritium from MP Biomedicals, Inc. with 100% specificity for rat CORT. PROG was assessed via RIA using I125 double antibody kits from MP Biomedicals (Solon, OH), with a specificity of 100% for rat PROG.
Data analysis
Four measures were collected during PCA: lever presses, nosepokes into the trough, CS trials, and unconditioned stimulus (US) trials. The numbers of lever presses during the 8-s intervals between lever presentation and reward delivery for each trial were summed across each daily session. Nosepokes into the food trough were likewise summed across the 25 trials for each day’s session. CS trials were defined as the number of trials in which the animal displayed sign-tracking behavior (i.e., a lever press) alone or prior to any goal-tracking behavior (a nosepoke). US trials consisted of trials where the animal displayed goal-tracking behavior alone or prior to any sign-tracking behavior. As there were only 25 trials per session, animals could only show a maximum of 25 CS or US trials. PCA data for both experiments were analyzed using analyses of variance (ANOVA). All significant effects were further analyzed using Tukey’s HSD post hoc tests. For hormone data, 1-way ANOVAs were conducted.
A PCA score was calculated and given to each animal, based on previous autoshaping procedures (Flagel et al., 2011). In short, a score was calculated for the average of the last 2 sessions by using the following formula: (Response Bias + Probability + Contact Latency)/3. Response bias was defined as: (amount of lever presses − nosepokes)/(lever presses + nosepokes). Probability was calculated as: lever press probability – nosepoke probability. Contact latency was determined using the formula: −(lever press latency − magazine entry latency)/8 s. The final score could range from −1.0 to 1.0, with an animal scoring −1.0 to −0.31 being labeled a goal tracker, −0.3 to 0.3 unpaired, or .031 to 1.0 a sign tracker.
Results
Experiment 1 (adolescent exposure)
Weight gain during exposure
Weight data for ethanol- or water-exposed animals were subjected to a 2 (ethanol- or water-exposed) × 11 (exposure days) repeated-measures ANOVA, which revealed a trend of group [F(1,18) = 3.520, p = .077], along with a significant effect of day [F(10,180) = 3998.672, p < .0001], as all animals continued to gain weight throughout exposure. Weight data were also analyzed including the NM animals on the first and last day of exposure (P28 and P48) using a 3 (ethanol-exposed, water-exposed, or NM animals) × 2 (exposure days) repeated-measures ANOVA. This ANOVA revealed a significant main effect of group [F(2,29) = 3.75, p = .035], as well as a significant interaction of group and exposure day [F(2,29) = 13.80, p < .001]. Tukey’s test was used to investigate the interaction between group and exposure day, revealing slight but significantly lower body weights among ethanol-exposed animals (264.1 ± 5.1) than water-exposed animals (283.4 ± 3.99; p = .005), as well as NM animals (284.67 ± 3.65; p = .002) on the last exposure day.
PCA
Sign tracking
Lever pressing data were subjected to a 3 (treatment) × 8 (session) repeated-measures ANOVA, which revealed significant main effects of group [F(2,29) = 5.139, p = .012] and session [F(7,203) = 6.764, p < .001] (see Fig. 1A). Tukey’s tests on the data collapsed across session revealed that ethanol-exposed animals had significantly higher lever pressing than each of the other groups (water-exposed: p = .03; NM: p = .02). Lever pressing also increased across session [F(7, 203) = 6.76, p < .0001]. The ANOVA of the CS trial data (Fig. 1B) similarly revealed significant main effects of group [F(2,29) = 6.469, p = .005] and session [F(7,203) = 10.168, p < .001]. Once again, ethanol-exposed animals exhibited significantly more CS trials than water-exposed (p = .02) and NM animals (p = .006), with the number of CS trials increasing across sessions [F(7,203) = 10.1677, p < .0001].
Figure 1.
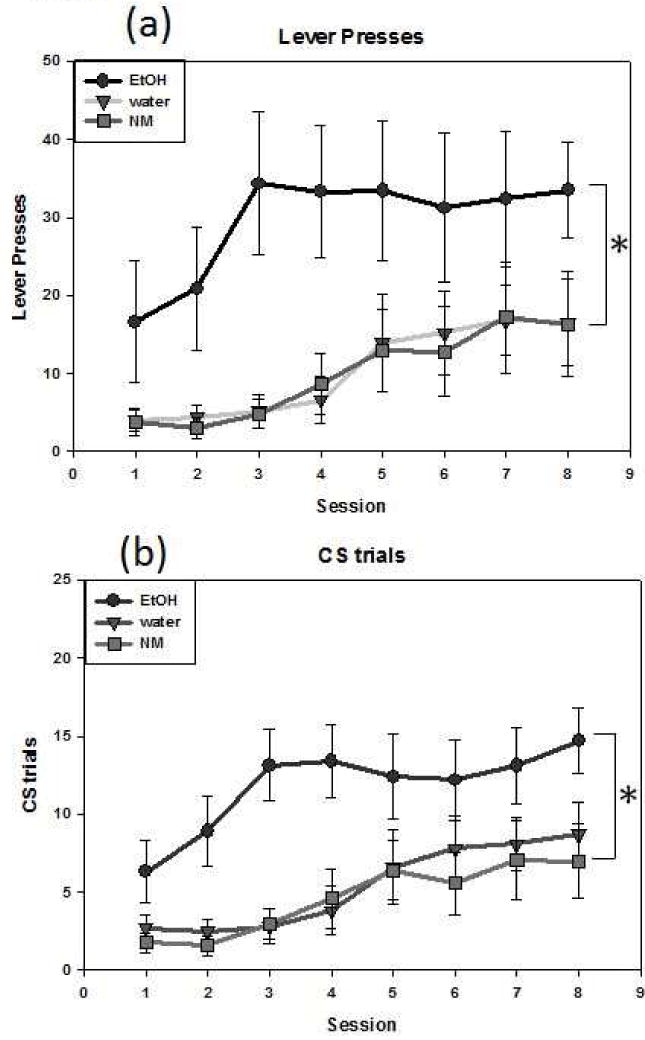
Sign-tracking behavior in adult animals exposed during adolescence as indexed by lever presses (a) and CS trials (b). Asterisks (*) denote significant differences between ethanol-exposed animals and water-exposed or NM animals. Data are expressed as mean ± SEM.
Goal tracking
The ANOVA of the nosepoking data (Fig. 2A) revealed that nosepokes generally increased across session [F(7,203) = 2.30, p = .03], with ethanol-exposed animals tending to exhibit fewer nosepokes than NM or water-exposed animals [F(2,29) = 2.805, p = .08]. The analysis of US trials per session (Fig. 2B) revealed significant main effects of group [F(2,29) = 4.18, p = .025] and session [F(7,203) = 2.30, p = .03]. The number of US trials was significantly higher among water-exposed animals than ethanol-exposed animals (p = .02).
Figure 2.
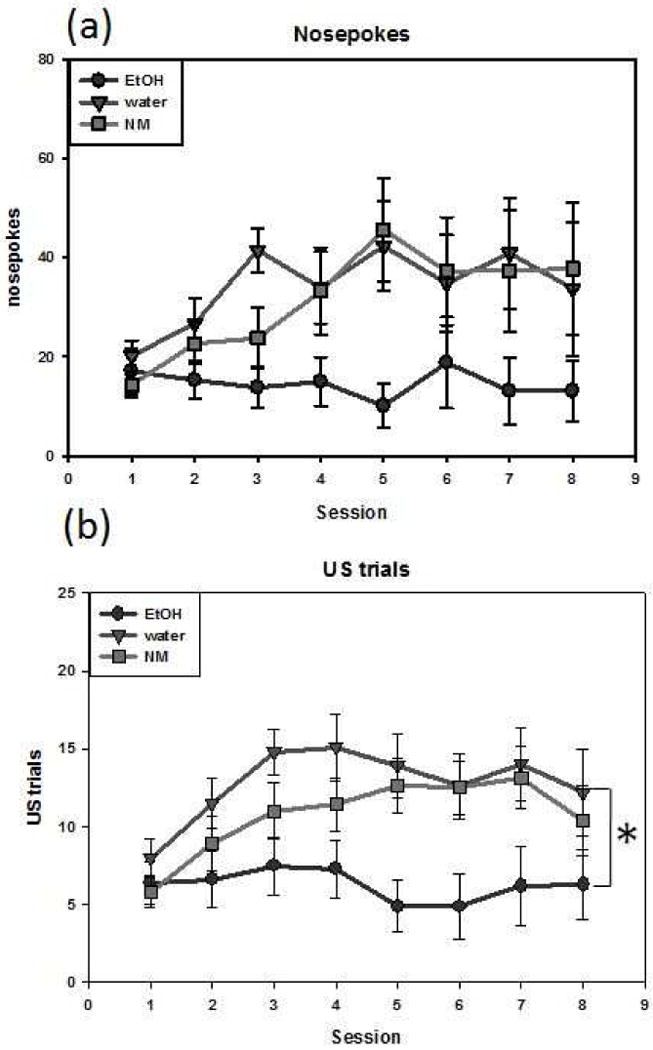
Goal-tracking behavior in adult animals exposed during adolescence as indexed by nosepokes (a) and US trials (b). The asterisk (*) denotes a significant difference in number of US trials between water- and ethanol-exposed groups. A similar trend with nosepoking did not reach significance. Data are expressed as mean ± SEM.
PCA score
A majority of the ethanol group was characterized as sign trackers in contrast to only 20% of the water-exposed and NM animals. Most water-exposed animals were found to be either unpaired or goal-trackers, whereas a majority of the NM animals were classified as goal trackers (see Table 2).
Table 2.
Number of animals of each phenotype expressed as a percentage of animals within an exposure group.
| PCA Scores | ||||
|---|---|---|---|---|
| ethanol | water | NM | ||
| Adolescents | Sign trackers | 60% | 10% | 25% |
| Unpaired | 20% | 50% | 8.3% | |
| Goal trackers | 20% | 40% | 66.7% | |
| Adults | Sign trackers | 30% | 40% | 50% |
| Unpaired | 30% | 10% | 10% | |
| Goal trackers | 40% | 50% | 40% | |
Hormone data
A 1-way ANOVA conducted on the post-session adolescent hormone data revealed a main effect of group only for the corticosterone data (Table 1) [F(2,25) = 4.392, p = .023], with a significant difference between ethanol-exposed and NM animals. A similar analysis of the progesterone data yielded a trend toward a significant group effect.
Table 1.
Levels of corticosterone and progesterone in blood following the last PCA session for both adolescent-exposed and adult-exposed animals. The asterisk (*) denotes a significant difference between animals in the ethanol-exposed and NM groups. Data are expressed as mean ± SEM.
| CORT | PROG | |||
|---|---|---|---|---|
| Age | Age | |||
| Exposure | Adolescent | Adult | Adolescent | Adult |
| ethanol | 132.63 ± 26.33* | 90.83 ± 26.27 | 1.98 ± 0.52 | 2.08 ± 0.40 |
| water | 106.88 ± 11.20 | 68.80 ± 13.99 | 1.94 ± 0.46 | 2.35 ± 0.29 |
| NM | 65.00 ± 12.37 | 93.57 ± 26.02 | 0.96 ± 0.26 | 2.43 ± 0.20 |
Experiment 2 (adult-exposed)
Weight gain during exposure
No significant effect emerged in the ANOVA of the weight data between ethanol-exposed and water-exposed animals [F(1,18) = .016, p = .89]. Both groups gained weight across the exposure period [F(10,180) = 68.950, p < .0001]. Weight data were also analyzed with NM animals on the first and last day of exposure (P28 and P48) using a 3 (ethanol-exposed, water-exposed, or NM animals) × 2 (exposure days) repeated-measures ANOVA, which revealed a significant interaction of group and exposure day [F(2,23) = 5.784, p = .009]. Tukey’s post hoc analyses, however, revealed no significant differences among the exposure groups on either assessment day, with means (± SEM) of 439.67 (± 16.43), 441.8 (± 11.95), and 444.7 (± 5.76) on the last exposure data for the ethanol, water, and NM groups, respectively. All groups showed significant gains in body weight across days.
PCA
Sign tracking
The lever pressing and CS trial data were subjected to separate repeated-measures ANOVAs with each revealing only a significant main effect of session, [F(7,175) = 9.188, p < .001], [F(7,175) = 11.976, p < .001], respectively. Both measures of sign tracking increased across sessions (see Fig. 3A and B).
Figure 3.
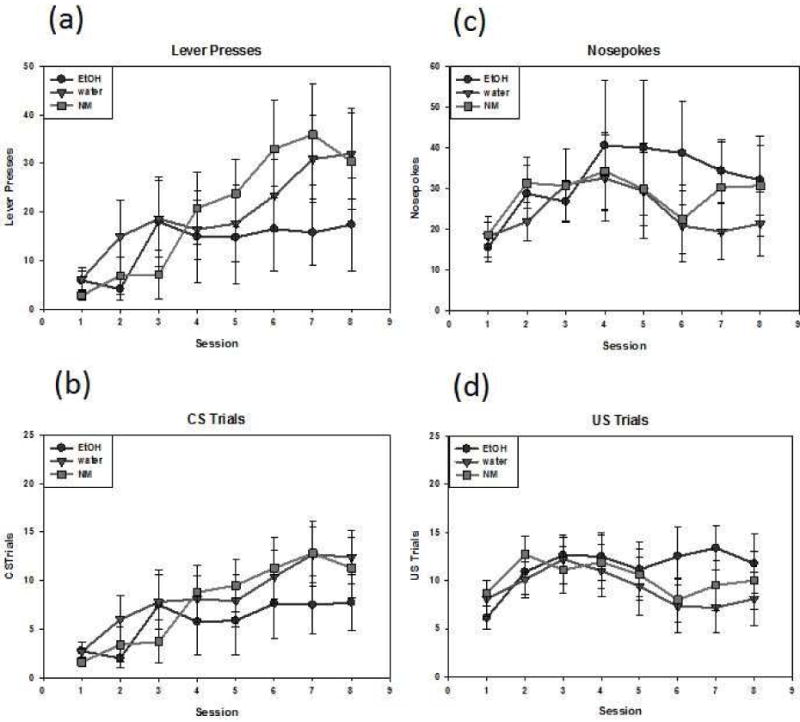
Sign-tracking and goal-tracking behavior among animals exposed as adults as indexed by lever presses (a), CS trials (b), nosepokes (c), and US trials (d). Data are expressed as mean ± SEM.
Goal tracking
No significant effects emerged in the ANOVAs of the nosepoke and US trial data, although there was a trend [F(7,175) = 1.938, p = .0662] for nosepokes to increase across session, whereas the analysis of the US trial data revealed no significant effects (see Fig. 3C and D).
Hormone data
No main effect of group emerged in the ANOVA of either the CORT or PROG data (see Table 1).
PCA score
Animals exposed to ethanol in adulthood showed nearly equal preference for sign tracking, goal tracking, and unpaired behavior during the final 2 sessions. Few animals in the water-exposed and NM groups were classified as unpaired, with most animals either being sign trackers or goal trackers (see Table 2).
Discussion
Animals exposed to ethanol during adolescence and tested in a Pavlovian conditioning paradigm beginning 3 weeks thereafter showed significantly more sign-tracking behavior than did water-exposed and NM control animals, along with significantly higher post-session CORT levels than NM animals. With goal-tracking behavior, an opposite pattern was seen, with both water-exposed and NM controls exhibiting significantly higher goal tracking than ethanol-exposed animals. In contrast, no differences across condition were seen in sign tracking, goal tracking, or postsession CORT levels when the chronic exposure period was delayed until adulthood. These data taken into conjunction suggest that the elevation in sign-tracking behavior seen weeks after termination of ethanol exposure is specific to ethanol exposure during adolescence. Given that sign tracking has previously been linked to behaviors often associated with drug abuse, including impulsivity (Flagel, Watson, Akil, & Robinson, 2008; Flagel, Watson, Robinson, & Akil, 2007; Tomie, Aguado, Pohorecky, & Benjamin, 1998), psychomotor sensitization (Tomie et al., 2008; Tomie, Wong, Apor, Patterson-Buckendahl, & Pohorecky, 2003), impoverished environments (Beckmann & Bardo, 2012; Gipson, Beckmann, El-Maraghi, Marusich, & Bardo, 2011; Lomanowska et al., 2011), and relapse-related behaviors such as reinstatement (Tomie, Kuo, Apor, Salomon, & Pohorecky, 2004), these data support the suggestion that intermittent exposure to ethanol during adolescence may increase later predisposition for the emergence of behaviors conducive to potential drug abuse. It should be noted that no pseudoconditioning control was present in these experiments, in which the CS and US are explicitly unpaired throughout a session. This could be important, as intermittent exposure to a lever may cause the rat to engage with the lever (Tomie, Cunha, et al., 1998). However, a previous study using a PCA task found that while pseudoconditioning can develop, levels of lever presses during a session remain low (Tomie, Cunha, et al., 1998).
This adolescent-specific effect adds to a growing list of findings of persistent effects of adolescent, but not adult, ethanol exposure. For example, Ehlers, Criado, Wills, Liu, & Crews (2011) found an increase in locomotor activity and a decrease in cholinergic neurons in Ch1–4 nuclei of the basal forebrain after adolescent ethanol exposure. There is also evidence of neuroinflammation and myelin disruption within the prefrontal cortex after chronic adolescent ethanol exposure in rats (Bava, Jacobus, Thayer, & Tapert, 2013; Pascual, Pla, Miñarro, & Guerri, 2013). This is consistent with previous research showing inflammatory brain damage and behavioral alterations caused by adolescent ethanol exposure (Pascual et al., 2007; Vallés, Blanco, Pascual, & Guerri, 2004). There is also evidence of changes in the glutamatergic system within the brain after exposure to ethanol. Ethanol acts in a non-competitive manner on NMDA receptors, and paired with an increase in GABA-mediated inhibitory tone, may cause long-lasting plasticity changes within the brain that help precipitate addiction (Chandler, 2003). These results in conjunction suggest that there are numerous brain and behavioral changes seen following adolescent ethanol exposure, which collectively could increase propensity for the development of later ethanol abuse and addiction.
Neural contributors to the selective increase in sign tracking after adolescent (but not adult) ethanol exposure have yet to be explored. Alterations in the dopamine system are a possibility, given that increases in brain dopamine activity have been associated with increases in the motivation for a drug or food reward, when indexed via lever pressing for a reward (Berridge & Robinson, 1998). There is also evidence for notable changes in the brain’s dopaminergic systems during adolescence, with the basal rate of firing in the striatum being decreased in adolescents relative to adults, although the adolescents are able to release more dopamine when challenged (Laviola, Pascucci, & Pieretti, 2001). Receptors also undergo developmental changes through adolescence, with post mortem human adolescents showing significantly higher levels of D1 and D2 receptors in the striatum compared to their adult counterparts (Seeman et al., 1987). This, in conjunction with the fact that drug use onset coincides with these changes (Johnston et al., 2013), raises the possibility that normal development of reward systems during adolescence may be altered by the initiation of alcohol and drug use.
Significantly higher post-session CORT levels were found in adults after adolescent ethanol exposure than in NM adults. A similar, albeit non-significant, tendency was seen with progesterone, another presumptive stress-related hormone (Auger & Forbes-Lorman, 2008). These CORT-related findings are consistent with previous work that has shown associations between CORT and sign tracking, with, for instance, adrenalectomies found to reduce sign-tracking performance (Thomas & Papini, 2001). Indeed, sign tracking has been reported to increase corticosterone levels, first reported by Tomie, di Poce, Derenzo, & Pohorecky (2002). The same group then showed increases in post-session CORT levels when the CS was linked to a rewarding stimulus, as opposed to stimuli that were presented at random (Tomie et al., 2003). Even more telling was the finding that animals that had elevated CORT levels following the first session (during which significant group differences in sign tracking had yet to emerge) showed greater sign tracking on later trials (Tomie et al., 2002), suggesting that elevations in CORT may have preceded the acquisition of sign-tracking behavior. Results of the present study, however, do not support a simple direct relationship between heightened CORT and sign tracking.
Adolescent water- and ethanol-exposed animals did not differ significantly in post-session CORT levels, although these groups differed significantly in levels of sign tracking. It is possible that ethanol given via gavage may be more stressful than water given via gavage during the exposure period, with those differences perhaps contributing to the elevations in ST seen during PCA testing among adolescent ethanol-exposed animals. Ethanol itself is well known to increase CORT levels (e.g., Willey, Anderson, Morales, Ramirez, & Spear, 2012), and hence it is likely that ethanol-exposed animals may have been exposed to greater CORT levels during the exposure period than animals receiving water, with CORT levels elevated in both of these groups relative to NM controls. Although CORT is but one measure of the HPA axis, it is possible that differential stress levels across groups during the adolescent exposure period may have contributed to differences in ST and post-session CORT levels observed in the present study. Without careful assessment of stress levels during the exposure period and at test using assessments of CORT as well as other measures of HPA activation and action, it is difficult to draw conclusions about the nature of the potential relationship between exposure-related stress and performance/CORT levels at the time of PCA testing.
It should be noted that, to avoid additional perturbation of the animals, BECs were not collected during the exposure phase of the study. Thus, it is possible that age differences in BEC levels produced during the exposure period may have been present across exposure ages, potentially contributing to the effects observed. In previous work examining age differences in the time course of BECs following acute ethanol, adolescents were observed to generally have significantly lower ethanol levels than adults following challenge with 1.5 g/kg ethanol intraperitoneally (i.p.) (Willey et al., 2012). Similar age differences may be evident with the higher i.g. dose used in the present study, given that i.g. administration generally produces about half the BEC as does i.p. administration at the same dose (Livy, Parnell, & West, 2003). To the extent that a similar age difference would be seen following the i.g. dose used here, the lower ethanol burden during adolescence than evident in adulthood would have been associated with more pronounced effects (i.e., elevated sign tracking) later in life, thereby arguing for an even more marked sensitivity of adolescents than adults to this long-term consequence of ethanol exposure. It also should be noted that greater consequences of adolescent than adult ethanol exposure were also observed in work using a vapor inhalation protocol that produced similar exposure BECs across age (e.g., Diaz-Granados & Graham, 2007). Thus, the greater sensitivity of adolescents than adults to the lasting effects of ethanol does not appear to be related to greater ethanol burdens during adolescence, and indeed is apparent even when exposure BECs are equated across age or perhaps even lower than those seen in adulthood.
Adolescence has previously been characterized as a period of heightened risk to develop a problem with substance abuse and addiction (Brown & Tapert, 2004; Oesterle, Hill, Hawkins, & Abbott, 2008). The present results add to the emerging list of findings suggesting that ethanol exposure during adolescence, but not comparable exposure in adulthood, may exert lasting neurobehavioral consequences. Given prior associations of sign tracking with other addiction-related behaviors, the present results are consistent with the suggestion that these consequences may include an increased propensity for the later emergence of abuse disorders. Much remains to be discerned, however, regarding the generality of these findings and their neural substrates.
Acknowledgments
The research presented in this paper was supported by NIH grant UO1 AA019972.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson RI, Spear LP. Autoshaping in adolescence enhances sign-tracking behavior in adulthood: impact on ethanol consumption. Pharmacology, Biochemistry, and Behavior. 2011;98:250–260. doi: 10.1016/j.pbb.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger CJ, Forbes-Lorman RM. Progestin receptor-mediated reduction of anxiety-like behavior in male rats. PLoS One. 2008;3:e3606. doi: 10.1371/journal.pone.0003606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Thayer RE, Tapert SF. Longitudinal changes in white matter integrity among adolescent substance users. Alcoholism: Clinical and Experimental Research. 2013;37(Suppl 1):E181–189. doi: 10.1111/j.1530-0277.2012.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Bardo MT. Environmental enrichment reduces attribution of incentive salience to a food-associated stimulus. Behavioural Brain Research. 2012;226:331–334. doi: 10.1016/j.bbr.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research. Brain Research Reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends in Neurosciences. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Broadwater MA, Spear LP. Tone conditioning potentiates rather than overshadows context fear in adult animals following adolescent ethanol exposure. Developmental Psychobiology. 2013;56:1150–1155. doi: 10.1002/dev.21186. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF. Adolescence and the trajectory of alcohol use: basic to clinical studies. Annals of the New York Academy of Sciences. 2004;1021:234–244. doi: 10.1196/annals.1308.028. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Tate SR, Abrantes AM. The role of alcohol in adolescent relapse and outcome. Journal of Psychoactive Drugs. 2000;32:107–115. doi: 10.1080/02791072.2000.10400216. [DOI] [PubMed] [Google Scholar]
- Chandler LJ. Ethanol and brain plasticity: receptors and molecular networks of the postsynaptic density as targets of ethanol. Pharmacology & Therapeutics. 2003;99:311–326. doi: 10.1016/s0163-7258(03)00096-2. [DOI] [PubMed] [Google Scholar]
- Coleman LG, Jr, He J, Lee J, Styner M, Crews FT. Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcoholism: Clinical and Experimental Research. 2011;35:671–688. doi: 10.1111/j.1530-0277.2010.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day AM, Celio MA, Lisman SA, Johansen GE, Spear LP. Acute and chronic effects of alcohol on trail making test performance among underage drinkers in a field setting. Journal of Studies on Alcohol and Drugs. 2013;74:635–641. doi: 10.15288/jsad.2013.74.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Granados JL, Graham DL. The effects of continuous and intermittent ethanol exposure in adolesence on the aversive properties of ethanol during adulthood. Alcoholism: Clinical and Experimental Research. 2007;31:2020–2027. doi: 10.1111/j.1530-0277.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Barreto M, Spear LP. Age-related differences in impulsivity among adolescent and adult Sprague-Dawley rats. Behavioral Neuroscience. 2012;126:735–741. doi: 10.1037/a0029697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Amphetamine-induced incentive sensitization of sign-tracking behavior in adolescent and adult female rats. Behavioral Neuroscience. 2011;125:661–667. doi: 10.1037/a0023763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR, Wills DN, Liu W, Crews FT. Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: correlation with behavioral pathology. Neuroscience. 2011;199:333–345. doi: 10.1016/j.neuroscience.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56(Suppl 1):139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Cameron CM, Pickup KN, Watson SJ, Akil H, Robinson TE. A food predictive cue must be attributed with incentive salience for it to induce c-fos mRNA expression in cortico-striatal-thalamic brain regions. Neuroscience. 2011;196:80–96. doi: 10.1016/j.neuroscience.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Behavioural Brain Research. 2008;186:48–56. doi: 10.1016/j.bbr.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology. 2007;191:599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- Fleming RL, Acheson SK, Moore SD, Wilson WA, Swartzwelder HS. In the rat, chronic intermittent ethanol exposure during adolescence alters the ethanol sensitivity of tonic inhibition in adulthood. Alcoholism: Clinical and Experimental Research. 2012;36:279–285. doi: 10.1111/j.1530-0277.2011.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Karanikas CA, Richardson HN. Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PLoS One. 2012;7:e31466. doi: 10.1371/journal.pone.0031466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Beckmann JS, El-Maraghi S, Marusich JA, Bardo MT. Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology. 2011;214:557–566. doi: 10.1007/s00213-010-2060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of Substance Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future: National results on adolescent drug use; Overview of key findings, 2011. Ann Arbor: Institute for Social Research, The University of Michigan; 2012. [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future: National results on drug use: 2012 Overview, Key Findings on Adolescent Drug Use. Ann Arbor: Institute for Social Research, The University of Michigan; 2013. [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. The Journal of Neuroscience. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Pascucci T, Pieretti S. Striatal dopamine sensitization to D-amphetamine in periadolescent but not in adult rats. Pharmacology, Biochemistry, and Behavior. 2001;68:115–124. doi: 10.1016/s0091-3057(00)00430-5. [DOI] [PubMed] [Google Scholar]
- Livy DJ, Parnell SE, West JR. Blood ethanol concentration profiles: a comparison between rats and mice. Alcohol. 2003;29:165–171. doi: 10.1016/s0741-8329(03)00025-9. [DOI] [PubMed] [Google Scholar]
- Lomanowska AM, Lovic V, Rankine MJ, Mooney SJ, Robinson TE, Kraemer GW. Inadequate early social experience increases the incentive salience of reward-related cues in adulthood. Behavioural Brain Research. 2011;220:91–99. doi: 10.1016/j.bbr.2011.01.033. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Tinsley KL, Diaz-Granados JL, Tokunaga S, Silvers JM. Chronic intermittent exposure to ethanol during adolescence produces tolerance to the hypnotic effects of ethanol in male rats: a dose-dependent analysis. Alcohol. 2008;42:617–621. doi: 10.1016/j.alcohol.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Oesterle S, Hill KG, Hawkins JD, Abbott RD. Positive functioning and alcohol-use disorders from adolescence to young adulthood. Journal of Studies on Alcohol and Drugs. 2008;69:100–111. doi: 10.15288/jsad.2008.69.100. [DOI] [PubMed] [Google Scholar]
- Pascual M, Blanco AM, Cauli O, Miñarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. The European Journal of Neuroscience. 2007;25:541–550. doi: 10.1111/j.1460-9568.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. Journal of Neurochemistry. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Pascual M, Pla A, Miñarro J, Guerri C. Neuroimmune activation and myelin changes in adolescent rats exposed to high-dose alcohol and associated cognitive dysfunction: a review with reference to human adolescent drinking. Alcohol and Alcoholism. 2013;49:187–192. doi: 10.1093/alcalc/agt164. [DOI] [PubMed] [Google Scholar]
- Peciña S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: map based on microinjection Fos plumes. Brain Research. 2000;863:71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annual Review of Psychology. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Seeman P, Bzowej NH, Guan HC, Bergeron C, Becker LE, Reynolds GP, et al. Human brain dopamine receptors in children and aging adults. Synapse. 1987;1:399–404. doi: 10.1002/syn.890010503. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescents and alcohol: acute sensitivities, enhanced intake, and later consequences. Neurotoxicology and Teratology. 2013;41:51–59. doi: 10.1016/j.ntt.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. (NSDUH Series H-46, HHS Publication No. (SMA) 13-4795). [Google Scholar]
- Thomas BL, Papini MR. Adrenalectomy eliminates the extinction spike in autoshaping with rats. Physiology & Behavior. 2001;72:543–547. doi: 10.1016/s0031-9384(00)00448-0. [DOI] [PubMed] [Google Scholar]
- Tomie A, Aguado AS, Pohorecky LA, Benjamin D. Ethanol induces impulsive-like responding in a delay-of-reward operant choice procedure: impulsivity predicts autoshaping. Psychopharmacology. 1998;139:376–382. doi: 10.1007/s002130050728. [DOI] [PubMed] [Google Scholar]
- Tomie A, Cunha C, Mosakowski EM, Quartarolo NM, Pohorecky LA, Benjamin D. Effects of ethanol on Pavlovian autoshaping in rats. Psychopharmacology. 1998;139:154–159. doi: 10.1007/s002130050700. [DOI] [PubMed] [Google Scholar]
- Tomie A, di Poce J, Derenzo CC, Pohorecky LA. Autoshaping of ethanol drinking: an animal model of binge drinking. Alcohol and Alcoholism. 2002;37:138–146. doi: 10.1093/alcalc/37.2.138. [DOI] [PubMed] [Google Scholar]
- Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Research Reviews. 2008;58:121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A, Kuo T, Apor KR, Salomon KE, Pohorecky LA. Autoshaping induces ethanol drinking in nondeprived rats: evidence of long-term retention but no induction of ethanol preference. Pharmacology, Biochemistry, and Behavior. 2004;77:797–804. doi: 10.1016/j.pbb.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Tomie A, Wong K, Apor K, Patterson-Buckendahl P, Pohorecky LA. Autoshaping of ethanol drinking in rats: effects of ethanol concentration and trial spacing. Alcohol. 2003;31:125–135. doi: 10.1016/j.alcohol.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Acerbo MJ, Jones SA, Robinson TE. The attribution of incentive salience to a stimulus that signals an intravenous injection of cocaine. Behavioural Brain Research. 2006;169:320–324. doi: 10.1016/j.bbr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Vallés SL, Blanco AM, Pascual M, Guerri C. Chronic ethanol treatment enhances inflammatory mediators and cell death in the brain and in astrocytes. Brain Pathology. 2004;14:365–371. doi: 10.1111/j.1750-3639.2004.tb00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcoholism: Clinical and Experimental Research. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol and Alcoholism. 2009;44:547–554. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey AR, Anderson RI, Morales M, Ramirez RL, Spear LP. Effects of ethanol administration on corticosterone levels in adolescent and adult rats. Alcohol. 2012;46:29–36. doi: 10.1016/j.alcohol.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


