Abstract
Peripheral artery disease (PAD) is recognized as a public health issue because of its prevalence, functional limitations, and increased risk of systemic ischemic events. Current treatments for claudication, the primary symptom in PAD patients, have limitations. Cells identified usingcytosolic enzyme aldehyde dehydrogenase (ALDH) may benefit patients with severe PAD but has not been studied in patients with claudication. PACE is a randomized, double-blind, placebo-controlled clinical trial conducted by the Cardiovascular Cell Therapy Research Network (CCTRN) to assess the safety and efficacy of autologous bone marrow–derived ALDHbr cells delivered by direct intramuscular injections in 80 patients with symptom-limiting intermittent claudication. Eligible patients will have a significant stenosis or occlusion of infrainguinal arteries and a resting ankle-brachial index <0.90 and will be randomized 1:1 to cell or placebo treatment with a 1-year follow-up.
The primary endpoints are the change in peak walking time and leg collateral arterial anatomy, calf muscle blood flow, and tissue perfusion as determined by magnetic resonance (MR) imaging at 6 months compared to baseline. The latter 3 measurements are new physiologic lower extremity tissue perfusion and PAD imaging-based endpoints that may help to quantify the biologic and mechanistic effects of cell therapy.
This trial will collect important mechanistic and clinical information on the safety and efficacy of ALDHbr cells in patients with claudication and provide valuable insight into the utility of advanced MR imaging endpoints.
Introduction
Clinical studies of cell therapy have suggested that delivery of bone marrow-derived cells to ischemic leg muscles might improve angiogenesis and clinical outcomes in patients with peripheral artery disease (PAD).(1, 2) These studies have primarily evaluated cell therapy in patients with critical limb ischemia (CLI), the most severe manifestation of PAD. However, the most common clinical presentation of PAD is intermittent claudication, anddata are limited in this group.
The evaluation of the physiologic and clinical effects of cell therapy in individuals with claudication presents considerable challenges to trial design. The Cardiovascular Cell Therapy Research Network (CCTRN) has significant expertise in both cell therapy trial design and PAD therapeutic outcome assessment. This study integrates knowledge from 3 distinct fields—cell therapy, vascular medicine, and magnetic resonance imaging (MRI)—into the planning of the Patients with Intermittent Claudication Injected with ALDH Bright Cells (PACE) trial. This prospective, multi-center, placebo-controlled randomized trial will investigate the effects of a subset of bone marrow mononuclear cells (BMMNCs) on leg perfusion and limb symptoms in individuals with symptom-limiting intermittent claudication. We anticipate these results will improve future PAD clinical trial design and ultimately lead to novel stem cell therapies for these patients.
Background
Peripheral Artery Disease
In the United States, at least 8–10 million people have PAD(3, 4) and up to 12% of adults with atherosclerosis risk factors in developed nations have evidence of PAD. Five clinical presentations of PAD are recognized: asymptomatic disease (in 50% of patients), atypical leg symptoms (40–50%), typical (classic) exercise-induced claudication (in 8–12%), and the 2 severe manifestations (<2–5%) of acute limb ischemia and CLI.
Individuals with lifestyle-limiting claudication symptoms may benefit from several evidence-based therapies that lower cardiovascular ischemic risk and diminish the functional limitations associated with claudication.(5, 6) Supervised exercise is an effective therapeutic option that is durable and cost-effective. Claudication pharmacotherapy (e.g., cilostazol) improves walking distance and quality of life in many low-risk individuals but is significantly underutilized. Invasive open surgical and endovascular revascularization techniques are useful in patients who do not improve with other options. However, each of these approaches has limitations. Supervised exercise cannot be used successfully in patients who have significant non-PAD exertional co-morbidities, whose healthcare providers do not offer this treatment in cardiac rehabilitation programs, or who lack motivation or social support. Cilostazol is not universally tolerated, cannot be used in patients with systolic heart failure, and may not yield an adequate therapeutic response. Revascularization may not be feasible in patients with unfavorable anatomy, has associated risks to the index limb and renal function, and may increase heart attack and stroke risk.(3) Therefore, novel therapeutic options are needed for patients with PAD and claudication. Cell therapy may become an attractive treatment option for individuals with claudication if clinical benefits are proven.
Cell Type
BMMNCs have enhanced neoangiogenesis in ischemic tissues in preclinical studies,(7, 8) and clinical studies have demonstrated that these cells may benefit patients with PAD and CLI.(9–11) However, only 1 small non-randomized trial has been conducted in patients with intermittent claudication.(12)
BMMNCs are a heterogeneous population of cells that includes stem cells and non-stem cells with varying degrees of therapeutic potential.(13, 14) Selected cell populations from the bone marrow enriched for angiogenic activities may provide a superior therapeutic effect(15) A subtype of hematopoietic progenitor cells has been isolated based on the presence of the cytosolic enzyme ALDH, a marker for stem and progenitor cells (Lin- CD34+CD38−).(16) This subpopulation of progenitor cells, called ALDHbr cells, represents about 1% of total BMMNCs and contains a heterogeneous mixture of cell types thought to be needed for ischemic repair, including hematopoietic, endothelial, and mesenchymal progenitor cells. Preclinical studies have shown that ALDHbr cells promote angiogenesis and restore blood flow in hind-limb ischemia,(17) and quantitative real-time polymerase chain reaction has shown gene expression of multiple angiogenic factors, including interleukin-8, transforming growth factor-β, vascular endothelial growth factor, and midkine in bone marrow-derived ALDHbr cells.(18) Moreover, in a phase I trial, Perin and colleagues demonstrated the safety and potential efficacy of ALDHbr cells in CLI patients.(10) In addition, in a pilot study of patients with heart failure, ALDHbr cells injected into the left ventricle showed a similar safety profile and improvement in surrogate efficacy endpoints.(19)
Magnetic Resonance Imaging
Recent advances in magnetic resonance (MR) imaging techniques may provide new, more accurate methods to noninvasively quantify the angiogenic response to cell administration. Both arterial anatomy and skeletal muscle perfusion may be evaluated by administering exogenous contrast media to image arterial and microvascular structures. The benefits of MR imaging for this study include the absence of ionizing radiation (making it safe for repeated measurements), a tomographic 3-dimensional field of view unencumbered by acoustic windows, excellent soft-tissue contrast, the ability to measure bulk vascular (and tissue) morphology and flow patterns within major vessels, and the capability to assess microvascular flow and tissue metabolism. This anatomic and physiologic evaluation of a stem cell intervention in vivo will enable clinical determination of the angiogenic effects in ischemic lower limbs. In addition, MR imaging can provide cross-sectional imaging of the skeletal muscle to identify and quantify pathophysiological alterations in the skeletal muscle of PAD patients.
CCTRN
In its second NIH-funded cycle, the CCTRN comprises 8 clinical research centers (University of Florida, Indiana University, University of Louisville, University of Miami, Minneapolis Heart Institute, the University of Minnesota, Texas Heart Institute, and Stanford University), a data coordinating center (DCC), a cell processing quality assurance center, and 3 core laboratories including a biorepository.(13, 20) The organizational structure and oversight of the CCTRN are described in the Appendix.
Study Design
PACE is a randomized, double-blind, placebo-controlled clinical trial designed to evaluate the effect of ALDHbr cells versus placebo in individuals with PAD and intermittent claudication. The investigation is funded by the National Heart, Lung, and Blood Institute (NHLBI). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents, with assistance in editing by Rebecca Bartow at the Texas Heart Institute.
The hypothesis of PACE is that ALDHbr cells will increase peak walking time (PWT) in patients with PAD and intermittent claudication by increasing blood flow in the treated limb. Additional hypotheses to be tested are that (1) ALDHbr cells increase the number of new vessels in the treated limb; (2) ALDHbr cells increase hyperemic vascular flow; (3) ALDHbr cells increase perfusion; and (4) changes in PWT will correlate with changes in the number of new vessels, vascular flow, or perfusion.
Study Population
A total of 80 patients will be randomly assigned to receive either active (n=40) or placebo (n=40) therapy. The study group will comprise patients who have atherosclerotic lower extremity PAD with symptom-limiting intermittent claudication identified as greater in 1 leg. Patients will be selected on the basis of their PAD anatomy to include the presence of a significant (≥50%) stenosis or occlusion of the infrainguinal arteries, including the superficial femoral artery, popliteal artery and/or infrapopliteal arteries. Patients will not have documented aorto-iliac inflow stenoses and will have a resting ankle-brachial index (ABI) <0.90 or a toe-brachial index <0.70 (for patients with non-compressible pedal pulses) (Table 1).
Table I.
Inclusion Criteria
| 1. | Patients with atherosclerotic peripheral artery disease with asymmetrical (i.e., greater in one leg) classic claudication or atypical leg pain (exertional leg pain that does not begin at rest or does not resolve consistently with rest) as defined by the San Diego Claudication Questionnaire. |
| 2. | Age ≥40 years |
| 3. | Resting ankle-brachial index <0.90 or a resting toe-brachial index of <0.70 |
| 4. | Presence of significant stenosis or occlusion of infrainguinal arteries including the superficial femoral artery, popliteal artery and/or infrapopliteal arteries as determined by Duplex ultrasound imaging (occlusion or focal doubling of peak systolic velocity of one or more affected segments) OR lower extremity CTA OR lower extremity MRA OR lower extremity catheter-based contrast arteriography. Each of these noninvasive and invasive anatomic assessments will identify patients with at least a 50% stenosis in the affected segment. |
Patients initially considered for PACE will undergo a preliminary review of their medical records to determine if they meet the inclusion and exclusion criteria (Table I and Supplementary Table I). Once deemed eligible, patients will meet with the research team to review the possible risks of participation and informed consent documents, which will provide information regarding standard alternative claudication therapies.
Baseline Screening and Randomization
Baseline evaluations will be conducted during the screening period extending from the date informed consent is signed until the day of treatment (not to exceed 60 days before treatment).
Participants will be randomized through a web access database created and maintained by the DCC.
Cell Processing
To isolate ALDHbr cells, we will collect 180 mL (±10mL) of bone marrow from the patient’s posterior superior iliac spine; the level of sedation (conscious sedation or general anesthesia) for this procedure will be determined by local institutional guidelines. The final study product must meet specific release criteria before distribution to the clinical sites (Table II). If the study product does not meet release criteria, the patient will not be treated but will continue in the trial as part of the intention-to-treat analysis. The cell product will be delivered to the local cell-processing laboratories from either Aldagen (North Carolina) or the Center for Cell and Gene Therapy (Baylor College of Medicine, Houston, Texas) and must be administered within 96 hours of bone-marrow aspiration. The placebo product comprises phenol red-free CellGro SCGM serum-free medium (CellGenix Technologie Transfer GmbH, Frieburg, Germany) supplemented with 1% human serum albumin. Both placebo and cell product are supplied in packaging identical in appearance.
Table II.
Study Product Release Criteria
| Assay | Specification |
|---|---|
| Phenotyping | ≤ 12% ALDHdim/CD3+ ≤ 15% ALDHdim/CD14+ ≤ 12% ALDHdim/CD19+ ≤ 15% ALDHdim/CD15+ ≤ 15% ALDHdim/CD235a+ |
| ALDHbr content | Entire sample must meet ≥70% purity specification |
| Gram stain | Negative |
| Endotoxin | ≤33.3 Eu/mL |
| Sterility | Sterility results pending at the time of release. Sites are notified if a positive culture is identified or at the conclusion of the culture period for a negative result. |
Cell Delivery
The objective of delivery is to place the cells or placebo at a depth corresponding to the muscular mass of the calf (gastrocnemius) and lower thigh (semimembranosus and biceps femoris). After the patient is made as comfortable as possible, the injection leg will be confirmed, and the 10 injection sites will be marked with a template to standardize injections across centers. Anesthetic cream will be applied, and the needle will be inserted at a 90° angle to the skin for 8 injections into the bulk of the gastrocnemius and 2 into the thigh adductors. After insertion, the syringe will be aspirated to avoid intravascular injection, and then a 1-mL volume of study product will be slowly delivered at each injection site. After injections, we will apply gentle pressure and a bandage over each injection site, if necessary (Figure 1).
Figure 1.
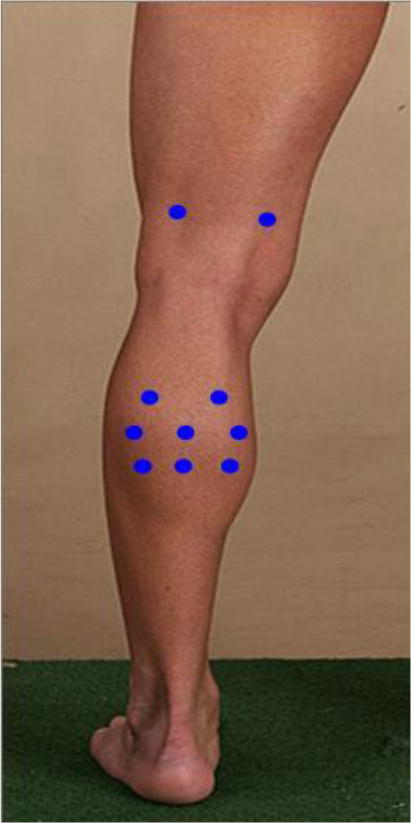
Injection template
Follow-up Evaluations
Follow-up will begin on the injection day (Day 0). Randomized patients will be followed for safety and efficacy for 180 days, with evaluations on days 7, 30, 90, and 180 (primary endpoint assessment) (Figure 2). Patients will be contacted by telephone at 12 months to assess long-term leg symptoms, physical limitations, and quality of life. Participants will be considered lost to follow-up after 3 consecutive failed telephone contacts and 1 undeliverable certified letter.
Figure 2.
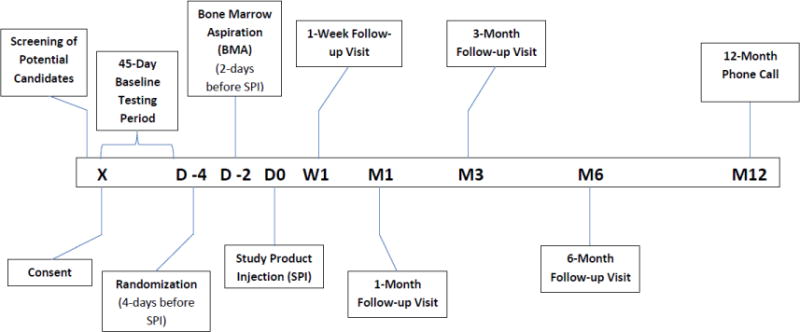
Study Timeline
Safety Monitoring
All study participants will have adverse events transmitted to each center’s IRB and the DCC, the Food and Drug Administration (FDA), and the NHLBI-sponsored DSMB. The DSMB will meet at least twice yearly to review the performance of the participating sites, assess recruitment, evaluate study progress, and report to the NHLBI. PACE will be placed on temporary hold until a thorough investigation is conducted if: 1 case of malignant tumor growth at the site of study product injection; 3 cases of cellulitis/vasculitis in a treated (index) limb; 3 cases of deep vein thrombosis (DVT); 1 case of pulmonary embolism; 1 case of disease progression to CLI; or 1 case of compartment syndrome. The DCC will oversee regulatory and safety complianceof all participating units.
Biorepository
For patients who consent to sample donation, any bone marrow that was not used to make the study product will be shipped to the Biorepository. In addition, the following samples will be shipped to the Biorepository for phenotyping and functional analyses: whole peripheral blood, plasma, and buffy coat obtained before bone marrow harvest, 30 minutes after product injection, and at follow-up (1 week, 1 month, and 6 months). Bone marrow from patients will be analyzed by flow cytometry for 14 cell surface markers and by 2 colony-forming assays (CFU-F and hematopoietic CFC-assay) to determine functional potency.
Magnetic Resonance Imaging
An MRI core lab (Johns Hopkins University) will assess MRI quality among sites. Endpoints were selected based on published reproducibility assessments.(21, 22) Lower extremity peripheral artery anatomy and function will be assessed by using time-resolved contrast-enhanced magnetic resonance angiography (CE-MRA) after the injection (0.05 mmol/kg) of an extravascular gadolinium-based contrast agent (GBCA). For upper leg MRA, 3D CE-MRA data acquisition will be triggered when the injected contrast bolus (0.1 mmol/kg) is seen at the target anatomy by using a real-time monitoring scan. Bulk macrovascular blood flow to the calf muscle will be assessed by using a phase-contrast MR sequence in conjunction with cardiac gating. Peak arterial blood flow and the time-resolved flow curve across the RR interval will be measured for the vessel of interest. Arterial flow reserve will be measured by using the flow measured at resting and hyperemic states. Reactive hyperemia will be induced in the symptomatic leg with the use of inflatable cuffs. The cuff will be inflated for 300 seconds (for 180–300 seconds for patients who cannot tolerate a 5-minute cuff inflation) at the mid-thigh level with supra-systolic pressures to completely occlude flow to the calf (>25–50 mm Hg above brachial systolic blood pressure). Hyperemic flow will be measured for up to 10 minutes after cuff deflation. Microvascular function will be determined in the calf musculature by performing perfusion measurements using dynamic contrast-enhanced (DCE) MRI. DCE-MRI will be preceded by the measurement of spin-lattice relaxation time (T1) of the tissues of interest (i.e., over the field of view of DCE-MRI measurement) using a multiple-flip angle T1 measurement. A similar cuff inflation and deflation protocol described above will be used. In addition, GBCA (0.05 mmol/kg) will be administered immediately after the cuff is fully inflated. A rapid release of cuff pressure (<1s) will ensure a bolus infusion of contrast agent, and the DCE-MRI sequence will be run in a dynamic fashion with an effective temporal resolution of about 5 seconds per dynamic. The acquired slice orientation will be transverse to encompass both legs. A total number of 60 dynamics will be acquired (3–4 minutes) to observe the first passage of contrast bolus. The signal intensity curves will be converted to contrast agent concentration curves to quantify microvascular blood flow. After the dynamic scan, the T1 measurement sequence will be repeated to measure post-contrast T1 times. We will use the pre- and post-contrast T1 times to calculate the extracellular volume of the calf muscle.
Statistical Analysis Plan
PACE will test the hypothesis that ALDHbr cells improve PWT and lower limb blood flow compared to placebo. Its 4 primary endpoints to be assessed at 6 months (1 clinical and 3 MR endpoints) are: 1) PWT; 2) leg collateral artery anatomy (MR); 3) macrovascular flow (MR); and 4) calf muscle perfusion (MR) (Table III). Each endpoint will be assessed as the difference between follow-up and baseline The effect of therapy on each primary evaluation will be assessed at the 0.05 level with no adjustment for multiple comparisons. All primary analyses will be conducted under the “intention-to-treat” principle and an additional “as treated” analysis.
Table III.
Study Endpoints
| Multiple Primary Endpoints (reflecting change from baseline to 6 months between groups) |
|
| Secondary Endpoints (reflecting change from baseline) |
|
ABI=ankle brachial index; COT=claudication onset time; PWT=peak walking time
Treadmill Core Lab (Colorado Prevention Center) will oversee PWT quality. Using data from the CLEVER trial, we computed that 78 patients would be required to detect a 2.5 minute difference in the increase in PWT from baseline in cell-treated patients compared to the change from baseline in placebo-treated patients, assuming a standard deviation of the difference of 3 minutes, 90% power, and 15% loss to follow-up.(23) PWT will be assessed at both 3 and 6 months. At this sample size, the power for each of the change in number of new vessels (Mann-Whitney U statistic, 90% power), vascular flow (2-tailed t-test, 95% power), and perfusion (2-tailed t-test, 90% power) is adequate based on Verslius.(21, 22)
Secondary endpoints are change in resting ABI, change in post-exercise ABI, and change in claudication onset time (COT). Each will be obtained at baseline and at 3 and 6 months. Wwe will formally assess the relationship between PWT and the 3 imaging-based primary endpoints adjusting for important baseline covariates. Specific analyses and subgroup evaluations are provided in the appendix.
Discussion
The PACE trial design is unique on several levels. First, the results of this study will fill a current knowledge gap by collecting efficacy data from a randomized clinical study of cell therapy in patients with intermittent claudication. Second, we are using a novel cell population that has shown promise in improving limb perfusion in a pilot study in CLI patients. Finally, this protocol evaluates new MR anatomic and physiologic endpoints that may be applied more broadly in future PAD clinical trials and may provide mechanistic insights that can link anatomic arterial structure, physiologic flow, and clinical endpoints in cell-treated patients.
The typical endpoint of PWT (also expressed as maximal walking distance) on a graded treadmill has many physiological determinants, including the severity of the conduit artery stenosis, the extent of arterial collaterals, microvascular function, capillary density, mitochondrial function, skeletal muscle fiber type and innervation, and neurobehavioral determinants such as motivation and alterations in gait.(24) Measurement of PWT is a clinically relevant endpoint as it defines functional status, is approvable by the FDA, and permits comparison of the net treatment effect of cell therapy to currently approved and future investigational therapeutic approaches. However, in evaluating a novel therapy that may have differential mechanistic effects, using an endpoint that also assesses specific anatomic and functional flow changes due to the therapy is desirable. Thus, we considered the use of MR imaging endpoints to evaluate the changes in perfusion, flow, and the vasculature that might be induced, allowing a more direct demonstration of the biological effect of stem cell therapy on collateral channels (arteriogenesis) or capillary density (angiogenesis). Since, the use of MR imaging endpoints presents additional challenges in a traditional phase III clinical trial paradigm in which only 1 primary endpoint is appropriate we are operating in a different type I error paradigm.(25)
Previous PAD trials have also assessed limb perfusion with the use of imaging modalities such as digital subtraction angiography (DSA). DSA has shown improvement in collateral circulation after cell therapy in CLI patients(11, 26, 27); however, in a randomized, double-blind cell therapy trial that showed significant increase in pain-free walking distance and ABI (n=25),(28, 29) 2 groups of experienced radiologists and vascular surgeons were unable to distinguish any significant difference in the arterial anatomy measured from the DSA of responders as compared to non-responders. The authors concluded that DSA is not sensitive and reliable enough to quantify the changes in blood flow or clinical benefits that might occur after cell therapy. In considering these findings, we sought to investigate the role of a potentially more sensitive imaging modality.
The data collected in this trial can offer new insights into how lower extremity arterial structure and blood flow physiology may alter key PAD clinical outcomes. For example, the relative concordance (or discordance) between the traditional functional PAD endpoints (PWT) and the novel MR endpoints is unknown. A therapeutic beneficial PWT response may be observed in the absence of anatomic collateral or perfusion changes and would therefore imply a need to re-evaluate current mechanistic hypotheses of cell therapy benefits. Such discordance may lead to new efforts to improve the sensitivity of the MR imaging measurements. Alternatively, an identifiable change in imaging endpoints without associated changes in traditional PAD assessments would advance the field by expanding the ability to evaluate therapeutic effects and identify the mechanisms of benefit.
By combining varied Network-based expertise, the PACE trial design offers significant benefits in advancing the knowledge base for PAD therapies. These results may lead to the identification of new, reliable MR imaging endpoints and increase the options for safe, effective cell treatments for PAD patients. Our study addresses an unmet need by identifying a potentially therapeutic stem cell regimen for patients with intermittent claudication.
Acknowledgments
We acknowledge the contributions of Dr. Sonia Skarlatos (1953–2013) for her insight, expertise and support of the CCTRN, which continues to propel the cell therapy field forward, and Rebecca A. Bartow, PhD, of the Texas Heart Institute for editorial assistance. Dr. Bartow was not financially compensated for her contribution.
Funding Source: Funding for the Cardiovascular Cell Therapy Research Network was provided by the National Heart, Lung, and Blood Institute under cooperative agreement UM1 HL087318-07.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: The authors have completed Conflict of Interest Disclosures and have reported the following: Alan T. Hirsch has 3 research grants at the University of Minnesota from Viromed, AstraZeneca, and Pluristem. He also serves as a consultant for Merck, Bayer, Anges, and Novartis. Joao A. Lima serves is funded as the MRI core lab through a grant from CAPRICOR. William R. Hiatt manages all industry funding through CPC Clinical Research, a non-profit clinical trials research center affiliate of the University of Colorado. He has grants from the following sponsors related to studies relevant to vascular disease research: AstraZeneca, Janssen, CSI, DNAVEC, Kowa, Kyushu University, Pluristem, ReNeuron, Rigel, Takeda. All remaining authors had no financial interests to disclose.
References
- 1.Gupta R, Losordo DW. Cell therapy for critical limb ischemia: Moving forward one step at a time. Circ Cardiovasc Interv. 2011;4:2–5. doi: 10.1161/CIRCINTERVENTIONS.110.960716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Losordo DW, Kibbe MR, Mendelsohn F, et al. A randomized, controlled pilot study of autologous CD34+ cell therapy for critical limb ischemia. Circ Cardiovasc Interv. 2012;5:821–30. doi: 10.1161/CIRCINTERVENTIONS.112.968321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics–2008 update: A report from the american heart association statistics committee and stroke statistics subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 4.Mahoney EM, Wang K, Keo HH, et al. Vascular hospitalization rates and costs in patients with peripheral artery disease in the united states. Circ Cardiovasc Qual Outcomes. 2010;3:642–51. doi: 10.1161/CIRCOUTCOMES.109.930735. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report. Circulation. 2006;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 6.Olin JW, Sealove BA. Peripheral artery disease: Current insight into the disease and its diagnosis and management. Mayo Clin Proc. 2010;85:678–92. doi: 10.4065/mcp.2010.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 8.Ziegelhoeffer T, Fernandez B, Kostin S, et al. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94:230–8. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 9.Higashi Y, Kimura M, Hara K, et al. Autologous bone-marrow mononuclear cell implantation improves endothelium-dependent vasodilation in patients with limb ischemia. Circulation. 2004;109:1215–8. doi: 10.1161/01.CIR.0000121427.53291.78. [DOI] [PubMed] [Google Scholar]
- 10.Perin EC, Silva G, Gahremanpour A, et al. A randomized, controlled study of autologous therapy with bone marrow-derived aldehyde dehydrogenase bright cells in patients with critical limb ischemia. Catheter Cardiovasc Interv. 2011;78:1060–7. doi: 10.1002/ccd.23066. [DOI] [PubMed] [Google Scholar]
- 11.Tateishi-Yuyama E, Matsubara H, Murohara T, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: A pilot study and a randomised controlled trial. Lancet. 2002;360:427–35. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 12.Bartsch T, Brehm M, Zeus T, et al. Transplantation of autologous mononuclear bone marrow stem cells in patients with peripheral arterial disease (the TAM-PAD study) Clin Res Cardiol. 2007;96:891–9. doi: 10.1007/s00392-007-0569-x. [DOI] [PubMed] [Google Scholar]
- 13.Zierold C, Carlson MA, Obodo UC, et al. Developing mechanistic insights into cardiovascular cell therapy: Cardiovascular cell therapy research network biorepository core laboratory rationale. Am Heart J. 2011;162:973–80. doi: 10.1016/j.ahj.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gee AP, Richman S, Durett A, et al. Multicenter cell processing for cardiovascular regenerative medicine applications: The cardiovascular cell therapy research network (CCTRN) experience. Cytotherapy. 2010;12:684–91. doi: 10.3109/14653249.2010.487900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawamoto A, Iwasaki H, Kusano K, et al. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–9. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 16.Storms RW, Trujillo AP, Springer JB, et al. Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proc Natl Acad Sci U S A. 1999;96:9118–23. doi: 10.1073/pnas.96.16.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capoccia BJ, Robson DL, Levac KD, et al. Revascularization of ischemic limbs after transplantation of human bone marrow cells with high aldehyde dehydrogenase activity. Blood. 2009;113:5340–51. doi: 10.1182/blood-2008-04-154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White HS, Smith L, Gentry T, et al. Mechanisms of action of human aldehyde dehydrogenase bright cells in therapy of cardiovascular diseases: Expression analysis of angiogenic factors and aldehyde dehydrogenase isozymes. Journal of Stem Cell Research & Therapy. 2011;S1:1–9. [Google Scholar]
- 19.Perin EC, Silva GV, Zheng Y, et al. Randomized, double-blind pilot study of transendocardial injection of autologous aldehyde dehydrogenase-bright stem cells in patients with ischemic heart failure. Am Heart J. 2012;163:415, 21–421.e1. doi: 10.1016/j.ahj.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Simari RD, Moye LA, Skarlatos SI, et al. Development of a network to test strategies in cardiovascular cell delivery: The NHLBI-sponsored cardiovascular cell therapy research network (CCTRN) J Cardiovasc Transl Res. 2010;3:30–6. doi: 10.1007/s12265-009-9160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Versluis B, Backes WH, van Eupen MG, et al. Magnetic resonance imaging in peripheral arterial disease: Reproducibility of the assessment of morphological and functional vascular status. Invest Radiol. 2011;46:11–24. doi: 10.1097/RLI.0b013e3181f2bfb8. [DOI] [PubMed] [Google Scholar]
- 22.Versluis B, Dremmen MH, Nelemans PJ, et al. Dynamic contrast-enhanced MRI assessment of hyperemic fractional microvascular blood plasma volume in peripheral arterial disease: Initial findings. PLoS One. 2012;7:e37756. doi: 10.1371/journal.pone.0037756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy TP, Cutlip DE, Regensteiner JG, et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: Six-month outcomes from the claudication: Exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012;125:130–9. doi: 10.1161/CIRCULATIONAHA.111.075770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brass EP, Hiatt WR, Green S. Skeletal muscle metabolic changes in peripheral arterial disease contribute to exercise intolerance: A point-counterpoint discussion. Vasc Med. 2004;9:293–301. doi: 10.1191/1358863x04vm572ra. [DOI] [PubMed] [Google Scholar]
- 25.Hare JM, Bolli R, Cooke JP, et al. Phase II clinical research design in cardiology: Learning the right lessons too well: Observations and recommendations from the cardiovascular cell therapy research network (CCTRN) Circulation. 2013;127:1630–5. doi: 10.1161/CIRCULATIONAHA.112.000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang P, Li S, Han M, et al. Autologous transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care. 2005;28:2155–60. doi: 10.2337/diacare.28.9.2155. [DOI] [PubMed] [Google Scholar]
- 27.Huang PP, Yang XF, Li SZ, et al. Randomised comparison of G-CSF-mobilized peripheral blood mononuclear cells versus bone marrow-mononuclear cells for the treatment of patients with lower limb arteriosclerosis obliterans. Thromb Haemost. 2007;98:1335–42. doi: 10.1160/th07-02-0137. [DOI] [PubMed] [Google Scholar]
- 28.Van Tongeren RB, Hamming JF, Fibbe WE, et al. Intramuscular or combined intramuscular/intra-arterial administration of bone marrow mononuclear cells: A clinical trial in patients with advanced limb ischemia. J Cardiovasc Surg (Torino) 2008;49:51–8. [PubMed] [Google Scholar]
- 29.van Tongeren RB, Hamming JF, le Cessie S, et al. Limited value of digital subtraction angiography in the evaluation of cell-based therapy in patients with limb ischemia. Int J Cardiovasc Imaging. 2010;26:19–25. doi: 10.1007/s10554-009-9507-5. [DOI] [PubMed] [Google Scholar]


