Abstract
Objective
Previous studies have found that family-based psychosocial treatments are effective adjuncts to pharmacotherapy among adults and adolescents with bipolar disorder (BD). The objective of this study was to compare the efficacy of adjunctive Child- and Family-Focused Cognitive Behavioral Therapy (CFF-CBT) to psychotherapy as usual (control) for mood symptom severity and global functioning in children with BD.
Method
Sixty-nine youth, aged 7–13 (M = 9.19, SD = 1.61) with DSM-IV-TR bipolar I, II, or not otherwise specified (NOS) disorder were randomly assigned to CFF-CBT or control groups. Both treatments consisted of 12 weekly sessions followed by 6 monthly booster sessions delivered over a total of 9 months. Independent evaluators assessed participants at baseline, week 4, week 8, week 12 (posttreatment), and week 39 (6-month follow-up).
Results
Participants in CFF-CBT attended more sessions, were less likely to drop out, and reported greater satisfaction with treatment than controls. CFF-CBT demonstrated efficacy compared to the control in reducing parent-reported mania at posttreatment and depression symptoms at posttreatment and follow-up. Global functioning did not differ at posttreatment but was higher among CFF-CBT participants at follow-up.
Conclusion
CFF-CBT may be efficacious in reducing acute mood symptoms and improving long-term psychosocial functioning among children with BD.
Keywords: Pediatric bipolar disorder, cognitive-behavioral therapy, family-focused intervention, randomized clinical trial
INTRODUCTION
Pediatric bipolar disorder (PBD) describes bipolar spectrum illness among children and preadolescents. Affecting approximately 1–2% of the population 1, PBD is characterized by extreme episodic mood dysregulation accompanied by symptoms (e.g., decreased need for sleep, hypersexuality, impulsivity) that significantly impair multiple domains of functioning. PBD is differentiated from adult-onset bipolar disorder (BD) by unique characteristics such as rapid cycling, mixed mood states, psychiatric comorbidity, and developmentally-specific psychosocial impairment 2, 3. Compared to healthy peers, children with PBD demonstrate neurocognitive deficits, academic underperformance 4, 5, and disruptive school behavior 6. Peer relationships are characterized by limited peer networks, peer victimization, and poor social skills 7, 8. Compared to families unaffected by PBD, family functioning is often characterized by strained relationships 8, 9, low levels of cohesion, and increased conflict 10–12; family stress and dysfunction increase with symptom levels 13, 14. The accumulation of psychosocial risk renders PBD a significant public health concern as evidenced by high rates of repeated hospitalization and suicide attempts 15. In adulthood, people with BD demonstrate greater mental health care utilization, elevated rates of other health conditions, lower rates of graduation, and decreased career productivity 15–17. Recent data from the World Health Organization indicate that BD is the fourth leading cause of disability in youth ages 10–24 worldwide 18.
Pharmacotherapy is the first-line treatment for PBD but is complicated by low response rates, poor tolerability 19, and inability to address the full range of impairments associated with PBD. Thus, adjunctive psychosocial intervention is considered essential for effective treatment 20. Despite this, few psychosocial treatments for youth with BD have been studied systematically. Adaptations of dialectical behavior therapy (DBT 21) and interpersonal and social rhythm therapy (IPSRT 22) for adolescents with BD have been tested in pilot studies. However, only two interventions have demonstrated efficacy in randomized controlled trials (RCT): multi-family psycho-education group psychotherapy (MF-PEP) for youth with BD or unipolar depression aged 8–12 23, and family-focused treatment (FFT) for adolescents 13–18 years old24. These treatments demonstrated effects on mood severity in children 23 and depression relapse in adolescents 24, respectively. It is not yet known whether a treatment model like CFF-CBT, which more specifically targets the unique symptoms and impairments in childhood BD, will improve outcomes compared to these other treatments.
CFF-CBT was developed to target the unique developmental needs of the PBD population in a comprehensive family-focused format. CFF-CBT integrates CBT with psychoeducation and complementary mindfulness-based and interpersonal/family therapy techniques tailored to address the range of therapeutic needs in families affected by PBD. The components of CFF-CBT are driven by three areas of research: (1) developmentally specific symptoms of PBD (e.g. rapid cycling, mixed mood states, comorbid disorders); (2) affective circuitry brain dysfunction in PBD (e.g. poor problem-solving during affective stimulation via ventral frontostriatal and dorsolateral prefrontal circuitry dysfunction and deficits in superior temporal and visual cortices 25–27; and (3) the impact of PBD on interpersonal/family functioning. CFF-CBT is delivered via 12 manualized weekly 60–90 minute sessions with the child, parent, and/or family. It includes seven components that comprise the treatment acronym “RAINBOW”: Routine (developing consistent daily routines); Affect Regulation (psychoeducation about feelings; mood monitoring; coping strategies to improve mood regulation); I Can Do It! (improving child self-esteem and parent self-efficacy); No Negative Thoughts/Live in the Now (cognitive restructuring and mindfulness techniques to reduce negative thoughts); Be a Good Friend/Balanced Lifestyle (social skill-building and improving parent self-care); Oh How Do We Solve this Problem? (family problem-solving and communication training); and Ways to Find Support (enhancing support networks; see 28 for details).
This was the first RCT testing the efficacy of CFF-CBT in treating PBD. Open trials have established the feasibility, acceptability, and preliminary efficacy of CFF-CBT with promising outcomes 28–30. The goal of this trial was to test the efficacy of the individual family format of CFF-CBT compared to patients receiving psychotherapy as usual (control) on outcomes of symptom control and global functioning. We hypothesized that CFF-CBT would improve the symptoms and global functioning of children at posttreatment compared to the control condition. We also hypothesized that treatment effects would be maintained at follow-up, evidenced by differences in longitudinal trajectories of symptoms and functioning from baseline through the follow-up assessment point.
METHOD
Participants
Participants were children (N = 69) diagnosed with PBD recruited from a specialty pediatric mood disorders clinic (PMDC) in an urban academic medical center between 2010 and 2013. Children meeting DSM-IV-TR criteria for bipolar spectrum disorders (I, II, and not otherwise specified [NOS]) aged 7–13 were eligible to participate. Inclusion criteria encompassed the following: stabilized on medication, parental consent, and youth assent. Stabilization on medication was defined by a Young Mania Rating Scale (YMRS 31) score ≤ 20 and Children’s Depression Rating Scale-Revised (CDRS-R 32) score < 80 (indicating no severe symptoms requiring immediate more intensive care). These criteria were intended to exclude children who needed acute stabilization before being able to participate in psychotherapy but still include children who were actively symptomatic. Thus, children scoring above threshold on these measures (n=4) were included if their psychiatrist determined they were stable enough to engage in treatment. Exclusion criteria for the study included: youth IQ < 70, as measured by the Kaufman Brief Intelligence Scale-2 (KBIT-2 33), active psychosis, active substance abuse/dependence, neurological or other medical problems that significantly complicate child’s psychiatric symptoms as assessed via the Washington University Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS34), and active suicidality requiring hospitalization as measured by the Columbia Suicide Severity Rating Scale (C-SSRS35). Additionally, youth whose primary caretakers were experiencing current depressive or manic episodes, indicated by a Beck Depression Inventory (BDI36) score ≥28 and an Altman’s Self-Report of Mania (ASRM 37) score > 6, were excluded. Youth with comorbid disorders, including high-functioning autism, were included to ensure a clinically relevant sample.
Procedures
Diagnosis and Randomization
All study procedures were approved by the Institutional Review Board at the University of Illinois at Chicago. Eligibility was assessed by trained study personnel. Potential participants were educated about the demands of the treatment protocol and the importance of adherence, regardless of treatment assignment. After providing informed consent and completing inclusion/exclusion measures, parents were interviewed using the WASH-U-KSADS 34 and portions of the Kiddie-SADS-Present and Lifetime Version (K-SADS-PL; 38) to define mood episodes, with corroborating information coming from child-report. Trained clinical interviewers (n=6) included licensed clinical psychologists and clinical psychology doctoral students who were trained via in-person observation of expert raters and rating of recorded expert diagnostic interviews until adequate reliability was reached for bipolar diagnosis. Diagnostic interviews were reviewed during study meetings for final determination. Patients who met diagnostic criteria for PBD completed the baseline assessment and were randomized to study condition using Research Randomizer software 39 via a customized randomization algorithm.
Study Design
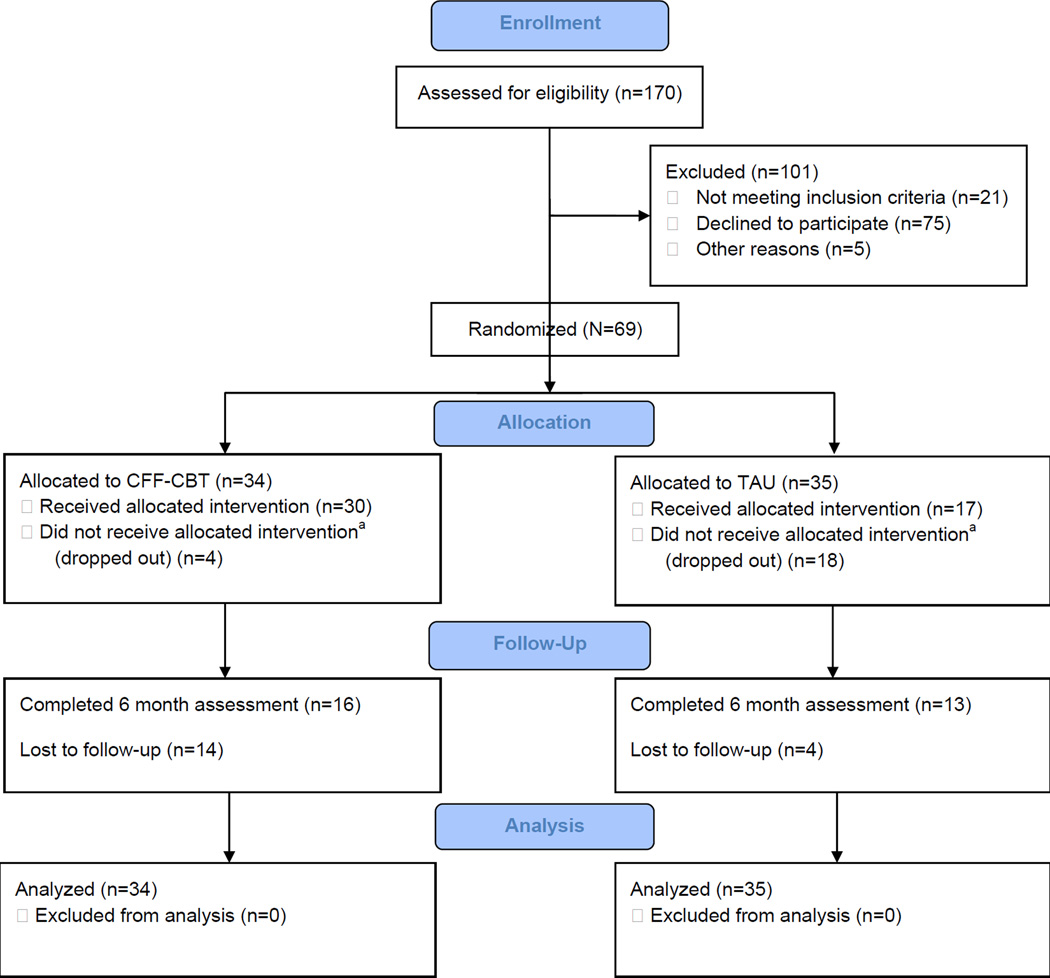
Sixty-nine study participants meeting eligibility criteria were randomly assigned to either CFF-CBT or the control group. See Figure 1 for the Consolidated Standards of Reporting Trials (CONSORT) diagram. Participants in both conditions received 12 weekly sessions (acute phase) and 6 monthly follow-up sessions (maintenance phase). Those in CFF-CBT were assigned a therapist from PMDC who had been trained on the CFF-CBT manual. Study therapists included 23 clinical psychology pre- and postdoctoral trainees with limited experience treating PBD and no previous experience with the CFF-CBT manual. Training on CFF-CBT involved one three-hour training session on the CFF-CBT theoretical foundation and manual and weekly supervision from the CFF-CBT developer and first (A.E.W) or second author (S.M.W).
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) Diagram. Note: CFF-CBT = Child- and Family-Focused Cognitive Behavioral Therapy; TAU = treatment as usual.
a did not receive all 12 sessions of treatment.
Participants randomized to control were assigned a therapist in the General Psychiatry Clinic. These sessions were otherwise unstructured by the study design. Control therapists were clinical psychology doctoral students, postdoctoral fellows, psychiatry fellows, and social work interns who had not been trained in CFF-CBT but were given a one-hour training on PBD. All sessions in both conditions were audiotaped to assess treatment content (see below).
All participants were treated in the same outpatient psychiatry program, and thus logistics associated with treatment that might impact access or engagement (e.g., clinic times, location, parking, scheduling) did not differ by condition. Participants in both conditions received medication management by a psychiatrist in the PMDC following an evidence-based algorithm 40. Although patients were required to be stabilized on their medication (stable dosages for 4+ weeks) prior to baseline and randomization, medication management was delivered as it would be in regular practice and was not manipulated as part of the study design. Medication changes over the course of the study were carefully tracked at all assessments. Assessments occurred at baseline (pre-randomization); 4 and 8 weeks (during treatment); 12 weeks (posttreatment); and 39 weeks (follow-up assessment at 6-months posttreatment).
Measures
Diagnosis
The WASH-U-KSADS 34, a semistructured interview specifically designed to assess for PBD, was used to make a DSM-IV diagnosis41. Research assistants were trained to administer the interview and demonstrated adequate inter-rater reliability (kappa >.74).
Symptom Severity
The Child Mania Rating Scale (CMRS; 42), a parent-rated measure to assess DSM-IV-TR mania symptoms, was our primary outcome measure of mania symptoms. Scores are calculated by summing across 21 items, each rated on a Likert scale ranging from 0 (never) to 3 (very often). Scores ≥ 20 are considered clinically significant. Reliability in this sample was strong (Cronbach’s alpha [α] =0.90). The CMRS was chosen as the method for assessing mania symptoms because of its ability to capture symptom changes over time by reporters (parents) that have more comprehensive access to the child’s behavior throughout the course of treatment and across many different contexts. Research suggests that parent report may result in more accurate assessment of mania 43, and the CMRS demonstrates strong psychometric properties, concurrent validity with the YMRS, and sensitivity to symptom change across treatment 42, 44.
The Child Bipolar Depression Rating Scale (CBDRS; Unpublished data, August, 2003) is a companion measure to the Child Mania Rating scale. This parent report includes 22 items that assess for DSM-IV-TR depressive symptoms. The scale is face valid, demonstrated strong reliability (α =0.88), and was significantly correlated with clinician-reported depression (CDRS-R26) in this sample at baseline (r=.32, p=.008).
The Children’s Depression Rating Scale-Revised (CDRS-R32). The CDRS-R is a clinician-rated instrument for measuring depression in children. Scores are summed across 17 items rated on a 5-point Likert scale. Internal reliability and rater reliability across assessments in this sample were strong (α =0.84; intraclass correlation [ICC] = .78).
Global Functioning
The Children’s Global Assessment Scale (C-GAS; 45) is a clinician-rated measure of child functioning based on impairment in family, social, school, and work areas due to psychiatric symptoms. Scores range from 1–100, with higher scores indicating better functioning. Rater reliability across assessments was moderate in this sample (ICC = 0.76).
The Children’s Global Impressions Scales for Bipolar Disorder (CGI-BP; 46) is a clinician report of child’s overall psychiatric illness severity. Scores are summed across the subscales (mania, depression, attention deficit/hyperactivity, psychosis, and aggression). Rater reliability across assessments in this sample was strong (ICC= 0.81).
Treatment Measures
The Treatment Satisfaction Scale is a 21-item scale that was completed posttreatment to assess caregiver satisfaction with treatment. A total score is calculated by averaging item responses; responses range from 1 (not true) to 3 (very true). This measure was developed and piloted for the preliminary study 28 and demonstrated strong reliability in this sample (α = .94).
Therapist Fidelity to CFF-CBT was assessed via a fidelity checklist developed and piloted in the preliminary study 28, which asked raters to record the key CFF-CBT elements delivered in each treatment session (defined as number of elements delivered/total elements to be delivered × 100). Ten CFF-CBT participants were randomly selected, and a trained rater completed the fidelity checklist for audio recordings of all 12 CFF-CBT sessions for that participant. In addition, 10 control participants were randomly selected, and a trained rater completed the CFF-CBT fidelity checklist for all 12 control sessions to assess for potential overlap with CFF-CBT ingredients.
Analytic Approach
Mixed-effects regression (growth curve) models (MRMs 47) were conducted via SPSS MIXED to examine youth response to treatment on key outcomes. MRMs are well-suited for longitudinal data analysis: they are robust to the data dependency that occurs with repeated assessments of individuals over time. Additionally, MRMs are efficient in handling missing data by using all available data for a given participant to estimate group trends at each time point. Separate MRMs were evaluated for each outcome measure and included effects for treatment (CFF-CBT [coded as 0], control), time (baseline, 4, 8, 12, and 39 weeks) and treatment × time. Models included both linear and quadratic effects for time; if the quadratic effect was not significant, the model was refitted without this term. Time was centered at 12 weeks to test our hypotheses about group differences at posttreatment (12 weeks) 48. Thus, treatment effects tested hypotheses related to group differences at posttreatment (i.e., elevation of response trajectory between groups), and the treatment by time interactions tested hypotheses related to group differences in symptom response trajectories (i.e., slopes) from baseline to 4, 8, 12, and 39 week assessments. Models were evaluated for the intent-to-treat sample and included all randomized participants to provide the most conservative test of hypotheses given differences in treatment completers across conditions (see below). Participants who dropped out of the study were contacted for follow-up assessments and were included in the analyses if available. For participants with outstanding assessment sessions at study completion (n=8), all available data was included.
RESULTS
Descriptive Statistics
The intent-to-treat sample included 69 participants. Mean age of the sample was 9.19 years (SD=1.61; range 7–13); n=29 (42.0%) were female. The majority of the sample met criteria for BP-NOS (n=43, 62.3%), with n=22 (31.9%) diagnosed as BP-I and n=4 (5.8%) diagnosed as BP-II. Medication status (e.g., type and dose) was reported by parents at baseline. Although 97% of participants (n=67) were taking medication at treatment outset, only 24 (35%) parents reported on type and dose at baseline; thus it is not possible to accurately characterize the types of medication and dosages across the entire sample at baseline. Table 1 presents demographic information and descriptive statistics for all outcome measures at baseline for participants assigned to CFF-CBT (n=34) and control (n=35). The sample presented with a complex clinical picture at baseline: youth experienced high rates of comorbidity, particularly attention-deficit/hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD), as well as variability in their index mood episode. In addition, the sample was diverse in terms of financial status and family composition. A series of t-tests and chi-square analyses confirmed the equivalence of conditions at baseline, with the exception of higher parent-reported mania scores among youth in the control condition relative to CFF-CBT (t[66] = −2.90, p = .005). Parents noted on a medication change self-report form whether any changes in the medication regimen had occurred since the previous study assessment. Forty-two percent (n=29) of children reported that a medication change occurred over the course of the study; 58% (n = 40) of children had unchanged medication regimens throughout treatment. Of those with any medication change, 18 participants specified the nature of the change: six reported a dose change, seven reported a medication switch, and five reported both. Medication changes did not differ between CFF-CBT (23%, n=15) and control groups (22%, n=14; χ2 = .229, n=64, p=.63).
Table 1.
Baseline Demographics and Clinical Characteristics of Youth Assigned to the Child- and Family-Focused Cognitive Behavioral Therapy (CFF-CBT) and Control
| CFF-CBT (n = 34) | Control (n = 35) |
|||
|---|---|---|---|---|
| Variable | M | SD | M | SD |
| Age | 9.26 | 1.93 | 9.11 | 1.25 |
| Young Mania Rating Scale | 13.21 | 5.01 | 13.20 | 5.70 |
| Child Mania Rating Scale* | 19.82 | 8.59 | 26.82 | 11.18 |
| Children’s Depression Rating Scale | 42.26 | 12.47 | 40.71 | 10.65 |
| Children’s Bipolar Depression Rating Scale | 17.92 | 9.72 | 20.59 | 10.59 |
| Clinical Global Impressions Scale-Severity | 4.06 | 0.67 | 4.12 | 0.48 |
| Children’s Global Assessment Scale | 50.50 | 5.87 | 48.74 | 4.86 |
| Medication Doses Missed Past Week | 0.93 | 1.52 | 0.72 | 1.05 |
| n | % | n | % | |
| Female Sex | 16 | 47 | 13 | 37 |
| Ethnicity | ||||
| Caucasian | 18 | 53 | 18 | 52 |
| African American | 11 | 32 | 10 | 29 |
| Hispanic | 4 | 12 | 3 | 8 |
| American Indian or Alaskan | 0 | 0 | 3 | 8 |
| Native American or Pacific Islander | 0 | 0 | 1 | 3 |
| Other | 1 | 3 | 0 | 0 |
| Family Income (<50,000/year) | 12 | 43 | 12 | 39 |
| Living Situation (single parent home) | 12 | 38 | 13 | 39 |
| Primary Diagnosis | ||||
| BP I | 8 | 24 | 14 | 40 |
| BP II | 2 | 6 | 2 | 6 |
| BP NOS | 24 | 70 | 19 | 54 |
| Index Mood Episode | ||||
| Manic | 8 | 23 | 8 | 24 |
| Hypomanic | 1 | 3 | 2 | 6 |
| Mixed | 9 | 27 | 12 | 37 |
| Depressed | 6 | 18 | 5 | 15 |
| Remitted | 10 | 29 | 6 | 18 |
| Co-morbid Disorders | ||||
| Anxiety Disorder | 13 | 39 | 8 | 27 |
| ADHD | 22 | 65 | 31 | 89 |
| ODD | 20 | 61 | 22 | 63 |
| CD | 3 | 9 | 3 | 9 |
Note: ADHD = attention-deficit/hyperactivity disorder; BP = bipolar disorder (I, II, and not otherwise specified [NOS]); CD = conduct disorder; ODD = oppositional defiant disorder.
Denotes group differences, p < .05 on t-test or chi-square analyses.
Treatment Completion
Core treatment completion differed by condition (Figure 1), with significantly more dropouts in the control group at posttreatment (n=18) than CFF-CBT (n=4; χ2=13.46, p<.001). Overall attrition by the 39-week (follow-up) assessment did not differ by condition (n=10 in CFF-CBT, n=19 in control; χ2=2.51, not significant). CFF-CBT participants attended an average of 11.34 (SD=2.39) of the 12 core treatment sessions, compared with 6.91 (SD=5.37) of 12 control sessions (t[60]=4.10, p<.0001). Participants in CFF-CBT completed an average of 2.88 maintenance sessions (SD=2.55, range 0–6), which was marginally greater than maintenance session completion in the control group (M=1.67, SD=30, range 0–6; t[57]=.193, p=.059).
Treatment Feasibility and Acceptability of CFF-CBT
Feasibility of CFF-CBT was examined via treatment adherence, consumer satisfaction, and treatment fidelity. Adherence was defined as attending 12 scheduled treatment sessions; as described above, families were adherent with CFF-CBT. Treatment satisfaction with CFF-CBT was high (M = 2.95, SD=0.22, range 2–3); of note, parents/caregivers were significantly more satisfied with CFF-CBT than the control (M=2.67, SD=0.49, range 2–3; t[30]= 2.24, p=.03). Last, findings indicated high fidelity to the CFF-CBT manual, with 93% of elements delivered across therapy sessions. Data also supported the specificity of CFF-CBT and limited overlap with the control group: only 4% of CFF-CBT elements were delivered across the control sessions.
PBD Symptom Outcomes
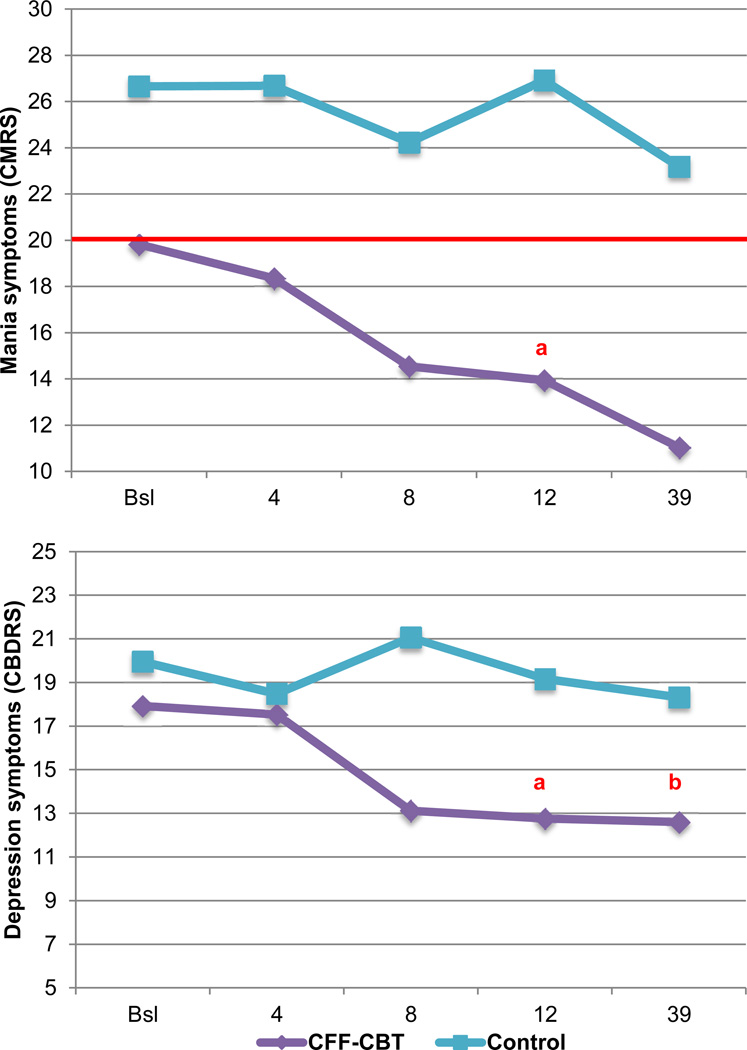
Separate MRMs, as specified above, examined treatment outcomes for child mania (CMRS) and depression symptoms (CBDRS and CDRS). Given the significant pretreatment group differences in mania symptoms (Table 1), baseline CMRS scores were included as a covariate; thus the time term in this model excluded baseline. For ease of presentation, only the hypothesized effects are presented; for further details, including estimates and standard errors, see Table S1 (available online). For mania symptoms, the significant and medium/large-sized treatment effect indicated that youth in CFF-CBT had significantly lower mania symptoms at posttreatment versus the control group, adjusting for baseline values (F[49] = 7.90, p=.007, d=0.69). As illustrated in Figure 2 (upper portion), youth in CFF-CBT experienced consistent improvement in mania symptoms across treatment and through follow-up. However, symptom trajectories over time did not significantly differ between conditions (treatment × time F[120]=0.95, p>.05, d=0.24). Notably, all youth experienced mania symptoms at (CFF-CBT) or above (control) clinical threshold at baseline (Table 1), but only youth in CFF-CBT achieved mean subthreshold mania symptoms by posttreatment and follow-up. Specifically, 88% (n=2) of youth in CFF-CBT scored below threshold at posttreatment and 93% (n=14) were below threshold by follow-up, versus 21% (n=2) posttreatment and 46% (n=5) at follow-up in the control group. In addition, youth in CFF-CBT also experienced significantly reduced parent-reported depression at posttreatment and a steeper symptom response trajectory across the entire study period (Figure 2, lower portion; F[67]=4.92, p=.03, d=0.55 and F[162]=3.82, p<.05, d=0.48); both effects were medium-sized. For clinician-reported depression symptoms, however, neither the treatment nor treatment × time effect was significant (F[62]=0.06, d=0.06 and F[170]=2.5, d=0.39, respectively; p>.05)
Figure 2.
Estimated symptom scores – mania (Child Mania Rating Scale [CMRS]), upper portion and depression (Child Bipolar Depression Rating Scale [CBDRS]), lower portion – over time (weeks) by treatment condition. Note: red line reflects the clinical cutoff on the CMRS. Bsl = baseline; CFF-CBT = Child- and Family-Focused Cognitive Behavioral Therapy.
aReflects significant posttreatment differences (treatment effect).
breflects significant differences in overall trajectory (treatment × time effect).
Global Functioning Outcomes
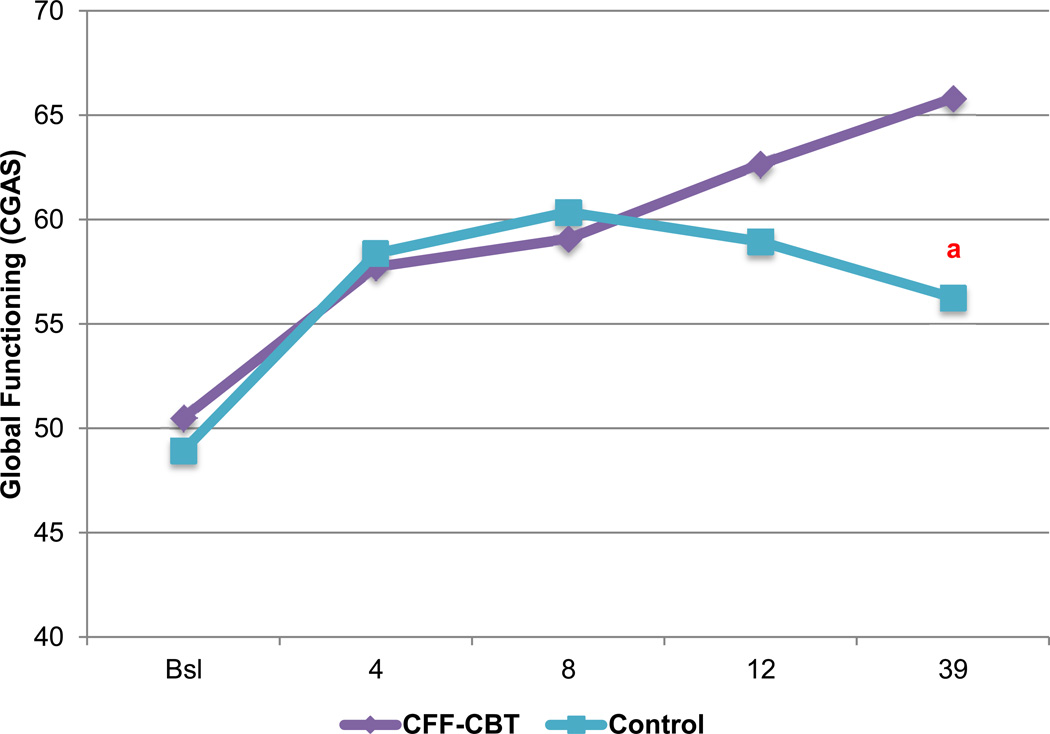
As shown in Figure 3, CFF-CBT resulted in significantly greater improvement in global psychosocial functioning (CGAS) across treatment as compared to control (treatment × time F[191]=4.26, p=.04, d=0.50), with medium-sized effects (higher scores indicate better functioning). Treatment differences were not significant at posttreatment but became pronounced by follow-up (treatment F[74]=2.23, p>.05, d=0.36). Results for overall psychiatric severity (CGI-BP-S) indicated that youth in CFF-CBT experienced marginally greater posttreatment effects and steeper symptom trajectories versus the control group, with effects that approached medium size (treatment F[73]=2.89, p=.09, d=0.42; treatment × time F[167]=3.26, p=.07, d=−.45).
Figure 3.
Estimated global psychosocial functioning (Children’s Global Assessment Scale [CGAS]) over time (weeks) by treatment condition. Note: Bsl=Baseline; CFF-CBT=Child- and Family-Focused Cognitive Behavioral Therapy.
areflects significant differences in overall trajectory (treatment × time effect).
DISCUSSION
The development and rigorous testing of psychosocial interventions is essential to address the significant public health burden associated with PBD and improve long-term outcomes for these youth. To our knowledge, CFF-CBT is the only single family psychosocial treatment developed exclusively for children and preadolescents with BD that has been tested through an RCT. Data from this study were consistent with previous open trials 28–30, suggesting the feasibility of delivering CFF-CBT in an academic clinical setting. CFF-CBT families attended more sessions, were less likely to drop out, and reported higher levels of satisfaction with treatment content, structure, and impact. Thus, it appears that compared to usual psychotherapy, CFF-CBT may better engage and retain participants with PBD. Treatment models that effectively engage families may be particularly important in light of recent findings that youth with PBD have low rates of psychosocial treatment utilization 49, 50.
CFF-CBT demonstrated excellent treatment fidelity, with 93% of core ingredients being delivered across the sessions. This finding is especially remarkable given the relatively basic training model (3-hour workshop and ongoing supervision) and limited experience of study therapists (psychology trainees with limited experience treating PBD). CFF-CBT appears feasible to implement with minimal initial training, despite incorporating multiple treatment modalities, concepts, and skills. Clinician training for CFF-CBT will be explored further in community-based effectiveness research; however, results with regard to fidelity suggest that CFF-CBT may be transportable to usual care settings where the majority of youth with PBD are treated 51, 52. The study sample lends further support for transportability; drawn from the university patient population, our sample was comprised of ethnically-diverse, predominantly inner-city, low socioeconomic status (SES), and difficult-to-engage patients, typical of those seen in community mental health settings. Finally, the limited overlap between the content of CFF-CBT and the control group suggests that the core components of specialized treatment for PBD are not typically delivered in usual treatment, even in a university-based clinic with well-trained and supervised clinicians.
CFF-CBT demonstrated efficacy compared to the control in reducing parent-reported mania symptoms. Youth in CFF-CBT exhibited significantly lower mania symptoms after treatment. By follow-up, groups did not differ significantly in mania symptoms, but only youth in CFF-CBT achieved below-threshold clinical symptoms. Thus, although the groups' mania symptom trajectories did not significantly differ over the course of the study, there appears to be a strong treatment effect for CFF-CBT, as group differences were significant at posttreatment and only those in CFF-CBT had sub-clinical mania symptoms at follow-up. It is important to note that the youth in CFF-CBT began treatment with significantly lower mania symptoms, which may have influenced their positive outcomes relative to the control group by posttreatment and follow-up.
Youth in CFF-CBT also experienced significantly reduced parent-reported depression symptoms at posttreatment and a steeper symptom response trajectory across the entire study period. For clinician-rated depression symptoms, however, groups did not differ at post-treatment or across the follow-up period. The lack of findings for clinician-reported depressive symptoms relative to parent-report is interesting. It is likely that parents are more sensitive to smaller changes in depressive symptoms over time as compared to a blinded rater with limited data to contextualize the child’s current symptom experience. In addition, findings may reflect rater “noise” due to multiple blind raters rating each case over time; unfortunately, we did not have a sufficiently powered sample to add a rater term to analytic models to further investigate these effects. Alternatively, because parents were involved in the treatment and not blind to condition, their reports may have been biased towards an overestimate of change in the CFF-CBT condition.
Youth in CFF-CBT also demonstrated improved global functioning compared to youth in the control condition across treatment, with pronounced differences in psychosocial functioning by follow-up. This suggests that psychosocial functioning may improve gradually yet steadily as treatment effects are internalized and consolidated, in contrast to the acute improvement observed in symptoms. Importantly, youth in CFF-CBT achieved a rating level by the follow-up that suggested these youth were functioning well at home and school, had meaningful social relationships, and any behavioral problems were sporadic or isolated. This finding was in contrast to youth in the control condition, whose levels of global functioning indicated moderate impairment in most social domains. Additionally, findings indicated a steeper trajectory of improvement for overall bipolar symptom severity in the CFF-CBT group versus control that approached significance. Marginally significant effects may be driven, in part, by measurement issues given the constricted range on the CGI-BP (i.e., a seven-point rating scale); thus, statistical models that better account for this scaling may be considered with a larger sample.
Overall, findings suggest that CFF-CBT is superior to psychotherapy as usual (even when enhanced via brief PBD training) in addressing symptoms and functional impairment in PBD. Results are consistent with extant studies involving youth with mood disorders more broadly and adolescents with BD indicating that targeted evidence-based treatment for PBD may optimize outcomes for these families 53. These results are also consistent with findings from randomized trials of adults with BD, which have found that adaptations of CBT, family-focused therapy (FFT), group psychoeducation, IPSRT, and systematic care management programs can be effective in hastening stabilization, delaying relapses, reducing symptom severity over time, or enhancing psychosocial and family functioning 54. Interestingly, findings from the largest RCT of psychosocial treatment for adult BD 55 to date, which support the efficacy of intensive psychotherapy (CBT, FFT, or IPSRT) relative to a 3-session control intervention (collaborative care), correspond to only a small effect size estimate for intensive psychotherapy over control in recovery rates. In the current study, we found medium/large effect sizes for CFF-CBT compared to a more intensive dose-matched, psychotherapy-as-usual control, suggesting that CFF-CBT may contribute unique and powerful content relative to usual care.
Strengths of this study include a novel treatment model, rigorous RCT design, a sample size adequately powered for primary analyses, well-validated assessment instruments and outcome measures, and a diverse and clinically representative sample of youth with PBD. One of the most significant strengths of the study is the use of an active, dose-matched, psychotherapy control condition delivered in the same competitive academic medical setting, thus delivering a powerful test of our manual-based treatment model for PBD. However, findings from this study must be interpreted in the context of study limitations. First, there was greater core treatment completion in CFF-CBT versus the control group. Although this finding is important for feasibility and acceptability, it limited the ability for equivalent measurement across conditions. The drop-out rate in the control group was consistent with that of our university psychiatry clinic, which serves an inner-city, low SES patient population; potential factors specifically related to treatment assignment may include logistical and emotional barriers not addressed well in treatment, and disappointment in receiving the control condition. Second, and related, participants were not blind to treatment condition. Although the study protocol enhanced perceived equipoise by emphasizing CFF-CBT as experimental, presenting the control condition as standard-of-care child and family treatment, using language and tone that expressed equivalency, and communicating the importance of engagement and participation to all participants during the consent process, treatment expectancy may have affected engagement and outcomes. This weakness was minimized by the use of clinician raters blinded to treatment condition. Third, while the use of blinded clinician raters is considered a design strength, their accuracy may have been hindered by the fact that they had no other interaction with the patients and were unable to assess the overall context of the participants’ symptom experiences over time. Fourth, youth in the control condition exhibited significantly higher mania symptoms at baseline than youth in CFF-CBT. Although we controlled for this difference in analyses, it still indicates that the two groups were not entirely equivalent in terms of clinical characteristics at baseline. Fifth, the primary outcome of mania symptoms was measured by parent report only. This decision was made based on the fact that the CMRS has demonstrated excellent psychometric properties 42, 44 and parent reports of mania are considered most accurate 56. In addition, our own clinical experience with these two measures suggests that the CMRS contributes more comprehensive, nuanced, and contextualized data on mania symptoms, while the YMRS demonstrates limited range and restricted variability in our patient population. However, a multiple-informant approach would have strengthened outcome measurement for mania. Sixth, while medication changes were captured in the measurement plan and did not differ by condition, it is possible that unmeasured medication effects might explain symptom improvement for some study participants. Finally, the interpretation of findings from the overall treatment trajectory (core treatment phase and follow-up together) is hindered by the large amount of attrition during the follow-up phase (n=29 dropped out prior to the 6 month follow-up). For this reason, posttreatment (treatment effect) was our primary outcome point, and results for the full 9-month trajectory (i.e., treatment × time effect) are regarded as exploratory.
Despite these limitations, this study makes an important contribution to the literature in providing rigorous evidence for a novel family-based psychosocial treatment model for children with PBD, a difficult-to-treat population for whom there are few, if any, effective evidence-based treatments. CFF-CBT proved efficacious compared to psychotherapy-as-usual in this study. Additional strengths with regard to future dissemination may include its short duration (12 weeks), efficient training model (3-hour workshop plus initial supervision), and the ability to engage difficult-to-treat families (e.g., severe symptoms; urban, low-SES clinic population with significant barriers to treatment). Future studies may establish whether CFF-CBT can be transported successfully to community settings and factors (e.g., training, organizational context) related to its successful implementation. In addition, we hope to examine potential moderators of treatment response, such as baseline family functioning, that can inform more individualized treatment approaches. Finally, we plan to explore potential treatment mechanisms, such as improved parental wellbeing and efficacy, and to incorporate novel neurobiological measures of treatment response that approximate these changes at the brain level (e.g., changes in neural circuitry related to arousal and emotional processing) to inform specific treatment targets.
Clinical Guidance
The psychosocial impairments and morbid consequences associated with PBD, along with limited efficacy of medication to address broad domains of functioning, has led to consensus that psychosocial intervention is a necessary component of treatment for PBD.
Children with BD may benefit from structured manual-based treatment specialized for PBD. In this trial, CFF-CBT demonstrated efficacy versus dose-matched control treatment for improving symptoms of PBD as well as global psychosocial functioning. CFF-CBT is a 12-session manualized therapy that focuses on improving the key domains of difficulty associated with PBD, including affect regulation, self-efficacy, social/family functioning, and youth/parent coping with the disorder.
CFF-CBT was feasible to implement even with minimal initial training and non-expert clinicians, thus demonstrating promise for dissemination into community practice settings.
Future studies may be able to determine the specific factors associated with positive treatment outcomes in PBD.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Mental Health (NIMH) K23 grant MH079935 (A.E.W.).
Dr. Henry served as the statistical expert for this research.
The authors thank Patrick Tolan, PhD, from the University of Virginia and David Miklowitz, PhD, from the University of California at Los Angeles for mentorship and consultation; Christine Celio, PhD, and Jackie Doxie, MA, from University of Illinois Chicago for serving as study coordinators. The authors would like to thank all of the psychology, psychiatry, and social work trainees and staff at the University of Illinois at Chicago that served as study therapists and/or supervisors. The authors would also like to thank all of the undergraduate and graduate psychology trainees from the University of Illinois at Chicago who served as study raters and research assistants. The authors thank the University of Illinois Center for Clinical and Translational Science (CCTS) for resources to support this study. The CCTS is supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through grant UL1TR000050. The work of the Center is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Disclosures: Dr. West has received honoraria from the NIH (grant review), Northwestern University (Grand Rounds), and University of Lund (academic talk). Dr. Weinstein has received funding from the American Foundation for Suicide Prevention (AFSP) and honoraria from the University of Lund (academic talk). Dr. Henry has received funding from the National Institute of Justice, National Institute of Drug Abuse, NIMH, Center for Disease Control and Prevention, and the McCormick Foundation. Dr. Henry has received honoraria and consultancies from Rush University, the Center for Alaska Native Health Research, and the University of Virginia Curry School of Education. Dr. Pavuluri receives or has received grant support from the following sources: NIH, NIMH, the National Alliance for Research on Schizophrenia and Depression (NARSAD), the American Foundation for Suicide Prevention, and the Marshall Reynolds Foundation. She is the recipient of the Berger-Colbeth Term Chair in Child Psychiatry and participated in the Otsuka Pharmaceuticals National Advisory Board meeting once. Mr. Cruz has received grant support from the National Institute of Drug Abuse. He has consulted for Mental Health Data Services, Inc. He was previously employed by the University of Washington.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Van Meter AR, Moreira AL, Youngstrom EA. Meta-analysis of epidemiologic studies of pediatric bipolar disorder. The Journal of clinical psychiatry. 2011;72(9):1250–1256. doi: 10.4088/JCP.10m06290. [DOI] [PubMed] [Google Scholar]
- 2.Geller B, Sun K, Zimerman B, Luby J, Frazier J, Williams M. Complex and rapid-cycling in bipolar children and adolescents: a preliminary study. Journal of affective disorders. 1995;34(4):259–268. doi: 10.1016/0165-0327(95)00023-g. [DOI] [PubMed] [Google Scholar]
- 3.Pavuluri MN, Birmaher B, Naylor MW. Pediatric bipolar disorder: a review of the past 10 years. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(9):846–871. doi: 10.1097/01.chi.0000170554.23422.c1. [DOI] [PubMed] [Google Scholar]
- 4.Henin A, Mick E, Biederman J, et al. Can bipolar disorder-specific neuropsychological impairments in children be identified? Journal of consulting and clinical psychology. 2007;75(2):210–220. doi: 10.1037/0022-006X.75.2.210. [DOI] [PubMed] [Google Scholar]
- 5.Pavuluri MN, O'Connor MM, Harral EM, Moss M, Sweeney JA. Impact of neurocognitive function on academic difficulties in pediatric bipolar disorder: A clinical translation. Biological psychiatry. 2006;60(9):951–956. doi: 10.1016/j.biopsych.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 6.Geller B, Zimerman B, Williams M, Delbello MP, Frazier J, Beringer L. Phenomenology of prepubertal and early adolescent bipolar disorder: examples of elated mood, grandiose behaviors, decreased need for sleep, racing thoughts and hypersexuality. Journal of child and adolescent psychopharmacology. 2002;12(1):3–9. doi: 10.1089/10445460252943524. [DOI] [PubMed] [Google Scholar]
- 7.Geller B, Craney JL, Bolhofner K, Nickelsburg MJ, Williams M, Zimerman B. Two-year prospective follow-up of children with a prepubertal and early adolescent bipolar disorder phenotype. The American journal of psychiatry. 2002;159(6):927–933. doi: 10.1176/appi.ajp.159.6.927. [DOI] [PubMed] [Google Scholar]
- 8.Wilens TE, Biederman J, Forkner P, et al. Patterns of comorbidity and dysfunction in clinically referred preschool and school-age children with bipolar disorder. Journal of child and adolescent psychopharmacology. 2003;13(4):495–505. doi: 10.1089/104454603322724887. [DOI] [PubMed] [Google Scholar]
- 9.Geller B, Bolhofner K, Craney JL, Williams M, DelBello MP, Gundersen K. Psychosocial functioning in a prepubertal and early adolescent bipolar disorder phenotype. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(12):1543–1548. doi: 10.1097/00004583-200012000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Rucklidge JJ. Psychosocial functioning of adolescents with and without paediatric bipolar disorder. Journal of affective disorders. 2006;91(2–3):181–188. doi: 10.1016/j.jad.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Schenkel LS, West AE, Harral EM, Patel NB, Pavuluri MN. Parent-child interactions in pediatric bipolar disorder. Journal of clinical psychology. 2008;64(4):422–437. doi: 10.1002/jclp.20470. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein TR, Miklowitz DJ, Mullen KL. Social skills knowledge and performance among adolescents with bipolar disorder. Bipolar disorders. 2006;8(4):350–361. doi: 10.1111/j.1399-5618.2006.00321.x. [DOI] [PubMed] [Google Scholar]
- 13.Keenan-Miller D, Peris T, Axelson D, Kowatch RA, Miklowitz DJ. Family functioning, social impairment, and symptoms among adolescents with bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(10):1085–1094. doi: 10.1016/j.jaac.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim EY, Miklowitz DJ, Biuckians A, Mullen K. Life stress and the course of early-onset bipolar disorder. Journal of affective disorders. 2007;99(1–3):37–44. doi: 10.1016/j.jad.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewinsohn PM, Seeley JR, Klein DN. Bipolar Disorder in Adolescents: Epidemiology and Suicidal Behavior. New York: Guilford Press; 2003. [Google Scholar]
- 16.Kessler RC, Akiskal HS, Ames M, et al. Prevalence and effects of mood disorders on work performance in a nationally representative sample of U.S. workers. The American journal of psychiatry. 2006;163(9):1561–1568. doi: 10.1176/appi.ajp.163.9.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kupfer DJ. The increasing medical burden in bipolar disorder. JAMA : the journal of the American Medical Association. 2005;293(20):2528–2530. doi: 10.1001/jama.293.20.2528. [DOI] [PubMed] [Google Scholar]
- 18.Gore FM, Bloem PJ, Patton GC, et al. Global burden of disease in young people aged 10–24 years: a systematic analysis. Lancet. 2011;377(9783):2093–2102. doi: 10.1016/S0140-6736(11)60512-6. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein BI, Sassi R, Diler RS. Pharmacologic treatment of bipolar disorder in children and adolescents. Child and adolescent psychiatric clinics of North America. 2012;21(4):911–939. doi: 10.1016/j.chc.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 20.McClellan J, Kowatch R, Findling RL. Work Group on Quality I. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(1):107–125. doi: 10.1097/01.chi.0000242240.69678.c4. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein TR, Fersch-Podrat RK, Rivera M, et al. Dialectical Behavior Therapy (DBT) for Adolescents with Bipolar Disorder: Results from a Pilot Randomized Trial. Journal of child and adolescent psychopharmacology. 2014 Jul 10; doi: 10.1089/cap.2013.0145. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hlastala SA, Kotler JS, McClellan JM, McCauley EA. Interpersonal and social rhythm therapy for adolescents with bipolar disorder: treatment development and results from an open trial. Depression and anxiety. 2010;27(5):457–464. doi: 10.1002/da.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fristad MA, Verducci JS, Walters K, Young ME. Impact of multifamily psychoeducational psychotherapy in treating children aged 8 to 12 years with mood disorders. Archives of general psychiatry. 2009;66(9):1013–1021. doi: 10.1001/archgenpsychiatry.2009.112. [DOI] [PubMed] [Google Scholar]
- 24.Miklowitz DJ, Axelson DA, Birmaher B, et al. Family-focused treatment for adolescents with bipolar disorder: results of a 2-year randomized trial. Archives of general psychiatry. 2008;65(9):1053–1061. doi: 10.1001/archpsyc.65.9.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garrett AS, Reiss AL, Howe ME, et al. Abnormal amygdala and prefrontal cortex activation to facial expressions in pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(8):821–831. doi: 10.1016/j.jaac.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rich BA, Carver FW, Holroyd T, et al. Different neural pathways to negative affect in youth with pediatric bipolar disorder and severe mood dysregulation. Journal of psychiatric research. 2011;45(10):1283–1294. doi: 10.1016/j.jpsychires.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Passarotti AM, Pavuluri MN. Brain functional domains inform therapeutic interventions in attention-deficit/hyperactivity disorder and pediatric bipolar disorder. Expert review of neurotherapeutics. 2011;11(6):897–914. doi: 10.1586/ern.11.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavuluri MN, Graczyk PA, Henry DB, Carbray JA, Heidenreich J, Miklowitz DJ. Child-and family-focused cognitive-behavioral therapy for pediatric bipolar disorder: development and preliminary results. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(5):528–537. doi: 10.1097/00004583-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 29.West AE, Henry DB, Pavuluri MN. Maintenance model of integrated psychosocial treatment in pediatric bipolar disorder: A pilot feasibility study. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(2):205–212. doi: 10.1097/01.chi.0000246068.85577.d7. [DOI] [PubMed] [Google Scholar]
- 30.West AE, Jacobs RH, Westerholm R, et al. Child and family-focused cognitive-behavioral therapy for pediatric bipolar disorder: pilot study of group treatment format. Journal of the Canadian Academy of Child and Adolescent Psychiatry = Journal de l'Academie canadienne de psychiatrie de l'enfant et de l'adolescent. 2009;18(3):239–246. [PMC free article] [PubMed] [Google Scholar]
- 31.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. The British journal of psychiatry : the journal of mental science. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 32.Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the children's depression rating scale. Journal of the American Academy of Child Psychiatry. 1984;23(2):191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test–Second Edition (KBIT-2) Circle Pines, MN: American Guidance Service; 2004. [Google Scholar]
- 34.Geller B, Williams M, Zimerman B, Frazier J. Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) St Louis: Washington University; 1996. [DOI] [PubMed] [Google Scholar]
- 35.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. The American journal of psychiatry. 2011;168(12):1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 37.Altman E, Hedeker D, Peterson JL, Davis JM. A comparative evaluation of three self-rating scales for acute mania. Biological psychiatry. 2001;50(6):468–471. doi: 10.1016/s0006-3223(01)01065-4. [DOI] [PubMed] [Google Scholar]
- 38.Primakoff L, Epstein N, Covi L. Homework compliance: An uncontrolled variable in cognitive therapy outcome research. Behavior Therapy. 1986;17(4):433–446. [Google Scholar]
- 39.Urbaniak GC, Plous S. Research randomizer: The Creators. 1999 [Google Scholar]
- 40.Pavuluri MN, Henry DB, Devineni B, Carbray JA, Naylor MW, Janicak PG. A pharmacotherapy algorithm for stabilization and maintenance of pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(7):859–867. doi: 10.1097/01.chi.0000128790.87945.2f. [DOI] [PubMed] [Google Scholar]
- 41.American Psychiatric Association. Diagnostic and statistical manual of mental health disorders (4th ed) Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 42.Pavuluri MN, Henry DB, Devineni B, Carbray JA, Birmaher B. Child mania rating scale: development, reliability, and validity. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(5):550–560. doi: 10.1097/01.chi.0000205700.40700.50. [DOI] [PubMed] [Google Scholar]
- 43.Youngstrom E, Meyers O, Demeter C, et al. Comparing diagnostic checklists for pediatric bipolar disorder in academic and community mental health settings. Bipolar disorders. 2005;7(6):507–517. doi: 10.1111/j.1399-5618.2005.00269.x. [DOI] [PubMed] [Google Scholar]
- 44.West AE, Celio CI, Henry DB, Pavuluri MN. Child Mania Rating Scale-Parent Version: A valid measure of symptom change due to pharmacotherapy. Journal of affective disorders. 2011;128(1):112–119. doi: 10.1016/j.jad.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaffer D, Gould MS, Brasic J, et al. A children's global assessment scale (CGAS) Archives of general psychiatry. 1983;40(11):1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 46.Spearing MK, Post RM, Leverich GS, Brandt D, Nolen W. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BP. Psychiatry research. 1997;73(3):159–171. doi: 10.1016/s0165-1781(97)00123-6. [DOI] [PubMed] [Google Scholar]
- 47.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 48.Singer JDaW JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford: Oxford University Press; 2003. [Google Scholar]
- 49.Merikangas KR, He J-p, Burstein M, et al. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A) Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(10):980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rizzo CJ, Esposito - Smythers C, Swenson L, et al. Factors associated with mental health service utilization among bipolar youth. Bipolar disorders. 2007;9(8):839–850. doi: 10.1111/j.1399-5618.2007.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Youngstrom E, Meyers O, Demeter C, et al. Comparing diagnostic checklists for pediatric bipolar disorder in academic and community mental health settings. Bipolar disorders. 2005;7(6):507–517. doi: 10.1111/j.1399-5618.2005.00269.x. [DOI] [PubMed] [Google Scholar]
- 52.Case BG, Olfson M, Marcus SC, Siegel C. Trends in the inpatient mental health treatment of children and adolescents in US community hospitals between 1990 and 2000. Archives of general psychiatry. 2007;64(1):89. doi: 10.1001/archpsyc.64.1.89. [DOI] [PubMed] [Google Scholar]
- 53.Weinstein SM, West AE, Pavuluri M. Psychosocial intervention for pediatric bipolar disorder: current and future directions. Expert review of neurotherapeutics. 2013;13(7):843–850. doi: 10.1586/14737175.2013.811985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cakir S, Ozerdem A. [Psychotherapeutic and psychosocial approaches in bipolar disorder: a systematic literature review] Turk psikiyatri dergisi = Turkish journal of psychiatry. 2010;21(2):143–154. [PubMed] [Google Scholar]
- 55.Miklowitz DJ, Otto MW, Frank E, et al. Psychosocial treatments for bipolar depression: a 1-year randomized trial from the Systematic Treatment Enhancement Program. Archives of general psychiatry. 2007;64(4):419–426. doi: 10.1001/archpsyc.64.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar disorders. 2008;10(1 Pt 2):194–214. doi: 10.1111/j.1399-5618.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.