Abstract
Head and neck squamous cell carcinomas (HNSCC) are malignant tumors that arise from the surface epithelium of the oral cavity, oropharynx and larynx, primarily due to exposure to chemical carcinogens or the human papilloma virus. Due to their location, dental practitioners are well-positioned to detect the lesions. Deadlier than lymphoma or melanoma, HNSCC is incompletely understood. For these reasons, dental practitioners and researchers are focused on understanding HNSCC and the processes driving it. One of these critical processes is invasion, the degradation of the basement membrane by HNSCC cells with subsequent movement into the underlying connective tissue, blood vessels or nerves. Cancer cells metastasize to distant sites via the blood vessels, lymphatics and nerves. Metastasis is associated with poor survival. Since invasion is essential for development and metastasis of HNSCC, it is essential to understand the mechanism(s) driving this process. Elucidation of the mechanisms involved will facilitate the development of targeted treatment, thereby accelerating development of precision/ personalized medicine to treat HNSCC. Robust in vitro and in vivo assays are required to investigate the mechanistic basis of invasion. This review will focus on in vitro and in vivo assays used to study invasion in HNSCC, with special emphasis on some of the latest assays to study HNSCC.
Keywords: squamous cell carcinoma, in vitro, in vivo, invasion, HNSCC
Introduction
Head and neck squamous cell carcinomas (HNSCC) are malignant neoplasms arising from surface epithelium of the oral cavity, oropharynx and larynx. HNSCC may be due to human papilloma virus (HPV) or tobacco and alcohol exposure [1]. genetic factors and patient behavior are also relevant to development and prognosis [1]. The American Cancer Society estimated that HNSCC accounted for 39,000 new cases and 8,000 deaths in the United States in 2011 [2]. Globally, ~600,000 new cases of HNSCC are diagnosed each year [1]. At ~50%, the five-year survival rate is lower than breast cancer or melanoma [3].
HNSCC tumors are heavily vascularized and metastasize through blood vessels, nerves or lymphatic vessels [4] to regional lymph nodes, lungs, bones and the liver [5, 6]. Treatment of metastatic HNSCC has poor success even if aggressive, whereas patients with non-metastatic cancer are treated more effectively [5]. Metastases and related complications are leading sources of cancer-related mortality and morbidity [7]. Since metastasis is distant invasion [8], it is critical to understand the mechanisms of invasion. The review highlights approaches used to study invasion, including recently developed in vitro and in vivo invasion assays.
Understanding HNSCC
Figure 1 provides a graphical illustration of HNSCC. HNSCC develops when genetic abnormalities accumulate in non-malignant epithelial cells. Abnormal cells initially cluster within the surface epithelium (pre-cancer or epithelial dysplasia/ carcinoma-in-situ) above the basement membrane. Pre-cancer progresses to HNSCC when the basement membrane is disrupted and transformed cells from the surface epithelium invade the underlying connective tissue [9]. Exposure to chemical carcinogens causes the accumulation of genetic abnormalities, which can result in cancer developing [4]. Multiple primary tumors or recurrent tumors may develop in oral tissues exposed to chemical carcinogens, a phenomenon referred to as “field cancerization” [4].
Figure 1. HNSCC invasion.
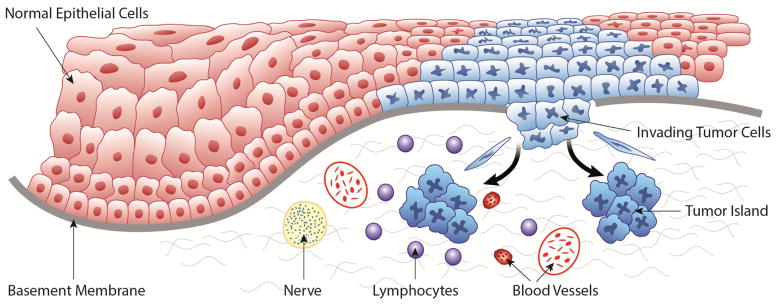
In this image, transformed cells (blue) are invading through the basement membrane (gray). Elongated mesenchymal cells represent the importance of EMT in invasion. Invasive tumor islands are present in the connective tissue. Elements of the tumor microenvironment are depicted, including nerves, vasculature and the inflammatory infiltrate.
Metastases of HNSCC may originate from a small population of primary tumor cells [10]. Epithelial to mesenchymal transition (EMT) occurs during HNSCC progression and is characterized by the transition of non-motile epithelial cells into motile mesenchymal-like cells [9]. EMT occurs in wound healing, embryonic development and cancer [9]. In HNSCC, EMT facilitates tumor progression, including invasion and metastasis, and potentially increases the population of cancer stem cells [9, 11]. After EMT, cells become more capable of initiation of the “invasion-metastasis cascade” [10, 12].
Understanding Invasion
The terms invasion and migration are often used interchangeably. However, in experimental cellular biology, invasion is defined as disruptive, proteolytic, cellular movement through three-dimensional (3D) barriers, distinct from migration which is defined as non-disruptive, non-proteolytic, movement of cells through tissues [11, 13]. “Migration” can also refer to two-dimensional directed movement on a substrate [13].
Despite intense study, the complex and heterogenous mechanisms of invasion and metastasis remain imperfectly understood [14]. Collective or individual invasion of cancer cells occurs via several mechanisms [15, 16]. Cells invading collectively exhibit preserved inter-cellular junctions with leader cells paving the way for the collective. In individual cell invasion, each cell invades the basement membrane via highly proteolytic mesenchymal cell movement or plasma membrane blebbing. In HNSCC, disruption of the basement membrane usually occurs via proteolytic degradation of extracellular matrix using matrix metalloproteases (MMPs) [16, 17]. Plasma membrane blebbing uses mechanical means to deform and penetrate the basement membrane [15]. Cancer cells may switch from a proteolytic to a mechanical invasion mechanism if matrix degradation by MMPs has been inhibited through protease inhibition [13, 15, 16]. These invasion patterns have been observed in cancer, wound healing and development [11].
Invasion is essential to metastasis in HNSCC. Invasion and metastasis require penetration of the basement membrane of the surface epithelium and blood vessels to access tissues at proximal and distant sites [8]. Since metastasis is a leading cause of cancer-related mortality and morbidity [7], targeting invasion should slow or prevent metastasis and improve survival in HNSCC.
Invasion Assays
Many invasion assays are used, some adapted from migration assays. In vitro invasion assays quantify cells invading through structures such as synthetic Basement Membrane Equivalents (BMEs). The category of BME includes a wide range of materials intended to mimic the structure and composition of the extracellular matrix, from reconstituted collagen gels to more complex materials. These in vitro assays may encourage invasion via chemotaxis, which is the process of cell movement in response to chemical gradients (towards an attractant or away from a repellant). Therefore, assays using chemical gradients can be referred to as chemotaxis-based invasion assays. In vivo invasion assays quantify cells invading through biological membranes that are analogous to the basement membrane.
Since invasion through the basement membrane of the surface epithelium transforms pre-cancer to HNSCC, understanding the mechanism of invasion will identify treatment targets to prevent malignant transformation [9, 18]. Unfortunately, there are gaps in traditional experimental models. For example, in vitro assays do not reproduce the complexity of invasion in living tissues, such as the heterogeneity of the extracellular matrix. Moreover, in vivo tumor studies typically feature injection of tumor cells into subcutaneous tissues, which circumvents penetration of the basement membrane of the surface epithelium, preventing investigations of this early event in transformation of pre-cancer to HNSCC [9, 18]. Therefore, there is an imperative to develop new and improved invasion assays and understand the traditional assays.
Materials
Due to easy availability, the earliest invasion studies used in vivo or organotypic models such as rabbit ear or amniotic tissue. Difficulties associated with using these materials and quantifying the data produced by these models lead to the development of in vitro alternatives, or BMEs [19]. BMEs include reconstituted collagen with supplements, from sources including rat-tail collagen [17, 20]. Reconstituted collagen BMEs require multiple steps to purify and monomerize collagen from an animal, with more extensively cross-linked collagens requiring more extensive processing before being re-polymerized under basic conditions [17]. Matrigel™, produced from secretions of Engelbreth-Holm-Swarm mouse sarcomas, is highly uniform and likely the most widely used BME [20, 21]. Matrigel™ contains a range of inherent growth factors, which could potentially confound experiments. Some Matrigel™ advocates argue that this is an advantage rather than a problem because normal in vivo extracellular matrix can also contain growth factors [21, 22]. Given the tendency of extracellular matrix to contain bound cytokines, including growth factors, this opinion seems defensible. However, companies provide growth factor-depleted Matrigel™ to avoid these potential problems [21]. Other, less commonly used, BMEs exist and are typically generated from cellular secretions or reconstituted extracellular matrix [21, 23]. Many BMEs, containing a range of proteins and other materials normally found in the extracellular matrix, are more structurally heterogenous than simple reconstituted collagen. However, since collagen makes up a large percentage of the extracellular matrix proteins and because it has a crucial structural role, any material that is intended to mimic the extracellular matrix should contain some collagen.
The complexities that made early assays difficult to quantify have major roles in invasion in vivo [24]. This is particularly true of pores in commonly used BMEs, which are more uniform than those found in vivo [17]. Experiments showing that invasion is encouraged by irregularities in a collagen lattice have illustrated the importance of disorganized regions or vessels in the extracellular matrix [25]. This has renewed interest in complex BMEs that mimic in vivo conditions, and in in vivo invasion assays. Ideal materials for invasion studies need to replicate the structural and constitutional complexity of the extracellular matrix. For example, these materials should include non-uniform pores and disorganized regions and recreate the interactions of the various laminins, collagens and other extracellular matrix proteins [18, 21, 25]. In this review, new approaches and more complex materials under development are described in the context of in vitro and in vivo invasion assays, including models to study invasion in 3D. The assays most commonly used to study invasion in HNSCC are described here, while less commonly used assays are not included.
In vitro assays
The most widely used in vitro assays of invasion may be sub-classified into three categories: wound healing, transwell and organotypic assays.
Wound Healing Assays
Wound Healing Assays (WHAs) trace their origin to the scratch assay for migration. Although the terms scratch assay and WHA have been used interchangeably, the use of different approaches to generate wounds has led some to use the phrase “scratch assay” to refer only to the traditional scratch, while Wound Healing Assays refer to the entire category including scratch assays [11]. In WHAs, a cellular monolayer is grown to confluence on a substrate and a linear or circular gap is created in the monolayer. The original WHA, the scratch assay, studied the rate at which cells migrated into a gap created by scratching a “wound” into the cellular monolayer with a pipette tip. Subsequently, WHAs were modified to study invasion in HNSCC by adding a BME in the gap and assessing the rate at which cells invade the BME, while controlling for cellular proliferation [19]. In this modified WHA, cells must invade through the BME instead of migrating across the substrate.
Modified Scratch Assay
The most basic WHA is the scratch migration assay, where a gap is created by scratching away the monolayer [19]. This sort of assay can be modified to study invasion by adding BME [11]. While the assay seems simple, creation of the gap is a challenge. These gaps are of non-standard dimensions, lined by cells with reduced motility due to damage from the scratch, and the substrate in the scratch may also be damaged [26, 27]. Efforts to improve the assay include creating devices that allow simultaneous, identical, scratches in multiple wells thereby increasing throughput and lowering variability [27]. While this approach reduces within-batch variability, it recapitulates the traditional scratch assay so difficulties remain.
Other Wound Healing Assays
Other WHAs focus on producing a uniform wound or reducing damage to cells or the underlying substrate. The Circular Invasion Assay (based on the established Circular WHA for migration) accomplishes the first goal by using a commercially available drill press with a blunt silicone tip to create standardized circular wounds in the cellular monolayer that are filled with BME so that the rate of closure can be studied using time-lapse microscopy to quantify and characterize invasion (i. e. collective versus individual) [26]. This assay produces damaged cells and while time-lapse microscopy and related imaging techniques yield useful results, these approaches are also used with other WHAs.
Wound Healing Assays with Inserts
WHAs may feature a variety of inserts that are used to generate uniform gaps. These inserts were first employed in migration assays, but were modified for invasion assays [11]. As with other “wound” assays, these gaps can be overlaid with BME to convert them into invasion assays. The Oris™ (Platypus Technologies) cell invasion assay features circular silicone inserts. The inserts are placed on cell culture wells coated with BME and a cellular monolayer is grown around the silicone plug. After the cells have adhered to the BME, the plugs are removed and a second layer of the same BME is overlaid, leaving the cells in a BME sandwich with a central cell-free zone [28]. Invasion of cells into the cell-free area is monitored and quantified by microscopy [28]. Like the circular invasion assay, this creates a circular, BME-filled wound but also avoids damaged cells around the gap. Similarly, the CytoSelect™ (Cell Biolabs, Inc.) WHA uses a linear insert placed on the substrate to produce a uniform “wound” surrounded by a cellular monolayer without damaged cells at the edge of the wound. These insert-based WHAs are examples of how WHAs for migration are easily adapted for invasion by addition of BME. While there are differences in wound generation procedure, the main functional difference is that the CytoSelect™ insert creates a uniform linear wound and the Oris™ insert creates a uniform circular wound. The inserts are reusable. Another approach to creating uniform wounds without damaging surrounding cells is a plate with pre-placed drops of biocompatibility gel in the wells, instead of inserts, which dissolve to leave behind circular gaps for cell invasion [29].
Transwell Invasion Assay
Transwell invasion assays (TIAs) are based on the Boyden Chamber migration assay Figure 2). The most commonly used TIA setup resembles the Boyden Chamber with an added insert for invasion [11]. This assay features two chambers. The cells are placed on a collagen matrix over a porous filter in the top chamber. Chemo-attractant is placed in the lower chamber. Invasion is assessed by quantifying the cells that penetrate the insert, either through visual quantification after staining with dye, or by quantification of fluorescence after lysing invading fluorescent-labeled cells [19]. In the FluoroBlok™ TIA, fluorescent-labeled invasive cells penetrate the same materials as in other TIAs, but since the insert is opaque to visible light, fluorescent cells are detected with bottom-reading plate readers [30]. Migration is controlled by quantifying cell migration in the absence of collagen matrix in the transwell chamber and calculating a “percent invasion” from the ratio of invaded cells over migrated cells; cell proliferation should also be controlled [20, 21, 31].
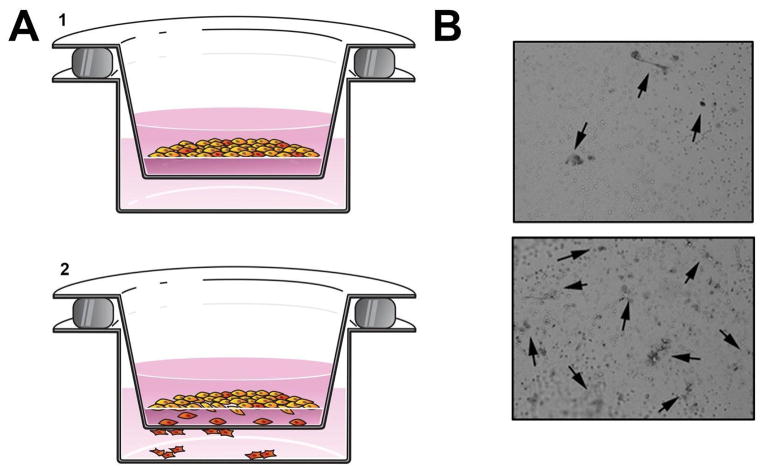
Figure 2. Boyden chamber assay.
(A) Graphical depiction of an invasion assay. 1. HNSCC cells seeded on BME in the upper chamber. 2. Cells invade toward a chemoattractant. (B) Photographs show HNSCC cells (highlighted by arrows) that have penetrated the BME. The upper image shows less invasion than the lower image.
While WHAs and TIAs are informative, they do not fully model in vivo invasion. The extent to which they mimic invasion depends on the type of insert, but in vivo invasion is more complicated than in vitro assays. In vivo, invading cells in biological tissues encounter structural and constitutional heterogeneity including disorganized regions, blood vessels, nerves, and mesenchymal and inflammatory cells, all of which can impact invasion [18, 21, 25]. Moreover, biological basement membranes have a complex composition including various laminins, collagens and other extracellular matrix proteins [18, 21, 25]. This complexity is greater than that observed in BMEs used for in vitro assays. Additionally, although Boyden chamber assays provide change-over-time results, they focus on end-point data, not continuous observation [32]. As such, they cannot provide a full range of information about the behavior of invading cells during invasion. This information is available from assays that allow continuous observation of invading cells, which can illustrate invasion mechanisms and paths through the matrix. This mechanistic and functional information is important in identifying methods to target invasion.
Spheroid Invasion Assays
Other in vitro assays include interaction assays like the spheroid/monodispersed cell invasion assay, where spheroids of a non-invasive cell type are co-cultured with an invasive cell type in single-cell suspension [11, 33]. Invasion into these spheroids is quantified by fluorescence imaging or immunohistochemistry.
The spheroid confrontation assay features pre-formed spheroids of two cell types, invasive and non-invasive, cultured adjacently leading to their fusion. At this stage, invasive cells invade the non-invasive spheroid and are quantified through appropriate imaging or immunohistochemistry [11, 34]. The spheroid-based invasion assays measure invasion into a tight, multi-cellular, 3D structure where cell-cell interactions occur. However the cells being invaded must be able to form spheroids. Moreover, quantification of invaded cells may be difficult due to the spheroid structure.
Three Dimensional Invasion Assays
Since invasion occurs in three dimensions in vivo, 3D in vitro invasion assays are important. Some of the earliest 3D invasion models were blocks of reconstituted collagen, with invasion assessed by tracking the movement of radioactively labeled cells into the collagen block. Although such materials are still in use today, there has been a great deal of experimentation with alternatives in order to create 3D materials that more closely resemble the complexity of the tissues and the invasive process [35–38]. Because of the prevalence and structural significance of collagen in biological extracellular matrices, any structure intended to model extracellular matrix structure is likely to contain some collagen. Two of the more recently developed materials of particular interest for the study of invasion in 3D, are human skin equivalent and oral cancer equivalent.
Human Skin Equivalent (HSE): In HSE, keratinocytes are added to human collagen that has been pre-incubated with fibroblasts for five to seven days. The HSE is incubated for another seven days, resulting in a stratified epidermis at the surface, featuring layers normally found in human epithelium [39]. HSE was developed to study wound healing, but is also used to study cancer invasion. This is a general skin equivalent lacking some collagen cross-links found in human tissue [39, 40].
Oral Cancer Equivalent (OCE): OCE, using human cadaveric acellular dermal matrix (AlloDerm™) [18], was inspired by its use in generating autologous human oral mucosa equivalent grafts from oral keratinocytes [41]. OCE is an HNSCC-specific 3D model to study invasion. Like HSE, OCE is an example of the ongoing shift towards improved approximations of in vivo extracellular matrix [32]. OCE is generated by seeding human HNSCC cells on decellularized human dermal tissue coated with human collagen IV. OCE mimics the HNSCC invasion environment better than simpler BMEs such as reconstituted collagen [18]. OCE can be adapted for a variety of assays and viewed cross-sectionally to illustrate depth of invasion, an important treatment selection factor in some HNSCC such as tongue cancer [42]. Two main types of images are generated: cross-sectional and en face images used either for surface assessment or to generate 3D models through non-linear optical microscopy or, potentially, through confocal microscopy and confocal imaging stacks [18]. The number of invasive cells and invasive islands [43], and the depth of invasion are quantified on tissue sections [18].
The materials created for 3D assays of invasion in skin cancer and HNSCC are adapted in a variety of ways to assess different aspects of invasion. For example some use stromal fibroblasts implanted in collagen gel to simulate interactions between stromal cells and cancer cells [38, 44]. With 3D approaches, the results are typically quantified with either invasive area measurements from image analysis software or by inspection of perpendicular cuts into formalin-fixed, paraffin-embedded (FFPE) samples. While FFPE requires additional equipment and expertise, it offers insights into cellular morphology and invasive patterns. Additional quantification is done with specialized microscopes for 3D cell tracking, tracing the paths and activities of individual labeled or unlabeled cells [45]. These assays have illustrated the importance of stromal-epithelial interactions in collective invasion, with one study showing that embedded fibroblasts break paths for collective invasion when interacting with tumor cells [46].
These new materials have improved understanding of the roles of individual components of the extracellular matrix and provided additional insights into the mechanisms of invasion in vitro. It is still necessary to establish in vivo models of invasion that recapitulate the complexity of the basement membrane and the supporting tissues in HNSCC.
In vivo assays
Traditionally, invasion in HNSCC has been studied with mouse models summarized in Figure 3. Work with mice includes both human HNSCC xenografts injected into immunocompromised animals, and immunocompetent animals injected with syngeneic cells [47]. Once tumors are established, they are assessed for growth or proliferation with bioluminescence imaging, 3D visualization through micro-computed topography, or excision followed by light microscopy [48, 49]. The more commonly used approaches will be discussed here, as well as some new in vivo assays.
Figure 3. HNSCC models in mouse.
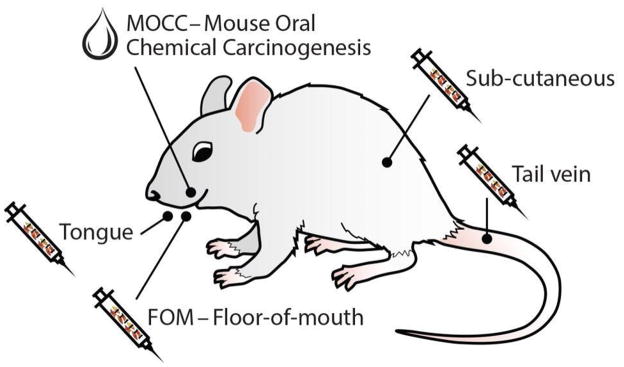
Several HNSCC models are available, including those indicated.
Sub-Cutaneous and Tail Vein Assays
The classical in vivo assay is a mouse injected with cells to produce tumors, which are assessed for size, mass and other histologic parameters of tumor progression (e.g. vascular invasion).
HNSCC cells may be injected into mice sub-cutaneously or in the tail vein. In sub-cutaneous or sub-mucosal injections, in vitro cultured tumor cells are injected in the back or hind-leg of the mouse to produce tumors at the injection site. Since tumor cells are injected directly into the connective tissue, bypassing the basement membrane, the crucial early steps of invasion that transform a pre-cancerous lesion to HNSCC cannot be investigated [9, 50]. However, these models are used to investigate other oncogenic phenotypes including angiogenesis, metastasis and the impact of proteins or other chemicals on tumor size [51–53]. Intra-vital imaging has improved understanding of the interactions between tumor cells and surrounding host tissues [19, 54]. Some highly complex approaches allow the indirect study of invasion using tumors generated through these methods. One particular approach features the insertion of a collagen-filled micro-needle with a chemoattractant gradient into the tumor of an anesthetized animal for a defined period of time [55]. Aggressive tumor cells that invade far into the collagen matrix are collected and their gene expression profiles are compared to those tumor cells that did not invade. The cells that do not invade are surgically collected from areas of tumor around the site of the micro-needle’s insertion. This approach allows comparison of gene expression profiles of the invasive/aggressive sub-population of tumor cells with that of the whole tumor cell population, and as such is a potential source of information on the genes involved in invasion. However, this is in effect an in vitro invasion assay (microneedle with chemoattractant gradient) performed on an in vivo tumor [55].
The injection of tumor cells into the tail vein has been used to study metastasis, which often form in organs such as the lungs and liver [51]. This approach skips several early steps in the metastatic cascade including invasion from the surface epithelium, invasion into the adjacent tissues, extracellular matrix interactions and angiogenesis, making it a sub-optimal approach for studying invasion in HNSCC.
Oral Tumor Assays
Other common HNSCC mouse models include tongue and floor-of-mouth models [47, 49]. In the tongue model, tumor cells are injected sub-mucosally and may require debulking as the tumor enlarges [48, 56]. Floor-of-mouth HNSCC models have traditionally used extraoral sub-cutaneous injections to establish tumors [57, 58]. These approaches use up to 2×106 cells, suspended in large volumes of buffer when injected [57, 58]. A newer approach features the intraoral sub-mucosal injection of 1×105 to 2×106 tumor cells suspended in ~100 μl media into the anterior floor-of-mouth [49]. For cell lines that have difficulty forming tumors under xenograft conditions, collagen matrix is added to the media (1:1; ~100 μl total volume) to assist in tumor development by facilitating HNSCC cell localization [49]. Due to outward growth of the tumors in the floor-of-mouth HNSCC model, tumors are more easily monitored and, due to the typically smaller number of cells used, can be studied for longer periods before tumor growth requires either killing the animal or debulking the tumor [49]. Tumor size is assessed in two dimensions using calipers in the sub-mandibular region or after removal from the euthanized animal. Tumor volume may also be assessed by bioluminescence [49]. Micro-computed topographical examination has been used to assess bone involvement [49].
Due to the tumor microenvironment, vascularity and anatomy of the region, including the adjacent mandible, the oral cavity orthotopic models mimic human HNSCC better than the subcutaneous model of the back [49]. Indeed, when the same HNSCC cell lines are injected into the backs and mouths of mice, the intraoral tumors resemble the original human HNSCC tumors more closely histologically, and typically show greater invasion, compared to sub-cutaneous tumors on the back [59].
Intra-Vital Assays
Intra-vital assays observe tumor activities within the host. Tumor progression is typically evaluated by Computed Topography or Magnetic Resonance Imaging scans or fluorescence-based bioluminescence imaging (BLI) to assess tumors [49, 60]. For example, in BLI, HNSCC in the floor-of-mouth is visualized by luciferase expression ten minutes after D-luciferin is injected intraperitoneally [49].
Surgical options such as modified skin fold chambers use intra-vital microscopy (IVM) to assess invasion [17, 61]. Common variations including dorsal and mammary skin fold chambers tend to be complex structures featuring observation through implanted windows. During setup, a skin fold is pushed together and a frame implanted around it; one layer of skin is replaced with a transparent panel for observation. After a chamber is implanted, tumor xenografts are established by either injection into or dropping onto the exposed tissue [61].
Intra-vital microscopy techniques allow researchers to observe invading cells in real-time, thereby improving knowledge of mechanisms of invasion (collective versus individual, proteolytic activity versus blebbing). Non-surgical intra-vital imaging in research is relevant and significant, but is a recent development. Surgical assays using IVM allow continuous monitoring of tumor development but have some problems. Implantation of these chambers produces a wound, and since invasion naturally occurs during wound healing this could cause interference. Meanwhile, direct injection into the connective tissue limits utility in studying the early steps of invasion, and with the drop-on approach, the tumor can interact with the observation coverslip, encouraging its dispersal [61]. Moreover, both IVM and non-surgical approaches to intra-vital analysis are technically complex, requiring specialized equipment and expertise. Finally, the high cost of these in vivo experiments, in combination with limited funding, restricts sample sizes.
Mouse Oral Chemical Carcinogenesis (MOCC)
Another novel in vivo assay, mouse oral chemical carcinogenesis (MOCC) induces HNSCC in mice through exposure to carcinogens in their water [62]. Chemical carcinogens are used to induce HNSCC in the hamster cheek-pouch model, where the cheek-pouch is “painted” with dimethylbenzoaanthracene (DMBA) to produce a tumor [47]. MOCC uses the carcinogen 4-nitroquinoline-1 oxide to mimic the impact of tobacco, producing field cancerization as seen in human HNSCC, including the formation of multiple pre-neoplastic lesions, some of which progress to HNSCC [62]. Unlike traditional models where cancer cells are injected directly into the connective tissue, MOCC simulates pre-neoplastic to neoplastic progression in HNSCC. Invasion can be assessed and quantified when tumors are harvested. This assessment can be based on the extent to which the basement membrane in the harvested tissue has been degraded and compromised by invasion or the assessment of tumor islands in the underlying tissue. Because chemical carcinogen exposure can result in the formation of multiple primary tumors (field cancerization), multiple distinct sites of invasion may be noted.
While MOCC models the development of HNSCC from chemical exposure, it cannot be used to study HPV-related cancers. Additionally, it studies mouse tumors instead of transplanted human cancer. This is an issue when investigating the impact of a particular treatment on human HNSCC.
Chick Chorioallantoic Membrane (CAM)
The CAM in chicken eggs may be used to study invasion of human HNSCC in vivo. The CAM consists of the chorionic epithelium separated from the underlying connective tissue by a basement membrane. The basement membrane of the CAM simulates the basement membrane that separates epithelium from the mesenchymal tissues in oral mucosa. The CAM has been used to study angiogenesis and metastasis [63, 64]. Studying HNSCC invasion with CAM is a recent innovation [50].
In CAM assays, a small “window” is drilled into the shell at the top of a fertilized chicken egg, maintaining the integrity of the eggshell’s outer membrane. This membrane is separated from the CAM, which is “dropped” with Hank’s buffered salt solution (HBSS) and suction [50]. Subsequently, the window is enlarged and the outer membrane removed to allow HNSCC cells to be seeded on the CAM [50]. Finally, the window is covered to prevent desiccation and the egg is incubated for multiple days for tumor development on the CAM. Then the CAM, liver and lower CAM are harvested. Tumor growth and angiogenesis are visualized and quantified [50, 52]
Basement membrane degradation and invasion are assessed in tissue sections [50], and metastasis is quantified in the liver and lower CAM. CAM-related assays are useful and versatile. Recently, significant attention is directed to research with fewer or “lower” animals. CAM assays provide in vivo data on tumor growth, invasion, metastasis and angiogenesis at a lower cost and in less time than mouse experiments. Additionally, larger sample sizes are possible due to lower costs. This addresses concerns about the reliability of studies with small samples. Some statistical models suggest that many seemingly significant results from tiny samples are effectively meaningless [65].
Conclusion
Invasion is required for tumor progression, including metastasis. Metastasis and related complications are a leading cause of cancer-related mortality and morbidity [7]. Therefore, it is essential to understand steps leading to metastasis, especially those that may represent tipping points where progression can be halted and prognosis improved. This is particularly important for HNSCC, where little progress has been made in treatment in decades [5]. This stagnation is possibly linked to limitations of traditional methods used to study HNSCC. Newer assays and materials may enable researchers to better understand HNSCC and its key mechanisms. Figure 4 shows histology from some of these assays and human HNSCC histology for comparison. Table 1 summarizes and provides references for the invasion assays discussed in this review. Some of these references do not use HNSCC cells specifically, but provide information about the assays.
Figure 4. Comparison of the histology of in vitro and in vivo models of invasion and human HNSCC.
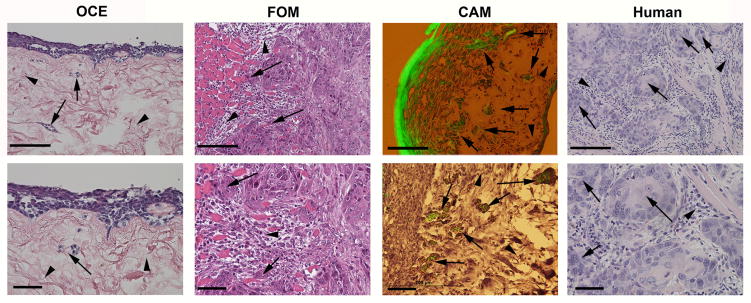
Histological comparison of the oral cancer equivalent (OCE, in vitro), floor-of-mouth (FOM, in vivo) and chick chorioallantoic membrane (CAM, in vivo) models of HNSCC, and human HNSCC, at low magnification (upper panels, scale bar=200um) and high magnification (lower panels, scale bar=50um). Arrows and arrowheads highlight invasive islands and mesenchymal tissue, respectively.
Table 1.
Invasion assay methods and references.
| Invasion Assay | Methods Papers |
|---|---|
| Circular Invasion Assay | (Kam et al., 2008) |
| Wound Healing Insert Assays | (Carragher & Frame, 2011; Cell Biolabs, Inc., 2008; Freytag et al., 2010; Kramer et al., 2013) |
| Transwell Invasion Assay |
Traditional (Albini et al., 1987; Albini and Noonan, 2010) FluoroBlok™(Partridge and Flaherty, 2009) |
| Spheroid Cell Invasion Assays |
Spheroid/Monodispersed (Ghosh et al., 2007) Spheroid Confrontation (Hattermann, Held-Feindt & Mentlein, 2011) |
| Organotypic 3-D Invasion Assays |
HSE (Egles et al., 2010) OCE (Scanlon et al., 2013a) |
| Sub-Cutaneous Injection Assays | (Wang et al., 2003) |
| Tail Vein Injection Assays | (Elkin and Vlodavsky, 2001) |
| Tongue Assays | (Fitch et al., 1988; Myers et al., 2002) |
| Floor of Mouth Assays |
Extra-oral (Dinesman et al., 1990; O’Malley et al., 1997) Sub-mucosal (Henson et al., 2007) |
| Intra-Vital Assays |
CT and MRI (Kato et al., 2014) BLI (Henson et al., 2007) Skin fold chamber (Alexander et al., 2008) |
| Mouse Oral Chemical Carcinogenesis | (Czerninski et al., 2009) |
| Chick Chorioallantoic Membrane | (Liu et al., 2013) |
Required improvements include better models of pre-cancer and metastasis. In particular, the MOCC model facilitates investigations on de novo HNSCC, although this model is restricted to carcinogen-induced murine HNSCC. The CAM model is excellent for investigations on invasion and metastasis in human HNSCC, since it includes the basement membrane. Like many of the models described here, the CAM is used to test potential therapeutic agents, and can spur advancements in HNSCC treatment.
This review focused on reporting invasion assays for HNSCC, with an emphasis on new techniques for improved mechanistic studies of invasion. Recent major advances in investigations of cancer mechanisms include live imaging, mathematical modeling and statistics, superior materials and biological models. These new techniques, materials and assays have facilitated studies on EMT and invasion. The identification of biomarkers associated with HNSCC progression will improve diagnostic tests and precision personalized medicine by suggesting treatment options and targets. Although patient survival has shown limited improvement in several decades, discovery of the mechanisms of HNSCC progression will change this.
Highlights.
We discuss invasion in Head and Neck Squamous Cell Carcinoma (HNSCC).
We review in vitro invasion assays commonly used to study HNSCC.
We review in vivo invasion assays commonly used to study HNSCC.
We highlight new and promising invasion assays being used to study HNSCC.
Acknowledgments
Elizabeth A. Van Tubergen, John Westman, Kenneth Reiger.
Financial support: This work was supported by NIDCR DE019513, DE018512, DE022567 (NJD), DE021293 (CSS) and the Elizabeth Caroline Crosby Fund.
Role of the Funding Source: This work was supported by NIDCR DE019513, DE018512, DE022567 (NJD), DE021293 (CSS) and the Elizabeth Caroline Crosby Fund. These grants supported the time dedicated to assembling this review and the generation of the figures.
Footnotes
Conflicts of interest: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11(1):9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 2.ACS. American cancer society report of cancer facts and figures. American Cancer Society; Atlanta, GA: 2011. [Google Scholar]
- 3.Siegel R, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 4.Walsh JE, et al. Mechanisms of tumor growth and metastasis in head and neck squamous cell carcinoma. Curr Treat Options Oncol. 2007;8(3):227–38. doi: 10.1007/s11864-007-0032-2. [DOI] [PubMed] [Google Scholar]
- 5.Bhave SL, Teknos TN, Pan Q. Molecular parameters of head and neck cancer metastasis. Crit Rev Eukaryot Gene Expr. 2011;21(2):143–53. doi: 10.1615/critreveukargeneexpr.v21.i2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takes RP, et al. Distant metastases from head and neck squamous cell carcinoma. Part I. Basic aspects. Oral Oncology. 2012;48(9):775–779. doi: 10.1016/j.oraloncology.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Loberg RD, et al. The lethal phenotype of cancer: the molecular basis of death due to malignancy. CA Cancer J Clin. 2007;57(4):225–41. doi: 10.3322/canjclin.57.4.225. [DOI] [PubMed] [Google Scholar]
- 8.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Scanlon CS, et al. Biomarkers of epithelial-mesenchymal transition in squamous cell carcinoma. J Dent Res. 2013;92(2):114–21. doi: 10.1177/0022034512467352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010;70(14):5649–69. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramer N, et al. In vitro cell migration and invasion assays. Mutat Res. 2013;752(1):10–24. doi: 10.1016/j.mrrev.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol. 2010;188(1):11–9. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9(4):274–84. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 15.Fackler OT, Grosse R. Cell motility through plasma membrane blebbing. J Cell Biol. 2008;181(6):879–84. doi: 10.1083/jcb.200802081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyckoff JB, et al. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr Biol. 2006;16(15):1515–23. doi: 10.1016/j.cub.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 17.Wolf K, et al. Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol. 2009;20(8):931–41. doi: 10.1016/j.semcdb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scanlon CS, et al. Characterization of squamous cell carcinoma in an organotypic culture via subsurface non-linear optical molecular imaging. Exp Biol Med (Maywood) 2013;238(11):1233–41. doi: 10.1177/1535370213502628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eccles SA, Box C, Court W. Cell migration/invasion assays and their application in cancer drug discovery. Biotechnol Annu Rev. 2005;11:391–421. doi: 10.1016/S1387-2656(05)11013-8. [DOI] [PubMed] [Google Scholar]
- 20.Albini A, et al. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47(12):3239–45. [PubMed] [Google Scholar]
- 21.Albini A, Noonan DM. The ‘chemoinvasion’ assay, 25 years and still going strong: the use of reconstituted basement membranes to study cell invasion and angiogenesis. Curr Opin Cell Biol. 2010;22(5):677–89. doi: 10.1016/j.ceb.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Liotta LA, Kohn EC. The microenvironment of the tumour–host interface. Nature. 2001;411(6835):375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 23.Siegal GP, et al. Development of a novel human extracellular matrix for quantitation of the invasiveness of human cells. Cancer Letters. 1993;69(2):123–132. doi: 10.1016/0304-3835(93)90164-5. [DOI] [PubMed] [Google Scholar]
- 24.Even-Ram S, Yamada KM. Cell migration in 3D matrix. Curr Opin Cell Biol. 2005;17(5):524–32. doi: 10.1016/j.ceb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Ilina O, et al. Two-photon laser-generated microtracks in 3D collagen lattices: principles of MMP-dependent and -independent collective cancer cell invasion. Phys Biol. 2011;8(1):015010. doi: 10.1088/1478-3975/8/1/015010. [DOI] [PubMed] [Google Scholar]
- 26.Kam Y, et al. A novel circular invasion assay mimics in vivo invasive behavior of cancer cell lines and distinguishes single-cell motility in vitro. BMC Cancer. 2008;8:198. doi: 10.1186/1471-2407-8-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yarrow JC, et al. A high-throughput cell migration assay using scratch wound healing, a comparison of image-based readout methods. BMC Biotechnol. 2004;4:21. doi: 10.1186/1472-6750-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freytag J, et al. PAI-1 Mediates the TGF-[beta]1+EGF-Induced [ldquo]Scatter[rdquo] Response in Transformed Human Keratinocytes. J Invest Dermatol. 2010;130(9):2179–2190. doi: 10.1038/jid.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carragher NO, Frame MC. Modelling distinct modes of tumour invasion and metastasis. Drug Discovery Today: Disease Models. 2011;8(2–3):103–112. [Google Scholar]
- 30.Partridge J, Flaherty P. An in vitro FluoroBlok tumor invasion assay. J Vis Exp. 2009;(29) doi: 10.3791/1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.BD Biosciences. BD BioCoat™ Matrigel™ Invasion Chamber. BD Biosciences; Bedford, MA: 2001. [Google Scholar]
- 32.Tong Z, et al. Chemotaxis of cell populations through confined spaces at single-cell resolution. PLoS One. 2012;7(1):e29211. doi: 10.1371/journal.pone.0029211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh S, et al. Use of multicellular tumor spheroids to dissect endothelial cell-tumor cell interactions: a role for T-cadherin in tumor angiogenesis. FEBS Lett. 2007;581(23):4523–8. doi: 10.1016/j.febslet.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 34.Hattermann K, Held-Feindt J, Mentlein R. Spheroid confrontation assay: a simple method to monitor the three-dimensional migration of different cell types in vitro. Ann Anat. 2011;193(3):181–4. doi: 10.1016/j.aanat.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Rocha B, Haston WS, Freitas AA. Lymphocyte migration into collagen gels: role of lymph. Scand J Immunol. 1984;19(4):297–305. doi: 10.1111/j.1365-3083.1984.tb00934.x. [DOI] [PubMed] [Google Scholar]
- 36.Schor SL, Allen TD, Winn B. Lymphocyte migration into three-dimensional collagen matrices: a quantitative study. J Cell Biol. 1983;96(4):1089–96. doi: 10.1083/jcb.96.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang W, et al. E-cadherin loss promotes the initiation of squamous cell carcinoma invasion through modulation of integrin-mediated adhesion. Journal of cell science. 2006;119(2):283–291. doi: 10.1242/jcs.02738. [DOI] [PubMed] [Google Scholar]
- 38.Garlick JA. Tissue Engineering II. Springer; 2007. Engineering skin to study human disease–tissue models for cancer biology and wound repair; pp. 207–239. [DOI] [PubMed] [Google Scholar]
- 39.Egles C, Garlick JA, Shamis Y. Three-dimensional human tissue models of wounded skin. Methods Mol Biol. 2010;585:345–59. doi: 10.1007/978-1-60761-380-0_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eyre DR, Paz MA, Gallop PM. Cross-linking in collagen and elastin. Annu Rev Biochem. 1984;53:717–48. doi: 10.1146/annurev.bi.53.070184.003441. [DOI] [PubMed] [Google Scholar]
- 41.Izumi K, et al. Development and characterization of a tissue-engineered human oral mucosa equivalent produced in a serum-free culture system. J Dent Res. 2000;79(3):798–805. doi: 10.1177/00220345000790030301. [DOI] [PubMed] [Google Scholar]
- 42.Alkureishi LW, et al. Does tumor depth affect nodal upstaging in squamous cell carcinoma of the head and neck? Laryngoscope. 2008;118(4):629–34. doi: 10.1097/MLG.0b013e31815e8bf0. [DOI] [PubMed] [Google Scholar]
- 43.Van Tubergen EA, et al. Inactivation or loss of TTP promotes invasion in head and neck cancer via transcript stabilization and secretion of MMP9, MMP2, and IL-6. Clin Cancer Res. 2013;19(5):1169–79. doi: 10.1158/1078-0432.CCR-12-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto E, et al. Mode of invasion, bleomycin sensitivity, and clinical course in squamous cell carcinoma of the oral cavity. Cancer. 1983;51(12):2175–80. doi: 10.1002/1097-0142(19830615)51:12<2175::aid-cncr2820511205>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 45.Hamilton N. Quantification and its applications in fluorescent microscopy imaging. Traffic. 2009;10(8):951–61. doi: 10.1111/j.1600-0854.2009.00938.x. [DOI] [PubMed] [Google Scholar]
- 46.Gaggioli C, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9(12):1392–400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 47.Mognetti B, Di Carlo F, Berta GN. Animal models in oral cancer research. Oral Oncol. 2006;42(5):448–60. doi: 10.1016/j.oraloncology.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 48.Fitch KA, Somers KD, Schechter GL. The development of a head and neck tumor model in the nude mouse. In: Wolf GT, Carey TE, editors. Head and Neck Oncology Research. Kugler Publications; Amsterdam: 1988. pp. 187–190. [Google Scholar]
- 49.Henson B, et al. An orthotopic floor-of-mouth model for locoregional growth and spread of human squamous cell carcinoma. J Oral Pathol Med. 2007;36(6):363–70. doi: 10.1111/j.1600-0714.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- 50.Liu M, et al. The Histone Methyltransferase EZH2 Mediates Tumor Progression on the Chick Chorioallantoic Membrane Assay, a Novel Model of Head and Neck Squamous Cell Carcinoma. Transl Oncol. 2013;6(3):273–81. doi: 10.1593/tlo.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elkin M, Vlodavsky I. Tail vein assay of cancer metastasis. Curr Protoc Cell Biol. 2001;Chapter 19:Unit 19 2. doi: 10.1002/0471143030.cb1902s12. [DOI] [PubMed] [Google Scholar]
- 52.Banerjee R, et al. The G Protein–Coupled Receptor GALR2 Promotes Angiogenesis in Head and Neck Cancer. Molecular cancer therapeutics. 2014;13(5):1323–1333. doi: 10.1158/1535-7163.MCT-13-0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Banerjee R, et al. The tumor suppressor gene rap1GAP is silenced by miR-101-mediated EZH2 overexpression in invasive squamous cell carcinoma. Oncogene. 2011;30(42):4339–4349. doi: 10.1038/onc.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nat Rev Cancer. 2003;3(12):921–30. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- 55.Wang W, et al. Gene expression analysis on small numbers of invasive cells collected by chemotaxis from primary mammary tumors of the mouse. BMC Biotechnol. 2003;3:13. doi: 10.1186/1472-6750-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Myers JN, et al. An orthotopic nude mouse model of oral tongue squamous cell carcinoma. Clin Cancer Res. 2002;8(1):293–8. [PubMed] [Google Scholar]
- 57.Dinesman A, et al. Development of a new in vivo model for head and neck cancer. Otolaryngol Head Neck Surg. 1990;103(5 Pt 1):766–74. doi: 10.1177/019459989010300517. [DOI] [PubMed] [Google Scholar]
- 58.O’Malley BW, Jr, et al. A new immunocompetent murine model for oral cancer. Arch Otolaryngol Head Neck Surg. 1997;123(1):20–4. doi: 10.1001/archotol.1997.01900010022003. [DOI] [PubMed] [Google Scholar]
- 59.Kawashiri S, et al. Development of a new invasion and metastasis model of human oral squamous cell carcinomas. European Journal of Cancer Part B: Oral Oncology. 1995;31(4):216–221. doi: 10.1016/0964-1955(95)00027-f. [DOI] [PubMed] [Google Scholar]
- 60.Kato H, et al. CT and MR imaging findings of palatal tumors. Eur J Radiol. 2014;83(3):e137–46. doi: 10.1016/j.ejrad.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 61.Alexander S, et al. Dynamic imaging of cancer growth and invasion: a modified skin-fold chamber model. Histochem Cell Biol. 2008;130(6):1147–54. doi: 10.1007/s00418-008-0529-1. [DOI] [PubMed] [Google Scholar]
- 62.Czerninski R, et al. Targeting mammalian target of rapamycin by rapamycin prevents tumor progression in an oral-specific chemical carcinogenesis model. Cancer Prev Res (Phila) 2009;2(1):27–36. doi: 10.1158/1940-6207.CAPR-08-0147. [DOI] [PubMed] [Google Scholar]
- 63.Levi-Montalcini R, Meyer H, Hamburger V. In vitro experiments on the effects of mouse sarcomas 180 and 37 on the spinal and sympathetic ganglia of the chick embryo. Cancer Res. 1954;14(1):49–57. [PubMed] [Google Scholar]
- 64.Ossowski L, Reich E. Experimental model for quantitative study of metastasis. Cancer Res. 1980;40(7):2300–9. [PubMed] [Google Scholar]
- 65.Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2(8):e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]