SUMMARY
Monoamine serotonin (5HT) has been linked to aggression for many years across species [1–3]. However, elaboration of the neurochemical pathways that govern aggression has proven difficult because monoaminergic neurons also regulate other behaviors [4, 5]. There are about 100 serotonergic neurons in the Drosophila nervous system and they influence sleep [6], circadian rhythms [7], memory [8, 9] and courtship [10]. In the Drosophila model of aggression [11] the acute shut down of the entire serotonergic system yields flies that fight less, while induced activation of 5HT neurons promotes aggression [12]. Using intersectional genetics we restricted the population of 5HT neurons that can be reproducibly manipulated to identify those that modulate aggression. Although similar approaches were used recently to find aggression-modulating dopaminergic [13] and FruM –positive peptidergic [14] neurons, the downstream anatomical targets of the neurons that make up aggression-controlling circuits remain poorly understood. Here we identified a symmetrical pair of serotonergic PLP neurons that are necessary for the proper escalation of aggression. Silencing these neurons reduced, and activating them increased aggression in male flies. GFP reconstitution across synaptic partners (GRASP) [15] analyses suggests that 5HT-PLP neurons form contacts with 5HT1A receptor - expressing neurons in two distinct anatomical regions of the brain. Activation of these 5HT1A receptor-expressing neurons, in turn, caused reductions in aggression. Our studies, therefore, suggest that aggression may be held in check, at least in part, by inhibitory input from 5HT1A receptor-bearing neurons, which can be released by activation of the 5HT-PLP neurons.
RESULTS and DISCUSSION
Isolation and anatomical characterization of individual serotonergic neurons
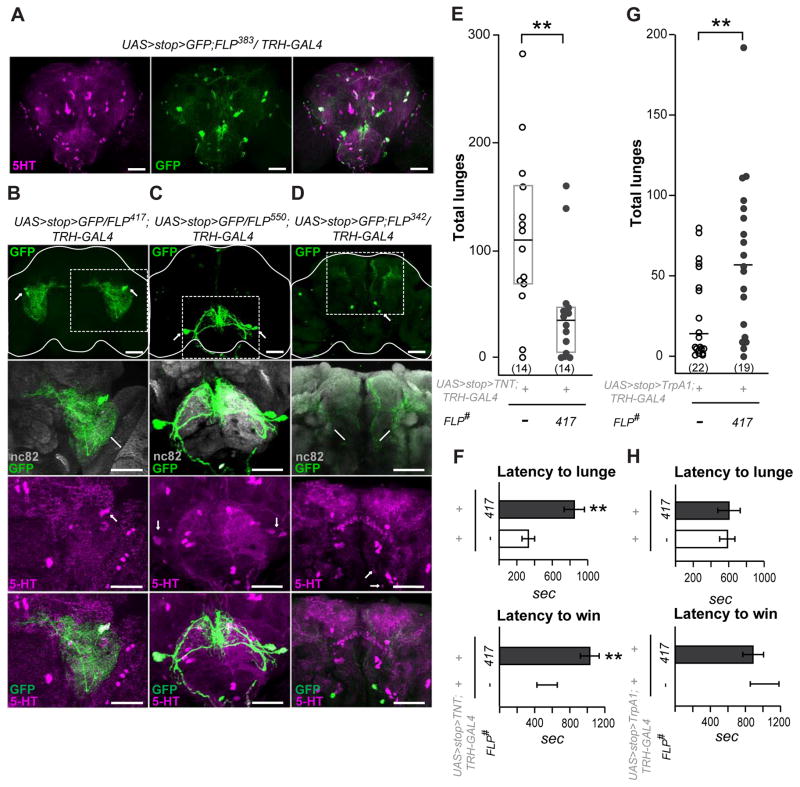
As in other species, serotonergic neurons in the fly nervous system display arbors of processes that ramify widely in multiple neuropil areas, through which they affect virtually all aspects of behavior. We therefore have used an intersectional genetics approach to isolate restricted sets of serotonergic neurons and manipulate their function in order to ask whether they are involved in the regulation of specific behaviors like aggression, or exert multiple modulatory actions on many behaviors. We screened 65 enhancer-trap FLP recombinase transgenic lines (et-FLP) [13] with a serotonin-specific TRH-Gal4 driver [12] and a UAS>stop>mCD8::GFP reporter seeking to find combinations that resulted in reproducible GFP expression in small subsets of 5HT neurons. While several broadly expressed FLP lines displayed a major overlap between the GFP-positive neurons and the total populations of 5HT neurons (for example, line FLP383, see Figure 1A), only three FLP lines reproducibly targeted very restricted sets of 5HT neurons. We further characterized each type of the isolated 5HT neurons by identifying the areas of their arborization within known neuropil regions throughout the brains. The line FLP417 (Figure 1B) targeted 1–2 5HT-positive neurons from the PLP cluster on each side of the brain. The PLP cell bodies are located on the posterior surface of the brain, but their arbors form a dense neuropil throughout the ventrolateral protocerebrum and also ramify towards the central complex structures (Figure 1B). A second line, FLP550, in combination with the TRH-Gal4 driver, consistently labeled two large serotonergic neurons from the SE1 cluster (Figure 1C). These neurons arborize in the dorsal region of the suboesophageal ganglion and send thick descending projections to the ventral nerve cord. The last of the selected 5HT-specific lines, FLP342, targeted neurons from the posterior PMP cluster (Figure 1D) that send projections to the superior medial protocerebrum.
Figure 1. A single pair of serotonergic PLP neurons enhances aggression.
A–D. Serotonergic neurons identified by the enhancer trap (et)-FLP screen.
A. Example of a broadly expressed FLP line that targets most of the 5HT neurons in the fly brain. The anti-5HT immunostaining pattern is shown in magenta, the membrane-tethered GFP signal driven by a combination of FLP383, TRH-Gal4 and UAS>stop>mCD8::GFP is shown in green. The full z-stack frontal projection is shown, scale bar represents 50 μm. B–D. Individual 5HT neurons targeted by the use of different et-FLP lines. The mCD8::GFP signal amplified by anti-CD8 antibody staining is shown in green, the neuropil areas stained by an nc82 (anti-Bruchpilot) antibody are shown in gray, and anti-5HT immunostaining is shown in magenta. Dotted boxes outline the magnified fields shown in the lower panels. The upper panels show full frontal projections, scale bar represents 50 μm. Different frontal z-stacks through either the anterior or posterior areas of the same triple-stained brains were created when required to view the processes or cell bodies shown in the lower panels. B. The FLP417 line restricts GFP expression to 1–2 bilateral neurons from the PLP cluster (green). These neurons arborize within the ventrolateral protocerebrum (VLP) and send a midline directed process toward the central complex (see Figure 3A for more details). C. The FLP550 line restricts GFP expression to 1–2 bilateral neurons from the SE1 cluster (green). These arborize within the suboesophageal ganglion (SOG) and send descending projections to the ventral nerve cord. D. The FLP342 line restricts GFP expression to 1–2 bilateral neurons from the PMP cluster (green) that arborize in the superior medial protocerebrum.
For et-FLP lines reproducibility and cell count data see Table S1.
E–H. Manipulation of individual 5HT neurons from the PLP cluster targeted by FLP417 changes aggression. E. Total numbers of lunges performed by pairs of males with TNT-inactivated 5HT-PLP neurons. F. Latency to the first lunge and to the establishment of dominance in flies with TNT-inactivated 5HT-PLP neurons.
In E and F both genetic control and experimental flies were reared and fought at constant +25°C conditions. Note, that the reduction in lunge numbers was not due to the increased latency to lunge, because the number of lunges counted for 30 min after the first lunge rather than from the time of landing on the food surface, was also reduced [FLP417: 74.2 ± 17.5; controls: 134.9 ± 22.0, Mann-Whitney U=54, P=0.043].
G. Total numbers of lunges performed by pairs of males with dTrpA1-activated 5HT-PLP neurons. H. Latency to the first lunge and to the establishment of dominance in flies with dTrpA1-activated 5HT-PLP neurons.
In G and H both genetic control and experimental flies were reared at +19°C and transferred to a +27°C experimental room 15 min before the aggression assay.
Each dot in panels E and G represents the lunge count for an individual pair of flies. Data are presented as boxplots with a median line. The bottom and top of the box show the 25th and 75th percentile. Latencies in panels F and H are presented as means ± SEM. ** -p<0.01 vs. controls (white bar or white dots), analyzed by nonparametric two-independent-sample Mann-Whitney U-test. See Figure S1C for FLP parental control aggression data.
Serotonergic PLP neurons enhance aggression
Our previous findings [12] demonstrated that acute disruption of serotonergic neurotransmission yielded male flies that could fight but displayed a dramatic reduction in the number of higher intensity aggressive interactions. Here, we asked whether silencing of any of the genetically isolated 5HT neurons using the tetanus neurotoxin light chain (TNT) [16] had effects on aggression. TNT cleaves the synaptic vesicle associated protein, synaptobrevin, thereby chronically blocking transmitter release [17]. For these experiments, we paired socially naïve males in multiwell plate aggression chambers [18]. The most important pattern in a Drosophila male aggressive attack is the lunge, a high intensity behavioral pattern required for the establishment of dominance relationships. To demonstrate the dynamics of fights, we measured how long it takes to initiate higher intensity attacks (the latency to the first lunge), the intensity levels displayed by the pair of flies (the numbers of lunges), and whether and when a dominance relationship was established as an outcome of a fight (the latency to establish dominance).
Early on we noticed that chronic silencing of large populations of 5HT neurons produced unhealthy flies that had difficulty landing on the food cup in the fight chamber and had profound locomotion deficits. As a consequence, they did not fight. This phenotype was observed with several broadly expressed lines, which targeted many 5HT neurons (FLP303, FLP402, FLP383, Figure S1A). Thus, any possible effects of individual 5HT neurons on aggression probably were masked by a major locomotion deficiency in these cases. Then, we checked the general activity of the flies with inactivated individual 5HT neurons and found that one of the restricted lines (FLP550) also produced unhealthy flies with noticeable locomotion deficit (Figure S1A). These flies did not fight either. In contrast, inactivation of neurons from the PMP cluster targeted by FLP342 had no effects on either locomotion or aggression (data not shown). Only the PLP neurons targeted by FLP417 yielded an aggression phenotype that was not accompanied by substantial deficits in other behaviors. Therefore, for the rest of the study we focused on the serotonergic PLP neurons. Inactivation of these neurons produced flies that not only lunged less often than controls (Figure 1E), but also took longer to start lunging and to establish dominance relationships (Figure 1F). To confirm the specificity of the observed aggression phenotype of the PLP neurons we acutely activated them using the UAS>stop>dTrpA1Myc transgene [19]. dTrpA1 is a temperature-sensitive cation channel that, when expressed in neurons, allows activation of the cells by small temperature increases [20]. We verified that the dTrpA1Myc transgene actually was expressed in the neurons of interest by dissecting the experimental fly brains and processing them for anti-Myc staining after completion of the aggression assays. In contrast to the TNT silencing results, activation of the PLP neurons produced significant increases in the numbers of lunges (Figure 1G). This phenotype was similar to our previously demonstrated effects of induced activation of the entire population of 5HT neurons on the numbers of lunges [12]. However, activation of the PLP neurons did not reduce the latency to lunge, unlike what was observed with activation of the entire 5HT system. This suggests that other, yet unidentified, 5HT neurons might also be involved in the modulation of aggression. Activation of the 5HT neurons targeted by either FLP550 or FLP342 had no effects on aggression (data not shown).
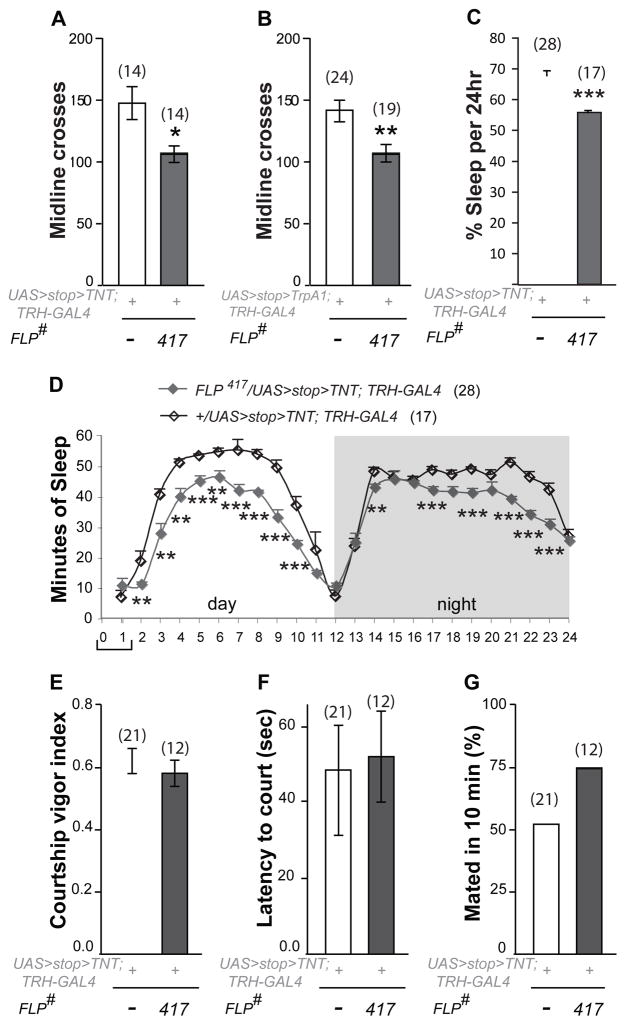
To ask whether the 5HT-PLP neurons played roles in behaviors other than aggression we expressed the TNT transgene in them and examined: (i) locomotion and sleep as indicators of general activity; and (ii) courtship as an example of a different social behavior. Flies with inactivated PLP neurons showed a small but significant deficit in locomotion (Figure 2A). It raises the question of whether the aggression-attenuating phenotype described above might be a consequence of the fact that these flies are simply “slower” then controls. However, a similar locomotion deficit was observed after dTrpA1-induced activation of the PLP neurons (Figure 2B), along with an increase of aggression (Figure 1G). These results indicate that the opposing aggression phenotypes caused by inhibition and activation of the 5HT-PLP neurons are not an indirect effect of a change in locomotor activity. An examination of 24hr sleep patterns (Figure 2C–D) revealed that these flies slept on average less than controls during both day and night. They did, however, show normal diurnal sleep profiles and also the expected circadian anticipation of light and dark phases of the cycles. Importantly, their amount of sleep was not different from controls during the first hour of morning activity peak when aggression assays were performed (Figure 2D, bracket). Next, we examined male courtship, as a different social behavior. We measured courtship vigor index, latency to court and copulation success, and found no deficits in flies with inactivated PLP neurons (FLP417, Figure 2E–G) - they performed courtship rituals as efficiently as controls. Thus, manipulation of 5HT-PLP neurons produced mild effects on activity-related behaviors that did not correlate with the observed effects on aggression or interfered with courtship behavior.
Figure 2. Manipulation of 5HT-PLP neurons has selective effects on behavior.
A. Inactivation of 5HT-PLP neurons produces a mild locomotion deficit. B. Induced activation of 5HT-PLP neurons produced a mild locomotion deficit. C. The inactivation of 5HT-PLP neurons produced sleep deficit measured by average percentage of sleep per 24 h. The data in A–C are presented as means ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 vs. controls (white bar) analyzed using a nonparametric two-independent-sample Mann–Whitney U test. D. Distribution of sleep during averaged 24-h periods in flies with inactivated 5HT-PLP neurons. Gray line - experimental flies; black line - controls. Data are presented as means ± SEM; **P < 0.01, ***P < 0.001 vs. the corresponding hour data point of the control group, analyzed by an unpaired t-test. The bracket shows the time of the day when aggression assays were performed.
E–G. Courtship behavior is unaffected by TNT inactivation of the aggression-modulating PLP (FLP417) neurons. The data for the courtship vigor index (E) and for the latency to court (F) are presented as means ± SEM. Courtship success is calculated as a percent of males that mated in 10 min of the assay (G).
Also see Figure S1B–E for FLP parental control data.
To rule out the possibility that FLP transgene insertion sites alone may contribute to the observed phenotypes, we examined flies carrying et-FLP transgenes without the Gal4 driver or UAS-effector. None of the progeny of these flies crossed to wild-type Canton-S replicated the locomotion (Figure S1B), aggression (Figure S1C), or sleep (Figure S1D–E) phenotypes seen with the full complement of corresponding transgenes.
Targets of the aggression-modulating 5HT-PLP neurons
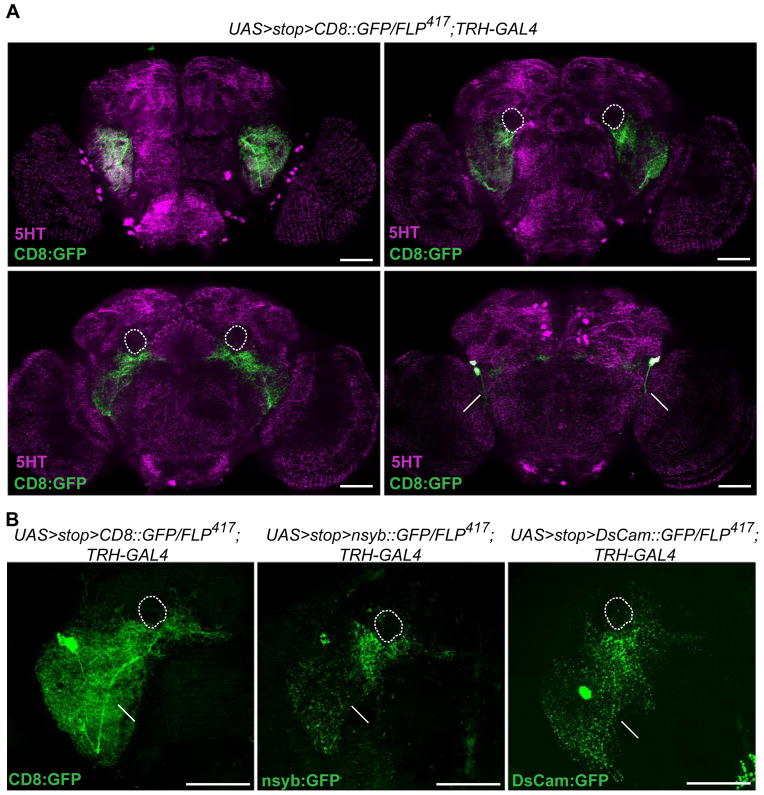
The detailed morphological analysis showed that the densest arborizations of the 5HT-PLP neurons are in the ventrolateral protocerebrum (Figure 3A, top left), a region previously characterized as an integrative center for auditory [21], visual [22] and olfactory processing [23]. The 5HT-PLP neurons also ramify around the peduncles of the mushroom body (Figure 3A, white dotted line) and near the fan-shaped body of the central complex (Figure 3A, bottom left), while their cell bodies and axons are located close to the posterior surface of the brain. However, our attempts to determine putative target areas of the 5HT-PLP neurons through their dendritic and axonal morphology failed since both the presynaptic nsyb::GFP and the dendritic DsCam::GFP markers [19] labeled much of the dense arborization fields of those neurons (Figure 3B). Thus, in contrast to the clearly identifiable axonal and dendritic fields of dopaminergic neurons concerned with aggression [13], the input to and output neuropil regions of the serotonergic PLP neurons remained unspecified.
Figure 3. Anatomical characterization of the aggression-modulating 5HT-PLP neurons.
A. Arborization patterns of the PLP neurons visualized by membrane-bound CD8::GFP (green) relative to anti-5HT labeled neuropil regions of the brain (magenta). These are displayed in frontal z-projections of an image stack through the ventrolateral protocerebrum and antennal lobes (top left), the ellipsoid body of the central complex and the peduncles of the mushroom bodies (top right), the fan-shaped body of the central complex (bottom left) and a posterior view of the brain where the PLP cell bodies and their axons are located (bottom right). Scale bar represents 50 μm. Short arrows point to cell bodies, long arrows to axons of the PLP neurons. A dotted line outlines the peduncles of the mushroom bodies that are not stained by anti-5HT antibodies.
B. Polarity of the serotonergic PLP neurons. (Left) The total arborization field of the PLP neurons visualized using membrane-bound CD8::GFP. (Center) The putative presynaptic terminals of the PLP neurons revealed using the presynaptic marker nsyb::GFP. (Right) The putative dendritic arbors of the PLP neurons visualized by expression of the postsynaptic marker DsCam:GFP. Full z-stack frontal projections are shown, scale bar represents 50 μm.
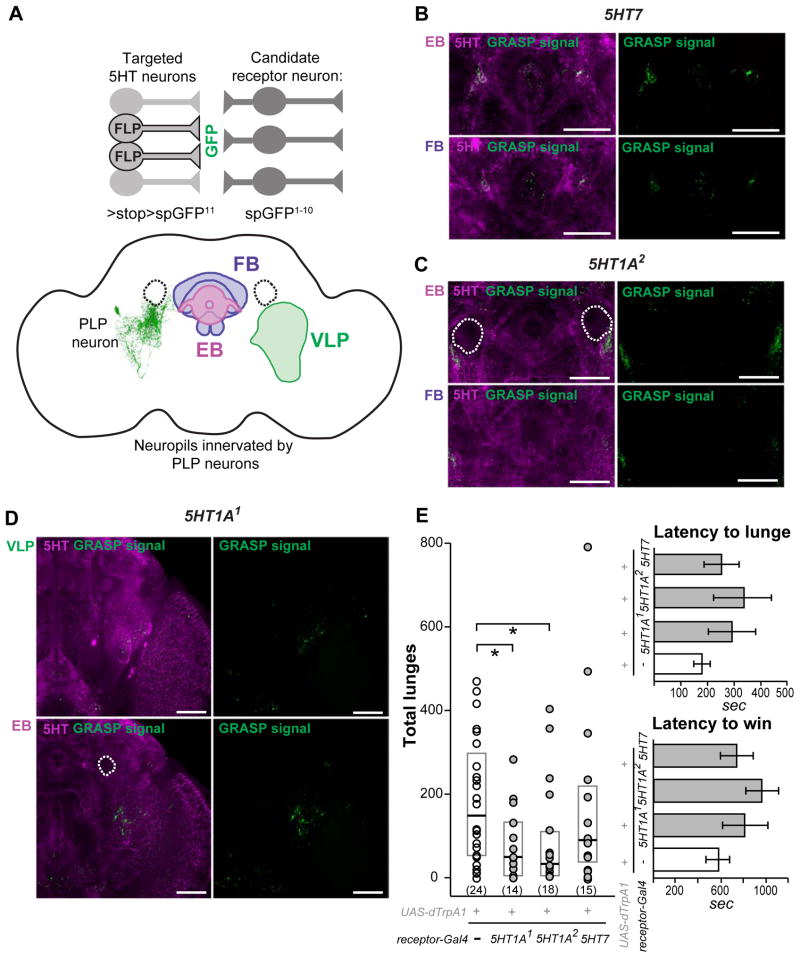
The actions of serotonin are mediated via distinct types of receptors expressed on the surface of target neurons, where they commonly modulate the firing properties of neurons and/or change the effects of excitatory and inhibitory signals to and from the cells [24]. There are four known types of Drosophila serotonin receptors, 5HT1A [6, 25], 5HT1B [7], 5HT2 [26] and 5HT7 [10], any of which could be expressed by neurons downstream of the 5HT-PLP cells. While a variety of Gal4 driver lines exist that presumably target 5HT receptor-bearing neurons, none has been fully evaluated since defined antibodies to subtypes of Drosophila serotonin receptors are unavailable. Therefore, we used the GFP reconstitution across synaptic partners (GRASP) technique [15] to identify downstream targets of the PLP neurons by visualizing the anatomical connections with their putative synaptic partners. The method is based on expressing two parts of GFP molecule by two different neurons. If both parts of the GFP are in very close proximity, as in synaptic regions, GFP is reconstituted and fluorescence is detected. We picked ten candidate 5HT receptor-Gal4 lines that targeted anatomical regions where arborization fields of the PLP neurons were observed (Table S2). We searched for possible connectivity or physical proximity between processes of those candidate receptor neurons and serotonergic neurons by the generation of a GRASP signal between them. To that end, we drove expression of one part of the GFP molecule (spGFP1–10) using candidate receptor-Gal4 lines, and we generated a TRH-LexA line to express the other portion of the GFP molecule (spGFP11) in 5HT neurons using the LexA/LexAop system. We found one 5HT1A1-Gal4 line that produced reconstituted GFP signal in the ventrolateral protocerebrum, a second 5HT1A2-Gal4 line that generated reconstituted GFP around the mushroom body peduncles, and 3 other lines (5HT7-Gal4, 5HT2-Gal4 and 5HT1A3-Gal4) that resulted in reconstituted GFP signals in central complex structures (Table S2).
The novel TRH-LexA line that we used to express the spGFP11 component of GRASP in serotonergic neurons has a broad expression pattern involving most of the 5HT neurons in the central brain area but also targets some non-serotonergic cells (Figure S2A). Therefore, we sought to combine the GRASP method with the intersectional strategy to express spGFP11 in restricted sets of 5HT neurons in order to identify their potential synaptic partners (cartoon in Figure 4A). We generated a LexAop>stop>spGFP11 line and used it first in combination with TRH-LexA and one of the broadly expressed lines, FLP210, which targets most of serotonergic neurons including the 5HT-PLP neurons (Figure S2E). This was to reconfirm the reconstituted GFP patterns described above when using the regular GRASP method. As expected, the combination of TRH-LexA and FLP210 resulted in a similar, but better defined reconstituted GFP signal between 5HT neurons and their close candidate downstream synaptic partners (Figure 4B–D). Detailed image analysis revealed that only three of the five candidate serotonin receptor GAL4 lines showed a GFP signal overlapping with or near areas of interest as potential targets of the 5HT-PLP neurons. These were the 5HT7-Gal4 line that showed putative synaptic contacts near the fan-shaped body of the central complex (Figure 4B), the 5HT1A2 -Gal4 line around the peduncles of the mushroom bodies (Figure 4C) and the 5HT1A1 -Gal4 line in the ventrolateral protocerebrum (Figure 4D). In some brain samples a 5HT1A1-derived GRASP signal was detected over the axons of the 5HT-PLP cells (Figure S3A–B), suggesting that close contacts might exist between 5HT1A1-bearing neurons and the axons of serotonergic PLP neurons. With the 5HT7 and 5HT1A2 -derived GRASP signals it was difficult to say whether the GFP puncta were derived from synaptic contacts with PLP neurons or with other serotonergic neurons branching in the same areas. Our further attempts to restrict the GRASP analysis to a single-cell level by utilizing a combination that should target only the 5HT-PLP neurons (e.g., using TRH-LexA and FLP417 - Figure S2F), yielded no GFP signal. Possibly, a successful reconstitution of GFP between individual neurons depends on the density and shape of their synaptic contacts, setting a limitation on the use of this approach at the present time.
Figure 4. Putative targets of 5HT-PLP neurons determined using the anatomical and functional analyses.
A. A schematic illustration of the combined use of the GRASP and FLP-recombinase techniques to find possible synaptic connections between serotonergic neurons and target neurons that express different subtypes of 5HT receptors. We used the FLP210 line that targets most of the serotonergic neurons in the brain to restrict the expression of the spGFP11 part of GFP driven by the TRH-LexA. The other part of GFP, spGFP1–10, was expressed under control of different 5HT-receptor Gal4 drivers. We found GRASP signals in 3 neuropil regions where arborization of 5HT-PLP neurons was observed: ventrolateral protocerebrum (VLP), around peduncles of the mushroom bodies at the ellipsoid body (EB) focal plane, and near fan-shaped body (FB).
The use of FLP417 line that further restricted the expression of the spGFP11 to aggression-modulating 5HT-PLP neurons yielded no detectable GRASP signals.
B–D: Patterns of reconstituted GFP (GRASP signal, green) between most of serotonergic neurons and candidate 5HT receptor neurons in the areas of interest, which are visualized by anti-5HT immunostaining (magenta). The experimental genotypes used were: w1118; LexAop>stop>spGFP11/FLP210; UAS-spGFP1–10, TRH-LexA/5HT receptor-Gal4. Different frontal z-projections of the image stack were created to view the corresponding neuropils of the same brain. The three neuropil regions (VLP, EB, and FB) were examined for each receptor type, but only regions that showed GRASP signal are shown. The dotted circles outline the peduncles of the mushroom bodies that were not stained by the anti-5HT antibody. White arrows point to areas in which GRASP signal is observed. Scale bar represents 50 μm. For positive and negative GRASP controls see Figure S2, for additional GRASP data see Figure S3. E. dTrpA1-induced activation of 5HT1A receptor neurons decreases the total numbers of lunges. Each dot represents the lunge count for an individual pair of flies. Data are presented as boxplots with a median line. The bottom and top of the box show the 25th and 75th percentile. *- P< 0.05 vs. corresponding control (white dots), analyzed by nonparametric two-independent-sample Mann-Whitney U-test. Latency to the first lunge and to the establishment of dominance in flies with dTrpA1-activated 5HT receptor neurons was not changed. The latencies are presented as means ± SEM.
Thus, the GRASP data showed that the processes of 5HT-PLP neurons are closely apposed to those of 5HT1A receptor-bearing neurons in the ventrolateral protocerebrum and near the peduncles of the mushroom body, and also to 5HT7 receptor-bearing neurons near the fan-shaped body.
Induced activation of 5HT1A receptor-bearing neurons reduces aggression
We next asked whether neurons that express 5HT1A receptors might serve as downstream targets in pathways involved with the serotonergic modulation of aggression. In mammalian systems [27] and in Drosophila [28] activation of 5HT1A receptors inhibits cAMP production, hyperpolarizes neurons and reduces neuronal excitability [27]. Activation of neurons bearing 5HT1A receptors in behaving animals, however, should yield opposite effects and might offer possible clues as to the normal behavioral roles served by some of these neurons. To test this hypothesis, we expressed the dTrpA1 channel in both populations of 5HT1A-bearing neurons and examined the aggressive behavior of flies shortly after thermal activation of the channel. We found that the numbers of lunges in flies with activated 5HT1A-bearing neurons using both the 5HT1A1-Gal4 (ventrolateral protocerebrum target area) and 5HT1A2-Gal4 (peduncle target area) drivers were significantly lower than in controls (Figure 4E). The magnitude of the effect on the numbers of lunges (~ 2 fold decrease) was similar to that observed with TNT inactivation of the 5HT-PLP neurons. This suggests that much of the PLP neuronal influence on aggression is mediated via 5HT1A receptor-bearing neurons. This, however, does not eliminate the possibility that other aggression-related neurons or circuits might also receive modulating influences from the 5HT-PLP neurons. Activation of the neurons expressing 5HT7 receptors had no effects on aggression (Figure 4E). These data combined with the fact that activation of the 5HT-PLP neurons results in an enhancement of aggression (see above) raise the possibility that 5HT released from activated PLP neurons might inhibit 5HT1A-bearing neurons that are key components of a descending aggression-suppressing pathway. Inhibition of an inhibitory pathway could subsequently lead to the display of higher levels of aggression This suggestion compares favorably with a model proposed in a vertebrate system in which activation of 5HT1A post-synaptic receptors, located on GABAergic interneurons, triggers hyperpolarizing responses to released 5HT. These hyperpolarizing responses reduce the postsynaptic neuronal excitability and firing rates thereby relieving the inhibition on the system [29]. Mammalian 5HT1A receptors show differential brain-region-specific transcriptional regulation [30] and are implicated in the regulation of mood, emotions and stress responses. In addition, they are candidate targets in the management of various neuropsychiatric disorders [31]. Moreover, similar inhibitory control mechanisms have been reported in Drosophila feeding circuits [32] and in a hierarchical inhibition switch observed in appetitive memory performance [33, 34].
Displays of appropriate levels of aggression rely on the ability of an animal to analyze many factors including: the correct identification and evaluation of the abilities of potential competitors; evaluation of the value of a territory and the likelihood of acquiring it; and the physiological state of the animal. Multiple sensory systems and circuits will be utilized in making such evaluations. The fixed numbers of neurons and neuronal circuits in nervous systems might limit the abilities of an animal to evaluate such a multiplicity of factors but great flexibility is introduced into the system by the availability of neuromodulators. These have the capability of rapidly, efficiently and reversibly reconfiguring the networks of neurons without changing the ‘hard-wiring’. The studies reported here illustrate the modulation by 1–2 pairs of serotonergic neurons that enhance aggression. Other modulatory neurons and systems have been identified previously in Drosophila that influence aggression including dopaminergic neurons [13], FruM –positive octopamine neurons that influence the behavioral choice between courtship and aggression [35], FruM –positive tachykinin [14] neurons that enhance aggression and neuropeptide F circuits that decrease aggression [36]. The arbors of processes of the 5HT-PLP neurons examined here densely innervate several integrative centers in the fly brain, but thus far they do not seem to overlap with the processes of the other reported aggression-influencing neuromodulatory neurons. The 5HT-PLP neurons do not co-express FruM or Dsx (unpublished observations). Thus, the modulatory control of the male-specific higher level aggression appears to involve both sex-specific regulatory factors [14, 35] and other as yet unidentified control elements. Our studies suggest further that going to higher intensity levels in fights may be held in check by inhibition, which can be released by activation of the 5HT-PLP neurons. Learning more about the neurons and neuronal circuits involved with a suggested downstream aggression-suppressing system and with the sensory systems that trigger aggression in the first place will be essential steps in further unraveling the complex circuitry that controls the release of aggression in Drosophila.
In summary, using a Drosophila model system and an intersectional genetic strategy, we identified a pair of serotonergic neurons in the PLP cluster that modulate aggressive behavior. These neurons arborize through several neuropil regions in the central brain where they influence the escalation of aggression, at least in part, via 5HT1A receptor-bearing neurons, and also independently influence locomotion and sleep. The single-cell resolution in identification of neuronal connections and explorations of their functioning in behaving animals provides an entry points into unraveling the circuitry associated with complex behaviors like aggression.
Supplementary Material
Figure S1, related to main Figures 1–2.
TNT inactivation of isolated 5HT neurons has selective effects on behavior.
A. Inactivation of large populations of 5HT neurons (light-gray bars) results in very low levels of locomotion, while inactivation of restricted 5HT neurons (dark-gray bars) produces varying effects. Data presented as means ± SEM, number of animals is indicated in parenthesis. ** -p<0.01; *** - p<0.001 vs. corresponding control (white bar), analyzed by nonparametric two-independent-sample Mann-Whitney U-test.
B. Chromosomal placement of FLP transgenes does not alter locomotion. Males carrying a single copy of various et-FLP transgenes (light and dark gray bars) have similar levels of locomotion as wild-type Canton-S males (white bar). Data are presented as means ± SEM, number of animals is indicated in parenthesis.
C. Chromosomal placement of FLP transgenes does not alter aggression. Numbers of lunges between pairs of males carrying a single copy of various FLP transgens are the same as in pairs of wild-type Canton-S males. Each dot represents the lunge count for an individual pair of flies. Number of tested pairs is indicated in parenthesis. The data are presented as boxplots with a median line. The lower and upper parts of the boxes are 25th and 75th percentiles, respectively.
D–E. Chromosomal placement of FLP417 transgene does not alter the average percentage of sleep (D) or the distribution of sleep (E). Gray line - males carrying a single copy of FLP417 transgene (FLP417/CS), black line - wild-type Canton-S males (CS). Data are presented as means ± SEM.
Figure S2, related to main Figure 4.
Positive and negative controls for GRASP combined with the intersectional strategy.
A. The TRH-LexA driven mCD8:GFP signal alone (green, upper panel) or counterstained with an anti-5HT antibody (magenta, lower panel).
B–D. GRASP negative controls. Flies carrying the two spGFP components of GRASP without either the Gal4 driver or the FLP-recombinase showed no detectable GFP signal in the ventrolateral protocerebrum (B), the ellipsoid body focal plane (C) or the fan-shaped body focal plane (D). The GRASP signal was visualized using a mouse anti-GFP-20 (Sigma) antibody (shown in green), while the anti-5HT immunostaining is shown in magenta.
E. Combination of the FLP210 line with the TRH-LexA line, which was used in GRASP experiments, targets a large population of 5HT neurons. The anti-5HT immunostaining pattern is shown in green, the mCherry signal driven by a combination of FLP210, TRH-LexA and LexAop>stop>BRP::mCherry is shown in magenta.
F. Combination of the FLP417 line with the TRH-LexA line, which was used in GRASP experiments, targets the individual 5HT-PLP neurons. The anti-5HT immunostaining pattern is shown in green, the mCherry signal driven by a combination of FLP417, TRH-LexA and LexAop>stop>Brp::mCherry is shown in magenta. The arrow points to a single targeted PLP neuron cell body.
G. 5HT1A1-Gal4 driven mCD8::GFP signal alone (green, upper panel) or counterstained with anti-5HT antibody (magenta, lower panel).
H. 5HT1A2-Gal4 driven mCD8::GFP signal alone (green, upper panel) or counterstained with anti-5HT antibody (magenta, lower panel).
A–H. Scale bar represents 50 μm.
Figure S3, related to the main Figure 4.
Additional GRASP data.
A–B. Two examples of the reconstituted GFP (GRASP signal, green) between most of the serotonergic neurons and the 5HT1A1-expressing neurons, visualized using the mouse anti-GFP-3E6 antibody. The large panels show full frontal projections and the small panels show projections of the posterior regions of the same brain for better view of the PLP cell bodies and their axons. Note that a GRASP signal is detected over the axons of the serotonergic PLP neurons. Scale bar represents 50 μm.
C–D. Patterns of reconstituted GFP (GRASP signal, green) between most of serotonergic neurons and candidate 5HT receptor neurons in the areas of interests, which are visualized by anti-5HT immunostaining (magenta). Different frontal z-projections of the image stack were created to view the corresponding neuropils of the same brain. White arrows point to areas in which GRASP signal is observed. Scale bar – 50 μm. Both 5HT2-GAL4 (C) and 5HT1A3-GAL4 (D) derived GRASP signal was observed in ellipsoid body, the region not innervated by aggression-modulation 5HT-PLP neurons. Scale bar represents 50 μm.
Table S1, related to main Figure 1.
Reproducibility of different enhancer trap (et-FLP) lines.
Table S2, related to main Figure 4.
Candidate serotonin receptor GAL4 lines used in GRASP experiments.
Acknowledgments
We thank Drs. Barry Dickson, Kristin Scott, Charles Nichols and Chi-Hon Lee for fly lines; past and present members of the Kravitz laboratory for helpful discussions (Jill Penn, Adelaine Leung, Sarah Certel, Joanne Yew); Michelle Ocana for her help with the 3D reconstruction of the PLP neuron, Alex Keene for his help with the setting up of the Activity Monitors and with the sleep data analysis; and Dr. Ravi Allada for kindly sharing the “Sleep Counting Macro” for sleep analysis.
This research was supported by grants from the National Institute of General Medical Sciences (GM099883 and GM074675) to E.A.K., by a departmental NIH training grant to O.V.A and by Deutsche Forschungsgemeinschaft to M.J.P. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Almeida RM, Ferrari PF, Parmigiani S, Miczek KA. Escalated aggressive behavior: dopamine, serotonin and GABA. Eur J Pharmacol. 2005;526:51–64. doi: 10.1016/j.ejphar.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Oliver B. Serotonin and Aggression. Ann NY Acad Sci. 2004;1036:382–392. doi: 10.1196/annals.1330.022. [DOI] [PubMed] [Google Scholar]
- 3.Raleigh MJ, McGuire MT, Brammer GL, Pollack DB, Yuwiler A. Serotonergic mechanisms promote dominance acquisition in adult male vervet monkeys. Brain Res. 1991;559:181–190. doi: 10.1016/0006-8993(91)90001-c. [DOI] [PubMed] [Google Scholar]
- 4.Huber RH, Kravitz EA. Aggression: towards an integration of gene, brain and behavior. In: Székely T, Moore AJ, Komdeur J, editors. Social behaviour: genes, ecology and evolution. Cambridge; New York: Cambridge University Press; 2010. p. xii.p. 562. [Google Scholar]
- 5.Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- 6.Yuan Q, Joiner WJ, Sehgal A. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr Biol. 2006;16:1051–1062. doi: 10.1016/j.cub.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 7.Yuan Q, Lin F, Zheng X, Sehgal A. Serotonin modulates circadian entrainment in Drosophila. Neuron. 2005;47:115–127. doi: 10.1016/j.neuron.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 8.Lee PT, Lin HW, Chang YH, Fu TF, Dubnau J, Hirsh J, Lee T, Chiang AS. Serotonin-mushroom body circuit modulating the formation of anesthesia-resistant memory in Drosophila. Proc Natl Acad Sci U S A. 2011;108:13794–13799. doi: 10.1073/pnas.1019483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sitaraman D, Zars M, Laferriere H, Chen YC, Sable-Smith A, Kitamoto T, Rottinghaus GE, Zars T. Serotonin is necessary for place memory in Drosophila. Proc Natl Acad Sci U S A. 2008;105:5579–5584. doi: 10.1073/pnas.0710168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becnel J, Johnson O, Luo J, Nassel DR, Nichols CD. The serotonin 5-HT7Dro receptor is expressed in the brain of Drosophila, and is essential for normal courtship and mating. PLoS One. 2011;6:e20800. doi: 10.1371/journal.pone.0020800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S, Lee AY, Bowens NM, Huber R, Kravitz EA. Fighting fruit flies: a model system for the study of aggression. Proc Natl Acad Sci U S A. 2002;99:5664–5668. doi: 10.1073/pnas.082102599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alekseyenko OV, Lee C, Kravitz EA. Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS One. 2010;5:e10806. doi: 10.1371/journal.pone.0010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alekseyenko OV, Chan YB, Li R, Kravitz EA. Single dopaminergic neurons that modulate aggression in Drosophila. Proc Natl Acad Sci U S A. 2013;110:6151–6156. doi: 10.1073/pnas.1303446110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asahina K, Watanabe K, Duistermars BJ, Hoopfer E, Gonzalez CR, Eyjolfsdottir EA, Perona P, Anderson DJ. Tachykinin-expressing neurons control male-specific aggressive arousal in Drosophila. Cell. 2014;156:221–235. doi: 10.1016/j.cell.2013.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feinberg EH, Vanhoven MK, Bendesky A, Wang G, Fetter RD, Shen K, Bargmann CI. GFP Reconstitution Across Synaptic Partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron. 2008;57:353–363. doi: 10.1016/j.neuron.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 16.Stockinger P, Kvitsiani D, Rotkopf S, Tirian L, Dickson BJ. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121:795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 17.Sweeney ST, Broadie K, Keane J, Niemann H, O’Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez MP, Chan YB, Yew JY, Billeter JC, Dreisewerd K, Levine JD, Kravitz EA. Pheromonal and behavioral cues trigger male-to-female aggression in Drosophila. PLoS Biol. 2010;8:e1000541. doi: 10.1371/journal.pbio.1000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Philipsborn AC, Liu T, Yu JY, Masser C, Bidaye SS, Dickson BJ. Neuronal control of Drosophila courtship song. Neuron. 2011;69:509–522. doi: 10.1016/j.neuron.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai JS, Lo SJ, Dickson BJ, Chiang AS. Auditory circuit in the Drosophila brain. Proc Natl Acad Sci U S A. 2012;109:2607–2612. doi: 10.1073/pnas.1117307109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otsuna H, Ito K. Systematic analysis of the visual projection neurons of Drosophila melanogaster. I. Lobula-specific pathways. J Comp Neurol. 2006;497:928–958. doi: 10.1002/cne.21015. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka NK, Endo K, Ito K. Organization of antennal lobe-associated neurons in adult Drosophila melanogaster brain. J Comp Neurol. 2012;520:4067–4130. doi: 10.1002/cne.23142. [DOI] [PubMed] [Google Scholar]
- 24.Ciranna L. Serotonin as a modulator of glutamate- and GABA-mediated neurotransmission: implications in physiological functions and in pathology. Curr Neuropharmacol. 2006;4:101–114. doi: 10.2174/157015906776359540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo J, Becnel J, Nichols CD, Nassel DR. Insulin-producing cells in the brain of adult Drosophila are regulated by the serotonin 5-HT(1A) receptor. Cell Mol Life Sci. 2011 doi: 10.1007/s00018-011-0789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nichols CD. 5-HT2 receptors in Drosophila are expressed in the brain and modulate aspects of circadian behaviors. Dev Neurobiol. 2007;67:752–763. doi: 10.1002/dneu.20370. [DOI] [PubMed] [Google Scholar]
- 27.Polter AM, Li X. 5-HT1A receptor-regulated signal transduction pathways in brain. Cell Signal. 2010;22:1406–1412. doi: 10.1016/j.cellsig.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saudou F, Boschert U, Amlaiky N, Plassat JL, Hen R. A family of Drosophila serotonin receptors with distinct intracellular signalling properties and expression patterns. EMBO J. 1992;11:7–17. doi: 10.1002/j.1460-2075.1992.tb05021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altieri SC, Garcia-Garcia AL, Leonardo ED, Andrews AM. Rethinking 5-HT1A receptors: emerging modes of inhibitory feedback of relevance to emotion-related behavior. ACS Chem Neurosci. 2013;4:72–83. doi: 10.1021/cn3002174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albert PR. Transcriptional regulation of the 5-HT1A receptor: implications for mental illness. Philos Trans R Soc Lond B Biol Sci. 2012;367:2402–2415. doi: 10.1098/rstb.2011.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Garcia AL, Newman-Tancredi A, Leonardo ED. P5-HT1A receptors in mood and anxiety: recent insights into autoreceptor versus heteroreceptor function. Psychopharmacology (Berl) 2014;231:623–636. doi: 10.1007/s00213-013-3389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pool AH, Kvello P, Mann K, Cheung SK, Gordon MD, Wang L, Scott K. Four GABAergic interneurons impose feeding restraint in Drosophila. Neuron. 2014;83:164–177. doi: 10.1016/j.neuron.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perisse E, Burke C, Huetteroth W, Waddell S. Shocking revelations and saccharin sweetness in the study of Drosophila olfactory memory. Curr Biol. 2013;23:R752–763. doi: 10.1016/j.cub.2013.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Waddell S. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–427. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Certel SJ, Leung A, Lin CY, Perez P, Chiang AS, Kravitz EA. Octopamine neuromodulatory effects on a social behavior decision-making network in Drosophila males. PLoS One. 2010;5:e13248. doi: 10.1371/journal.pone.0013248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dierick HA, Greenspan RJ. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat Genet. 2007;39:678–682. doi: 10.1038/ng2029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1, related to main Figures 1–2.
TNT inactivation of isolated 5HT neurons has selective effects on behavior.
A. Inactivation of large populations of 5HT neurons (light-gray bars) results in very low levels of locomotion, while inactivation of restricted 5HT neurons (dark-gray bars) produces varying effects. Data presented as means ± SEM, number of animals is indicated in parenthesis. ** -p<0.01; *** - p<0.001 vs. corresponding control (white bar), analyzed by nonparametric two-independent-sample Mann-Whitney U-test.
B. Chromosomal placement of FLP transgenes does not alter locomotion. Males carrying a single copy of various et-FLP transgenes (light and dark gray bars) have similar levels of locomotion as wild-type Canton-S males (white bar). Data are presented as means ± SEM, number of animals is indicated in parenthesis.
C. Chromosomal placement of FLP transgenes does not alter aggression. Numbers of lunges between pairs of males carrying a single copy of various FLP transgens are the same as in pairs of wild-type Canton-S males. Each dot represents the lunge count for an individual pair of flies. Number of tested pairs is indicated in parenthesis. The data are presented as boxplots with a median line. The lower and upper parts of the boxes are 25th and 75th percentiles, respectively.
D–E. Chromosomal placement of FLP417 transgene does not alter the average percentage of sleep (D) or the distribution of sleep (E). Gray line - males carrying a single copy of FLP417 transgene (FLP417/CS), black line - wild-type Canton-S males (CS). Data are presented as means ± SEM.
Figure S2, related to main Figure 4.
Positive and negative controls for GRASP combined with the intersectional strategy.
A. The TRH-LexA driven mCD8:GFP signal alone (green, upper panel) or counterstained with an anti-5HT antibody (magenta, lower panel).
B–D. GRASP negative controls. Flies carrying the two spGFP components of GRASP without either the Gal4 driver or the FLP-recombinase showed no detectable GFP signal in the ventrolateral protocerebrum (B), the ellipsoid body focal plane (C) or the fan-shaped body focal plane (D). The GRASP signal was visualized using a mouse anti-GFP-20 (Sigma) antibody (shown in green), while the anti-5HT immunostaining is shown in magenta.
E. Combination of the FLP210 line with the TRH-LexA line, which was used in GRASP experiments, targets a large population of 5HT neurons. The anti-5HT immunostaining pattern is shown in green, the mCherry signal driven by a combination of FLP210, TRH-LexA and LexAop>stop>BRP::mCherry is shown in magenta.
F. Combination of the FLP417 line with the TRH-LexA line, which was used in GRASP experiments, targets the individual 5HT-PLP neurons. The anti-5HT immunostaining pattern is shown in green, the mCherry signal driven by a combination of FLP417, TRH-LexA and LexAop>stop>Brp::mCherry is shown in magenta. The arrow points to a single targeted PLP neuron cell body.
G. 5HT1A1-Gal4 driven mCD8::GFP signal alone (green, upper panel) or counterstained with anti-5HT antibody (magenta, lower panel).
H. 5HT1A2-Gal4 driven mCD8::GFP signal alone (green, upper panel) or counterstained with anti-5HT antibody (magenta, lower panel).
A–H. Scale bar represents 50 μm.
Figure S3, related to the main Figure 4.
Additional GRASP data.
A–B. Two examples of the reconstituted GFP (GRASP signal, green) between most of the serotonergic neurons and the 5HT1A1-expressing neurons, visualized using the mouse anti-GFP-3E6 antibody. The large panels show full frontal projections and the small panels show projections of the posterior regions of the same brain for better view of the PLP cell bodies and their axons. Note that a GRASP signal is detected over the axons of the serotonergic PLP neurons. Scale bar represents 50 μm.
C–D. Patterns of reconstituted GFP (GRASP signal, green) between most of serotonergic neurons and candidate 5HT receptor neurons in the areas of interests, which are visualized by anti-5HT immunostaining (magenta). Different frontal z-projections of the image stack were created to view the corresponding neuropils of the same brain. White arrows point to areas in which GRASP signal is observed. Scale bar – 50 μm. Both 5HT2-GAL4 (C) and 5HT1A3-GAL4 (D) derived GRASP signal was observed in ellipsoid body, the region not innervated by aggression-modulation 5HT-PLP neurons. Scale bar represents 50 μm.
Table S1, related to main Figure 1.
Reproducibility of different enhancer trap (et-FLP) lines.
Table S2, related to main Figure 4.
Candidate serotonin receptor GAL4 lines used in GRASP experiments.