Abstract
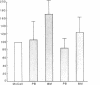
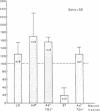
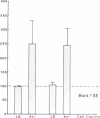
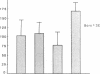
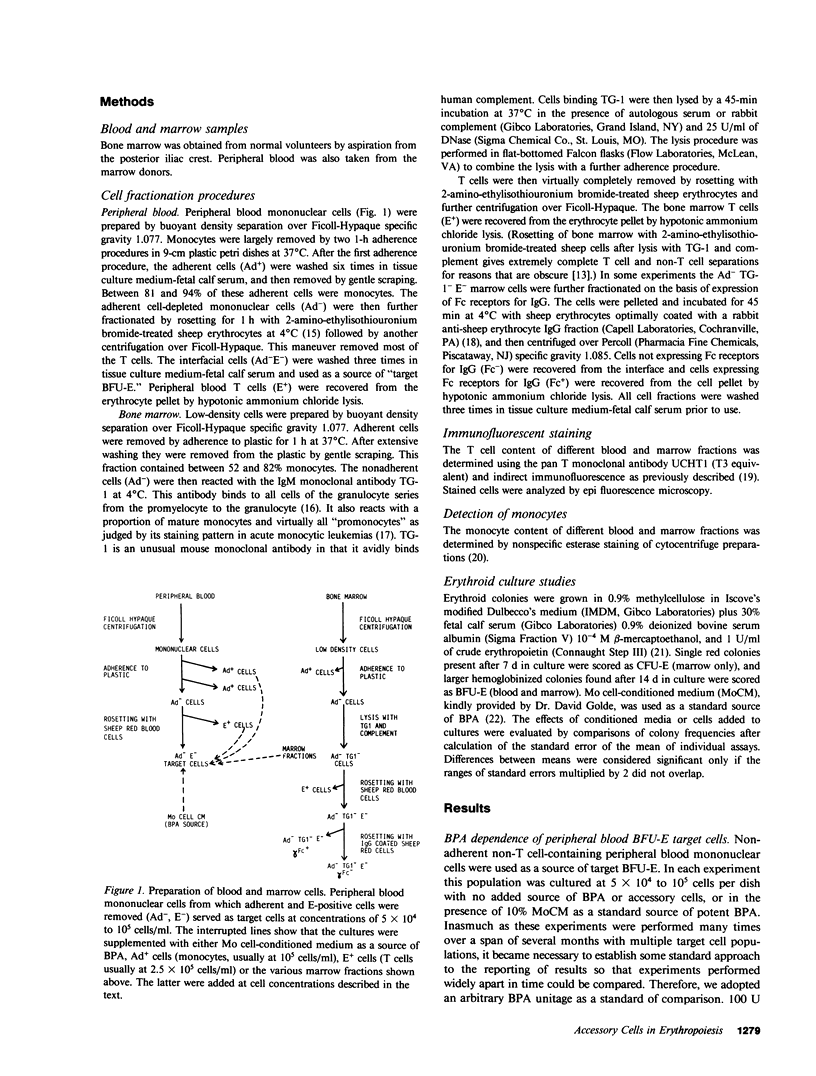
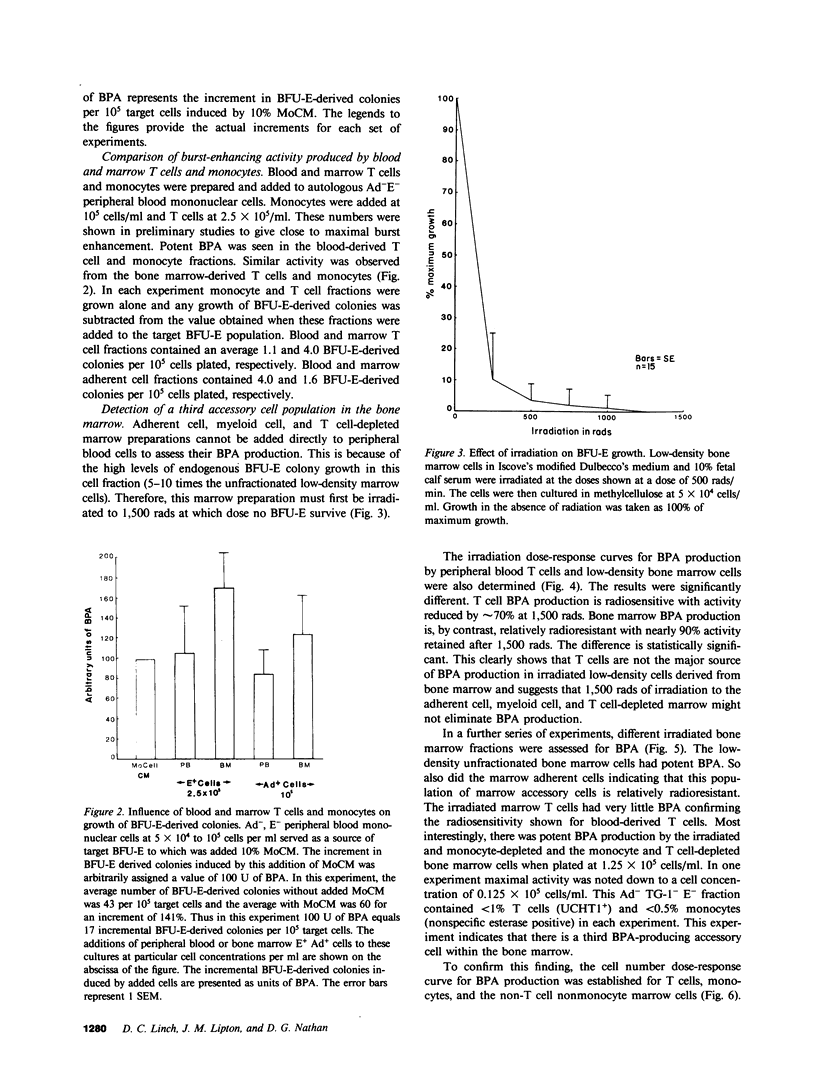
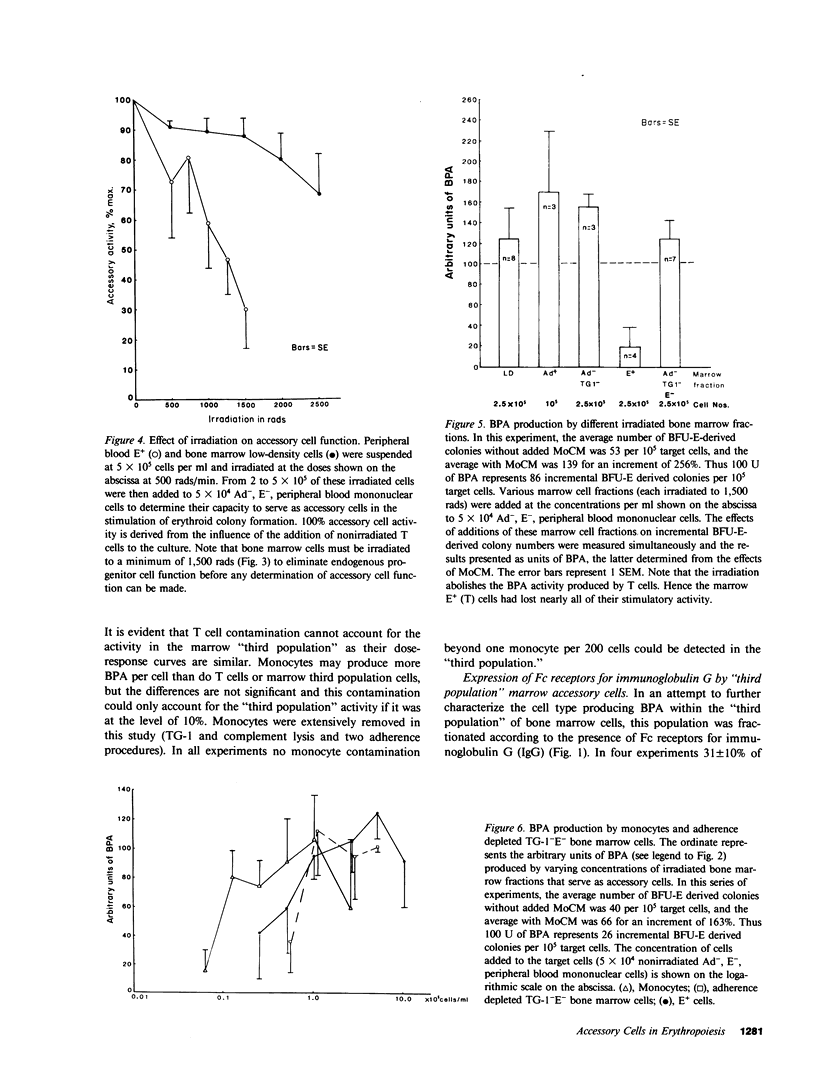
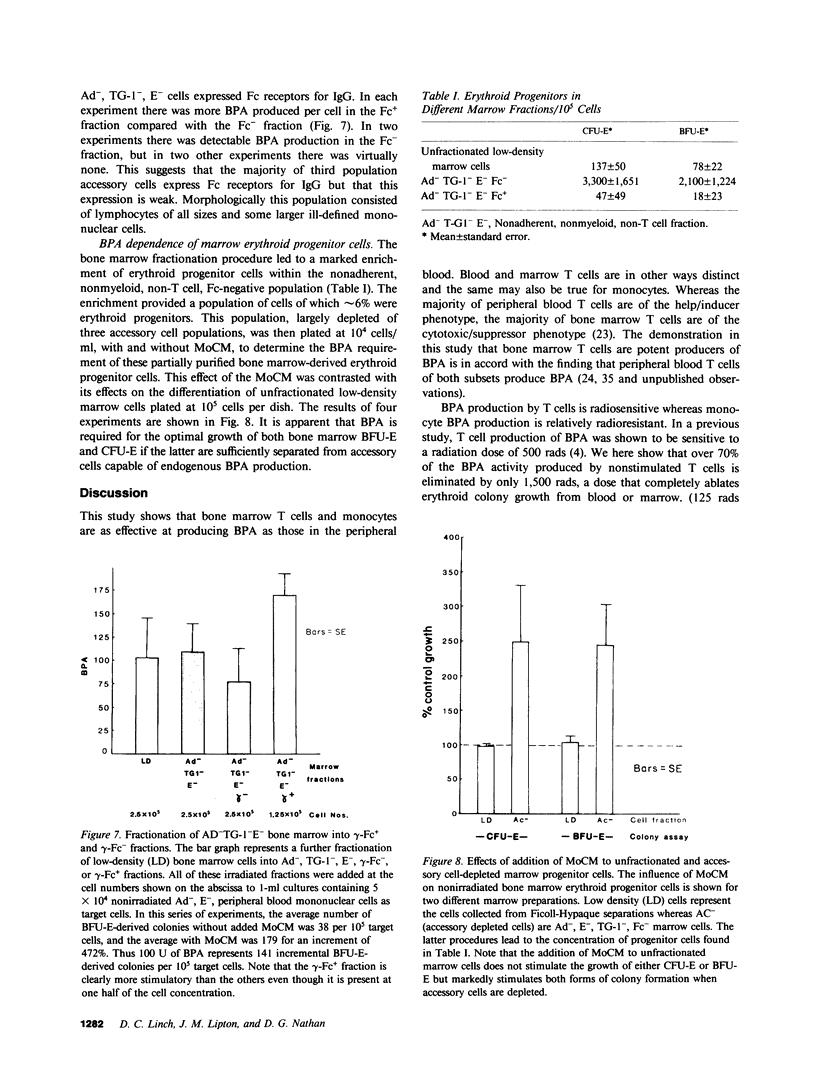
Several laboratories have demonstrated a requirement for burst-promoting activity (BPA), a product of T cells, or T cell/monocyte collaboration in the induction of differentiation of peripheral blood erythroid burst-forming units (BFU-E) in vitro. The physiologic significance of this finding is brought into question by patients with severe mature T cell deficiency who have normal in vivo erythropoiesis. The studies described here were designed to determine whether the burst-promoting effects of marrow T cells and adherent cells are similar to those of peripheral blood, to define whether a third population of marrow cells is capable of production of BPA, and to describe the BPA requirements of immature and mature marrow erythroid progenitors. To that end we prepared adherence- and E-depleted low-density peripheral blood mononuclear cells as a source of BFU-E and demonstrated that their optimal erythropoietin-induced differentiation requires BPA. We then determined that both bone marrow and peripheral blood T cells and monocytes could provide the necessary BPA to induce their erythropoietin dependent differentiation. BPA production by T cells was sensitive to irradiation, but that of the whole bone marrow low-density population was considerably less sensitive. This in itself demonstrated that BPA production in marrow is not T cell dependent. We further demonstrated a potent, albeit infrequent, third population of BPA-producing marrow cells. These proved to be nonadherent, E receptor-negative, granulocyte antigen-negative, and gamma-Fc receptor-positive. Finally, we separated all of these BPA-producing cells from marrow erythroid progenitors and concentrated the latter into a population in which they comprised 6% of the cells. With this population we demonstrated that both immature (BFU-E) and mature (colony-forming units [CFU-E]) erythroid progenitors require BPA in addition to erythropoietin to induce them to form erythroid colonies in vitro. These results may explain the normal erythropoiesis found in patients with mature T cell deficiency. Though the differentiation of both BFU-E and CFU-E requires BPA, this need can be met by a special class of nonadherent, radioresistant, E receptor-negative, granulocyte antigen-negative, and gamma-Fc-positive cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascensao J. L., Vercellotti G. M., Jacob H. S., Zanjani E. D. Role of endothelial cells in human hematopoiesis: modulation of mixed colony growth in vitro. Blood. 1984 Mar;63(3):553–558. [PubMed] [Google Scholar]
- Beverley P. C., Linch D., Delia D. Isolation of human haematopoietic progenitor cells using monoclonal antibodies. Nature. 1980 Sep 25;287(5780):332–333. doi: 10.1038/287332a0. [DOI] [PubMed] [Google Scholar]
- Clarke B. J., Nathan D. G., Alter B. P., Forget B. G., Hillman D. G., Housman D. Hemoglobin synthesis in human BFU-E and CFU-E-derived erythroid colonies. Blood. 1979 Oct;54(4):805–817. [PubMed] [Google Scholar]
- Dainiak N., Davies G., Kalmanti M., Lawler J., Kulkarni V. Platelet-derived growth factor promotes proliferation of erythropoietic progenitor cells in vitro. J Clin Invest. 1983 May;71(5):1206–1214. doi: 10.1172/JCI110869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessypris E. N., Krantz S. B. Effect of pure erythropoietin on DNA-synthesis by human marrow day 15 erythroid burst forming units in short-term liquid culture. Br J Haematol. 1984 Feb;56(2):295–306. doi: 10.1111/j.1365-2141.1984.tb03957.x. [DOI] [PubMed] [Google Scholar]
- Eaves C. J., Eaves A. C. Erythropoietin (Ep) dose-response curves for three classes of erythroid progenitors in normal human marrow and in patients with polycythemia vera. Blood. 1978 Dec;52(6):1196–1210. [PubMed] [Google Scholar]
- Ferrarini M., Moretta L., Abrile R., Durante M. L. Receptors for IgG molecules on human lymphocytes forming spontaneous rosettes with sheep red cells. Eur J Immunol. 1975 Jan;5(1):70–72. doi: 10.1002/eji.1830050115. [DOI] [PubMed] [Google Scholar]
- Golde D. W., Bersch N., Quan S. G., Lusis A. J. Production of erythroid-potentiating activity by a human T-lymphoblast cell line. Proc Natl Acad Sci U S A. 1980 Jan;77(1):593–596. doi: 10.1073/pnas.77.1.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq A. U., Rinehart J. J., Balcerzak S. P. T-cell subset modulation of blood erythroid burst-forming unit proliferation. J Lab Clin Med. 1983 Jan;101(1):53–57. [PubMed] [Google Scholar]
- Iscove N. N., Sieber F., Winterhalter K. H. Erythroid colony formation in cultures of mouse and human bone marrow: analysis of the requirement for erythropoietin by gel filtration and affinity chromatography on agarose-concanavalin A. J Cell Physiol. 1974 Apr;83(2):309–320. doi: 10.1002/jcp.1040830218. [DOI] [PubMed] [Google Scholar]
- Iscove N. N. The role of erythropoietin in regulation of population size and cell cycling of early and late erythroid precursors in mouse bone marrow. Cell Tissue Kinet. 1977 Jul;10(4):323–334. doi: 10.1111/j.1365-2184.1977.tb00300.x. [DOI] [PubMed] [Google Scholar]
- Janossy G., Tidman N., Selby W. S., Thomas J. A., Granger S., Kung P. C., Goldstein G. Human T lymphocytes of inducer and suppressor type occupy different microenvironments. Nature. 1980 Nov 6;288(5786):81–84. doi: 10.1038/288081a0. [DOI] [PubMed] [Google Scholar]
- Kanamaru A., Durban E., Gallagher M. T., Miller S. C., Trentin J. J. Augmentation of erythroid burst formation by the addition of thymocytes and other myelo-lymphoid cells. J Cell Physiol. 1980 Aug;104(2):187–197. doi: 10.1002/jcp.1041040208. [DOI] [PubMed] [Google Scholar]
- Kaplan M. E., Clark C. An improved rosetting assay for detection of human T lymphocytes. J Immunol Methods. 1974 Jul;5(2):131–135. doi: 10.1016/0022-1759(74)90003-9. [DOI] [PubMed] [Google Scholar]
- Linch D. C., Allen C., Beverley P. C., Bynoe A. G., Scott C. S., Hogg N. Monoclonal antibodies differentiating between monocytic and nonmonocytic variants of AML. Blood. 1984 Mar;63(3):566–573. [PubMed] [Google Scholar]
- Lipton J. M., Reinherz E. L., Kudisch M., Jackson P. L., Schlossman S. F., Nathan D. G. Mature bone marrow erythroid burst-forming units do not require T cells for induction of erythropoietin-dependent differentiation. J Exp Med. 1980 Aug 1;152(2):350–360. doi: 10.1084/jem.152.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan K. F., Chikkappa G., Bieler L. Z., Scharfman W. B., Parkinson D. R. Regulation of human blood erythroid burst-forming unit (BFU-E) proliferation by T-lymphocyte subpopulations defined by Fc receptors and monoclonal antibodies. Blood. 1982 May;59(5):990–996. [PubMed] [Google Scholar]
- Meytes D., Ma Ortega J. A., Shore N. A., Dukes P. P. Human erythroid burst-promoting activity produced by phytohemagglutinin-stimulated, radioresistant peripheral blood mononuclear cells. Blood. 1979 Nov;54(5):1050–1057. [PubMed] [Google Scholar]
- Nathan D. G., Chess L., Hillman D. G., Clarke B., Breard J., Merler E., Housman D. E. Human erythroid burst-forming unit: T-cell requirement for proliferation in vitro. J Exp Med. 1978 Feb 1;147(2):324–339. doi: 10.1084/jem.147.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistoia V., Ghio R., Nocera A., Leprini A., Perata A., Ferrarini M. Large granular lymphocytes have a promoting activity on human peripheral blood erythroid burst-forming units. Blood. 1985 Feb;65(2):464–472. [PubMed] [Google Scholar]
- Porter P. N., Ogawa M. Characterization of human erythroid burst-promoting activity derived from bone marrow conditioned media. Blood. 1982 Jun;59(6):1207–1212. [PubMed] [Google Scholar]
- Porter P. N., Ogawa M., Leary A. G. Enhancement of the growth of human early erythroid progenitors by bone marrow conditioned media. Exp Hematol. 1980 Jan;8(1):83–88. [PubMed] [Google Scholar]
- Tepperman A. D., Curtis J. E., McCulloch E. A. Erythropietic colonies in cultures of human marrow. Blood. 1974 Nov;44(5):659–669. [PubMed] [Google Scholar]
- Torok-Storb B., Martin P. J., Hansen J. A. Regulation of in vitro erythropoiesis by normal T cells: evidence for two T-cell subsets with opposing function. Blood. 1981 Jul;58(1):171–174. [PubMed] [Google Scholar]
- Westbrook C. A., Gasson J. C., Gerber S. E., Selsted M. E., Golde D. W. Purification and characterization of human T-lymphocyte-derived erythroid-potentiating activity. J Biol Chem. 1984 Aug 25;259(16):9992–9996. [PubMed] [Google Scholar]
- Wisniewski D., Strife A., Wachter M., Clarkson B. Regulation of human peripheral blood erythroid burst-forming unit growth by T lymphocytes and T lymphocyte subpopulations defined by OKT4 and OKT8 monoclonal antibodies. Blood. 1985 Feb;65(2):456–463. [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- Zuckerman K. S. Human erythroid burst-forming units. Growth in vitro is dependent on monocytes, but not T lymphocytes. J Clin Invest. 1981 Mar;67(3):702–709. doi: 10.1172/JCI110086. [DOI] [PMC free article] [PubMed] [Google Scholar]