SUMMARY
Central serotonin (5-HT) modulates somatosensory transduction, but how it achieves sensory modality-specific modulation remains unclear. Here we report that enhancing serotonergic tone via administration of 5-hydroxytryptophan potentiates itch sensation, whereas mice lacking 5-HT or serotonergic neurons in the brainstem exhibit markedly reduced scratching behavior. Through pharmacological and behavioral screening, we identified 5-HT1A as a key receptor in facilitating gastrin-releasing peptide (GRP)-dependent scratching behavior. Co-activation of 5-HT1A and GRP receptors (GRPR) greatly potentiates subthreshold, GRP-induced Ca2+ transients and action potential firing of GRPR+ neurons. Immunostaining, biochemical and biophysical studies suggest that 5-HT1A and GRPR may function as receptor heteromeric complexes. Furthermore, 5-HT1A blockade significantly attenuates, whereas its activation contributes to, long-lasting itch transmission. Thus, our studies demonstrate that the descending 5-HT system facilitates GRP-GRPR signaling via 5-HT1A to augment itch-specific outputs and a disruption of crosstalk between 5-HT1A and GRPR may be a useful anti-pruritic strategy.
Introduction
Somatosensory integration and transduction in the spinal cord and the trigeminal brainstem are subject to monoaminergic modulation. As an integral part of an animal’s adaptive response to an ever-changing environment, facilitatory and inhibitory modulation of somatosensory neural circuits is crucial for the maintenance of homeostasis. Monoaminergic modulation of neural circuits serves an important role in regulating behavioral responses to somatosensory inputs. One notable example is that an array of monoamine neuromodulators produced by supraspinal regions is important for the modulation of nociceptive transmission (Basbaum and Fields, 1984; Suzuki et al., 2004). Given the fundamental role of central modulation in sensory transduction, one can presume that itch sensation is similarly modulated at several levels. Imaging studies in human subjects indicated that pain-mediated inhibition of itch is associated with an activity level of the midbrain regions such as periaqueductal gray matter (PAG), an area known for pain modulation (Mochizuki et al., 2003). Experiments in humans also indicated that noxious counterirritants reduce itch sensation (Murray and Weaver, 1975; Ward et al., 1996; Yosipovitch et al., 2007). Interruption of the upper cervical spinal cord of rodents attenuated dry skin-induced itch, suggesting an involvement of the descending spinal pathway in itch responses (Akiyama et al., 2011).
G protein-coupled receptors (GPCRs), or seven trans-membrane proteins, constitute a large repertoire of cellular sensors required for transducing sensory signals from the skin to the brain (Jeffry et al., 2011; Julius and Nathans, 2012). Gastrin-releasing peptide receptor (GRPR) is a Gq protein-coupled receptor that belongs to the mammalian bombesin receptor family (Jensen et al., 2008; Kroog et al., 1995). GRPR is expressed in laminae I-II of spinal cord neurons and has an important role in the transmission of pruritogenic information (Liu et al., 2011; O‧Donohue et al., 1984; Sun and Chen, 2007; Sun et al., 2009). Gastrin-releasing peptide (GRP), an endogenous neuropeptide for itch, is expressed in a subset of dorsal root ganglion (DRG) and trigeminal ganglion (TG) cells and mediates GRPR activation in the spinal cord (Sun and Chen, 2007; Takanami et al., 2014; Zhao et al., 2013b). While confusion has arisen about GRP expression in DRG neurons, the issue has recently been clarified (Liu et al., 2014; Takanami et al., 2014; Zhao et al., 2013b; Zhao et al., 2014). The GRP-GRPR pathway is primarily engaged in transducing nonhistaminergic acute itch sensation, and may play a relatively minor role in histaminergic itch (Akiyama et al., 2013; Sun et al., 2009; Zhao et al., 2014). Enhanced GRP and GRPR expression is positively correlated with the intensity of chronic itch manifested by increased scratching bouts of animals (Nattkemper et al., 2013; Tominaga et al., 2009; Zhao et al., 2013b). Conversely, loss of GRPR or GRP markedly reduces chronic itch (Lagerstrom et al., 2010; Zhao et al., 2013b), demonstrating the importance of GRPR in the development and maintenance of chronic itch. Despite these studies, molecular mechanisms by which the function of GRPR is modulated remain elusive.
Serotonergic neurons reside principally in the brainstem raphe nuclei and modulate a myriad of behavioral and physiological functions through descending and ascending pathways (Barnes and Sharp, 1999; Dillon et al., 2004). Serotonergic neurons in the rostral ventromedial medulla (RVM), including nucleus raphe magnus (NRM) and surrounding reticular formation, project to both the trigeminal nucleus caudalis (SpVc) and the dorsal horn of the spinal cord and modulate nociceptive transmission in a bidirectional fashion (Basbaum and Fields, 1984; Millan, 2002; Ossipov et al., 2010; Suzuki et al., 2004). In contrast to numerous studies of serotonergic modulation of pain, little is known about the involvement of central 5-HT and receptor mechanisms in itch modulation. In this study, we tested the hypothesis that 5-HT signaling has a pivotal role in itch modulation.
RESULTS
Central Serotonin Facilitates Itch Transmission Elicited by Chloroquine
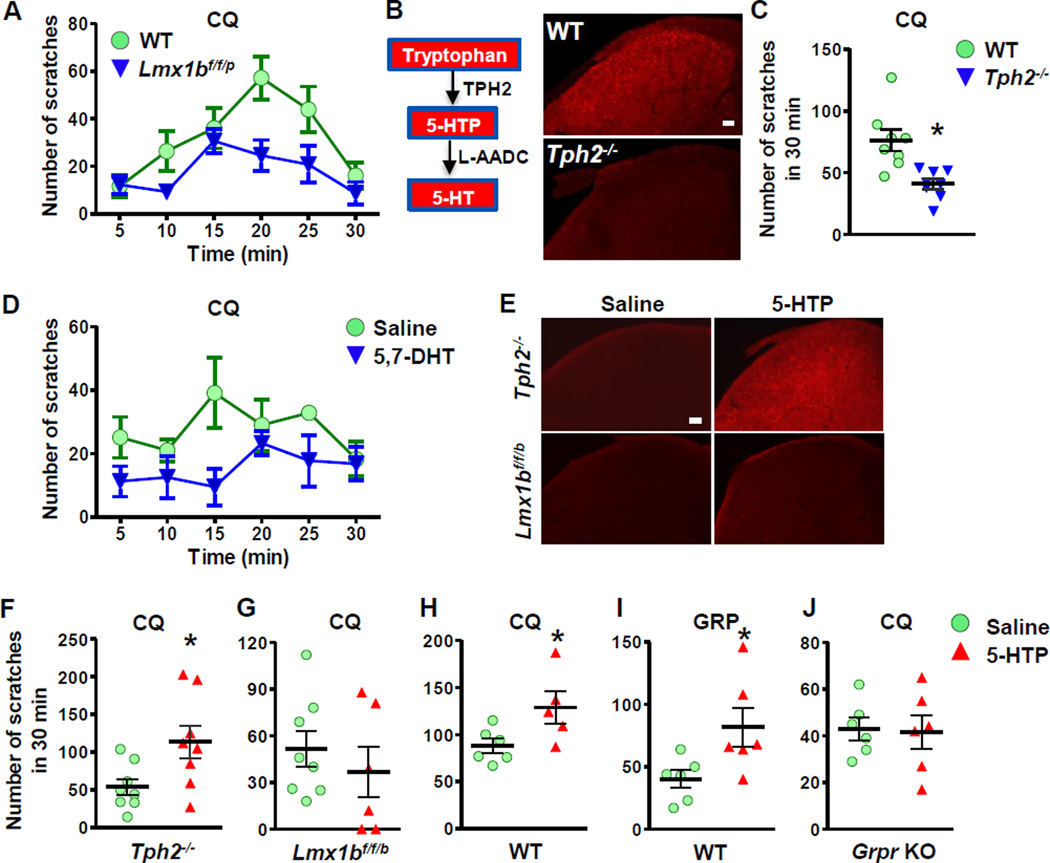
To evaluate the involvement of the central serotonergic system in modulation of itch transmission, we first examined the scratching behavior of Lmx1bf/f/p mice to intradermal injection (i.d.) of chloroquine (CQ), a representative nonhistaminergic pruritogen that acts, in part, via a Mas-related GPCR (MrgprA3) in sensory neurons and GRPR in the spinal cord (Liu et al., 2009; Sun and Chen, 2007). LMX1B is a LIM homeodomain-containing transcription factor that is required for the development of all central 5-HT neurons (Ding et al., 2003). We previously generated conditional Lmx1bf/f/p mice in which Lmx1b is conditionally ablated in Pet1-expressing serotonergic neurons in the raphe nuclei. As a result, all central 5-HT neurons of Lmx1bf/f/p mice were ablated due to lack of Lmx1b (Zhao et al., 2006). However, these mice survive and exhibit normal motor function and thus represent a unique genetic model for investigating the role of central 5-HT neurons in diverse physiological functions (Zhao et al., 2007a; Zhao et al., 2007b). Compared with wild-type (WT) mice, CQ-evoked scratching responses were significantly reduced in Lmx1bf/f/p mice (Figure 1A), demonstrating that central 5-HT neurons exert a constitutive tone for positively modulating itch transmission. To determine whether the decrease was specifically due to a loss of 5-HT or whether other factors originating in central 5-HT neurons were involved, we generated mice lacking tryptophan hydroxylase 2 (Tph2) that encodes the rate limiting enzyme for 5-HT synthesis in the brain (Walther et al., 2003) using gene targeting strategy (Figure 1B). Tph2−/− mice showed no expression of Tph2 and 5-HT in the central nervous system but maintained normal presumptive 5-HT neurons (Figure 1B) (Kim et al., 2014; Liu et al., 2011). Tph2−/− mice displayed a significant reduction in scratching response after i.d. injection of CQ in nape models (Figure 1C), recapitulating the phenotype of Lmx1bf/f/p mice. Since 5-HT signaling in the brain and the spinal cord may contribute differentially to the modulation of itch sensation, next we asked whether impaired itch sensation observed in Lmx1bf/f/p mice and Tph2−/− mice may be ascribed to a blockage of the descending 5-HT pathway. We selectively degenerated spinal 5-HT+ terminals in the spinal cord by an intrathecal injection (i.t.) of 5,7-dihydroxytryptamine (5,7-DHT) following pretreatment with desipramine to prevent the transport of 5,7-DHT into noradrenergic and dopaminergic terminals (Bjorklund et al., 1975). Two weeks after 5,7-DHT injection when spinal 5-HT+ fibers were depleted (Figure S1A), mice exhibited an attenuated scratching response to CQ (Figure 1D), mimicking the phenotype of Lmx1bf/f/p mice and Tph2−/− mice. These loss-of-function studies confirm the contribution of descending 5-HT signaling to the modulation of itch sensation.
Figure 1. Central Serotonin Facilitates Itch Transmission.
(A) Lmx1bf/f/p mice showed deficits in CQ-induced scratching behavior (p < 0.05).
(B) Diagram showing synthesis of 5-HT in the brain. IHC images show that spinal 5-HT was not detectable in Tph2−/− mice.
(C) Tph2−/− mice exhibited attenuated CQ-induced scratching responses.
(D) CQ-induced scratching responses were attenuated after 5,7-DHT injection in C57Bl/6J mice (p < 0.05).
(E) Injection of 5-HTP (10 mg/kg, i.p.) for 60 min restored dorsal spinal 5-HT in Tph2−/− mice, but not in Lmx1bf/f/p mice.
(F and G) Injection of 5-HTP for 30 min rescued CQ-induced scratching behavior in Tph2−/− mice (F) but not in Lmx1bf/f/p mice (G).
(H-J) Injection of 5-HTP facilitated scratching behaviors induced by CQ (H) and GRP (I) in C57Bl/6J mice, while Grpr KO mice did not respond to 5-HTP injection (J).
Error bars represent SEM. *p < 0.05, versus WT (C) or saline (F-J), by un-paired t test in C, F-J or Two-way repeated measures ANOVA in A, D. n = 6–9. Scale bars: 100 µm. See also Figure S1.
To complement the loss-of-function approaches, we next determined whether we could rescue the attenuated itch transmission of Tph2−/− mice and Lmx1bf/f/p mice using an intraperitoneal (i.p.) injection of the 5-HT precursor, 5-hydroxytryptophan (5-HTP), to boost 5-HT tone. Exogenous 5-HTP can be decarboxylated to 5-HT by aromatic L-amino acid decarboxylase (AADC), thereby by-passing the need for TPH2 for 5-HT synthesis (Figure 1B) (Birdsall, 1998). Indeed, immunohistochemical staining (IHC) revealed abundant 5-HT staining in the spinal cord of Tph2−/− mice after 5-HTP injection (Figure 1E, top panel). In contrast, Lmx1bf/f/p mice treated with 5-HTP failed to produce 5-HT in the brain (Figure 1E, lower row). High performance liquid chromatography (HPLC) analysis also revealed a significant increase in 5-HT concentrations and its metabolite, 5-hydroxyindoleacetic acid (5-HIAA), in the brain and spinal cord of Tph2−/− and wild-type mice treated with 5-HTP (Figure S1B-S1E). These studies demonstrate a successful conversion of 5-HT from 5-HTP in the absence of TPH2 and that 5-HT synthesis is critically dependent on 5-HT neurons which express AADC. We next examined scratching responses of Tph2−/− mice after 5-HTP injection and found that 5-HTP injection indeed restored normal scratching responses of Tph2−/− mice to CQ (Figure 1F). However, the same treatment failed to enhance CQ-elicited scratching in Lmx1bf/f/p mice (Figure 1G). This finding complements the results from the 5,7-DHT lesion study suggesting that 5-HT is important in the facilitation of CQ-induced itch and supports the notion that AADC in 5-HT neurons is required for catalyzing the conversion of 5-HTP into 5-HT. These results also demonstrate that peripheral 5-HT is not involved in modulation of itch transmission. Consistently, WT mice treated with 5-HTP showed a dramatic increase of CQ-elicited scratching (Figure 1H). Since CQ-elicited itch is dependent on GRP-GRPR signaling (Sun and Chen, 2007), we reasoned that 5-HT might synergistically act with GRP to enhance the function of GRPR. We tested this possibility by examining the GRP-induced scratching (GIS) with an intracisternal injection in mice treated with 5-HTP. Indeed, GIS was markedly potentiated by 5-HTP (Figure 1I), revealing a positive correlation between the activity of GRPR and central 5-HT tone. Importantly, 5-HTP injection did not affect the scratching response-induced by CQ in Grpr KO mice (Figure 1J). Taken together, we conclude that spinal 5-HT signaling facilitates itch transmission through GRPR.
5-HT1A Mediates 5-HT-Dependent Facilitation of GRPR Function
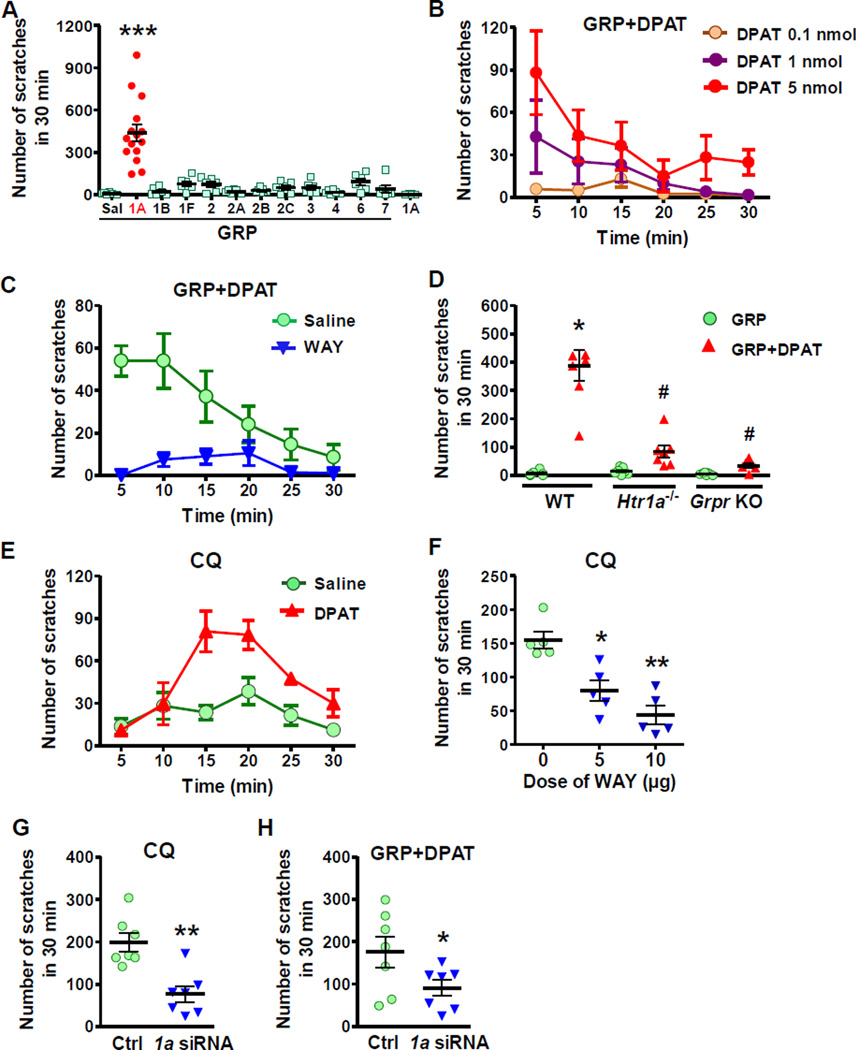
To identify the 5-HT receptor subtype that mediates the facilitating effect of descending 5-HT on itch transmission, we carried out pharmacological and behavioral screening by injecting a variety of 5-HT receptor agonists and GRP into the spinal cord of WT mice. GRP at 0.01 nmol was used because this dose was insufficient to elicit scratching behavior greater than vehicle (Figure 2A). The use of a minimal concentration of GRP serves to enhance the sensitivity of screening for identifying 5-HT receptor agonists that may potentiate GRP action. Of all the agonists tested for the 5-HT receptor subtypes, only R-(+)-8-OH-DPAT (DPAT), a 5-HT1A agonist, showed a robust facilitating effect on GIS (Figure 2A) and the effect of DPAT is dose-dependent (Figure 2B). Notably, i.t. DPAT alone failed to induce scratch behavior (Figure 2A). Although DPAT is also a partial agonist for 5-HT7 receptor, it is unlikely that 5-HT7 is involved since AS-19, an agonist for 5-HT7, did not enhance GIS significantly (Figure 2A). Furthermore, agonists for other 5-HT receptor subtypes failed to increase GIS (Figure 2A). We next employed WAY100635 (WAY), a highly specific 5-HT1A antagonist, to verify whether the robust scratching response elicited by DPAT/GRP is mediated by 5-HT1A. Pretreatment of mice with WAY (10 µg, i.t.) for 5 min nearly abolished scratching behavior evoked by DPAT/GRP co-injection (Figure 2C). Importantly, DPAT failed to enhance scratching behavior in Htr1a−/− mice and Grpr KO mice (Figure 2D). These data demonstrate that activation of spinal 5-HT1A is required for the facilitation of GRPR function in itch transmission.
Figure 2. Serotonin Facilitates Scratching Behavior Through 5-HT1A.
(A) Scratching behaviors evoked by co-injection of GRP and various 5-HT receptor agonists (5 nmol) in WT mice. Sal: saline, 1A: DPAT, 1B: CP 93129, 1F: LY344864, 2: α-ME-5-HT, 2A: DOI, 2B: BW 723C86, 2C: m-cpp, 3: m-CPBG, 4: RS 67506, 6: EMD 386088, 7: AS-19.
(B) Co-injection of DPAT dose-dependently facilitated GIS (p < 0.01).
(C) WAY blocked the facilitatory effect of DPAT on GIS (p < 0.01).
(D) Co-injection of DPAT and GRP failed to elicit scratching behavior in Htr1a−/− mice or Grpr KO mice. (E-G) CQ-induced scratching behavior was significantly enhanced by DPAT (p < 0.01) (E), while attenuated by WAY (F) and Htr1a siRNA (1a siRNA) (G).
(H) Facilitatory effect of DPAT on GIS was significantly reduced by Htr1a siRNA.
Error bars represent SEM. n = 5–9. *p < 0.05, **p < 0.01, ***p < 0.001, versus saline (A, D and F) or ctrl (G and H). #p < 0.05, versus DPAT in WT. One-way ANOVA followed by Dunnett‧s Multiple Comparison Test in A, D and F, two-way repeated measures ANOVA in B, C and E, un-paired t test in G and H.
Since an elevated 5-HT level facilitates itch elicited by CQ, it is likely that 5-HT1A activation may facilitate CQ-elicited itch as well. Indeed, CQ-induced scratching behavior was greatly enhanced by i.t. DPAT (Figure 2E), mimicking the effect of 5-HTP (Figure 1H). Conversely, pre-injection of WAY markedly attenuated CQ-induced scratching behavior (Figure 2F). Furthermore, CQ-induced scratching behavior was significantly attenuated after spinal siRNA knockdown of Htr1a (Figure 2G), which was functionally confirmed by reduced facilitatory effect of DPAT on GIS (Figure 2H). These results suggest that activation of spinal 5-HT1A is important for mediating descending 5-HT signaling to facilitate the function of GRPR in pruriceptive transmission.
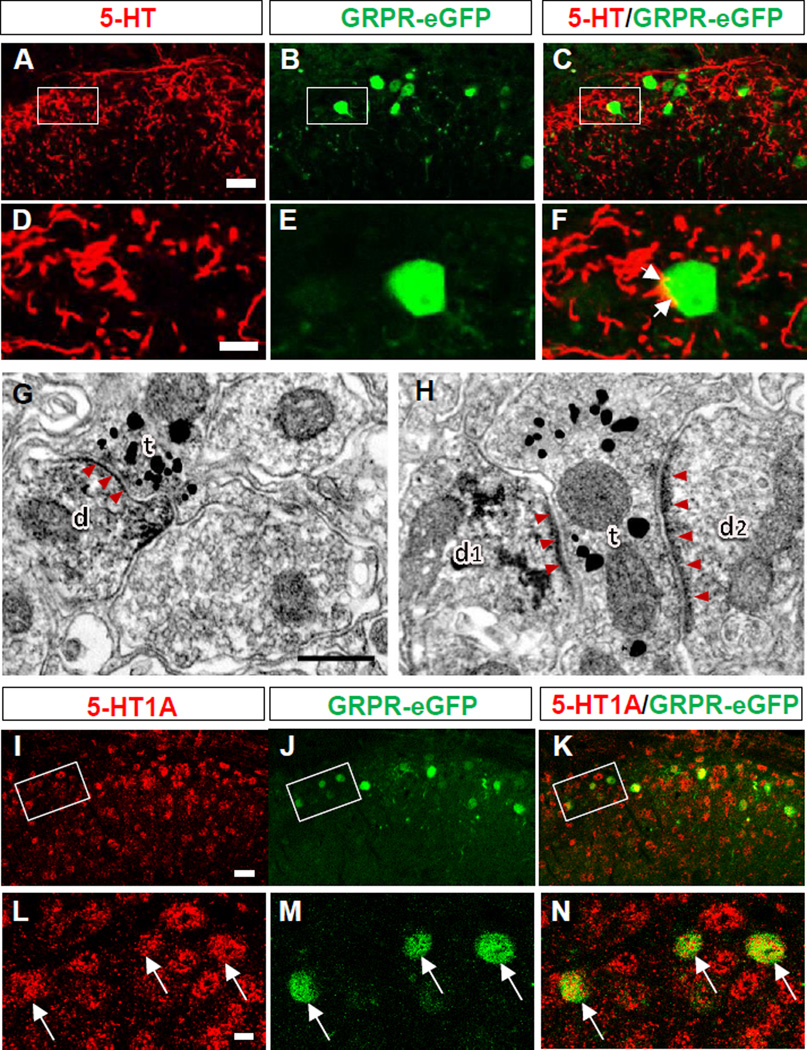
Descending 5-HT Terminals Contact GRPR+ Neurons and co-Expression of 5-HT1A and GRPR in the Spinal Cord
5-HT terminals mainly originated from the NRM are densely distributed in the superficial part (laminae I and IIo) of the dorsal spinal cord and SpVc of the brainstem (Li et al., 1997). To examine whether 5-HT+ terminals make connections with GRPR+ neurons in the dorsal spinal cord, we performed double immunohistochemistry (IHC) for 5-HT and eGFP in GRPR-eGFP mice. Indeed, numerous 5-HT+ fibers and GRPR+ neurons were detected in the superficial laminae of the dorsal spinal cord, and 5-HT+ fibers overlap with all GRPR+ neurons (Figure 3A-3F). Synaptic connections between 5-HT+ terminals and GRPR+ neurons were further examined by electron microscopy using a double-immunolabeling method (Li et al., 1997) for 5-HT and eGFP. In the lumbar cord, both 5-HT+ terminals identified by the nanogold–silver enhancement and eGFP (GRPR)+ neurons revealed by the immunoperoxidase products were observed to distribute in the same pattern as found by our double IHC (Figure 3A-3F). 5-HT+ terminals form synapses with GRPR+ dendritic profiles (Figures 3G and 3H). Of the synaptic types characterized (Uchizono, 1965), 85.5% (71/83) were symmetric synapses (Figure 3G), while 14.5% (12/83) were asymmetric ones (Figure 3H). Therefore, serotoninergic descending terminals predominantly make symmetrical synapses with GRPR+ neurons in the dorsal spinal cord.
Figure 3. Co-Expression of GRPR and 5-HT or 5-HT1A in the Spinal Cord.
(A-C) Double immunostaining of 5-HT+ terminals (red) with GFP (green) in the superficial dorsal horn.
(D-F) High power image of boxed area in A-C. Arrows indicate close contacts between 5-HT+ terminals and GRPR+ neuronal cell bodies.
(G, H) 5-HT+ axon terminals (t; silver grains) make symmetric (G) or asymmetric (H) synaptic contacts with GRPR+ dendritic profiles (d, d1, d2; DAB reaction products), respectively. Arrow heads indicate post-synaptic membranes.
(I-K) Double immunostaining of 5-HT1A (red) and GFP (green) in the superficial dorsal horn.
(L-N) High power image of boxed area in I-K. Arrows indicate double-stained cells.
Scale bars: 10 µm in A and I, 2.5 µm in D and L, 0.3 µm in G and H. See also Figures S2 and S3.
One prerequisite for 5-HT1A and GRPR crosstalk is the co-expression of two partner receptors in the same neurons (Pin et al., 2007). The expression of 5-HT1A is heavily concentrated in the dorsal horn of the spinal cord (Zhang et al., 2002). To examine whether 5-HT1A is also expressed in GRPR+ neurons, we performed double IHC of 5-HT1A and GFP in the spinal cord of GRPR-eGFP mice using anti-GFP antibody and anti-5-HT1A antibody (Figure S2). 5-HT1A is broadly expressed in the dorsal horn (Figures 3I-3N). Double IHC revealed that the overwhelming majority (91%) of GFP-expressing cells in the dorsal spinal cord were co-stained with 5-HT1A (Figure 3N). Moreover, we examined the co-expression of Htr1a and Grpr in GRPR-eGFP neurons using single cell RT-PCR (Figure S3B) and found Htr1a mRNA signals in 78% (7/9) of eGFP+ neurons (Table S1). Furthermore, all eGFP+ neurons expressed Grpr mRNA (Table S1), validating the usage of eGFP as a marker for GRPR expression in GRPR-eGFP mice.
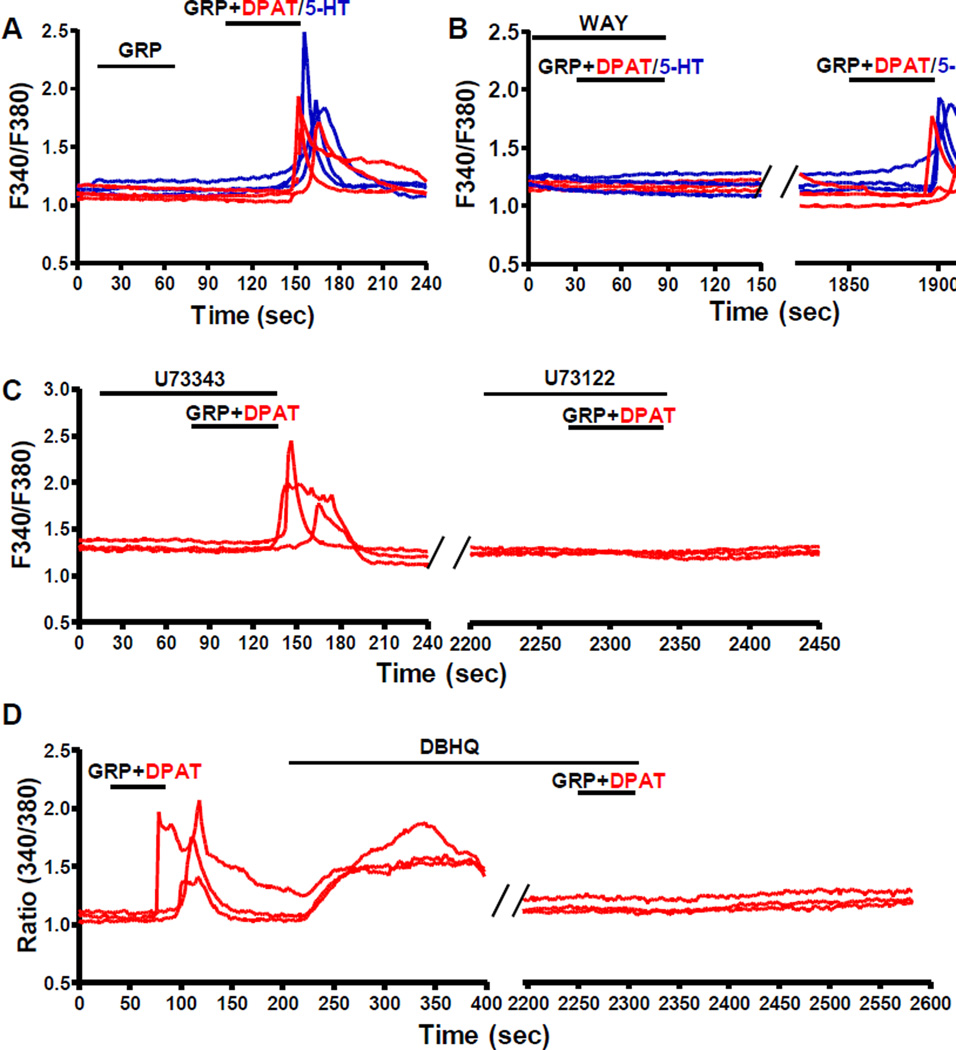
Facilitation of GRP-Induced Calcium Signaling by 5-HT1A in Spinal GRPR+ Neurons
GRPR mediates itch sensation through the PLCβ/IP3 pathway and intracellular Ca2+ release (Liu et al., 2011), whereas 5-HT1A signals predominantly through the Gi protein-coupled cAMP pathway (Hoyer et al., 2002). To investigate the functional cross-communication between 5-HT1A and GRPR, we examined intracellular Ca2+ mobilization in dissociated spinal dorsal horn neurons using calcium imaging. As expected, neither 5-HT or DPAT at 10 µM (Figure S4A) nor GRP at 5 nM evoked a Ca2+ response in spinal dorsal horn neurons (Figure 4A). In contrast, co-application of GRP (5 nM) and 5-HT (10 µM) produced Ca2+ transients in 6% of spinal neurons (30/487) (Figure 4A), which cannot be attributed to additive effect. Similarly, DPAT (10 µM) also greatly facilitated GRP-induced Ca2+ signaling, indicating that 5-HT1A mediated the effect of 5-HT (Figure 4A). Importantly, spinal neurons of Grpr KO mice did not respond to GRP at high concentrations (up to 20 nM) (Figure S4B), indicating that it is GRPR that mediated the intracellular Ca2+ mobilization in WT neurons. To further examine the involvement of 5-HT1A in the observed facilitating effect of 5-HT and DPAT, WAY was used to block 5-HT1A prior to GRP+DPAT/5-HT application. Indeed, WAY completely blocked Ca2+ responses of GRPR+ neurons upon GRP+DPAT/5-HT incubation, and the responses recovered after WAY was washed out (Figure 4B). WAY did not display a non-specific effect on intracellular Ca2+ mobilization evoked by GRP alone without 5-HT1A activation (Figure S4C). We previously showed that Gq/PLCβ/IP3R signaling pathway and intracellular Ca2+ store are essential for GRP-induced Ca2+ response in HEK 293 cells (Liu et al., 2011). To further understand the signaling events downstream of 5-HT1A/GRPR upon GRP+DPAT stimulation, we first tested the effect of U73122, a PLC inhibitor, and found that U73122, but not its inactive analog U73343, completely abolished GRP+DPAT-induced Ca2+ mobilization in GRPR+ neurons (Figure 4C). GRP+DPAT-responding neurons did not respond to the second incubation of GRP+DPAT after the intracellular Ca2+ store was depleted by 2,5-di-tert-butyl-hydroquinone (DBHQ), a selective and potent inhibitor of endoplasmic reticulum Ca2+-ATPase (SERCA) (Moore et al., 1987) (Figure 4D). The effects of U73122 and DBHQ were not due to ligand-induced desensitization of 5-HT1A/GRPR signaling pathway because GRPR+ neurons showed comparable Ca2+ responses upon two consecutive applications of GRP+DPAT with an interval of 30 min wash (Figure S4D). These results provide the first in vivo evidence indicating that co-activation of 5-HT1A by 5-HT or DPAT facilitates GRP/GRPR-mediated PLC-dependent intracellular Ca2+ signaling pathway.
Figure 4. Co-Activation of 5-HT1A Facilitates GRPR Ca2+ Signaling in Spinal Neurons.
(A) Representative traces showing that 5 nM GRP failed to evoke Ca2+ responses in dissociated WT spinal neurons, while co-application of 5-HT (blue) or DPAT (red) together with GRP (5 nM) induced intracellular Ca2+ mobilization.
(B) WAY completely blocked Ca2+ responses of spinal neurons evoked by GRP+5-HT (blue) or GRP+DPAT (red), which recovered after WAY was washed out.
(C) U73122, but not U73343 blocked intracellular Ca2+ mobilization induced by GRP+DPAT.
(D) GRP+DPAT-evoked intracellular Ca2+ mobilization vanished after depletion of intracellular Ca2+ store by DBHQ.
The experiments were repeated for 3 times and at least 200 neurons were analyzed for each experiment. See also Figure S4.
Heteromeric Interactions Between 5-HT1A and GRPR in vitro and in vivo
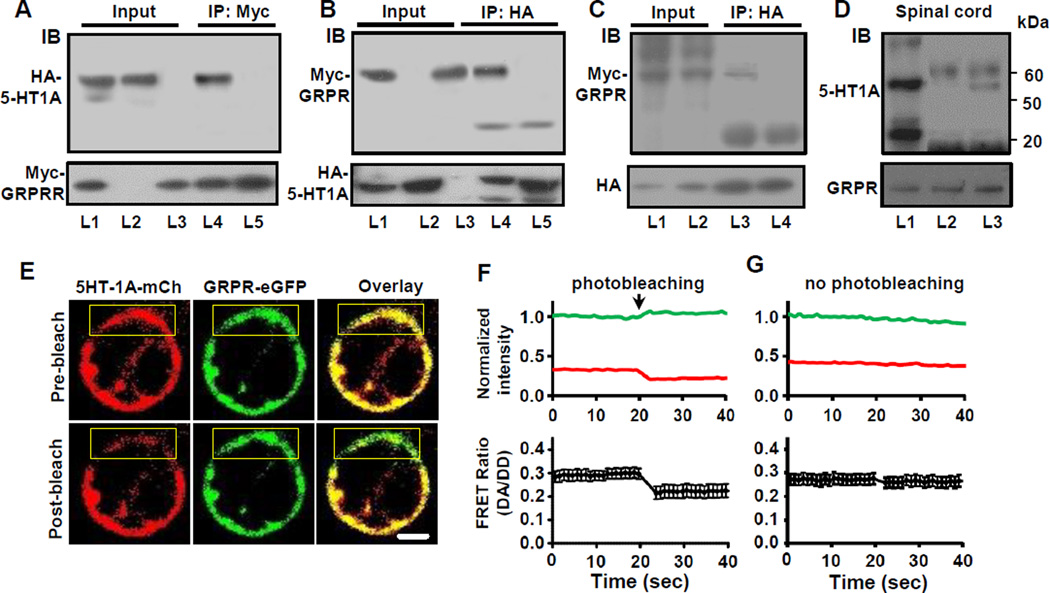
GPCR heteromeric interactions have been increasingly implicated in conferring GPCRs with expanded functionality (Bouvier, 2001 ; Milligan, 2013). Receptor crosstalk confers neuronal GPCRs with novel signaling and pharmacological properties enhancing the capacity of neural circuits to regulate a wide array of behavioral outputs (Prinster et al., 2005). The co-expression and synergistic effect of 5-HT1A and GRPR co-activation raised the possibility that these two receptors may function as receptor heteromeric complexes. To ascertain whether 5-HT1A and GRPR may physically interact through receptor heteromerization, we conducted co-immunoprecipitation (Co-IP) experiments using membrane proteins extracted from HEK 293 cells co-expressing HA-5-HT1A and Myc-GRPR. Anti-Myc antibody co-immunoprecipitated a band that corresponds to HA-5-HT1A (Figure 5A). In a reverse Co-IP experiment, a specific Myc-GRPR band was also detected in anti-HA precipitates (Figure 5B). In contrast, the specific bands were not observed in the precipitates from mixed membrane proteins that were prepared using cells expressing either HA-5-HT1A or Myc-GRPR (Figures 5A and 5B), suggesting the presence of constitutive 5-HT1A-GRPR complexes in the membrane when these two receptors are co-expressed in the same cells (Figures 5A and 5B). To verify the specificity of Co-IP between HA-5-HT1A and Myc-GRPR, we examined whether GRPR would interact with 5-HT1B, a 5-HT1 receptor subtype that is phylogenetically most closely related to 5-HT1A (Hoyer et al., 2002). Myc-GRPR immunoreactivity was not detectable in HA-5-HT1B precipitates using HEK 293 cells co-expressing HA-5-HT1B and Myc-GRPR (Figure 5C). These results suggest that the formation of 5-HT1A and GRPR heteromers is likely to be specific. Next, we examined whether 5-HT1A and GRPR interact with each other in vivo by Co-IP studies using the spinal cord membrane preparations (Liu et al., 2011). A specific 5-HT1A band was co-immunoprecipitated with GRPR using mouse anti-GRPR antibodies (Figure 5D), but was not detectable when an irrelevant mouse IgG was used (Figure 5D). Taken together, these results suggest the presence of receptor heteromeric complexes containing 5-HT1A and GRPR both in vitro and in vivo. Moreover, it further confirms that the two receptors are co-expressed in the same neurons in the spinal cord.
Figure 5. GRPR and 5-HT1A Form Heteromeric Complexes and Are within Close Proximity.
(A and B) Co-IP of HA-5-HT1A and Myc-GRPR. L1 and L4, HA-5-HT1A/Myc-GRPR cells; L2, HA-5-HT1A cells; L3, Myc-GRPR cells; L5, mixture of L2 and L3.
(C) Myc-GRPR was not co-immunoprecipitated with HA-5-HT1B. L1, L3, HA-5-HT1A/Myc-GRPR cells; L2, L4, HA-5-HT1B/Myc-GRPR cells.
(D) Co-IP of 5-HT1A and GRPR from the spinal cord membrane preparations. L1, input; L2, irrelevant mouse IgG; L3, mouse anti-GRPR.
(E) Representative confocal images show one HEK 293 cell co-expressing 5HT1A-mCherry (red) and GRPR-eGFP (green), before and after acceptor (mCherry) photobleaching in selected region of the cell (yellow box). Scale bar, 10 µm.
(F) Top: Plots showing background subtracted normalized fluorescence intensities of donor emission (green trace, 488 nm excitation, 515 nm emission: DD) and acceptor emission (red trace, 488 nm excitation, 630 nm emission: DA) from selected plasma membrane region in E. Bottom: Averaged FRET ratio (DA/DD) of the photobleached regions in E.
(G) Background subtracted normalized fluorescence intensities (top) and averaged FRET ratio (bottom) of non-photobleached plasma membrane regions in E.
Number of cells, n = 9–11. Error bars represent SEM. See also Figures S5 and S6.
5-HT1A and GRPR Are Located in Close Proximity
Although Co-IP data suggest a physical association between 5-HT1A and GRPR, it remained unclear whether the receptors themselves interact or if intermediaries such as scaffolding or anchoring proteins were required for receptor association or whether the two receptors may coexist in microdomains (Prezeau et al., 2010). To examine this possibility, we utilized confocal imaging and single-cell subcellular acceptor photobleaching fluorescence resonance energy transfer (FRET) analysis to assess the physical association between 5-HT1A and GRPR receptors (Karunarathne et al., 2013). A major advantage of our confocal set-up is that the analysis of FRET can be performed in subcellular compartments on a single cell level. This allows us to use a defined region of the same cell that is not photobleached as an internal control. The C-terminus of 5-HT1A and GRPR were tagged with fluorescent protein eGFP as the donor and mCherry as the acceptor, respectively. FRET is calculated by time-lapse imaging of donor GFP (488 excitation, 510 emission) and acceptor mCherry (488 excitation, 630 emission) before and after photobleaching the acceptor (mCherry) in a selected region of the plasma membrane (Figure 5E). A plasma membrane region in the same cell which was not photobleached served as controls (Figure 5E). Fast photobleaching (0.89 ms/µm2) and slow mobility of several transmembrane receptors on the plasma membrane ensured that during FRET analysis both the donor and acceptor fluorescent proteins remained stationary. After acceptor (5-HT1A-mCherry) photobleaching, donor (GRPR-eGFP) fluorescence intensity was increased due to the loss of energy transfer, suggesting the presence of FRET (Figure 5F and 5G). The FRET loss associated with acceptor photobleaching and subsequent increase in donor fluorescence intensity was 44.8 ± 11.2 % (Figure 5F and 5G). In contrast, no detectable FRET signal was observed between 5-HT1B-mCherry and GRPR-eGFP (Figure S6B). Lack of FRET between 5-HT1B and GRPR is consistent with Co-IP results, further supporting the notion that the 5-HT1A-GRPR association is specific. Taken together, these results suggest that 5-HT1A and GRPR are in close proximity on the plasma membrane, which may facilitate crosstalk between the two receptors.
Activation of 5-HT1A Potentiates the Excitability of GRPR+ Neurons
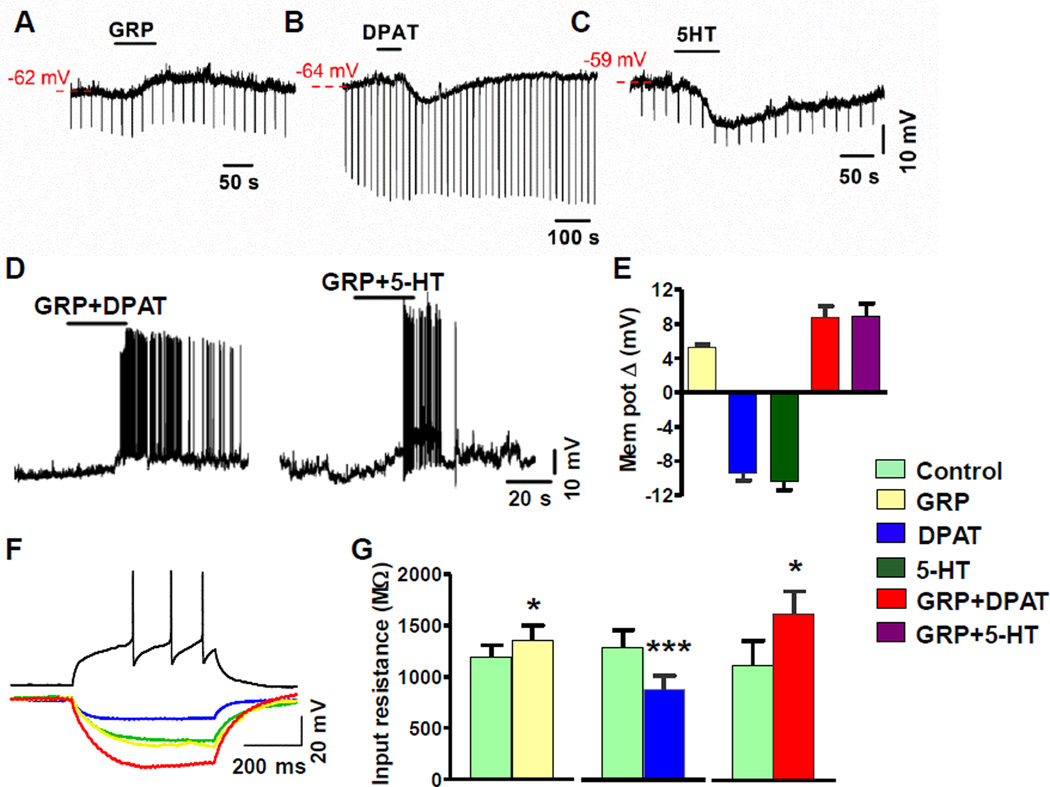
We next evaluated the effect of 5-HT1A activation on GRP-induced excitation of GRPR+ neurons by whole-cell patch-clamp recordings of on GRPR-eGFP neurons using spinal cord slice preparations, hereafter referred to as GRPR+ neurons (Zhao et al., 2013a). We first measured changes in membrane potential and input resistance of GRPR+ neurons in response to GRP, DPAT and 5-HT. A bath application of GRP induced a subthreshold membrane depolarization (Figures 6A and 6E). In contrast, DPAT and 5-HT hyperpolarized GRPR+ neurons as expected (Figures 6B, 6C and 6E). Thus, activation of GRPR and 5-HT1A alone appeared to have opposing effects on the excitability of GRPR+ neurons in the spinal cord. To determine whether DPAT or 5-HT could facilitate GRP-dependent excitation, we examined the effect of a co-application of GRP and DPAT or 5-HT on GRPR+ neurons. Importantly, a co-application of GRP+DPAT or GRP+5-HT not only masked the hyperpolarization observed in response to DPAT or 5-HT alone, but also induced a larger magnitude of depolarization that often resulted in action potential (AP) firing (Figure 6D). GRP and GRP + DPAT treatments significantly increased input resistance compared to control, whereas DPAT alone significantly decreased input resistance compared to control (Figures 6F and 6G). These data revealed that GRP-induced subthreshold membrane depolarization not only counteracted the hyperpolarizating effect of DPAT but was also potentiated by DPAT with a net increase in the excitability of GRPR+ neurons.
Figure 6. Co-Activation of 5-HT1A and GRPR Increased the Excitability of GRPR+ Neurons.
(A-C) Representative traces show membrane depolarization by GRP (A) and hyperpolarizing response induced by DPAT (B) and 5-HT (C) in current-clamped GRPR+ neurons.
(D) Representative traces showing that a co-application of GRP and DPAT or 5-HT evoked membrane depolarization and AP firing in current-clamped GRPR+ neurons. AP firing evoked by GRP+DPAT and GRP+5-HT ranged 0.167 to 3.5 Hz (n = 6) and 0.03 to 6 Hz (n = 17), respectively.
(E) Quantified data of A-D. GRP depolarized GRPR+ neurons by 5.3 ± 0.3 mV (n = 8). DPAT (7.5 µM, blue) and 5-HT (40 µM, dark green) hyperpolarized GRPR+ neurons by 9.4 ± 0.9 mV (n = 19) and 10.4 ± 1 mV (n =13), respectively. In contrast, co-application of GRP+DPAT depolarized GRPR+ neurons by 8.7 ± 1.4 mV (red) (n = 15). GRP+5-HT depolarized GRPR+ neurons by 8.9 ± 1.5 mV (purple) (n = 35 ).
(F) Representative traces illustrate the effect of GRP (yellow), DPAT (blue), and GRP+DPAT (red) on membrane input resistance in current-clamped GRPR+ neurons receiving negative current injections (−20 pA). Control (green): extracellular buffer only. For all recordings, neuronal health was verified by observing action potentials in response to positive current injection under control conditions (black).
(G) GRP (yellow) increased the membrane input resistance from 1,185 ± 124 MΩ to 1,350 ± 150 MΩ (n = 8), DPAT decreased the input resistance from 1,311 ± 168 MΩ to 894 ± 137 MΩ (n = 19) and GRP + DPAT increased the input resistance from 1,116 ± 247 MΩ to 1,616 ± 226 MΩ (n = 15), *p < 0.05, **p < 0.01, ***p < 0.001, paired t-test.
A Blockade of 5-HT1A Attenuates Chronic Itch
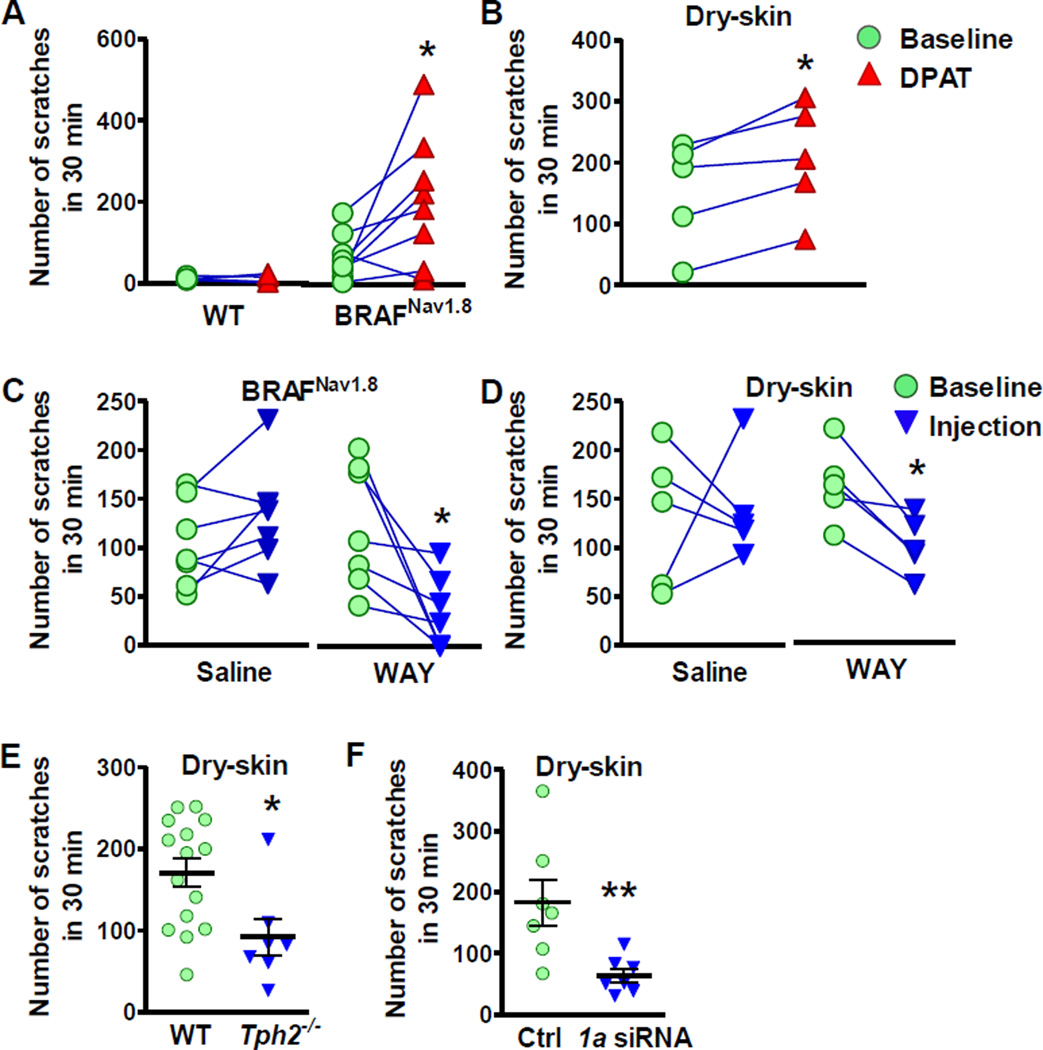
In light of the facilitatory effect of 5-HT1A on GRPR signaling and CQ-elicited itch, next we asked whether 5-HT1A could modulate long-lasting scratching behavior using two distinct chronic itch models that are dependent on enhanced GRP/GRPR signaling for maintaining long-lasting itch transmission (Zhao et al., 2013b). First, we tested the effect of 5-HT1A activation on spontaneous scratching behavior of BRAFNav1.8 mice, in which the BRAF kinase in sensory neurons expressing the sodium channel Nav1.8 was selectively activated by genetically replacing the WT Braf gene with a kinase activated one (V600E) (Zhao et al., 2013b). BRAFNav1.8 mice progressively developed spontaneous scratching behavior accompanied by skin lesions as a result from increased expression of a cohort of itch-related genes, including GRP in sensory neurons and GRPR in the spinal cord (Zhao et al., 2013b). Although WT mice failed to exhibit scratching behavior after i.t. DPAT alone, the spontaneous scratching behavior of BRAFNav1.8 mice was significantly enhanced (Figure 7A). Likewise, DPAT also significantly enhanced the chronic scratching behavior induced by dry-skin (xerosis) (Figure 7B), a skin condition often associated with pruritus (Miyamoto et al., 2002). The enhanced scratching responses in mice with chronic itch by DPAT suggests that 5-HT1A is likely to be constitutively primed for tonic activation by enhanced release of 5-HT. To test this, we measured the levels of 5-HT and 5-HIAA in mice with chronic itch using HPLC. Moreover, the levels of 5-HT were significantly elevated in the hindbrain of BRAFNav1.8 mice and cervical spinal cord of dry-skin mice (Figure S7A). The levels of 5-HIAA were also significantly elevated in hindbrain and cervical spinal cord of BRAFNav1.8 mice, suggesting enhanced activity of 5-HT in these regions (Figure S7B). Then, we examined whether WAY may attenuate chronic itch. Similar to its inhibitory effect on CQ-elicited itch, i.t. WAY significantly attenuated spontaneous scratching behaviors of BRAFNav1.8 mice (Figure 7C) and dry skin mice (Figure 7D). Importantly, injection of saline had no significant effect on spontaneous scratching behaviors of BRAFNav1.8 mice or dry skin mice (Figures 7C and 7D). Furthermore, Tph2−/− mice also displayed deficits in spontaneous scratching behaviors under dry-skin condition (Figure 7E) and WT mice showed attenuated scratching behavior after knock-down of spinal 5-HT1A by i.t. Htr1a siRNA (Figure 7F). Thus, these data support the notion that 5-HT1A is constitutively activated in the setting of chronic itch and its activation by descending 5-HT is required for maintaining long-lasting itch transmission.
Figure 7. Activation of 5-HT1A Facilitates Long-Lasting Itch Transmission.
(A and B) Spontaneous scratching behaviors of BRAFNav18 mice (A) and dry-skin mice (B) were facilitated after DPAT injection.
(C and D) WAY (10 µg) suppressed spontaneous scratching behavior of BRAFNav18 mice (C) and dry-skin mice (D).
(E) Tph2−/− mice showed attenuated spontaneous scratching behavior under dry-skin condition compared to WT littermates.
(F) Htr1a siRNA treatment significantly attenuated spontaneous scratching behavior under dry-skin condition compared to control siRNA (Ctrl).
Error bars represent SEM. n = 5–15. *p < 0.05, paired t test in (A-D), unpaired t test in (E and F). See also Figures S7.
DISCUSSION
The Role of 5-HT1A in the Modulation of Itch Transmission
Using both loss- and gain-of-function, genetic, pharmacological, behavioral and electrophysiological examinations, we demonstrate that central 5-HT signaling is essential for facilitating itch transmission and this function is mediated by the 5-HT1A receptor. Decreased itch transmission in mice lacking 5-HT is unlikely to be due to developmental deficits because we were able to restore normal itch transmission in Tph2−/− mice by an administration of exogenous 5-HTP. Furthermore, we demonstrate that central 5-HT neurons are essential for converting 5-HTP into 5-HT since mice lacking these neurons failed to be rescued by exogenous 5-HTP. Our study reveals a previously unrecognized function of 5-HT in sensory modality-specific modulation and demonstrate that 5-HT1A is a principal receptor for mediating 5-HT-dependent modulation of itch transmission. Importantly, the facilitatory role of 5-HT1A is required not only for acute itch but also for long-lasting itch transmission. Therefore, 5-HT1A has a broad role in the modulation of itch transmission.
5-HT1A-GRPR Crosstalk Amplifies Itch Signaling
A key finding of our work is that 5-HT1A facilitates itch modulation through its crosstalk with GRPR. Several pieces of evidence such as co-expression, co-IP and FRET data imply a physical association between 5-HT1A and GRPR in the same cells. While further studies are needed to explain whether the existence of 5-HT1A-GRPR heteromeric complexes is a prerequisite for the initiation of signaling crosstalk, it is tempting to speculate that the spatial proximity of 5-HT1A and GRPR may permit specific heteromeric crosstalk without accidental engagement of unrelated signaling pathways in GRPR+ neurons. A close proximity with 5-HT1A may enable GRPR to process information via pairing with specific GPCRs in a real time manner. 5-HT1A-dependent facilitation requires co-activation of GRPR by exogenous GRP. Although co-application of GRP is not required in chronic itch models for 5-HT1A-dependent facilitation, it is presumed that there is a constitutive release of GRP from primary afferents to prime GRPR for 5-HT modulation because GRP expression is significantly up-regulated in chronic itch conditions and GRP blocker attenuated spontaneous scratching behaviors (Liu et al., 2014; Nattkemper et al., 2013; Zhao et al., 2013b).
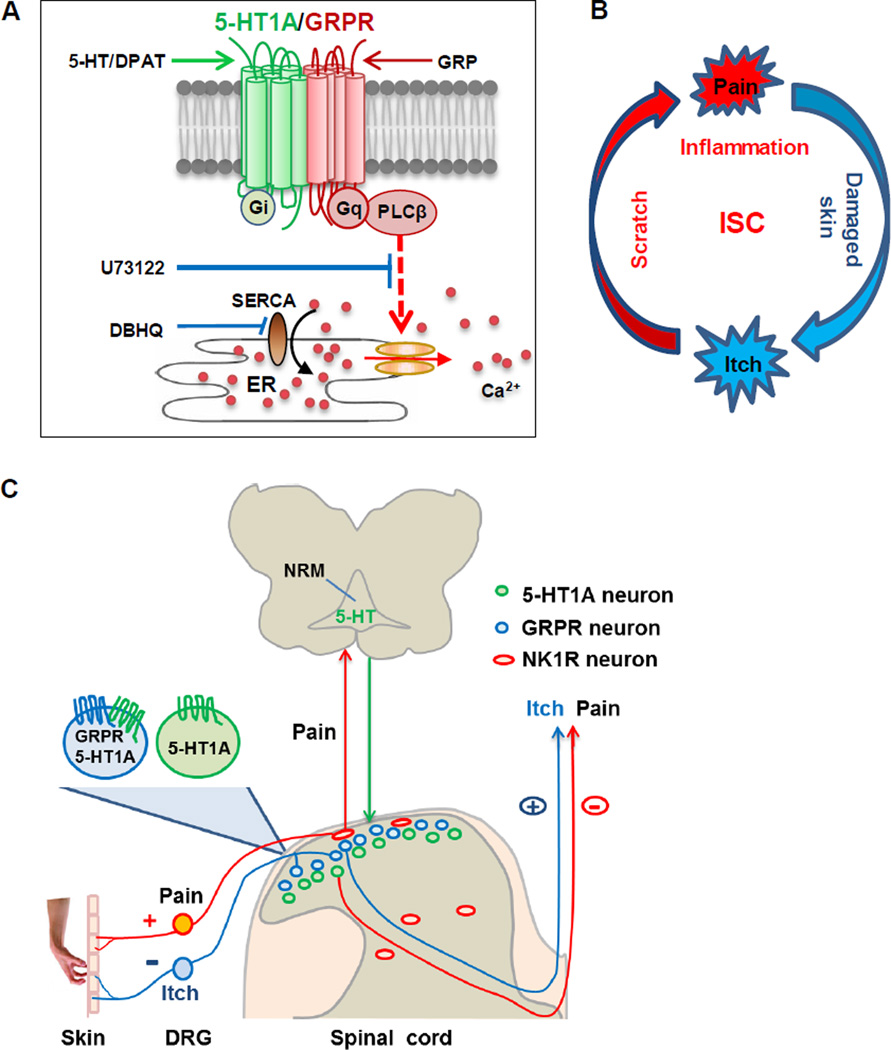
The mode of 5-HT1A or GRPR activation in a single receptor paradigm clearly differs from that of the two receptor paradigm. The fact that DPAT by itself is unable to elicit scratching behavior implies that 5-HT1A cannot transmit itch information directly. In contrast, GRP at a low dose that is insufficient to elicit either a calcium response (5 nM) or scratching behavior (0.01 nmol) can do so only if 5-HT1A is simultaneously activated. Since DPAT alone fails to produce calcium spikes, it is unlikely that DPAT alone is capable of exciting GRPR+ neurons. On the contrary, DPAT causes hyperpolarization of GRPR+ neurons. Postsynaptic 5-HT1A activation predominantly hyperpolarizes dorsal horn neurons to dampen neuronal excitability and synaptic transmission (Grudt et al., 1995; Yoshimura and Furue, 2006). Remarkably, the hyperpolarizing response of GRPR+ neurons produced by DPAT was completely masked by GRP. When GRP and DPAT were co-applied, together they were able to induce action potential firing. This response was not observed by an application of either GRP or DPAT alone. Thus, interactions between 5-HT1A and GRPR alter the pharmacological and physiological properties of either of the receptors and confer a novel functionality to 5-HT1A (Figure 8A). While activation of Gi-coupled 5-HT1A is usually inhibitory via the cAMP pathway, the receptor may facilitate the responsiveness of Gq-coupled receptors through signaling coupling. Our finding thus uncovers a novel paradigm for exploring facilitatory crosstalk mechanisms between Gi- and Gq-coupled receptor signaling transduction in a highly physiologically relevant context. It is important to note that the present study is only the first step towards understanding of central modulation of itch transmission. Since both heteromeric interactions and/or convergence of intracellular signaling pathways may occur (Prezeau et al., 2010), our studies raise several key questions concerning the underlying mechanisms: What are the interfacing regions for 5-HT1A-GRPR heteromeric interaction? Does the cross-talk between 5-HT1A and GRPR occur at the receptor or intracellular second messenger levels or both? If intracellular crosstalk is required, does 5-HT1A potentiate GRPR signaling by releasing Gβγ subunits to stimulate Gq-coupled PLC signaling? What is the respective role of Gi coupled signaling in activation vs. facilitation of GRPR? It will be of great interest to determine where the two signaling pathways might converge to amplify Gq-dependent itch signaling through either a “switch” or “turn off” of the signaling characteristic of Gi coupled receptors.
Figure 8. Hypothetic Models Illustrating the Role of 5-HT/5-HT1A in the Itch-Scratch Cycle.
(A) Schematic showing signaling interactions between 5-HT1A and GRPR. Co-activation of 5-HT1A and GRPR amplifies Gq-coupled PLCβ-dependent signaling pathway, resulting in the release of Ca2+ from ER through SERCA pumps. ER: endoplasmic reticulum.
(B) Schematic that illustrates the vicious ISC. Scratching induces skin inflammation, which in turn induces more vicious itching attacks.
(C) Schematic of dual modulation of descending 5-HT signaling in pain and itch during the ISC. Scratching evokes inflammatory/mechanical pain, which results in the activation of 5-HT neurons in NRM of the brainstem. Descending release of 5-HT acts on two distinct subpopulations of neurons: one expressing 5-HT1A alone (green cells) that inhibits nociceptive processing, while the other expressing both 5-HT1A and GRPR (blue cells) that facilitates pruriceptive transmission. NRM: nucleus raphe magnus.
Opposing Modulation of Itch and Pain by 5-HT Receptor Mechanisms
Pain and itch have been known as two opposing sensations (Ikoma et al., 2006; Lamotte et al., 2013; Ma, 2010) and, conceivably, may be subject to opposing descending modulation in the spinal cord. Despite some conflicting reports and the pain modality-dependent role of 5-HT1A (Bardin, 2011), most studies suggest an inhibitory function for postsynaptic 5-HT1A in spinal nociceptive processing, especially in thermal and inflammatory pain (Bardin, 2011; Millan, 2002; Yoshimura and Furue, 2006; Zhao et al., 2007a). The majority of substantia gelatinosa (lamina II) neurons of the dorsal horn and SpVc responded to 5-HT or 5-HT1A agonists with a hyperpolarizing membrane current, thereby inhibiting nociceptive processing (Abe et al., 2009; Grudt et al., 1995; Lu and Perl, 2007; Yoshimura and Furue, 2006). In contrast, 5-HT1A facilitates itch by enhancing the excitability of GRPR+ neurons and by inhibiting its canonical pathway. Taken together, 5-HT1A has opposing roles in mediating descending 5-HT modulation of itch and pain, and this dual role is exerted through distinct subsets of dorsal horn neurons which express 5-HT1A with or without GRPR (Figure 8C). Along with previous studies, our data, therefore, provide the first evidence indicating that itch and pain are subject to opposing modulation of descending 5-HT signaling in the spinal cord.
Contribution of Central 5-HT Signaling to Development of the Itch-Scratch Cycle
What is the probable physiological significance of 5-HT1A-mediated central modulation of itch and pain? A cardinal feature of chronic itch associated with a wide spectrum of skin diseases is a vicious itch-scratch cycle (ISC) (Paus et al., 2006; Yosipovitch and Papoiu, 2008)(Figure 8B). Scratching behavior, as a noxious mechanical stimulus, can inhibit itch sensation and spinal projection neurons (Davidson et al., 2009; Yosipovitch et al., 2007). However, it also intensifies skin inflammation which, in turn, provokes more intense itch sensation as well as an uncontrollable urge to scratch. Given the role of postsynaptic 5-HT1A in the inhibition of inflammatory pain and spinal nociceptive processing, we suggest a hypothetic model that explains the dual consequences of scratching behavior (Figure 8C). Past studies indicated that activation of the nociceptive pathway in response to somatic and/or noxious stimuli can promote 5-HT release in the spinal cord (Sorkin and McAdoo, 1993; Yaksh and Tyce, 1981). Likewise, vigorous scratching-induced pain can conceivably evoke 5-HT release to inhibit nociceptive processing through 5-HT1A-dependent negative feedback mechanisms (Figure 8B). Concurrently, tonic 5-HT release paradoxically activates 5-HT1A in GRPR+ neurons to potentiate itch transmission (Figure 8C). Thus, the positive and negative feedback controls of pain and itch outputs are likely to contribute to the vicious development of the ISC.
Because cross-signaling of 5-HT1A-GRPR heteromers is itch-specific (only when GRPR is activated), a disruption of an interface between 5-HT1A and GRPR may alleviate the urge to scratch in chronic itch conditions. The advantage of such a strategy is to permit the normal functioning of 5-HT1A and GRPR in other areas of the brain where the receptors are not co-expressed. Unraveling key interfaces required for 5-HT1A-GRPR cross-signaling could be a crucial next step. Since GPCR crosstalk may occur at multiple signaling levels, the converging points of Gi-Gq cross-signaling may also be substrates for a therapeutic blockade. As enhanced central 5-HT signaling is likely to exacerbate the ISC, an exploration of the disruption of modulatory function of 5-HT1A opens an additional avenue for designing novel therapeutics that ameliorate chronic pruritus.
EXPERIMENTAL PROCEDURES
Animal Behavior
Adult male C57BL/6J mice, Lmx1bf/f/p mice (Zhao et al., 2006), Tph2−/− mice (Kim et al., 2014), Htr1a−/− mice (Heisler et al., 1998), Grpr KO mice (Hampton et al., 1998) and BRAFNav1.8 mice (Zhao et al., 2013b) were used for the study. Scratching behaviors were performed as previously described (Sun and Chen, 2007; Zhao et al., 2013b). All animal experiments were performed in accordance with the guidelines of the National Institutes of Health and the International Association for the Study of Pain and were approved by the Animal Studies Committee at Washington University School of Medicine.
Immunohistochemistry and In Situ Hybridization
IHC and ISH were performed as described (Zhao et al., 2006). For the IHC study, sections were incubated with primary antibodies overnight at 4°C followed by the use of FITC or Cy3-conjugated secondary antibodies (Jackson ImmunoResearch). The following primary antibodies were used: rabbit anti-5-HT (1:5,000, Immunostar), rabbit anti-5-HT-1A (1:200, Santa Cruz) and chicken anti-GFP (polyclonal, 1:500, Aves Labs). For the ISH study, a digoxigenin-labeled cRNA probe was used as described earlier (Zhao et al., 2006). Images were taken using a Nikon Eclipse Ti-U microscope.
Small Interfering RNA Treatment
Htr1a siRNA (Sigma) were delivered to the lumbar region of the spinal cord, twice daily for 6 consecutive days as described previously (Liu et al., 2011). Behavioral testing was carried out 24 h after the last injection.
Immune-Electron Microscopy
Immune-electron microscopic studies were performed as described (Li et al., 1997; Pang et al., 2006). Briefly, cross sections of lumbar spinal cord of adult GRPR-eGFP mice were double immune-labeled by rabbit anti-5-HT antibody (1:2,000; Incstar Corporation, Stillwater, MN) and guinea pig anti-GFP antibody (1.5 µg/ml) using immunogold–silver and immunoperoxidase methods, respectively. Furthermore, 50-nm-thick ultrathin sections were examined with a JEM-1400 electron microscope (JEM, Tokyo, Japan). The digital micrographs were captured by VELETA (Olympus, Tokyo, Japan).
HPLC
The concentrations of monoamines were measured as previously described (Zhao et al., 2006). The resulting values were corrected for volume and expressed as pg of amine per mg of wet tissue or per 100 µL of plasma. For analyzing the effect of 5-HTP injections on indoleamine concentrations, samples were collected one hour after 5-HTP or saline administration.
Cell Culture and Transfections
Constructs were transfected into HEK 293 cell lines for selection as described (Liu et al., 2011). For FRET experiments, pcDNA3.1/GRPR-eGFP (1 µg/cm2) and pcDNA3.1/5-HT1A-mCherry (0.2 µg/cm2) were transiently transfected into HEK 293 cells using Lipofectamine 2000 (Invitrogen). FRET was done 24 h after transfection.
Calcium Imaging, Co-IP and Western Blot Analysis
Calcium imaging experiments were performed as described previously (Liu et al., 2011). Experiments were repeated three times and a minimum of 50 cells were included for analysis. Co-IP and Western Blot Analysis were performed as described (Liu et al., 2011). Briefly, solubilized membrane proteins (200 µg) were precipitated with rabbit anti-HA (BD bioscience), mouse anti-Myc (Covance) or mouse anti-GRPR (Abmart) antibodies and TrueBlot™ anti-rabbit or anti-mouse IgG bead slurry (eBioscience). After elution proteins were analyzed using western blot with mouse anti-Myc (1:1,000), rabbit anti-HA (1:1,000), mouse anti-GRPR (1:5,000) or rabbit anti-5-HT1A (1:5,000, Abcam).
Confocal Subcellular FRET Imaging
The FRET imaging and calculations were performed as described previously (Karunarathne et al., 2013). Basal FRET between eGFP (donor) and mCherry (acceptor) was measured by rapid photobleaching of the acceptor in a defined region of a single cell, whereas the unbleached region was used as the control. Before and after photobleaching, a series of time lapse images were captured with donor excitation-donor emission (DD) and donor excitation-acceptor emission (DA).
Electrophysiological Recording
Patch-clamp studies were performed as described previously (Jeffry et al., 2009). Slices of the lumbar spinal cord of GRPR-eGFP mice 3–4 weeks of age were prepared for patch clamp recording. Firing patterns were examined by injection of steps of positive current for 500 ms. Input resistance was tested every 20 s for drug induced changes by an injection of negative current (−20 pA). Series resistance was monitored in voltage clamp mode by measuring the instantaneous current in response to small voltage steps. Data were analyzed offline (ClampFit 10) and plotted in Origin 8 graphing software.
Statistical Analysis
Statistical analyses were performed using Prism 5 (version 5.03, GraphPad Software). p < 0.05 was considered statistically significant.
Supplementary Material
Highlights.
Central 5-HT signaling facilitates itch transmission
5-HT1A potentiates GRPR-mediated itch signaling
5-HT1A and GRPR are present in close proximity
Blockade of 5-HT1A function reduces chronic itch
ACKNOWLEDGMENTS
We are grateful to Y.Z Tian, AA. Kim, R. Kim, J. Yin, X. L Meng, Q. Meng, N. Han and Q. F. Li for technical help and members of the Chen laboratory for comments. The project was supported by a NIAMS grant AR056318-01 (Z.F.C), a NIH grant R01 DA019921 (K.J.R.), the National Natural Science Foundation of China grants No. 81371239 and 31371211 (H.L and Y.L) and NIH grants GM069027 and GM080558 (N.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information includes seven figures, one table and Supplemental Experimental Procedures
Figure S1. Reduced Spinal 5-HT Level after 5,7-DHT Injection and Enhanced Central 5-HT Concentrations by Systemic 5-HTP Administration, Related to Figure 1
Figure S2. 5-HT1A Antibody Is Specific, Related to Figure 3
Figure S3. Co-Expression of Grpr and Htr1a in GRPR-eGFP Neurons, Related to Figure 3
Figure S4. GRP, 8-OH-DPAT and WAY100635 Are Specific, Related to Figure 4
Figure S5. The Specificity Test of Mouse Anti-GRPR Monoclonal Antibody, Related to Figure 5
Figure S6. No Detectable FRET Was Observed Between GRPR-eGFP and 5-HT1B-mCh, Related to Figure 5
Figure S7. Enhanced 5-HT Levels in Mice with Chronic Itch, Related to Figure 7
REFERENCES
- Abe K, Kato G, Katafuchi T, Tamae A, Furue H, Yoshimura M. Responses to 5-HT in morphologically identified neurons in the rat substantia gelatinosa in vitro. Neuroscience. 2009;159:316–324. doi: 10.1016/j.neuroscience.2008.12.021. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Iodi Carstens M, Carstens E. Transmitters and pathways mediating inhibition of spinal itch-signaling neurons by scratching and other counterstimuli. PLoS One. 2011;6:e22665. doi: 10.1371/journal.pone.0022665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Tominaga M, Takamori K, Carstens MI, Carstens E. Roles of glutamate, substance P and gastrin releasing peptide as spinal neurotransmitters of histaminergic and non-histaminergic itch. Pain. 2013 doi: 10.1016/j.pain.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin L. The complex role of serotonin and 5-HT receptors in chronic pain. Behavioural pharmacology. 2011;22:390–404. doi: 10.1097/FBP.0b013e328349aae4. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Birdsall TC. 5-Hydroxytryptophan: a clinically-effective serotonin precursor. Altern Med Rev. 1998;3:271–280. [PubMed] [Google Scholar]
- Bjorklund A, Baumgarten HG, Rensch A. 5,7-Dihydroxytryptamine: improvement of its selectivity for serotonin neurons in the CNS by pretreatment with desipramine. Journal of neurochemistry. 1975;24:833–835. [PubMed] [Google Scholar]
- Bouvier M. Oligomerization of G-protein-coupled transmitter receptors. Nat Rev Neurosci. 2001;2:274–286. doi: 10.1038/35067575. [DOI] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ., Jr Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract neurons. Nature neuroscience. 2009;12:544–546. doi: 10.1038/nn.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, Haugen HS, Maurer M, Harder B, Johnston J, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–760. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Marklund U, Yuan W, Yin J, Wegman L, Ericson J, Deneris E, Johnson RL, Chen ZF. Lmx1b is essential for the development of serotonergic neurons. Nature neuroscience. 2003;6:933–938. doi: 10.1038/nn1104. [DOI] [PubMed] [Google Scholar]
- Grudt TJ, Williams JT, Travagli RA. Inhibition by 5-hydroxytryptamine and noradrenaline in substantia gelatinosa of guinea-pig spinal trigeminal nucleus. The Journal of physiology. 1995;485(Pt 1):113–120. doi: 10.1113/jphysiol.1995.sp020716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton LL, Ladenheim EE, Akeson M, Way JM, Weber HC, Sutliff VE, Jensen RT, Wine LJ, Arnheiter H, Battey JF. Loss of bombesin-induced feeding suppression in gastrin-releasing peptide receptor-deficient mice. Proc Natl Acad Sci U S A. 1998;95:3188–3192. doi: 10.1073/pnas.95.6.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci U S A. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- Jeffry J, Kim S, Chen ZF. Itch signaling in the nervous system. Physiology (Bethesda) 2011;26:286–292. doi: 10.1152/physiol.00007.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffry JA, Yu SQ, Sikand P, Parihar A, Evans MS, Premkumar LS. Selective targeting of TRPV1 expressing sensory nerve terminals in the spinal cord for long lasting analgesia. PLoS One. 2009;4:e7021. doi: 10.1371/journal.pone.0007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RT, Battey JF, Spindel ER, Benya RV. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol Rev. 2008;60:1–42. doi: 10.1124/pr.107.07108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D, Nathans J. Signaling by sensory receptors. Cold Spring Harb Perspect Biol. 2012;4:a005991. doi: 10.1101/cshperspect.a005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunarathne WK, Giri L, Kalyanaraman V, Gautam N. Optically triggering spatiotemporally confined GPCR activity in a cell and programming neurite initiation and extension. Proc Natl Acad Sci U S A. 2013;110:E1565–E1574. doi: 10.1073/pnas.1220697110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Kim A, Zhao ZQ, Liu XY, Chen ZF. Postnatal maintenance of the 5-Ht1a-Pet1 autoregulatory loop by serotonin in the raphe nuclei of the brainstem. Molecular brain. 2014;7:48. doi: 10.1186/1756-6606-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroog GS, Jensen RT, Battey JF. Mammalian bombesin receptors. Med Res Rev. 1995;15:389–417. doi: 10.1002/med.2610150502. [DOI] [PubMed] [Google Scholar]
- Lagerstrom MC, Rogoz K, Abrahamsen B, Persson E, Reinius B, Nordenankar K, Olund C, Smith C, Mendez JA, Chen ZF, et al. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron. 2010;68:529–542. doi: 10.1016/j.neuron.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamotte RH, Dong X, Ringkamp M. Sensory neurons and circuits mediating itch. Nat Rev Neurosci. 2013;15:19–31. doi: 10.1038/nrn3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JL, Kaneko T, Shigemoto R, Mizuno N. Distribution of trigeminohypothalamic and spinohypothalamic tract neurons displaying substance P receptor-like immunoreactivity in the rat. The Journal of comparative neurology. 1997;378:508–521. doi: 10.1002/(sici)1096-9861(19970224)378:4<508::aid-cne6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Liu ZC, Sun YG, Ross M, Kim S, Tsai FF, Li QF, Jeffry J, Kim JY, Loh HH, et al. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell. 2011;147:447–458. doi: 10.1016/j.cell.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Wan L, Huo FQ, Barry D, Li H, Zhao ZQ, Chen ZF. B-type natriuretic peptide is neither itch-specific nor functions upstream of the GRP-GRPR signaling pathway. Molecular pain. 2014;10:4. doi: 10.1186/1744-8069-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Perl ER. Selective action of noradrenaline and serotonin on neurones of the spinal superficial dorsal horn in the rat. The Journal of physiology. 2007;582:127–136. doi: 10.1113/jphysiol.2007.131565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. Labeled lines meet and talk: population coding of somatic sensations. J Clin Invest. 2010;120:3773–3778. doi: 10.1172/JCI43426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Milligan G. The prevalence, maintenance, and relevance of G protein-coupled receptor oligomerization. Mol Pharmacol. 2013;84:158–169. doi: 10.1124/mol.113.084780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Nojima H, Shinkado T, Nakahashi T, Kuraishi Y. Itch-associated response induced by experimental dry skin in mice. Jpn J Pharmacol. 2002;88:285–292. doi: 10.1254/jjp.88.285. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Tashiro M, Kano M, Sakurada Y, Itoh M, Yanai K. Imaging of central itch modulation in the human brain using positron emission tomography. Pain. 2003;105:339–346. doi: 10.1016/s0304-3959(03)00249-5. [DOI] [PubMed] [Google Scholar]
- Moore GA, McConkey DJ, Kass GE, O’Brien PJ, Orrenius S. 2,5-Di(tert-butyl)-1,4-benzohydroquinone--a novel inhibitor of liver microsomal Ca2+ sequestration. FEBS letters. 1987;224:331–336. doi: 10.1016/0014-5793(87)80479-9. [DOI] [PubMed] [Google Scholar]
- Murray FS, Weaver MM. Effects of ipsilateral and contralateral counterirritation on experimentally produced itch in human beings. J Comp Physiol Psychol. 1975;89:819–826. doi: 10.1037/h0077028. [DOI] [PubMed] [Google Scholar]
- Nattkemper LA, Zhao ZQ, Nichols AJ, Papoiu AD, Shively CA, Chen ZF, Yosipovitch G. Overexpression of the gastrin-releasing peptide in cutaneous nerve fibers and its receptor in the spinal cord in primates with chronic itch. The Journal of investigative dermatology. 2013;133:2489–2492. doi: 10.1038/jid.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donohue TL, Massari VJ, Pazoles CJ, Chronwall BM, Shults CW, Quirion R, Chase TN, Moody TW. A role for bombesin in sensory processing in the spinal cord. J Neurosci. 1984;4:2956–2962. doi: 10.1523/JNEUROSCI.04-12-02956.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest. 2010;120:3779–3787. doi: 10.1172/JCI43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang YW, Li JL, Nakamura K, Wu S, Kaneko T, Mizuno N. Expression of vesicular glutamate transporter 1 immunoreactivity in peripheral and central endings of trigeminal mesencephalic nucleus neurons in the rat. The Journal of comparative neurology. 2006;498:129–141. doi: 10.1002/cne.21047. [DOI] [PubMed] [Google Scholar]
- Paus R, Schmelz M, Biro T, Steinhoff M. Frontiers in pruritus research: scratching the brain for more effective itch therapy. J Clin Invest. 2006;116:1174–1186. doi: 10.1172/JCI28553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin JP, Neubig R, Bouvier M, Devi L, Filizola M, Javitch JA, Lohse MJ, Milligan G, Palczewski K, Parmentier M, et al. International Union of Basic and Clinical Pharmacology. LXVII. Recommendations for the recognition and nomenclature of G protein-coupled receptor heteromultimers. Pharmacol Rev. 2007;59:5–13. doi: 10.1124/pr.59.1.5. [DOI] [PubMed] [Google Scholar]
- Prezeau L, Rives ML, Comps-Agrar L, Maurel D, Kniazeff J, Pin JP. Functional crosstalk between GPCRs: with or without oligomerization. Current opinion in pharmacology. 2010;10:6–13. doi: 10.1016/j.coph.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Prinster SC, Hague C, Hall RA. Heterodimerization of g protein-coupled receptors: specificity and functional significance. Pharmacol Rev. 2005;57:289–298. doi: 10.1124/pr.57.3.1. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, McAdoo DJ. Amino acids and serotonin are released into the lumbar spinal cord of the anesthetized cat following intradermal capsaicin injections. Brain Res. 1993;607:89–98. doi: 10.1016/0006-8993(93)91492-b. [DOI] [PubMed] [Google Scholar]
- Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004;25:613–617. doi: 10.1016/j.tips.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Takanami K, Sakamoto H, Matsuda KI, Satoh K, Tanida T, Yamada S, Inoue K, Oti T, Sakamoto T, Kawata M. Distribution of gastrin-releasing peptide in the rat trigeminal and spinal somatosensory systems. The Journal of comparative neurology. 2014;522:1858–1873. doi: 10.1002/cne.23506. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Ogawa H, Takamori K. Histological characterization of cutaneous nerve fibers containing gastrin-releasing peptide in NC/Nga mice: an atopic dermatitis model. The Journal of investigative dermatology. 2009;129:2901–2905. doi: 10.1038/jid.2009.188. [DOI] [PubMed] [Google Scholar]
- Uchizono K. Characteristics of excitatory and inhibitory synapses in the central nervous system of the cat. Nature. 1965;207:642–643. doi: 10.1038/207642a0. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- Ward L, Wright E, McMahon SB. A comparison of the effects of noxious and innocuous counterstimuli on experimentally induced itch and pain. Pain. 1996;64:129–138. doi: 10.1016/0304-3959(95)00080-1. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Tyce GM. Release of norepinephrine and serotonin in cat spinal cord: direct in vivo evidence for the activation of descending monoamine pathways by somatic stimulation. J Physiol (Paris) 1981;77:483–487. [PubMed] [Google Scholar]
- Yoshimura M, Furue H. Mechanisms for the anti-nociceptive actions of the descending noradrenergic and serotonergic systems in the spinal cord. J Pharmacol Sci. 2006;101:107–117. doi: 10.1254/jphs.crj06008x. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Duque MI, Fast K, Dawn AG, Coghill RC. Scratching and noxious heat stimuli inhibit itch in humans: a psychophysical study. Br J Dermatol. 2007;156:629–634. doi: 10.1111/j.1365-2133.2006.07711.x. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Papoiu ADP. What causes itch in atopic dermatitis? Curr Allergy Asthma Rep. 2008;8:306–311. doi: 10.1007/s11882-008-0049-z. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Gao X, Ji GC, Huang YL, Wu GC, Zhao ZQ. Expression of 5-HT1A receptor mRNA in rat lumbar spinal dorsal horn neurons after peripheral inflammation. Pain. 2002;98:287–295. doi: 10.1016/S0304-3959(02)00026-X. [DOI] [PubMed] [Google Scholar]
- Zhao Z-Q, Huo F-Q, Jeffry J, Hampton L, Demehri S, Kim S, Liu X-Y, Barry DM, Wan L, Liu Z-C, et al. Chronic itch development in sensory neurons requires BRAF signaling pathways. The Journal of Clinical Investigation. 2013a;123:4769–4780. doi: 10.1172/JCI70528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Chiechio S, Sun YG, Zhang KH, Zhao CS, Scott M, Johnson RL, Deneris ES, Renner KJ, Gereau RWt, et al. Mice lacking central serotonergic neurons show enhanced inflammatory pain and an impaired analgesic response to antidepressant drugs. J Neurosci. 2007a;27:6045–6053. doi: 10.1523/JNEUROSCI.1623-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Gao YJ, Sun YG, Zhao CS, Gereau RWt, Chen ZF. Central serotonergic neurons are differentially required for opioid analgesia but not for morphine tolerance or morphine reward. Proc Natl Acad Sci U S A. 2007b;104:14519–14524. doi: 10.1073/pnas.0705740104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Huo FQ, Jeffry J, Hampton L, Demehri S, Kim S, Liu XY, Barry DM, Wan L, Liu ZC, et al. Chronic itch development in sensory neurons requires BRAF signaling pathways. J Clin Invest. 2013b;123:4769–4780. doi: 10.1172/JCI70528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Scott M, Chiechio S, Wang JS, Renner KJ, Gereau RWt, Johnson RL, Deneris ES, Chen ZF. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci. 2006;26:12781–12788. doi: 10.1523/JNEUROSCI.4143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Wan L, Liu XY, Huo FQ, Li H, Barry DM, Krieger S, Kim S, Liu ZC, Xu J, et al. Cross-Inhibition of NMBR and GRPR Signaling Maintains Normal Histaminergic Itch Transmission. J Neurosci. 2014;34:12402–12414. doi: 10.1523/JNEUROSCI.1709-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.