Abstract
Microglia undergo maturation during the third-trimester of human development (equivalent to the first 1–2 weeks of postnatal life in rodents), during which these cells may be particularly sensitive to insult. Alcohol exposure during this period can activate the neuroimmune system, an effect that may contribute to the pathophysiology of fetal alcohol spectrum disorders. Here, we investigated whether repeated alcohol exposure during the third-trimester-equivalent in rats has a priming effect on the neuroimmune response to injection of bacterial lipopolysaccharide (LPS). Pups were exposed to alcohol in vapor chambers for four hours daily from postnatal day (PD)2 to PD16 (peak blood alcohol concentrations ~ 150 mg/dL). On PD17, rats were injected with either saline or LPS (50 μg/kg) and the frontal cortex, cerebellar vermis, and dentate gyrus were collected 2 hours later. Messenger RNA (mRNA) levels for the pro-inflammatory agents interleukin 1β (IL-1β) and chemokine (C–C) motif ligand 2 (CCL2), as well as levels of the anti-inflammatory cytokine interleukin 10 (IL-10), were measured using reverse transcriptase-polymerase-chain reaction. LPS consistently increased IL-1β and CCL2 mRNA levels in the dentate gyrus, frontal cortex, and cerebellum of both male and female rats. Furthermore, the LPS-induced increase of IL-1β mRNA levels was significantly blunted in the frontal cortex of alcohol-exposed female rats. Conversely, LPS only minimally affected IL-10 mRNA expression and there were no significant differences between air and alcohol-exposed rats. Taken together with the literature regarding the effect of third trimester alcohol exposure on the neuroimmune system, our findings suggest that chronic exposure to lower levels is less disruptive to the neuroimmune system than binge-like exposure to high doses of alcohol.
Keywords: Fetal alcohol syndrome, cytokine, chemokine, neuroimmune, lipopolysaccharide, microglia
Introduction
Alcohol consumption during pregnancy is the most common cause of preventable birth defects. The term fetal alcohol spectrum disorders (FASDs) encompasses the molecular and functional consequences resulting from developmental alcohol exposure, ranging from isolated behavioral deficits, particularly in learning and memory, to a constellation of morphological and behavioral alterations characteristic of fetal alcohol syndrome. It is estimated that the prevalence of FASDs in the United States is approximately 2–5% (May et al., 2009). Recent studies have presented evidence for a role of the neuroimmune system in FASDs (Reviewed by Drew and Kane, 2013)
Immune responses in the central nervous system (CNS) are the outcome of interactions between many cell types such as glial cells (microglia, astrocytes, oligodendrocytes), neurons in the surrounding tissue, and invading peripheral components. Of these cells, microglia are the most well-characterized and widely studied with respect to neuroimmune system function. Microglia are dynamic cells that undergo a spectrum of changes in morphology, phenotype, and gene expression upon activation. The response generated by microglia is highly dependent on factors such as type of insult, duration, and individual features of the host including gender and age. Typical microglial activation involves the secretion of cytokines and chemokines which have been identified as powerful modulators of the CNS influencing neuronal plasticity (Nelson et al., 2012; Vereker et al., 2000), receptor activity and expression (Wang et al., 2012), neurogenesis (Bland et al., 2010), and maintenance of homeostasis (Ji et al., 2013; Nayak et al., 2014). The neuroimmune system plays a particularly critical role during development and infections in the early postnatal period can impair cognitive functions in adulthood (Bilbo et al., 2005b; Reviewed by Spencer et al., 2011) and increase susceptibility to neurodevelopmental disorders (Brown et al., 2004). Additionally, microglia undergo maturation during the rodent third-trimester equivalent of human pregnancy, which takes place postnatally, possibly leading to an increased vulnerability to insult during this time (Reviewed by Bilbo and Schwarz, 2009). It has been postulated that neonatal infection programs an exaggerated response in the developing neuroimmune system that could result in behavioral alterations such as memory impairment (Bilbo et al., 2005a).
Alcohol intake has been shown to activate microglia, resulting in a neurotoxic increase in pro-inflammatory cytokines (He and Crews, 2008; McClain et al., 2011; Qin and Crews, 2012) a pattern of activation known as the “classical” M1 response. Conversely, recent research has emphasized the complexity of this system by demonstrating that microglia may, under some conditions, instead assume a neuroprotective role (denoted as “alternative activation” or M2 response) (Reviewed by Colton, 2009) after alcohol exposure, characterized by production of anti-inflammatory factors such as interleukin (IL)-10 (Marshall et al., 2013). Additionally, Kane and colleagues have shown an age-dependent neuroimmune response to alcohol (Kane et al., 2014), highlighting the importance of discrete developmental stages in neuroimmune function. Additional studies have revealed that gestational alcohol exposure is associated with increases in microglial and astrocyte activation (Soscia et al., 2006; Watari et al., 2006; Wierzba-Bobrowicz et al., 2003). Furthermore, studies focusing on the third-trimester equivalent have shown increases in pro-inflammatory cytokines and microglial activation in several brain regions, including the cerebellum and hippocampus (Kane et al., 2011; Tiwari and Chopra, 2012).
Perturbation of the neuroimmune system by developmental alcohol exposure may contribute to the pathophysiology of FASD in multiple ways. It could directly disrupt CNS synaptic refinement by altering levels of cytokines known to effect neuronal plasticity (Katsuki et al., 1990; Nelson et al., 2012) or by changing microglial phenotype, thereby disrupting normal microglial-dependent processes such as synaptic pruning (Paolicelli et al., 2011). Conversely, developmental alcohol exposure could act by programming a permanently altered neuroimmune system. In support of this notion, FASD offspring have been shown to have impaired immune responses in adulthood (McGill et al., 2009). Furthermore, the prevalence of neonatal infection in FASD patients is increased dramatically, as even small amounts of alcohol during gestation are associated with a 2.5 fold increase in risk for infection, whereas excessive alcohol intake increases the risk by 3–4 times (Gauthier et al., 2005). A neuroimmune system altered by alcohol exposure during development could react differently to a second insult (such as neonatal infection) than that of a normal infant. The relationship between the co-exposure to alcohol and neonatal infection could have critical implications for the pathophysiology of FASD.
The objective of this study was to characterize neuroimmune function in response to repeated third-trimester-equivalent alcohol exposure after a “second insult” in the form of injection of a low dose of bacterial lipopolysaccharide (LPS). We included both male and female pups, and focused on the dentate gyrus (DG) subregion of the hippocampus as well as the cerebellum and frontal cortex. These brain regions have been shown to be susceptible to damage by developmental alcohol exposure (Brady et al., 2013; Galindo et al., 2004; Guerri, 1998; Hamilton et al., 2010; Uban et al., 2010) that could be mediated, in part, by neuroimmune alterations (Kane et al., 2011; Tiwari and Chopra, 2012). To assess the function of the neuroimmune system, we measured changes in mRNA levels for the pro-inflammatory markers, interleukin-1β (IL-1β) and chemokine (C–C motif) ligand 2 (CCL2) whose expression in the neonatal brain increases after LPS injections (Schwarz and Bilbo, 2011). Additionally, to determine if postnatal alcohol exposure (PAE) may induce an anti-inflammatory effect, we measured mRNA levels for IL-10 which has been shown to increase after LPS injections in the hippocampus of male rat pups (Schwarz and Bilbo, 2011).
Materials and Methods
Animal treatments and tissue collection
Animal procedures were approved by the Institutional Animal Care and Use Committee of the University of New Mexico Health Sciences Center and conformed to National Institutes of health guidelines. Time-pregnant Long-Evans rats (gestational day 14–15) were obtained from Harlan Laboratories Inc. (Indianapolis, IN) and allowed to acclimate for approximately one week before giving birth. Two days after litter birth, dams and their respective offspring were housed together in a vapor chamber apparatus. Starting at 10:00 am, litters were exposed to 3.2 ± 0.4 mg/dL alcohol via vapor inhalation for 4 hours daily from postnatal day (PD) 2 until PD 16 (as described previously by Zamudio-Bulcock et al., 2014). Control litters were housed similarly but received only air flow (no alcohol). Litters were culled to 10 pups on PD 5. Blood alcohol concentrations (BACs) were determined using an alcohol dehydrogenase-based assay, as previously described (Puglia and Valenzuela, 2009). Rats were given intraperitoneal injections of LPS (50 μg/kg body weight in ~90 μL) (Millipore 0111:B4, lot# 2089863) or endotoxin-free saline (Santa Cruz Biotechnology, sc286637, lot#F0513) ~24 hours after the last alcohol exposure (on PD17). LPS doses were chosen based on previous reports showing significant effects on immune system gene expression (Schwarz and Bilbo, 2011). Furthermore, this dose is on the lower end of what is reported in the literature and has been shown to produce submaximal activation of the neuroimmune system (Kendall et al., 2011; Lin et al., 2009), therefore avoiding possibly masking any effect of PAE on the response to LPS via a “ceiling” effect. Samples were collected 2 hours after LPS injections, as described below. Animals received ketamine (250 mg/kg) by intraperitoneal injection and brains were removed by decapitation. Brains were placed into ice cold phosphate buffered saline and whole dentate gyri were extracted from both hemispheres as described previously (Hagihara et al., 2009). In addition, frontal cortex and cerebellar vermis samples were collected. A minimum of 6 animals per experimental group were chosen based on an a priori power calculation performed using the standard deviation and effect size obtained in a previous study (Qin et al., 2008). This experimental sample size is also consistent with previous reports that have detected changes in LPS-induced neuroinflammation after exposure to insult, including alcohol (Bilbo et al., 2005a; Bilbo et al., 2005b; Qin et al., 2008).
Real-time PCR analysis
For RNA isolation, tissue was placed into Buffer RLT (Qiagen RNeasy Mini Kit, Cat. No. 74106) containing 40 mM dithiothreitol. Samples were homogenized by sonication and RNA was extracted immediately after tissue removal using an RNeasy mini Kit (SABiosciences/Qiagen) according to the manufacturer’s instructions. The RNA was frozen at −20 °C until use. cDNA was synthesized fr om 1 μg of RNA using the RT2 First Strand Kit (SABiosciences/Qiagen) and stored at −20 °C. Gene expression was measured using real-time PCR. All primers were purchased from SABiosciences/Qiagen including IL-1β (Cat No. PPR06480B) CCL2 (Cat. No. PPR06714B), IL-10 (Cat No. PPR06479A) and hypoxanthine phosphoribosyltransferase 1 (Hprt1; Cat. No. PPR42247F). PCR was carried out according to the RT2 qPCR Primer assay instructions (SABiosciences/Qiagen) in combination with the RT2 SYBR Green qPCR Master Mix. The threshold amplification number (CT) value for each gene of interest (GOI) was normalized to the housekeeping gene (HKG) Hprt1 following the formula . To validate the use of HPRT1 as a housekeeping gene, its expression was measured and found to be unaffected by vapor chamber treatment (alcohol versus air control), injection (LPS versus saline), or sex (data not shown). To avoid the potential of a floor effect, a cut-off CT value of 35 was used.
Statistics
Data analysis was carried out on Prism 6.03 (GraphPad Software, San Diego, CA). Whether the data followed a Gaussian distribution was determined with Shapiro-Wilk or Kolmogorov-Smirnov normality test, and statistics were determined by either parametric tests (one-sample t-test vs. a theoretical mean of 1 and two-tailed student’s t-test or by non-parametric tests (Wilcoxon sign test vs. a theoretical mean of 1 and Mann-Whitney test), as appropriate. The unit of determination is defined a results obtained with one pup per treatment condition for each litter.
Results
To model human fetal alcohol exposure during the third-trimester of pregnancy, rat pups were exposed daily to alcohol in vapor inhalation chambers between PD2 and PD16, as previously described (Zamudio-Bulcock et al., 2014). Chamber levels remained relatively stable during exposure and pups were exposed to alcohol vapor levels near 3 g/dL (data not shown). On PD2, prior to culling, approximately equal sized litters were assigned to either the postnatal alcohol exposure (PAE) or air control groups (Control group: 9.769 ± 2.048; PAE group: 11.08 ± 1.935; p = 0.1072, n = 13, by unpaired t-test). With the exception of cage changes on PD2, PD7 and PD14, pups were unhandled and no mortality occurred. Peak BACs immediately following exposure to alcohol were 155 ± 37.59 mg/dL and 164 ± 46.61 mg/dL on PD 6 (n = 4 litters) and PD16 (n = 3 litters), respectively. These levels reflect a BAC of approximately twice the legal limit of intoxication (80 mg/dL). Analysis of pup weight on PD17 showed a significant decrease in the PAE group compared to air control rats (Control group: 34.74 ± 3.616 g, n = 12 litters; PAE group: 32.01 ± 2.131 g, n = 11 litters; p = 0.0123, by Mann Whitney test).
Alcohol exposure does not significantly alter the neuroimmune response to LPS in the DG
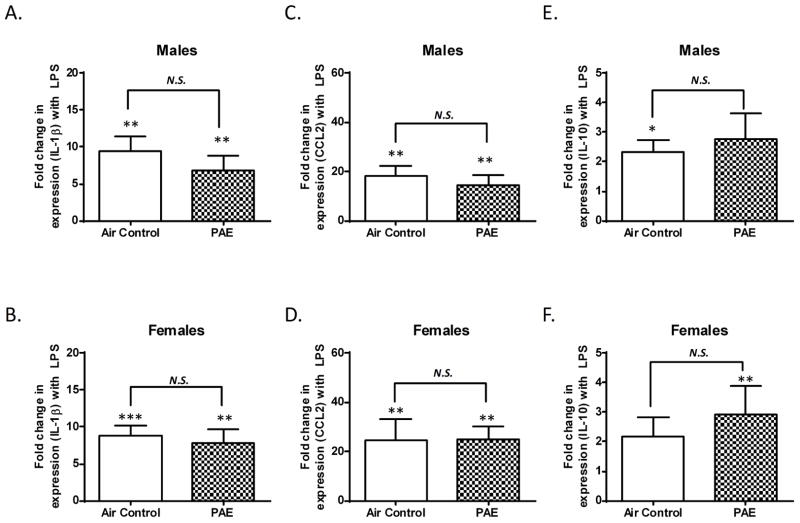
On PD 17, 24 hours after completion of alcohol exposure, two pups from each litter (one male, one female) received an LPS injection and two pups (one male, one female) received a saline injection. After 2 hours, mRNA was harvested and the levels of pro-inflammatory cytokine IL-1β and chemokine CCL2 (also known as monocyte chemoattractant protein-1) were examined. Additionally, anti-inflammatory cytokine IL-10 mRNA was assessed. With respect to saline injected rats, as expected, LPS injections significantly increased IL-1β mRNA levels in the DG of both control (p = 0.002, n = 10, by one sample t-test vs. one) and PAE (p = 0.0039, n = 10, by Wilcoxon Signed Rank Test vs. one) male rats (Fig 1A). Similarly, LPS injections significantly increased IL-1β mRNA levels in the DG of control (p = 0.0003, n = 10, by one sample t-test vs. one) and PAE (p = 0.0071, n = 9, by one sample t-test vs. one) female rats (Fig 1B). However, there was not a significant difference between the air control and PAE groups in the LPS-induced increase of IL-1β mRNA levels in either males (Fig 1A, p = 0.2443, by Mann-Whitney test) or females (Fig 1B, p =0.6828, by unpaired t-test).
Figure 1. Third trimester alcohol exposure did not alter mRNA expression of IL-1β CCL2, or IL-10 in the dentate gyrus of male or female pups after LPS injection.
Levels of IL-1β (A), CCL2 (C), and IL-10 (E) were determined in male pups after LPS injection compared to saline-injected littermates. Levels of IL-1β (B), CCL2 (D), and IL-10 (F) were determined in females after LPS injection compared to saline-injected littermates. The fold change in mRNA expression represents the ratio of levels in LPS-injected pups over saline-injected littermates. (N.S. = not significant, * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001 vs. a theoretical mean of 1).
LPS injections significantly increased CCL2 mRNA levels in the DG of both control (p = 0.0024, n = 10, by one sample t-test vs. one) and PAE (p = 0.0069, n = 10, by one sample t-test vs. one) male rats (Fig 1C). LPS injections also significantly increased CCL2 mRNA levels in the DG of both control (p = 0.002, n = 10, by Wilcoxon Signed Rank Test vs. one) and PAE (p = 0.0025, n = 9, by one sample t-test vs. one) female rats (Fig 1D). However, there was not a significant difference between the air control and PAE groups in the LPS-induced increase of CCL2 mRNA levels in the DG of either males (Fig 1C, p = 0.5339, by unpaired t-test) or females (Fig 1D, p = 0.7065, by Mann-Whitney test).
LPS injections significantly increased IL-10 mRNA levels in the DG of control (p = 0.0135, n = 8, by one sample t-test vs. one) but not PAE (p = 0.0805, n = 8, by one sample t-test vs. one) male rats (Fig 1E). In female rats LPS injections did not significantly affect IL-10 mRNA levels in controls (p = 0.0915, n = 10, by one sample t-test vs one), but did significantly increase levels in the PAE group (p = 0.0078, n = 8, by Wilcoxon Signed Rank Test vs. one) (Fig 1F). However, the fold change in IL-10 expression after LPS injection was not significantly different between control and PAE in male (Fig 1E, p = 0.6472, by unpaired t-test) and female (Fig 1F, p = 0.4038, by Mann-Whitney test) rats.
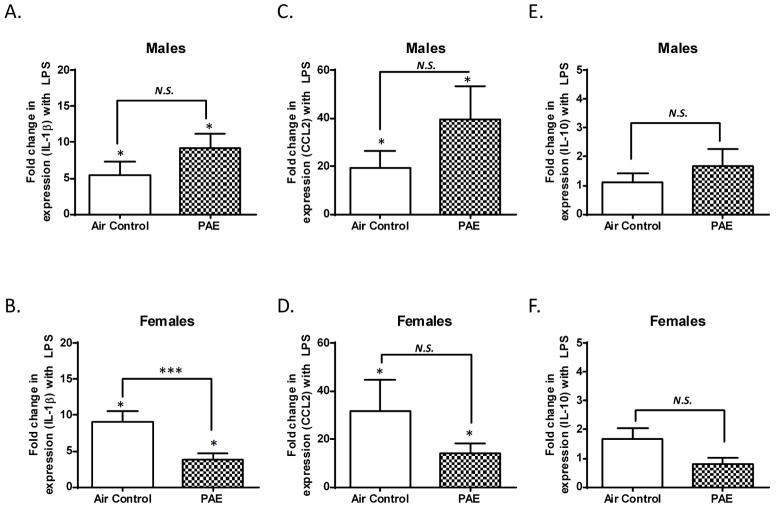
Alcohol exposure blunts the LPS-induced increase of IL-1β mRNA levels in the frontal cortex of female rats
With respect to saline injected rats, LPS injections significantly increased IL-1β mRNA levels in the frontal cortex of both control (p = 0.0466, n = 8, by one sample t-test vs. one) and PAE (p = 0.0156, n = 7, by Wilcoxon Signed Rank Test vs. one) male rats (Fig 2A). Similarly, LPS injections significantly increased the IL-1β mRNA levels in the frontal cortex of both control (p = 0.0156, n = 7, by Wilcoxon Signed Rank test vs. one) and PAE (p = 0.0106, n = 8, by one sample t-test vs. one) female rats (Fig 2B). PAE did not significantly alter the LPS-induced increase of IL-1β mRNA in the frontal cortex of male rats (Fig 2A, p = 0.1203, by Mann-Whitney test). Conversely, PAE significantly blunted the LPS-induced increase of IL-1β mRNA in female rats (Fig 2B, p = 0.0006, by Mann-Whitney test).
Figure 2. Third trimester alcohol exposure blunted the LPS-induced increase in IL-1β mRNA expression in the frontal cortex of female pups but not males.
The fold change in mRNA expression represents the ratio of levels in LPS-injected pups over saline-injected littermates. Data is displayed as in Figure 1. The LPS-induced fold change in IL-1β mRNA expression was significantly attenuated in female rats of the PAE group compared to female rats in the air control group. (N.S. = not significant, * p ≤ 0.05 vs. a theoretical mean of 1, *** p ≤ 0.001 vs. Air control).
LPS injections significantly increased CCL2 mRNA levels in the frontal cortex of control (p = 0.0330, n = 8, by one sample t-test vs. one) and PAE (p = 0.0304, n = 7, by one sample t-test vs. one) male rats (Fig 2C). Similarly, LPS injections significantly increased CCL2 mRNA levels in both control (p = 0.0156, n = 7, by Wilcoxon Signed Rank Test vs. one) and PAE (p = 0.0158, n = 8, by one sample t-test vs. one) female rats (Fig 2D). However, PAE did not significantly alter the LPS-induced increase of CCL2 mRNA levels in either males (Fig 2C, p = 0.1952, by unpaired t-test) or females (Fig 2D, p = 0.2785, by Mann-Whitney test).
LPS injections did not significantly increase IL-10 mRNA expression in the frontal cortex of control (p = 0.3828, n = 8, by Wilcoxon Signed Rank Test vs. one) or PAE (p = 0.2936, n = 7, by one sample t-test vs. one) male rats (Fig 2E). Similarly, LPS injections did not significantly increase IL-10 mRNA levels in control (p = 0.1157, n = 7, by one sample t-test vs. one) or PAE (p = 0.4119, n = 8, by one sample t-test vs. one) female rats (Fig 2F). There were no statistically significant differences in IL-10 mRNA levels after LPS in control or PAE samples from either male (Fig 2E, p >0.9999, by Mann-Whitney test) or female rats (Fig 2F, p = 0.0594, by unpaired t-test).
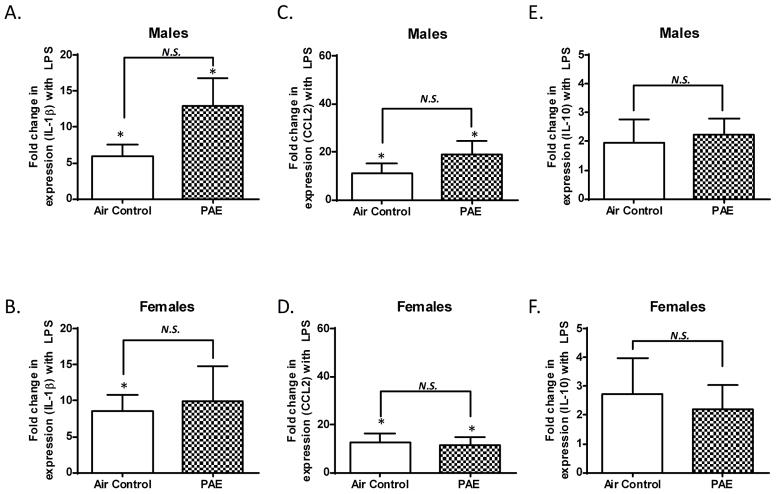
Alcohol exposure did not significantly affect IL-1β or CCL2 mRNA levels in the cerebellar vermis of male and female rats
With respect to saline injected rats, LPS injections significantly increased IL-1β mRNA levels in the cerebellar vermis of both control (p = 0.0160, n = 8, by one sample t-test vs. one) and PAE (p = 0.0200, n = 7, by one sample t-test vs. one) male rats (Fig 3A). In female rats, LPS significantly increased IL-1β mRNA levels in the cerebellar vermis of the control group (p = 0.0144, n = 7, by one sample t-test vs. one) but not the PAE (p = 0.1251, n = 6, by one sample t-test vs. one) group (Fig 3B). PAE did not significantly alter the LPS-induced increase of IL-1β mRNA levels in either males (Fig 3A, p = 0.0993, by unpaired t-test) or females (Fig 3B, p = 0.7949, by unpaired t-test).
Figure 3. Third trimester alcohol exposure did not alter mRNA expression of IL-1β CCL2, or IL-10 after LPS injection in the cerebellar vermis of male or female pups.
The fold change in mRNA expression represents the ratio of levels in LPS-injected pups over saline-injected littermates. Data is displayed as in Figure 1. (N.S. = not significant, * p ≤ 0.05 vs. a theoretical mean of 1)
LPS injections significantly increased CCL2 mRNA levels in the cerebellar vermis of both control (p = 0.0390, n = 8, by one sample t-test vs. one) and PAE (p = 0.0169, n =7, by one sample t-test vs. one) male rats (Fig 3C). Similarly, LPS injections significantly increased CCL2 mRNA levels in the cerebellar vermis of both control (p = 0.0202, n = 7, by one sample t-test vs. one) and PAE (p = 0.0305, n = 6, by one sample t-test vs. one) female rats (Fig 3D). PAE did not significantly alter the LPS-induced increase of CCL2 mRNA expression in either males (Fig 3C, p = 0.2643, by unpaired t-test) or females (Fig 3D, p = 0.8171, by unpaired t-test).
LPS injections did not significantly increase IL-10 mRNA expression in control (p = 0.2791, n = 8, one sample t-test vs. one) or PAE (p = 0.0863, n = 6, by one sample t-test vs. one) male rats (Fig 3E). Similarly, LPS injection did not increase IL-10 mRNA expression in controls (p = 0.2188, n = 7, by Wilcoxon Signed Rank Test vs. one) or in PAE (p = 0.2062, n = 6, by one sample t-test vs. one) female rats (Fig 3F). There were no statistically significant differences in IL-10 mRNA levels after LPS in control or PAE samples from either male (Fig 3E, p = 0.7979, by unpaired t-test) or female rats (Fig 3F, p > 0.9999, by Mann-Whitney test).
Discussion
This study examined the effect of a repeated third-trimester equivalent alcohol exposure on the developing neuroimmune system, concentrating on the interaction of developmental alcohol exposure with a LPS challenge in the rat neonatal period. Many previous studies have demonstrated that neuroimmune activation during the neonatal period can reprogram this system, resulting in long-term deficits that are subtle and essentially undetectable until unmasked by a second insult. The reprogrammed neuroimmune system then over-reacts to later insults with increased pro-inflammatory cytokine production eliciting behavioral deficits (Bilbo et al., 2005b; Bland et al., 2010; Schwarz and Bilbo, 2013; Reviewed by Spencer et al., 2011). Based on these studies, we hypothesized that ethanol could produce a similar effect; i.e., that exposure to moderate ethanol levels during late stages of pregnancy could program the neuroimmune system to have an exaggerated pro-inflammatory response to an infection, leading to secondary CNS damage. Contrary to our hypothesis, we found that exposure to relatively moderate alcohol levels during the third trimester-equivalent had little effect on neuroimmune function in response to LPS injections. Moreover, PAE had an effect opposite what we expected in the frontal cortex of female rats, where it actually blunted the LPS-induced production of IL-1β mRNA.
Using a previously characterized vapor chamber exposure paradigm (Zamudio-Bulcock et al., 2014), we exposed rats to alcohol through vapor inhalation for 2 weeks, from PD2 to 16, which encompasses several of the developmental events that occur in the human brain during the third trimester of human pregnancy (Clancy et al., 2007). Unlike humans, rodents undergo the brain growth spurt associated with the human third trimester of gestation during the early postnatal period. This period includes key brain developmental processes, such as synapse formation and elimination (Cudd, 2005). While our paradigm only models alcohol consumption during the third trimester equivalent without any exposure in the first or second trimesters, this has been identified as the third most common drinking pattern during pregnancy in the US (Ethen et al., 2009). Our laboratory has previously found that this alcohol model, using a slightly higher BAC, induces alterations in GABAergic subunit expression in the cerebellum (Diaz et al., 2014), GABAergic transmission (Everett et al., 2012) and LTP induction in the hippocampus (Puglia and Valenzuela, 2010). However, using lower doses, as in this study, ethanol did not significantly affect either LTP in the hippocampus (Puglia and Valenzuela, 2010) or complex spikes and long-term depression in cerebellar Purkinje neurons (Zamudio-Bulcock et al., 2014). Additionally, apoptotic neuronal cell death has not been shown to occur at the BACs used in this study (Ikonomidou et al., 2000). Therefore, we did not expect that the more moderate level of alcohol exposure in this model would cause large changes, but instead sought to investigate whether it might cause more subtle changes in the neuroimmune system that could be unmasked with a second insult (LPS).
Using this paradigm, we attained peak BACs that were approximately twice the legal limit of intoxication (80 mg/dL) and could be achieved by a pregnant woman consuming around 5–6 drinks (Burd et al., 2012). Therefore, these levels of alcohol exposure are considered high from the human perspective. However, previous studies have shown that rodents are more resistant to alcohol than humans and higher BACs are needed to elicit similar effects in the former (Gohlke et al., 2008). Using a similar paradigm, our laboratory has previously shown that after this type of exposure, BACs gradually return to zero over the course of approximately 8 hours (Diaz et al., 2014). The current study uses pointedly lower BACs, which likely return to baseline in less time. While we did not observe an increase in mortality, there was a significant decrease in body weight in PAE rat pups on PD17. Therefore, this paradigm models some of the characteristics of fetal alcohol syndrome which is, in part, characterized by decreased body weight.
Alcohol exposure did not affect the LPS-induced increase in IL-1β, CCL2, and IL-10 mRNA in the DG
We investigated the effect of PAE followed by immune challenge with a low dose of LPS in order to mimic an infant prenatally exposed to ethanol contracting an infection soon after birth. We gauged the neuroimmune pro-inflammatory response to LPS by measuring the expression of IL-1β and CCL2 mRNA, both of which have shown to be significantly elevated after LPS injection, and are regularly used as markers of neuroinflammation (Schwarz and Bilbo, 2011). Additionally, we measured the anti-inflammatory cytokine IL-10, which has been shown to be elevated in the hippocampus of male pups after LPS (Schwarz and Bilbo, 2011).We therefore expected that IL-10 mRNA would be elevated in other brain regions as well. Two hours after LPS injection, we observed a significant increase in both pro-inflammatory genes in the DG, with a larger increase in CCL2 expression (~20 fold) than in IL-1β (5–10 fold), consistent with previous findings (Schwarz and Bilbo, 2011). Conversely, we did not detect robust changes in IL-10 mRNA expression in rats injected with LPS.
Previous studies investigating the role of the neuroimmune response after PAE have focused primarily on male rats. Given the sexually dimorphic effects of PAE (Goodlett and Peterson, 1995; Uban et al., 2013; Weinberg et al., 2008), as well as the striking gender differences in microglia colonization during development (Schwarz et al., 2012), we included both males and females in this study. In the DG, we detected no difference in the LPS-induced pro-inflammatory response between males and females. However, there was a highly variable response to LPS across rats within the same treatment groups, with some animals having very little increase in pro-inflammatory gene production and others having a much more pronounced effect. This may be explained by non-overt differences in maternal care or environmental factors, such as prenatal exposure to stress, as these have been shown to affect the neuroimmune system (Diz-Chaves et al., 2012; Schwarz et al., 2011). Future studies should assess this possibility.
A peripheral injection of a low dose of LPS is expected to induce neuroinflammation indirectly given that it does not penetrate the CNS at significant levels (Banks and Robinson, 2010). LPS is known to increase both cytokine levels in the periphery and blood brain barrier permeability, potentially allowing cytokines from the periphery to enter the CNS (Erickson and Banks, 2011; Jaeger et al., 2009). Therefore, changes in CNS cytokine levels could be due in part to invasion of peripheral immune components, such as direct permeation by IL-1β or chemotactic macrophages. Additionally, peripheral immune system infiltration would likely activate neuroimmune cells and induce a local response. Importantly, with the exception of the female frontal cortex, our PAE paradigm did not alter the inflammatory response created by peripheral LPS injections, meaning that the pathway of activation is generally still intact.
Alcohol exposure during the third-trimester equivalent has been shown to cause neuroimmune activation in the hippocampus, including an increase in several pro-inflammatory markers such as IL-1β (Tiwari and Chopra, 2012); however, BACs were more than twice as high as those used in the current study. Additionally, the route (i.e. intragastic gavage vs. vapor inhalation) and duration (acute vs. chronic) of alcohol administration could contribute to the observed discrepancies between studies.
With regard to the possibility of an anti-inflammatory response, we found that IL-10 expression was increased after LPS in the DG of male controls, but in the females this increase was only significant in the PAE group. Futhermore, there were no significant differences in IL-10 expression after LPS between the PAE and air control rats in either males or females. Thus it is likely that no difference in anti-inflammatory IL-10 expression exists between the two groups in this exposure paradigm. Our findings in the males are consistent with a previous study showing a similar fold increase in IL-10 two hours after LPS injection in the hippocampus of male rat pups (Schwarz and Bilbo, 2011). It is unclear why IL-10 production after LPS injection did not also increase in the female rats. While the ability of IL-10 to block the production and function of many pro-inflammatory cytokines is well-established (Bogdan et al., 1991; de Waal Malefyt et al., 1991), it has also been suggested that pro-inflammatory cytokines may increase the expression of IL-10 (Foey et al., 1998; Platzer et al., 1995; Wanidworanun and Strober, 1993) acting as an inflammatory brake system. Additionally, IL-10 production has been shown to occur as a result of the activation of specific pathways, such as the p38 MAPK pathway, that are also associated with the production of pro-inflammatory cytokines, including IL-1β (Foey et al., 1998; Saraiva and O’Garra, 2010). Therefore, we expected the increased pro-inflammatory cytokine expression to be accompanied by an increase in IL-10. Surprisingly this was not typically the case, suggesting that our paradigm does not engage a strong anti-inflammatory response, at least at the time we performed our assays. This could be due, in part to the timing of our assay. We collected mRNA samples 2 hours after LPS expression, at which time pro-inflammatory cytokine expression is reliably increased. Conversely, IL-10 has been suggested to occur on a comparatively delayed timescale (de Waal Malefyt et al., 1991; Foey et al., 1998) and consequently it is possible that we missed the peak elevation in IL-10.
Alcohol exposure blunts the LPS-induced increase of IL-1β mRNA in the cortex of female rats
In the cortex of control animals, LPS injections produced a significant increase in production of IL-1β and CCL2 mRNA in both males and females. This response was very similar to that seen in the DG, with a slightly larger increase in CCL2 mRNA than for IL-1β. However, in PAE female rats the LPS-induced production of IL-1β mRNA was significantly blunted. These findings are consistent with previous studies showing that alcohol exposure has region-specific, selective effects on particular cytokines (Kane et al., 2014). While the IL-1β and CCL2 pathways are highly interconnected, the two have different functions. We consistently observed a more pronounced response in CCL2 than IL-1β, indicating that perhaps the subtle impact of PAE on this system only significantly affected the less robust IL-1β response to LPS.
In addition to blunting the response in a cytokine-selective manner, PAE also induced a gender-specific effect. As mentioned previously, the effects of PAE are sexually dimorphic and the development of the neuroimmune system is different between males and females. Specifically, microglia in juvenile female rats exhibit a more activated morphology than juvenile male rats, are slower to colonize the postnatal brain, and males and females exhibit different gene expression profiles of inflammatory cytokines and chemokines (Schwarz et al., 2012). A combination of these factors could contribute to sex-dependent differences in the effect of PAE observed in the current study. Finally, the effects being region-specific to the cortex may result in PAE offspring having problems with executive, higher functioning, as has been observed in patients with FASD (Rasmussen, 2005). These findings are also consistent with the literature on the effect of ethanol in the adult brain where region specific changes in neuroinflammation have been identified (Kane et al., 2014). Finally, while no significant increase in IL-10 was observed after LPS injection in the frontal cortex of either male or female rats pups, in the female pups there was a strong trend toward decreased IL-10 expression, suggesting that the decrease in IL-1β is not due to an increased anti-inflammatory response, but may instead be due to a diminished overall neuroimmune response, which may be related to toxic effects of PAE on developing microglia (Kane et al., 2011).
Based on the literature and the idea of neuroimmune system “priming”, we hypothesized that PAE would increase the response to a second insult, eliciting an over-reaction. However, we observed instead that PAE had the opposite effect, blunting the neuroimmune response in the female frontal cortex. Therefore the PAE-induced weakening of the neuroimmune response, could leave the CNS more susceptible to damage by infection, as activation of this system plays an important role in the destruction of pathogenic agents, removal of damaged cells, and stimulation of repair processes (Reviewed by Ousman and Kubes, 2012). Further studies are needed to address these possibilities.
Alcohol exposure does not affect the LPS-induced increase in IL-1β and CCL2 mRNA in the cerebellar vermis
In the cerebellar vermis, LPS injections elicited similar effects in control animals as seen in both the DG and frontal cortex. As expected, LPS induced a significant increase in mRNA expression of CCL2 and IL-1β in both males and females. This effect was not significantly altered by PAE for either sex. Additionally, IL-10 was not found to be significantly altered by LPS injections, suggesting that this anti-inflammatory pathway is not engaged in the cerebellar vermis in response to LPS injection, further highlighting the brain regional differences in neuroinflammation at this age. Previous studies utilizing a third trimester binge-like alcohol exposure paradigm have identified both increases in basal levels of pro-inflammatory markers (Tiwari and Chopra, 2012) and microglial activation (Kane et al., 2011) in the cerebellum. However, these studies employed higher doses of alcohol yielding BACs of more than twice the peak BACs found here, and used different methods of administration than the one used in our study (gavage versus inhalation). Differences in paradigm likely play a role in the discrepancies observed in the current report.
Overall Conclusion
To our knowledge, this is the first study of the impact of PAE on neuroimmune activation induced by LPS injection during the neonatal period. We found that, in most cases, PAE had very little effect on neuroimmune function as in response to a second insult. Importantly, PAE selectively blunted the LPS-induced production of IL-1β mRNA in the cortex of female rats. Taken together with the collective findings regarding third trimester alcohol exposure and the neuroimmune system (Reviewed by Drew and Kane, 2013), our findings suggest that chronic exposure to lower levels of alcohol is less disruptive to the neuroimmune system than binge-like exposure to high doses of alcohol.
We examine the effect of fetal alcohol exposure on neuroimmune function
A chronic, lower dose of alcohol exposure is used
Cytokine production is measured after injections of lipopolysaccharide
Pro and anti-inflammatory cytokines were included
This paradigm may not be as disruptive to the neuroimmune system as higher doses
Acknowledgments
The authors would like to thank Kiran Bhaskar, Erin Milligan, Russell Morton, and Marvin Diaz for their edits and comments on this manuscript. Supported by NIH grants R37 AA015614 and T32 AA014127.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banks WA, Robinson SM. Minimal penetration of lipopolysaccharide across the murine blood-brain barrier. Brain Behav Immun. 2010;24:102–109. doi: 10.1016/j.bbi.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Biedenkapp JC, Der-Avakian A, Watkins LR, Rudy JW, Maier SF. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J Neurosci. 2005a;25:8000–8009. doi: 10.1523/JNEUROSCI.1748-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Levkoff LH, Mahoney JH, Watkins LR, Rudy JW, Maier SF. Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav Neurosci. 2005b;119:293–301. doi: 10.1037/0735-7044.119.1.293. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland ST, Beckley JT, Young S, Tsang V, Watkins LR, Maier SF, Bilbo SD. Enduring consequences of early-life infection on glial and neural cell genesis within cognitive regions of the brain. Brain Behav Immun. 2010;24:329–338. doi: 10.1016/j.bbi.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174:1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady ML, Diaz MR, Iuso A, Everett JC, Valenzuela CF, Caldwell KK. Moderate prenatal alcohol exposure reduces plasticity and alters NMDA receptor subunit composition in the dentate gyrus. J Neurosci. 2013;33:1062–1067. doi: 10.1523/JNEUROSCI.1217-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, Susser ES. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- Burd L, Blair J, Dropps K. Prenatal alcohol exposure, blood alcohol concentrations and alcohol elimination rates for the mother, fetus and newborn. J Perinatol. 2012;32:652–659. doi: 10.1038/jp.2012.57. [DOI] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudd TA. Animal model systems for the study of alcohol teratology. Exp Biol Med (Maywood) 2005;230:389–393. doi: 10.1177/15353702-0323006-06. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MR, Vollmer CC, Zamudio-Bulcock PA, Vollmer W, Blomquist SL, Morton RA, Everett JC, Zurek AA, Yu J, Orser BA, et al. Repeated intermittent alcohol exposure during the third trimester-equivalent increases expression of the GABA(A) receptor delta subunit in cerebellar granule neurons and delays motor development in rats. Neuropharmacology. 2014;79:262–274. doi: 10.1016/j.neuropharm.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diz-Chaves Y, Pernia O, Carrero P, Garcia-Segura LM. Prenatal stress causes alterations in the morphology of microglia and the inflammatory response of the hippocampus of adult female mice. J Neuroinflammation. 2012;9:71. doi: 10.1186/1742-2094-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew PD, Kane CJM. Neuroimmune Mechanisms of Glia and Their Interplay with Alcohol Exposure Across the Lifespan. In: Changhai Cui LG, Noronha Antonio, editors. Neural-Immune Interactions in Brain Function and Alcohol Related Disorders. New York: Springer US; 2013. pp. 359–386. [Google Scholar]
- Erickson MA, Banks WA. Cytokine and chemokine responses in serum and brain after single and repeated injections of lipopolysaccharide: multiplex quantification with path analysis. Brain Behav Immun. 2011;25:1637–1648. doi: 10.1016/j.bbi.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethen MK, Ramadhani TA, Scheuerle AE, Canfield MA, Wyszynski DF, Druschel CM, Romitti PA. Alcohol consumption by women before and during pregnancy. Matern Child Health J. 2009;13:274–285. doi: 10.1007/s10995-008-0328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett JC, Licon-Munoz Y, Valenzuela CF. Effects of third trimester-equivalent ethanol exposure on Cl(−) co-transporter expression, network activity, and GABAergic transmission in the CA3 hippocampal region of neonatal rats. Alcohol. 2012;46:595–601. doi: 10.1016/j.alcohol.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foey AD, Parry SL, Williams LM, Feldmann M, Foxwell BM, Brennan FM. Regulation of monocyte IL-10 synthesis by endogenous IL-1 and TNF-alpha: role of the p38 and p42/44 mitogen-activated protein kinases. J Immunol. 1998;160:920–928. [PubMed] [Google Scholar]
- Galindo R, Frausto S, Wolff C, Caldwell KK, Perrone-Bizzozero NI, Savage DD. Prenatal ethanol exposure reduces mGluR5 receptor number and function in the dentate gyrus of adult offspring. Alcohol Clin Exp Res. 2004;28:1587–1597. doi: 10.1097/01.alc.0000141815.21602.82. [DOI] [PubMed] [Google Scholar]
- Gauthier TW, Drews-Botsch C, Falek A, Coles C, Brown LA. Maternal alcohol abuse and neonatal infection. Alcohol Clin Exp Res. 2005;29:1035–1043. doi: 10.1097/01.alc.0000167956.28160.5e. [DOI] [PubMed] [Google Scholar]
- Gohlke JM, Griffith WC, Faustman EM. Computational models of ethanol-induced neurodevelopmental toxicity across species: Implications for risk assessment. Birth Defects Res B Dev Reprod Toxicol. 2008;83:1–11. doi: 10.1002/bdrb.20137. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Peterson SD. Sex differences in vulnerability to developmental spatial learning deficits induced by limited binge alcohol exposure in neonatal rats. Neurobiol Learn Mem. 1995;64:265–275. doi: 10.1006/nlme.1995.0009. [DOI] [PubMed] [Google Scholar]
- Guerri C. Neuroanatomical and neurophysiological mechanisms involved in central nervous system dysfunctions induced by prenatal alcohol exposure. Alcohol Clin Exp Res. 1998;22:304–312. doi: 10.1111/j.1530-0277.1998.tb03653.x. [DOI] [PubMed] [Google Scholar]
- Hagihara H, Toyama K, Yamasaki N, Miyakawa T. Dissection of hippocampal dentate gyrus from adult mouse. J Vis Exp. 2009 doi: 10.3791/1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton GF, Whitcher LT, Klintsova AY. Postnatal binge-like alcohol exposure decreases dendritic complexity while increasing the density of mature spines in mPFC Layer II/III pyramidal neurons. Synapse. 2010;64:127–135. doi: 10.1002/syn.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Jaeger LB, Dohgu S, Sultana R, Lynch JL, Owen JB, Erickson MA, Shah GN, Price TO, Fleegal-Demotta MA, Butterfield DA, et al. Lipopolysaccharide alters the blood-brain barrier transport of amyloid beta protein: a mechanism for inflammation in the progression of Alzheimer’s disease. Brain Behav Immun. 2009;23:507–517. doi: 10.1016/j.bbi.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji K, Miyauchi J, Tsirka SE. Microglia: an active player in the regulation of synaptic activity. Neural Plast. 2013;2013:627325. doi: 10.1155/2013/627325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane CJ, Phelan KD, Douglas JC, Wagoner G, Johnson JW, Xu J, Phelan PS, Drew PD. Effects of ethanol on immune response in the brain: region-specific changes in adolescent versus adult mice. Alcohol Clin Exp Res. 2014;38:384–391. doi: 10.1111/acer.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane CJ, Phelan KD, Han L, Smith RR, Xie J, Douglas JC, Drew PD. Protection of neurons and microglia against ethanol in a mouse model of fetal alcohol spectrum disorders by peroxisome proliferator-activated receptor-gamma agonists. Brain Behav Immun. 2011;25(Suppl 1):S137–145. doi: 10.1016/j.bbi.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki H, Nakai S, Hirai Y, Akaji K, Kiso Y, Satoh M. Interleukin-1 beta inhibits long-term potentiation in the CA3 region of mouse hippocampal slices. Eur J Pharmacol. 1990;181:323–326. doi: 10.1016/0014-2999(90)90099-r. [DOI] [PubMed] [Google Scholar]
- Kendall GS, Hristova M, Horn S, Dafou D, Acosta-Saltos A, Almolda B, Zbarsky V, Rumajogee P, Heuer H, Castellano B, et al. TNF gene cluster deletion abolishes lipopolysaccharide-mediated sensitization of the neonatal brain to hypoxic ischemic insult. Lab Invest. 2011;91:328–341. doi: 10.1038/labinvest.2010.192. [DOI] [PubMed] [Google Scholar]
- Lin HY, Huang CC, Chang KF. Lipopolysaccharide preconditioning reduces neuroinflammation against hypoxic ischemia and provides long-term outcome of neuroprotection in neonatal rat. Pediatr Res. 2009;66:254–259. doi: 10.1203/PDR.0b013e3181b0d336. [DOI] [PubMed] [Google Scholar]
- Marshall SA, McClain JA, Kelso ML, Hopkins DM, Pauly JR, Nixon K. Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype. Neurobiol Dis. 2013;54:239–251. doi: 10.1016/j.nbd.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- McClain JA, Morris SA, Deeny MA, Marshall SA, Hayes DM, Kiser ZM, Nixon K. Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain Behav Immun. 2011;25(Suppl 1):S120–128. doi: 10.1016/j.bbi.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill J, Meyerholz DK, Edsen-Moore M, Young B, Coleman RA, Schlueter AJ, Waldschmidt TJ, Cook RT, Legge KL. Fetal exposure to ethanol has long-term effects on the severity of influenza virus infections. J Immunol. 2009;182:7803–7808. doi: 10.4049/jimmunol.0803881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D, Roth TL, McGavern DB. Microglia Development and Function. Annu Rev Immunol. 2014 doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TE, Olde Engberink A, Hernandez R, Puro A, Huitron-Resendiz S, Hao C, De Graan PN, Gruol DL. Altered synaptic transmission in the hippocampus of transgenic mice with enhanced central nervous systems expression of interleukin-6. Brain Behav Immun. 2012;26:959–971. doi: 10.1016/j.bbi.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousman SS, Kubes P. Immune surveillance in the central nervous system. Nat Neurosci. 2012;15:1096–1101. doi: 10.1038/nn.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Platzer C, Meisel C, Vogt K, Platzer M, Volk HD. Up-regulation of monocytic IL-10 by tumor necrosis factor-alpha and cAMP elevating drugs. Int Immunol. 1995;7:517–523. doi: 10.1093/intimm/7.4.517. [DOI] [PubMed] [Google Scholar]
- Puglia MP, Valenzuela CF. AMPAR-mediated synaptic transmission in the CA1 hippocampal region of neonatal rats: unexpected resistance to repeated ethanol exposure. Alcohol. 2009;43:619–625. doi: 10.1016/j.alcohol.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglia MP, Valenzuela CF. Repeated third trimester-equivalent ethanol exposure inhibits long-term potentiation in the hippocampal CA1 region of neonatal rats. Alcohol. 2010;44:283–290. doi: 10.1016/j.alcohol.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Crews FT. Chronic ethanol increases systemic TLR3 agonist-induced neuroinflammation and neurodegeneration. J Neuroinflammation. 2012;9:130. doi: 10.1186/1742-2094-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen C. Executive functioning and working memory in fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2005;29:1359–1367. doi: 10.1097/01.alc.0000175040.91007.d0. [DOI] [PubMed] [Google Scholar]
- Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Bilbo SD. LPS elicits a much larger and broader inflammatory response than Escherichia coli infection within the hippocampus of neonatal rats. Neurosci Lett. 2011;497:110–115. doi: 10.1016/j.neulet.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Bilbo SD. Adolescent morphine exposure affects long-term microglial function and later-life relapse liability in a model of addiction. J Neurosci. 2013;33:961–971. doi: 10.1523/JNEUROSCI.2516-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Hutchinson MR, Bilbo SD. Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression. J Neurosci. 2011;31:17835–17847. doi: 10.1523/JNEUROSCI.3297-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J Neurochem. 2012;120:948–963. doi: 10.1111/j.1471-4159.2011.07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soscia SJ, Tong M, Xu XJ, Cohen AC, Chu J, Wands JR, de la Monte SM. Chronic gestational exposure to ethanol causes insulin and IGF resistance and impairs acetylcholine homeostasis in the brain. Cell Mol Life Sci. 2006;63:2039–2056. doi: 10.1007/s00018-006-6208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, Galic MA, Pittman QJ. Neonatal programming of innate immune function. Am J Physiol Endocrinol Metab. 2011;300:E11–18. doi: 10.1152/ajpendo.00516.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari V, Chopra K. Attenuation of oxidative stress, neuroinflammation, and apoptosis by curcumin prevents cognitive deficits in rats postnatally exposed to ethanol. Psychopharmacology (Berl) 2012;224:519–535. doi: 10.1007/s00213-012-2779-9. [DOI] [PubMed] [Google Scholar]
- Uban KA, Comeau WL, Ellis LA, Galea LA, Weinberg J. Basal regulation of HPA and dopamine systems is altered differentially in males and females by prenatal alcohol exposure and chronic variable stress. Psychoneuroendocrinology. 2013;38:1953–1966. doi: 10.1016/j.psyneuen.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uban KA, Sliwowska JH, Lieblich S, Ellis LA, Yu WK, Weinberg J, Galea LA. Prenatal alcohol exposure reduces the proportion of newly produced neurons and glia in the dentate gyrus of the hippocampus in female rats. Horm Behav. 2010;58:835–843. doi: 10.1016/j.yhbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereker E, Campbell V, Roche E, McEntee E, Lynch MA. Lipopolysaccharide inhibits long term potentiation in the rat dentate gyrus by activating caspase-1. J Biol Chem. 2000;275:26252–26258. doi: 10.1074/jbc.M002226200. [DOI] [PubMed] [Google Scholar]
- Wang DS, Zurek AA, Lecker I, Yu J, Abramian AM, Avramescu S, Davies PA, Moss SJ, Lu WY, Orser BA. Memory deficits induced by inflammation are regulated by alpha5-subunit-containing GABAA receptors. Cell Rep. 2012;2:488–496. doi: 10.1016/j.celrep.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanidworanun C, Strober W. Predominant role of tumor necrosis factor-alpha in human monocyte IL-10 synthesis. J Immunol. 1993;151:6853–6861. [PubMed] [Google Scholar]
- Watari H, Born DE, Gleason CA. Effects of first trimester binge alcohol exposure on developing white matter in fetal sheep. Pediatr Res. 2006;59:560–564. doi: 10.1203/01.pdr.0000203102.01364.de. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinol. 2008;20:470–488. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzba-Bobrowicz T, Lewandowska E, Kosno-Kruszewska E, Lechowicz W, Skorzewska A, Gwiazda E, Pasennik E. Dendritic and microglial cells in pups of alcohol-treated female rats. Folia Neuropathol. 2003;41:131–137. [PubMed] [Google Scholar]
- Zamudio-Bulcock PA, Morton R, Valenzuela CF. Third Trimester-Equivalent Ethanol Exposure does not Alter Complex Spikes and Climbing Fiber Long-Term Depression in Cerebellar Purkinje Neurons from Juvenile Rats. Alcoholism: Clinical and Experimental Research. 2014 doi: 10.1111/acer.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]