Abstract
Local signals maintain adult stem cells in many tissues. Whether the sexual identity of adult stem cells must also be maintained was not known. In the adult Drosophila testis niche, local Jak-STAT signaling promotes somatic cyst stem cell (CySC) renewal through several effectors, including the putative transcription factor Chronologically inappropriate morphogenesis (Chinmo). Here, we find that Chinmo also prevents feminization of CySCs. Chinmo promotes expression of the canonical male sex determination factor DoublesexM (DsxM) within CySCs and their progeny, and ectopic expression of DsxM in the CySC lineage partially rescues the chinmo sex transformation phenotype, placing Chinmo upstream of DsxM. The Dsx homologue DMRT1 prevents the male-to female conversion of differentiated somatic cells in the adult mammalian testis, but its regulation is not well understood. Our work indicates that sex maintenance occurs in adult somatic stem cells, and that this highly conserved process is governed by effectors of niche signals.
Introduction
Male versus female fate is controlled by a variety of mechanisms across taxa (Kopp, 2012). In mammals, this choice was recently found to be labile even in adults; loss of sex-specific transcriptional regulators in the adult mouse gonad causes differentiated somatic cells to transdifferentiate into somatic cells of the opposite sex (Matson et al., 2011; Uhlenhaut et al., 2009). This indicates that sexual identity must continuously be maintained in specific differentiated cell types long after sex determination has occurred. Whether sexual identity is plastic in undifferentiated adult stem cells remains unknown. Since adult stem cells have the capacity to rebuild entire adult organ systems, altering a stem cell’s sexual identity could conceivably cause widespread changes to the tissue.
In Drosophila, a well-studied sex determination cascade culminates in the sex-specific splicing of mRNA encoding the conserved transcription factor Doublesex (Dsx); this binary choice cell-autonomously dictates somatic sexual identity (Whitworth et al., 2012). While upstream regulation of sex determination differs between flies and mammals, downstream control converges on Dsx/mab-3 related transcription factor (Dmrt) genes (Matson and Zarkower, 2012). The Janus kinase-Signal Transducer and Activator of Transcription (Jak-STAT) pathway is also required for the initial choice of somatic sexual identity in Drosophila embryos and promotes male germline sexual behavior in embryonic testes (Jinks et al., 2000; Wawersik et al., 2005). However, it is not known whether Jak-STAT signaling is required for sex maintenance in Drosophila, and the link between the Jak-STAT pathway and the canonical sex determination pathway is unknown.
The Drosophila ovary and testis provide excellent models for studying adult stem cell behavior in vivo (Fuller and Spradling, 2007; Matunis et al., 2012). In the testis, Jak-STAT signaling maintains two types of stem cells: sperm-producing germline stem cells (GSCs) and supporting somatic stem cells called cyst stem cells (CySCs). Both of these cell types attach to a single niche created by quiescent somatic hub cells at the testis apex and divide asymmetrically to produce differentiating progeny (spermatogonia and cyst cells, respectively) that are displaced from the niche (Matunis et al., 2012). Several factors, including the Jak-STAT targets Zinc-finger homeodomain-1 (Zfh-1) and Chinmo, are required for CySC self-renewal (Amoyel et al., 2013; Flaherty et al., 2010; Issigonis and Matunis, 2012; Leatherman and Dinardo, 2008; Michel et al., 2012). Here we reveal an unexpected function of Chinmo: it acts through the canonical sex determinant DsxM to maintain the male identity of adult CySCs.
Results
Reduction of Chinmo triggers the appearance of cells resembling ovarian follicle cells in the adult niche, then throughout the testis
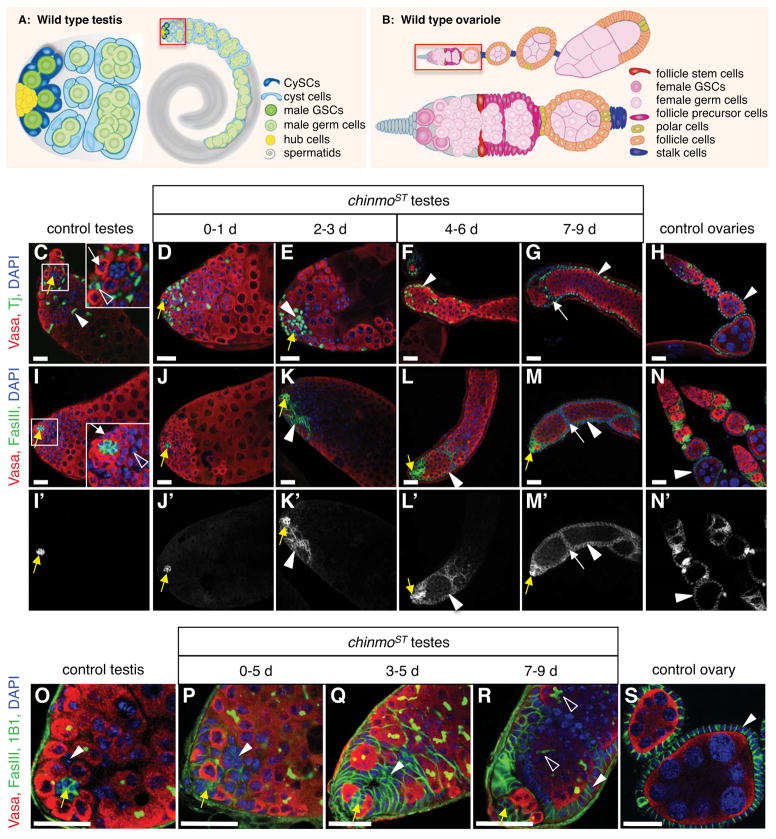
While screening for Drosophila testis phenotypes, we identified a spontaneous mutation causing a striking transformation of the adult testis. Adult mutant males are fertile, indicating testes develop normally. Consistent with this observation, testes from young males (0–1 day) are indistinguishable from wild type testes in overall morphology (Figures 1C–D, I–J). With age, however, a progressive change in the testis morphology occurs. Initially, subtle changes are detected at the testis apex, where aggregates of epithelial somatic cells (defined as 8 or more closely apposed cells expressing high levels of adhesion proteins) appear adjacent to the hub, while the remainder of the tissue is unaffected (Figures 1E, K, P–Q). With time, somatic cell aggregates acquire additional cells and extend away from the testis apex, while older differentiating germ cells and cyst cells are displaced toward the basal end of the testis (Figures 1F–G, L–M). In 7–9 day old males, an obvious transformation is apparent throughout the testis: somatic cell aggregates adjacent to the hub remain but now a monolayer of columnar epithelial cells lines the testis periphery, while germ cells are restricted to the lumen of the tissue (Figures 1G, M, R). The progression of this phenotype from the testis apex to the basal end suggests a stem cell origin. This testis phenotype had not been described before. However, the somatic cells bear a striking resemblance to the arrangement of somatic follicle cells within the Drosophila ovary, which form a columnar monolayer surrounding developing germ cells (Mahowald and Kambysellis, 1980) (Figures 1B, H, N, S). Therefore, we refer to these somatic cells in the mutant testes as “follicle-like cells”. We also find that germ cells in 7–9 day old mutant testes are arrested as early male germ cells (spermatogonia) based on their morphology, branching fusomes (de Cuevas et al., 1997; Hime et al., 1996; Lin and Spradling, 1994) (open arrowheads, Figure 1R), expression of the male-specific early germ cell marker M5-4 (Tran et al., 2000) (Figures S1A–C) and the undifferentiated germ cell marker Bam (data not shown) (McKearin and Spradling, 1990). By 2–3 weeks, degeneration of differentiation-arrested germ cells is observed (Figure S1D). Since germ cell arrest occurs when the germline and somatic sex are mismatched (Whitworth et al., 2012), this phenotype is consistent with a defect in somatic sexual identity maintenance in the adult testis.
Figure 1. Reduction of Chinmo causes somatic cells in adult testes to be gradually replaced by cells resembling ovarian follicle cells.
(A) Illustration of a wild-type Drosophila testis (right) with the apex magnified (left). Germline stem cells (GSCs, dark green) and somatic cyst stem cells (CySCs, dark blue) adhere to the hub (yellow). GSCs produce differentiating male germ cells (spermatogonia and spermatocytes, green) that are displaced from the hub and form elongated spermatids (grey) and mature sperm (not shown). Approximately two CySCs flank each GSC; CySCs produce squamous, quiescent cyst cells (light blue), which encase differentiating germ cells. (B) Illustration of a wild-type Drosophila ovariole (top) comprised of a germarium (magnified, bottom) followed by a series of developing egg chambers. In the germarium, anterior niche cells cap cells (grey) support GSCs (dark pink), which produce differentiating female germ cells (light pink). Two somatic follicle stem cells (red), located near the middle of the germarium, produce follicle precursor cells (magenta), which differentiate into follicle cells (orange), stalk cells (purple), and polar cells (yellow). Each egg chamber contains 16 germ cells surrounded by a monolayer of columnar epithelial follicle cells. Polar cells are located at each end; egg chambers are linked by chains of stalk cells. (C–S) Immunofluorescence detection in adult testes and ovaries of Tj (C–H, green) to visualize somatic cell nuclei, or FasIII (I–S, green) to highlight the hub in all testes (yellow arrows), and somatic cell membranes in ovaries and chinmo mutant testes (arrowheads). Panels I′-N′ show the FasIII signal alone. Vasa (red) marks germ cells and DAPI (blue) marks nuclei in all panels. In control testes (C, I, O), somatic CySC lineage cells (arrowheads) are squamous and interspersed among germ cells. Insets (C, I) show GSCs (white arrows) and CySCs (open arrowheads) surrounding the hub. In chinmoST testes (D–G, J–M, P–R), a distinct phenotype develops over time. Testes from young mutant males (D–E, J–K, P–Q) resemble those from controls except that most (~77%, n = 61) contain aggregates of 8 or more somatic cells (arrowheads); these always appear near the hub (yellow arrows). As flies age (F–G, L–M, R), aggregates expand beyond the testis apex and become columnar and peripheral (arrowheads) in 82% of testes (n = 545), forming FasIII-positive “follicle-like cells” that resemble somatic follicle cells (arrowheads) in control ovaries (H, N, S). Follicle-like cells occasionally invaginate (G, M, white arrows) to envelop groups of germ cells. 1B1 (O–R, green) marks fusomes; branching fusomes in older germ cells in chinmoST testes indicate spermatogonial arrest (R, open arrowheads). Scale bars = 20 μm. See also Figure S1.
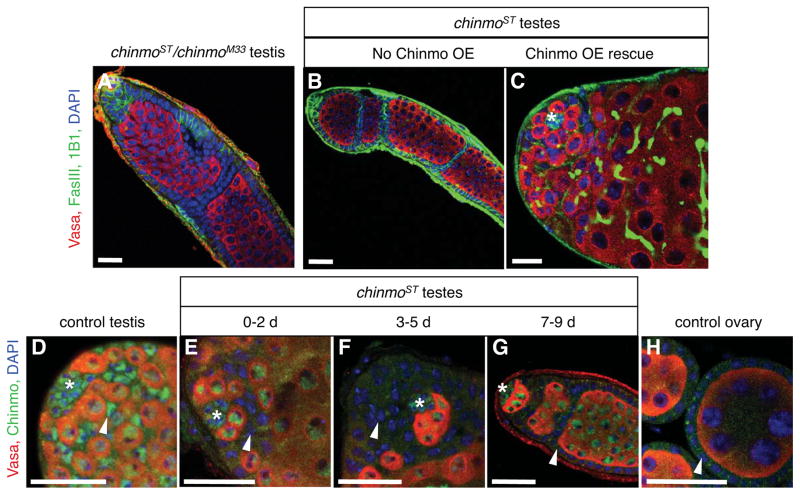
Sex maintenance has not been characterized previously in the adult Drosophila testis or ovary, but was recently found to occur in the adult mammalian testis and ovary (Matson et al., 2011; Uhlenhaut et al., 2009). The underlying mechanisms are of interest, since defects in this process may cause testicular cancer or disorders of sexual development (Hanson and Ambaye, 2011). Therefore, we sought to identify this spontaneous mutant. Our genetic complementation (Table S1, Figure 2A) and rescue experiments (Figures. 2B–C, Table S2) indicate that the mutation is a partial loss-of-function allele of the essential BTB-Zinc finger cell fate determinant chinmo (Flaherty et al., 2010; Zhu et al., 2006), which we denote chinmoSex Transformation, or chinmoST. Furthermore, Chinmo is specifically depleted in the CySC lineage in chinmoST mutant testes (Figures 2D–H, S1E–F). Together, these data support the hypothesis that Chinmo promotes male sexual identity in the adult testis soma.
Figure 2. Genetic complementation and rescue experiments indicate that chinmoST is a partial loss-of-function allele of chinmo.
(A) Immunofluorescence detection of FasIII (green at cell periphery) to visualize follicle-like cells in testes, and Vasa (red) to visualize germ cells. Testes from chinmoST chinmoM33 males resemble chinmoST homozygous testes (compare to Figure 1R). (B–C) The follicle-like cell phenotype in chinmoST testes (B) can be rescued by overexpressing chinmo in the CySC lineage (c587-Gal4; chinmoST; UAS-chinmo/tub-Gal80ts) (C). The position of the hub is marked with an asterisk. (D–H) Immunofluorescence detection of Chinmo (green) in testes and ovaries. In control testes (D), Chinmo is found in the nuclei of hub cells (asterisk), CySC lineage cells (arrowhead), and germ cells (Vasa, red) (Flaherty et al., 2010). In both young and old chinmoST testes (E–G), Chinmo is present in hub cells and germ cells but is absent from CySC lineage cells (arrowhead). Chinmo is absent from follicle cells (arrowhead) in control ovaries (H). DAPI marks nuclei (blue). Hubs marked by asterisk. Scale bars = 20 μm. See also Figure S1, Table S1, Table S2.
Reduction of Chinmo in adult testes causes somatic stem cells and their progeny to acquire female somatic identity
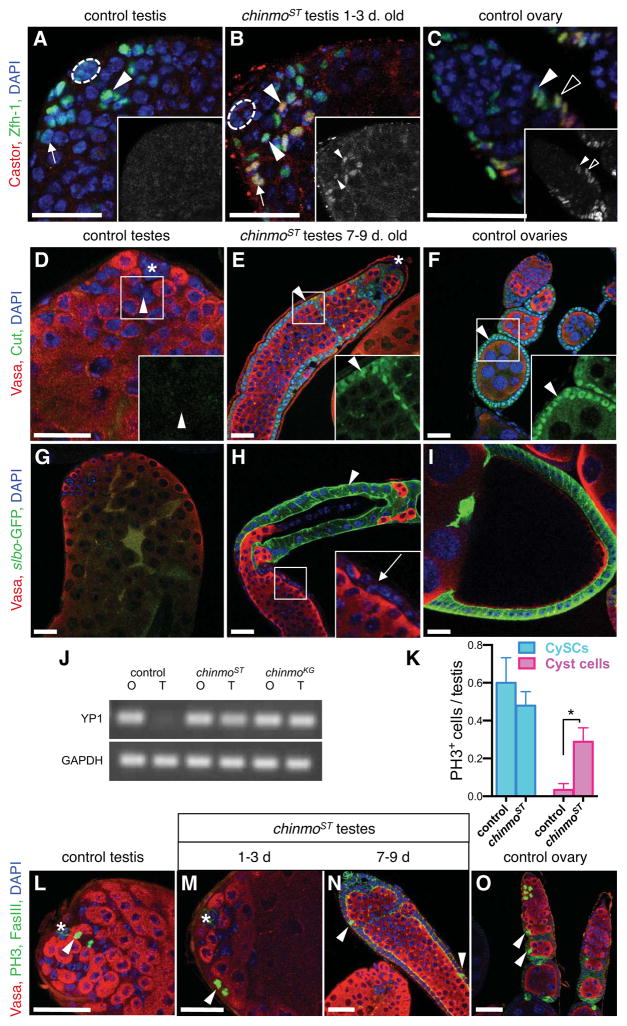
Since testes with reduced chinmo acquire somatic cells that morphologically resemble ovarian follicle cells, we directly tested whether these follicle-like cells underwent a male-to-female sexual transformation. Specifically, female-specific gene expression and cell behavior was examined in chinmoST testes. Castor, Cut, and a Slbo-GFP gene reporter are normally expressed in adult ovaries (Chang et al., 2013; Jackson and Blochlinger, 1997) (described below), but not testes (Figures 3A, D, G). Castor, the earliest marker in the ovarian follicle stem cell lineage, marks follicle stem cells and their earliest progeny (Figure 3C), while more differentiated follicle cells express high levels of Cut and then Slbo-GFP (Figures 3F, I). In chinmoST testes, these three markers are sequentially expressed as the phenotype progresses (Figures 3B, E, H). In testes from young (1–3 day) chinmoST males, follicle-like cells have not formed yet (described above). However, the earliest ovarian marker, Castor, is detected in most testes (61.7%, n=47). Importantly, Castor is always restricted to a subset of somatic cells within the testis at this time-point: CySCs and their earliest daughters (Figure 3B). To ask whether Castor expression originates in CySCs, their earliest daughters, or both, we next stained testes from even younger males (newly eclosed virgins). At this time-point, fewer testes contained Castor-positive cells (38.8% of testes, n=49), and the number of Castor-positive cells ranged from 3 to around 30 (data not shown). Castor was detected in at least one CySC in all testes where it was expressed, further supporting the hypothesis that CySCs, but not their differentiating progeny (cyst cells) which have exited the stem cell niche, are the cells within the testis that undergo sexual transformation. These data further suggest that follicle-like cells arise from sex transformed CySCs. Consistent with this hypothesis, the ovarian somatic cell marker Cut becomes strongly expressed in follicle-like cells in 7–9 day old chinmoST testes (Figure 3E). In contrast, the earlier marker, Castor, becomes depleted from follicle-like cells (described below, Figure 4), a pattern consistent with its absence in ovarian follicle cells (Chang et al., 2013). Finally, the Slbo-GFP reporter, which normally becomes highly expressed in follicle cells at stage 10 of oogenesis (Cai et al., 2014) (Figure 3I), is also expressed in a subset of follicle-like cells in older chinmoST testes (Figure 3H). Together these data suggest that follicle-like cells can progress through the normal follicle cell differentiation program to a surprising extent, in an otherwise male gonad. Consistent with this hypothesis, Yolk protein1 (Yp1) transcripts, which become highly expressed in follicle cells at stage 10 but are not transcribed in the testis (Brennan et al., 1982; Logan et al., 1989), are present in testes with reduced chinmo (Figure 3J).
Figure 3. Reduction of Chinmo in adult testes causes somatic stem cells and their progeny to acquire female somatic markers.
(A–C) Immunofluorescence detection of Zfh-1 (green) and Castor (red) in testes and ovaries. Insets, red channel only shown in grayscale. In control testes (A), Zfh-1 marks CySCs (arrowhead) and early cyst cells (arrow), but Castor is not detected. In testes from young (1–3 day) chinmoST males (B), some CySCs (arrowheads) and cyst cells (arrow) express both Zfh-1 and Castor, as do ovarian follicle stem cells (solid arrowhead) and their early progeny (follicle cell progenitors, open arrowhead) in control ovaries (C). (D–F) Immunofluorescence detection of Cut (green) and Vasa (red). Insets, green channel only, magnified. In control testes (D), Cut is absent from all cells including CySCs (arrowhead), but it is present in follicle-like cells in chinmoST testes (E, arrowhead) and follicle cells in control ovaries (F, arrowhead). (G–I) Immunofluorescence detection of slbo-GFP (green). slbo-GFP is not expressed in control testes (G) but marks later somatic follicle cells (around stage 10) in control ovaries (I). In chinmoST testes (H), slbo-GFP marks a subset of follicle-like cells (arrowhead); adjacent follicle-like cells (inset, arrow) are not marked. (J) Yolk protein 1 (YP1) expression is detected by RT-PCR in ovaries (O) and testes (T) from 7–9 day chinmoST and chinmoKG adults but not from control adults. GAPDH is used as a control. (K) Bar graph showing the number of CySCs and cyst cells containing the mitotic marker phospho-histone H3 (PH3) in testes from 1–3 day old control or chinmoST flies. Somatic cells within 2 cell diameters of the hub were scored as CySCs; those more than 2 cell diameters away were scored as cyst cells. Significantly more mitotic cyst cells are found in chinmoST testes than in control testes (*, p=0.0168). There is no significant difference in the number of mitotic CySCs between chinmoST and control testes. Data represented as mean ± standard error of the mean. (L–O) Immunofluorescence detection of PH3 (green in nuclei). FasIII (green) marks cell membranes in the hub (asterisk, L–M), follicle-like cells (N), and follicle cells (O). In control testes (L), PH3 is detected in CySCs (arrowhead); cyst cells away from the hub are non-mitotic and do not express PH3. Cyst cells in young chinmoST testes (M) and follicle-like cells in older chinmoST testes (N) express PH3 (arrowheads), as do follicle cells (arrowheads) in control ovaries (O). Hubs marked by dashed line (A–B) or asterisk (D–E, L–M). DAPI marks nuclei (blue, all panels). Scale bars = 20 μm.
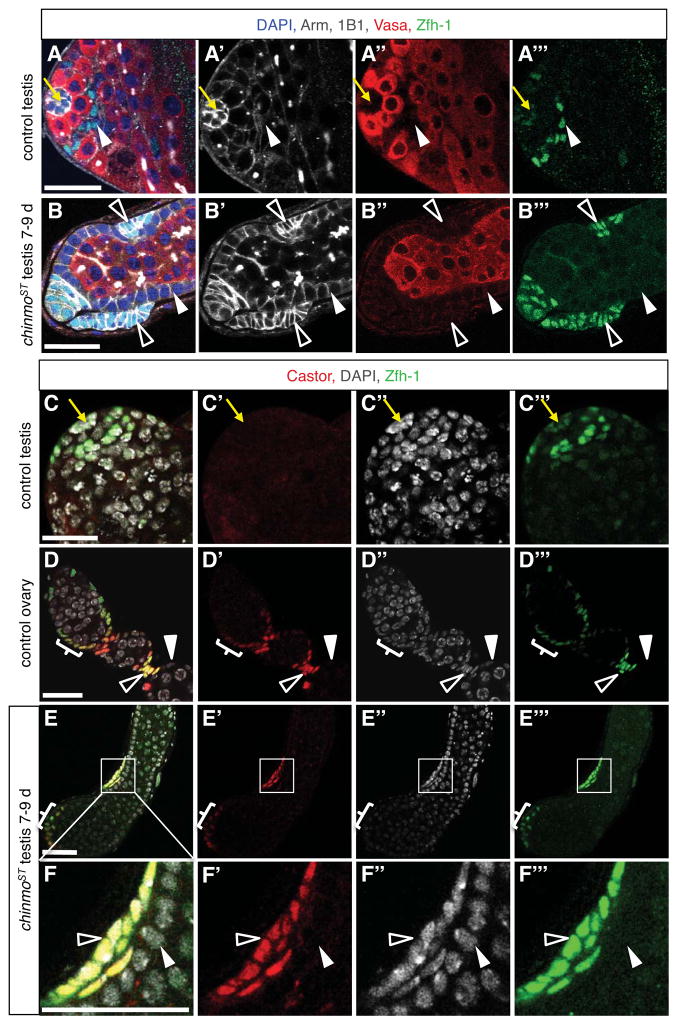
Figure 4. Ovarian stalk cell markers are expressed in a subset of somatic cells in chinmoST testes.
(A–B) Immunofluorescence detection of Arm (white) and Zfh-1 (green) reveals stalk-like cells in chinmoST testes. In control testes (A), hub cells (yellow arrow) express high levels of Arm and low levels of Zfh-1, and CySCs and their immediate daughters (arrowhead) express low levels of Arm and high levels of Zfh-1. In all other cyst cells, Arm is low and Zfh-1 is absent. In chinmoST testes (B), Arm is low and Zfh-1 is absent in follicle-like cells lining the periphery (solid arrowhead). Other somatic cells (open arrowheads) form aggregates that resemble ovarian stalk cells morphologically and express high levels of Arm and Zfh-1, which are characteristic of stalk cells (data not shown). These aggregates are typically located just beneath the testis sheath, sandwiched between follicle-like cells and the basement membrane. (C–F) Immunofluorescence detection of Castor (red) and Zfh-1 (green) reveals stalk-like cells in chinmoST testes. Castor is absent from control testes (C; also see Fig. 2A). Hubs marked by yellow arrow. In control ovaries (D), Castor is expressed in follicle stem cells and early follicle cell progenitors (bracket; also see Fig. 2C). After egg chamber formation, Castor is restricted to polar cells and Zfh-1+ stalk cells (open arrowhead) and is no longer expressed in main-body follicle cells (solid arrowhead). In chinmoST testes (E–F), Castor is expressed in Zfh-1+ cell aggregates at the testis apex that resemble follicle cell progenitors (bracket) and in stalk-like cells (open arrowheads) but not in Zfh-1− follicle-like cells (solid arrowhead). Panel F is an enlargement of the boxed area in panel E. Scale bars = 20 μm.
In addition to distinct morphological and molecular characteristics, somatic cells in the testis and ovary differ in potency and proliferation status. CySCs are unipotent, giving rise to cyst cells that exit the cell cycle (Gönczy and Dinardo, 1996). In contrast, follicle stem cells are multipotent, yielding both stalk cells and mitotically active follicle cells (Margolis and Spradling, 1995). By examining markers of cell proliferation in chinmoST testes, we find that follicle-like cells express mitotic markers (Figures 3K–O). Furthermore, somatic cells with the molecular and morphological characteristics of stalk cells become apparent in older chinmoST testes (Figure 4), suggesting that reduction of Chinmo causes CySCs to transdifferentiate into cells resembling multipotent female follicle stem cells. Together, these data support the hypothesis that chinmo actively maintains a male sexual identity in adult CySCs.
Chinmo autonomously maintains male sexual identity in adult somatic stem cells
The above data indicate that CySCs specifically require Chinmo for maintenance of male sexual identity. However, this requirement could be direct or indirect. To distinguish between these possibilities, we used cell-type specific RNA-interference (RNAi) knockdown of chinmo. Consistent with previous mosaic analysis (Flaherty et al., 2010), knockdown of chinmo in the germ line during development does not yield a testis phenotype (Figure 5F), ruling out a germline requirement for chinmo. We next allowed testes to develop normally, and then conditionally induced chinmo RNAi in subsets of adult somatic cells. Knockdown of chinmo in adult hub cells does not yield testes with the chinmoST phenotype (Figure 5G). In contrast, the chinmoST phenotype is fully recapitulated by knockdown of chinmo in all adult CySCs and early cyst cells (Figures 5A–E, S2A–D, Table S3a and b). To determine whether chinmo is required primarily in CySCs or in their cyst cell progeny, we compared the phenotypes that developed when various CySC lineage drivers were used to induce chinmo RNAi. Drivers expressed in CySCs and their immediate cyst cell daughters are sufficient to recapitulate the chinmoST phenotype, while drivers expressed solely in later cyst cells are not (Figures 5H, S2E–I, Table S3a). Together these data indicate that chinmo is required in CySCs, but not in their differentiated progeny, for the maintenance of adult somatic sexual identity.
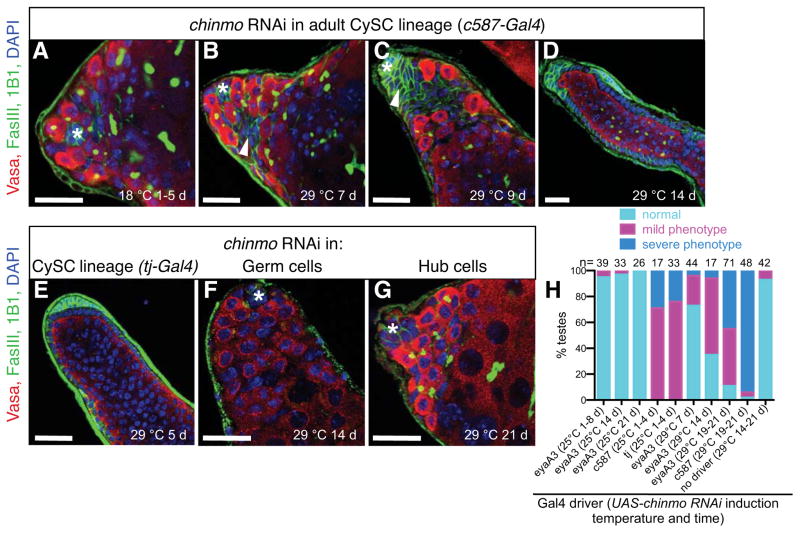
Figure 5. Chinmo is required autonomously in adult CySC lineage cells to prevent their transformation into female soma.
(A–G) Immunofluorescence detection of FasIII (green at cell periphery) and the germ cell marker Vasa (red) to visualize the morphology of adult testes after expression of chinmo-RNAi in different cell types. Before RNAi induction in the CySC lineage (A), testes appear normal. After RNAi induction in the CySC lineage, testes resemble chinmoST testes: somatic cells initially form aggregates (97% of testes, n = 36/37) (B–C, arrowheads) and then follicle-like cells (76% of testes, n = 68/90) (D). RNAi induction in the CySC lineage with a different Gal4 driver also phenocopies chinmoST testes (E). RNAi induction in germ cells (F) or in hub cells (G) does not phenocopy chinmoST. Hubs marked by asterisk. Scale bars = 20 μm. (H) Composite bar graph showing the percentage of testes with normal, mild, or severe phenotypes after expression of chinmo-RNAi with different somatic drivers. Testes remain morphologically wild type after chinmo-RNAi expression only in cyst cells (eyaA3-Gal4 at 25 °C), but after expression in both CySCs and cyst cells (eyaA3-Gal4 at 29 °C, c587-Gal4, or tj-Gal4), testes display a range of chinmoST phenotypes, as defined in Methods. See also Figure S2, Table S3.
Follicle-like cells arise from the adult somatic stem cell lineage, not hub cells
The fact that CySCs autonomously require chinmo for maintenance of adult sexual identity suggests that follicle-like cells arise directly from CySC lineage cells. However, it is also possible that chinmo is required in CySCs to prevent hub cells, the only other somatic cells in the testis niche, from giving rise to follicle-like cells. To distinguish between these possibilities, we used genetic lineage tracing to permanently mark hub cells or CySCs and early cyst cells in chinmoST males during development, and then assayed for the presence of permanently marked cells in adult testes. Permanently marked hub cells do not produce marked follicle-like cells in chinmoST testes (Figures 6A–B, Table S4), ruling out hub cells as a source of follicle-like cells. In contrast, testes with permanently marked CySCs and early cyst cells acquire marked follicle-like cells over time (Figures 6C–E). Since follicle-like cells do not express the CySC and early cyst cell driver used in this experiment (Figures S2J–L), the marked follicle-like cells must be descendants of CySCs and early cyst cells. We conclude that CySCs and early cyst cells, but not hub cells, give rise to follicle-like cells when chinmo is reduced. Consistent with this finding, hub cells within chinmoST testes remained quiescent when assayed for proliferation using extended in-vivo BrdU labeling (Figures 6F–H). Since all CySC drivers are also expressed in early cyst cells, we cannot exclusively mark CySCs via lineage tracing. However, since CySCs but not cyst cells require chinmo, and CySCs are the earliest cells to express ovarian somatic markers, our data strongly support the hypothesis that somatic stem cells, rather than differentiated cells, undergo sex transformation when levels of Chinmo are reduced.
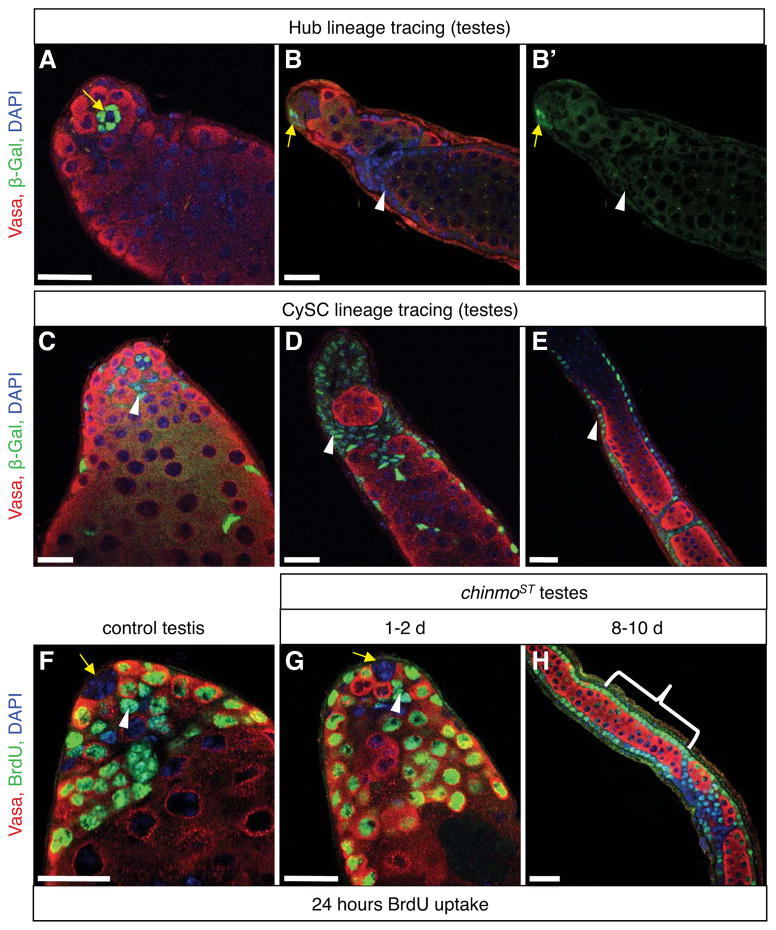
Figure 6. Follicle-like cells come from the cyst stem cell lineage but not from hub cells.
(A–E) Immunofluorescence detection of β-gal (green), which permanently marks either hub cells alone (A–B) or CySC lineage and hub cells (C–E) in chinmoST testes. Somatic cell aggregates and follicle-like cells (B, D–E, arrowheads) are derived from CySC lineage cells (C, arrowhead) but not from hub cells (A–B, arrows) because they express β-gal in testes with marked CySC lineage cells but not in testes with only marked hubs. (F–H) Immunofluorescence detection of the thymidine analog bromodeoxyuridine (BrdU, green). Adult males were fed BrdU for 24 hr prior to dissection to label all cells that traversed S-phase during this time. In control (F) and young chinmoST (G) testes, BrdU is not found in any hub cells (arrows), but many germ cells (red) and CySCs (arrowheads) are BrdU+. BrdU is also found in most follicle-like cells in older chinmoST testes (H, bracket). In all panels, DAPI marks nuclei (blue) and Vasa marks germ cells (red). Scale bars = 20 μm. See also Table S4.
Chinmo maintains male sexual identity through the canonical sex determination pathway
Sex determination in both mammals and Drosophila converges on the transcription factor Dsx/Dmrt1. Our finding that chinmo promotes adult somatic sex maintenance prompted us to ask whether Chinmo works in concert with the canonical sex determination pathway, in which a splicing cascade mediated by Sex-lethal (Sxl) and Transformer (Tra) promotes the formation of either a female or male isoform of Dsx (DsxF or DsxM, respectively) (Whitworth et al., 2012). Although female forms of Sxl, tra and dsx mRNA were not detected in chinmoST testes (Figure S3A), changes in DsxM protein expression were observed. DsxM is absent from the ovary (Figure S3B) but present in the somatic cells of the testis including hub cells, CySCs and cyst cells (Hempel and Oliver, 2007) (Figure 7A). Testes from young chinmoST males closely resemble wild type testes, but a few CySCs and their immediate progeny lack DsxM, while older cyst cells retain this male determinant. As somatic cell aggregates and follicle-like cells arise, they all lack DsxM, and the remaining older DsxM-positive somatic cells become displaced from the testis apex (Figures 7B–D). Similar results were obtained in testes with chinmo RNAi knockdown in the CySC lineage (data not shown). Although DsxM protein is decreased in the CySC lineage in chinmoST testes, a decrease in dsxM mRNA is not detectable at the whole testis level (Figure S3A), suggesting that a decrease in dsxM mRNA in CySCs and cyst cells might be masked by the presence of dsxM mRNA from other cells in the testis, such as sheath, basal epithelial, and seminal vesicle cells. Therefore, we used dsx-Gal4 to detect changes in dsx transcription levels specifically in CySC lineage cells (Robinett et al., 2010). Two independent dsx-Gal4 lines are active in CySC lineage cells in control testes (Figure 7E, G) but not in follicle cells in normal ovaries (Figure S3C–D). In chinmoST testes, however, somatic aggregates and follicle-like cells no longer express these transgenic reporters (Figure 7F, H). These results suggest that Chinmo either directly or indirectly regulates dsxM transcription levels. Since DsxM directly represses Yp1 expression (Burtis et al., 1991; Coschigano and Wensink, 1993), the appearance of ectopic Yp1 transcripts in chinmoST testes likely reflects loss of DsxM (Figure 3J). Taken together, these results suggest that Chinmo is required for maintenance of dsxM expression in the CySC lineage, and that loss of DsxM contributes to the male-to-female somatic sex transformation in chinmoST testes. In support of this hypothesis, expression of DsxM in the CySC lineage in chinmo-RNAi testes partially rescues the phenotype (Table 1). We have also found, using two independent dsx-RNAi lines, that knockdown of dsx in the CySC lineage partially phenocopies the chinmo mutant phenotype. In most dsx-RNAi testes, germ cells overproliferate and arrest at early spermatogonial stages (Figure 7J, Figures S3I–J) as do germ cells in chinmoST testes (Figure 1R), consistent with a mismatch of germline and somatic sex. Some testes also contain small aggregates of somatic cells (Figure S3J, arrows) that resemble the follicle-like cells in chinmo mutant testes, but they never develop a full layer of follicle-like cells. Together, these results suggest that Chinmo has targets in addition to DsxM that maintain other aspects of CySC fate and prevent them from transforming into follicle stem cell-like cells. We conclude that male sexual identity is actively maintained in the CySC lineage of the adult testis, and that this requires the concerted action of Chinmo and DsxM.
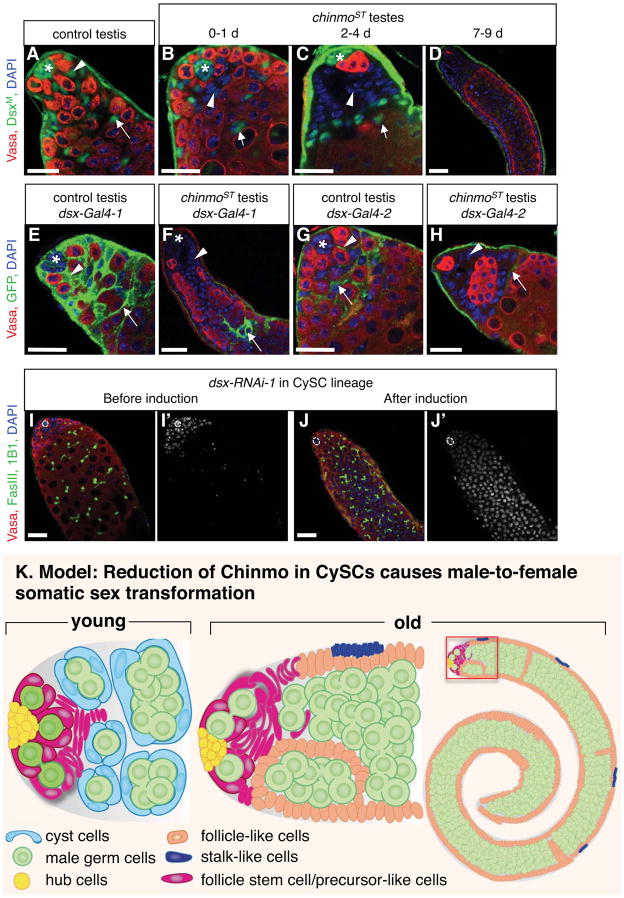
Figure 7. Chinmo maintains the male identity of adult somatic stem cells through the canonical sex determination pathway.
(A–D) Immunofluorescence detection of DsxM (green) in adult testes. In control testes (A), DsxM is expressed in the nuclei of hub cells (asterisk), CySCs (arrowhead), and cyst cells (arrow) (n=59 testes). In young chinmoST testes that are still morphologically normal (B), DsxM levels are low in some CySCs and early cyst cells (arrowhead) but remain high in differentiated cyst cells away from the apex (arrow) (n=35 testes). In chinmoST testes containing somatic cell aggregates (C), DsxM levels are low in aggregates (arrowhead) but remain high in differentiated cyst cells (arrow) (n=37 testes). In chinmoST testes with follicle-like cells (D), DsxM is absent from all follicle-like cells (n=42 testes). (E–H) Immunofluorescence detection of cytoplasmic GFP (green) in adult testes to reflect the transcription of dsx-Gal4 (two different lines). dsx-Gal4-1 is expressed in control testes (E) at high levels in CySCs (arrowhead) and cyst cells (arrow), and at lower levels in some hub cells (asterisk) (n=15 testes). In chinmoST testes (F), dsx-Gal4-1 expression is absent from somatic aggregates and follicle-like cells (arrowhead) but remains high in differentiated cyst cells away from the apex (arrow) (n=19 testes). dsx-Gal4-2 is expressed in control testes (G) in early cyst cells (arrow) and in some CySCs (arrowhead) but is not detectable in hub cells (asterisk) (n=21 testes). In chinmoST testes (H), dsx-Gal4-2 expression is absent from somatic aggregates (arrowhead) but present in some differentiated cyst cells away from the apex (arrow) (n=28 testes). (I–J) Immunofluorescence detection of FasIII and 1B1 (green) and DAPI (blue) in adult testes before and after induction of dsx-RNAi in the CySC lineage. Panels I’ and J’ show the blue channel (DAPI) only in grayscale. Before RNAi induction (I), testes look normal, and cells that stain brightly with DAPI (early germ cells and somatic cells) are restricted to the apex of the testis (n = 32 testes). After RNAi induction (29 °C for 10 days) (J), testes fill with germ cells that arrest as early spermatogonia, based on the expansion of the bright DAPI zone and presence of branching fusomes (94% of testes, n = 51). In all panels, nuclei are marked with DAPI (blue); germ cells are marked with Vasa (red); Hubs marked by asterisk (A–C, E–G) or dashed line (I–J). scale bars = 20 μm. (K) Schematic drawings of a young (left) and older (center) chinmo mutant testis apex, and an older chinmo mutant testis (right) with the apex indicated (red box). In young chinmo mutant testes, CySCs and their early progeny lose male fate and adopt a follicle stem cell/progenitor-like cell identity (magenta). As flies age, follicle stem cell/progenitor-like cells produce follicle-like cells (orange) and stalk-like cells (purple), which gradually displace normal cyst cells from the niche. The germ cells, which become restricted to the lumen of the tissue, maintain characteristics of their male identity, but over-proliferate and arrest as spermatogonia since the germline and somatic sex are mismatched. See also Figure S3.
Table 1.
Overexpressing DsxM in the CySC lineage rescues Chinmo knock down phenotype
| Use c587-Gal4 to express: [1] | % testes with FasIII+ somatic aggregates [2] | |||||
|---|---|---|---|---|---|---|
| 0 d | 4 d | 7 d | 9 d | 11 d | 14d | |
| UAS-chinmoRNAi-1 | 0 (n=31) | 6.3 (n=64) | 73.8 (n=103) | 80.5 (n=133) | 97.1 (n=35) | 100 (n=59) |
| UAS-chinmoRNAi-1, UAS-DsxM | 0 (n=25) | 5.3 (n=76) | 36.6 (n=153) | 55.9 (n=145) | 39.4 (n=33) | 86.3 (n=124) |
| UAS-DsxM | 0 (n=18) | N/A | 0 (n=24) | 5.3 (n=19) | 0 (n=21) | N/A |
UAS-chinmoRNAi-1 = chinmoHM04048
All flies were raised at 18 °C, which suppresses RNAi induction. After eclosion, adult flies were shifted to 29 °C for the indicated amount of time to induce RNAi
For DsxM rescue, p<0.0001 at 7, 9, 11days, P<0.01 at 14 days
Discussion
The male-to-female sex transformation phenotype that we observe in Drosophila testes with reduced Chinmo demonstrates that adult somatic stem cells actively maintain their sexual identity in a cell autonomous manner (Figure 7K). In vertebrates, sexual phenotypes were thought to be determined exclusively by systemic hormones, but recent studies have found exceptions to this rule. In birds, for example, somatic sexual identity may be largely cell autonomous, as illustrated by lateral gynandromorph chickens; these male:female chimeras have one side that appears male and the other female, even though both sides are exposed to the same blood system and hormone environment (Zhao et al., 2010). Another example is the adult mouse gonad, where loss of sex-specific transcriptional regulators can cause differentiated somatic cells to transdifferentiate into cells of the opposite sex (Matson et al., 2011; Uhlenhaut et al., 2009). Other recent work has found that adult stem cells can respond differently to the different levels of hormones found in male and female mice: hematopoietic stem cells, which were thought to function similarly in both sexes, respond to the higher levels of estrogen in females, resulting in differences in self-renewal ability (Nakada et al., 2014). We now show, using the Drosophila testis as a model, that adult somatic stem cells can also autonomously maintain their sexual identity. Chinmo regulates sexual identity by promoting expression of the male sex determination factor DoublesexM (DsxM) in CySCs and their progeny. Since dsx-family members play central roles in the maintenance of somatic sexual identity in diverse organisms including mice, fish and amphibians (Masuyama and Matsuda, 2012; Matson et al., 2011; Shibata et al., 2002), but their upstream regulators are not well understood, it will be interesting to determine whether Chinmo is required for sex maintenance at other times in development and in other tissues in Drosophila, and whether Chinmo homologues function more broadly in sex maintenance in other organisms.
Our data suggest that Chinmo is the central regulator of CySC sex maintenance and implicate DsxM is an important target of Chinmo in this process. However, since expression of DsxM only partially rescues the sex maintenance phenotype in chinmo mutant testes, and dsx knockdown only partially phenocopies the chinmo mutant phenotype, DsxM is unlikely to be the only target of Chinmo. Together, our data support a model in which Chinmo promotes expression of DsxM, which in turn regulates its own set of target genes that are critical for male sex identity. In addition to promoting DsxM expression, Chinmo likely affects the expression of Dsx-independent target genes that are important for additional aspects of CySC identity. In this case, resupplying DsxM does not fully rescue the chinmo-RNAi phenotype because these testes still lack Chinmo targets that are independent of Dsx but required for CySC self-renewal. Similarly, CySCs lacking Dsx may only partially recapitulate the Chinmo loss-of-function phenotype because they lack expression of male determinants downstream of Dsx. However, expression of Dsx-independent Chinmo targets may permit maintenance of CySCs lacking Dsx, and may also prevent the complete conversion of these mutant CySCs into Follicle Stem or progenitor-like cells. It is also possible that additional sex determination factors, such as the female determinant DsxF, will be required for a full conversion of CySCs lacking DsxM into follicle stem or progenitor-like cells. Assessing the role of this and other sex determination pathway members in sex maintenance will be of interest. Furthermore, since our data suggest that Chinmo regulates DsxM at the level of transcription, comparison of targets of Chinmo and DsxM in the CySC lineage should be informative.
It is intriguing that removing chinmo clonally in a few CySCs leads to rapid loss of the mutant CySCs (Flaherty et al., 2010), while depleting chinmo from all CySCs (via RNAi or the chinmoST mutation) allows them to remain but become feminized. We hypothesize that CySC clones lacking chinmo (either weak or strong alleles) are out-competed by wild type CySCs, and that the full sex transformation phenotype only develops when the possibility of competition is removed. (i.e., when most or all CySCs lack chinmo). Individual CySCs lacking Chinmo may also undergo sex transdifferentiation, but we have not been able to determine whether or not this is the case, since such CySCs are lost quite rapidly. Comparing testes that contain either a few or many CySCs that lack chinmo could be informative, as it should reveal the number of stem cells that must be mutant in order to allow the sex transformation of the testis soma to occur. The ability of stem cells to compete for niche access is a poorly understood yet important aspect of adult stem cell biology in general (Stine and Matunis, 2013), and this work provides an avenue for studying the underlying mechanisms.
Our finding that stem cells undergo sexual transformation may provide unique insight into how transdifferentiation is regulated at a cellular and molecular level more generally. Sex transformation of stem cells in adult Drosophila testes provides a highly tractable genetic system to study cellular mechanisms by which highly conserved dsx-related transcription factors and their target genes actively maintain somatic sexual identity. This may provide insight into human testicular cancers, such as granulosa cell tumors, that may be linked to altered somatic sexual identity (Hanson and Ambaye, 2011). Analysis of Chinmo and Dsx/Dmrt1-mediated sex maintenance pathways in somatic stem cells may also yield insight into the maintenance of sexual dimorphism in other organs, such as the mammalian liver, and provides a unique model to study adult stem cell transdifferentiation in vivo.
Experimental procedures
Fly stocks and cultures
Fly stocks were raised at 25 °C on standard molasses/yeast medium unless otherwise indicated. The following fly stocks were used: UAS-FL-chinmo and chinmo1 (Zhu et al., 2006), eyaA3-Gal4 (Leatherman and DiNardo, 2008), M5-4 (Gönczy and DiNardo, 1996), c587-Gal4 (Kai and Spradling, 2003), nanos-Gal4-VP16 (Van Doren et al., 1998), tj-Gal4 (Drosophila Genetic Resource Center), UAS-dsxM (Lee et al., 2002), and UAS-lifactin-GFP and slbo-lifactin-GFP (from X. Wang and D. Montell), UAS-dsx RNAi-1 (P[KK111266]VIE-260B, From Vienna Drosophila RNAi Center (VDRC)), UAS-dsx RNAi-2 (from T. Shirangi and M. Mckeown), dsx-Gal4-1 (w1118; P[GMR40A05-Gal4]attP2, From Bloomington Drosophila Stock Center (BDSC)), dsx-Gal4-2 (w−; dsx-Gal4/TM3, Sb, Ser, twist-gal4, UAS-2xEGFP, from B. Baker). y w and chinmoST/CyO flies were used as control flies. Other fly stocks were from the VDRC or BDSC including the SOD[x39]/TM3 line from which chinmoST was isolated.
Immunostaining
Testes and ovaries were dissected, fixed, and stained as described previously (Matunis et al., 1997). Tyramide signal amplification (Invitrogen) was used to increase sensitivity of rat anti-DsxM (from B. Oliver, 1:500 dilution). Antibodies used are described in Supplemental Experimental Procedures.
Lineage analysis
For lineage analysis, chinmoST; actin>stop>lacZ or chinmoKG05386; actin>stop>lacZ males were mated to E132-Gal4; chinmoST; UAS-FLP or c587-Gal4; chinmoST; UAS-FLP females to permanently mark hub cells or the CySC lineage, respectively. Newly eclosed flies were then aged prior to testis dissection and immunostaining. To determine whether the c587-Gal4 and E132-Gal4 drivers become re-expressed in follicle-like cells in aged chinmoST testes, chinmoST; UAS-lifactin-GFP males were crossed to E132-Gal4; chinmoST; tub-Gal80[ts] or c587-Gal4; chinmoST; tub-Gal80[ts] females at 18 °C. Newly eclosed progeny were aged at 18 °C for 1 week to allow development of the follicle-like cell phenotype, and then switched to 29 °C to assess Gal4 activity in chinmoST testes.
mRNA extraction and PCR
mRNA extraction and reverse transcription-PCR were performed as previously described (Issigonis and Matunis, 2012). Primer sequences are in Supplemental Experimental Procedures.
In vivo BrdU incorporation
Groups of about 20 age-matched adult males were incubated at 25 °C in empty vials for 4 hours, and then transferred to vials of fly food overlaid with disks of filter paper soaked with 120 μl of 2.5 mM 5-bromo-2′-deoxyuridine (BrdU) (Life Techniologies) and 5 μl of green food coloring (McCormick). After 24 hours, flies that had not eaten the BrdU (and therefore did not have green guts) were discarded, and BrdU was detected in testes from the remaining flies as described (Brawley and Matunis, 2004).
Quantification of cell division and severity of the chinmoST phenotype
CySC mitoses were quantified by counting the number of PH3+ Vasa− cells within two cell diameters of the hub. Cyst cell mitoses were quantified by counting the number of PH3+ Vasa− cells more than two cell diameters from the hub. To categorize the severity of the chinmoST phenotype, we used the following criteria. Morphologically wild-type testes were scored as normal. Testes with epithelial aggregates and/or follicle-like cells at the apex near the hub, but not away from the apex, were scored as having a mild phenotype; these testes often contained overproliferating germ cells. Testes with epithelial aggregates and/or follicle-like cells throughout the entire testis were scored as having a severe phenotype; germ cells in these testes were often arresting at early spermatogonial stage or degenerating. The severity of the chinmoST and chinmo RNAi phenotypes both progress with age. Therefore, the presence of a mild vs. severe phenotype was used to estimate testis age when not clearly indicated.
Microscopy and Image Analysis
Fixed testes were mounted in Vectashield (Vector Labs), imaged with a Zeiss LSM 5 Pascal or LSM 510 Meta, and analyzed using the Zeiss LSM Image Browser software; panels are single confocal sections unless stated otherwise. Statistical analysis of pH3 positive cell number was performed with GraphPad Prism 5 software and averages were compared using a two-tailed Student’s t test assuming unequal variances. Percentages were analyzed for statistical significance using a chi-squared test.
Supplementary Material
Figure S1. (Related to Figure 1 and 2) Reduction of Chinmo causes somatic cells in adult testes to be gradually replaced by cells resembling ovarian follicle cells.
(A–C) Immunofluorescence detection of β-galactosidase (green) to visualize M5-4, a male-specific escargot enhancer trap, and Vasa (red) to visualize germ cells. In control testes (A), M5-4 is expressed in hub cells (yellow arrow), GSCs and early differentiated germ cells (yellow arrowheads), but not in CySC lineage cells (white arrowhead) or later differentiated germ cells (white arrow). After RNAi-mediated depletion of chinmo from the CySC lineage (c587-Gal4; UAS-chinmo-RNAi) (B), hub cells and germ cells continue to express M5-4, suggesting that they maintain characteristics of their male identity. Germ cells away from the testis apex fail to downregulate M5-4 (yellow arrowhead), indicating that they do not differentiate properly. M5-4 is absent from ovaries (C). (D) Immunofluorescence detection of FasIII (green at cell periphery) to visualize follicle-like cells in testes, and Vasa (red) to visualize germ cells. At 2–3 weeks of age, germ cells in chinmoST testes degenerate; only somatic cells remain at the testis apex. (E–F) Immunofluorescence detection of Chinmo (red) in adult testes containing chinmo1 or control clones verifies the specificity of anti-Chinmo antisera in adult testes. Hubs marked by dashed line. Chinmo levels are decreased in a chinmo1 germ cell clone (white arrowhead) and somatic clones (white arrows), identified by the absence of GFP (green), compared to neighboring GFP-positive heterozygous germ cells (one indicated, open arrowhead) and somatic cells (yellow arrow) (E). Wild-type control somatic clones and neighboring cells (white and yellow arrows, respectively) (F) have similar levels of Chinmo. DAPI marks nuclei (blue). Scale bars = 20 μm.
Figure S2. (Related to Figure 5) Chinmo is required autonomously in adult CySC lineage cells to prevent their transformation into female soma.
(A–B) Immunofluorescence detection of nuclear-localized UAS-GFP (green) in adult testes reveals that c587-Gal4 (A) and tj-Gal4 (B) are expressed in early CySC lineage cells but not in hub cells (asterisk) or germ cells (red). (C–D) Immunofluorescence detection of Chinmo (green) in adult testes verifies the efficacy of chinmo RNAi in the CySC lineage (genotype: c587-Gal4; UAS-chinmo RNAi/tub-Gal80ts). Before expression of chinmo RNAi (C), Chinmo is detected in hub cells (asterisk), CySC lineage cells (arrowheads), and germ cells (red). After 6 days of chinmo-RNAi expression in CySC lineage cells (D), Chinmo is no longer detected in CySC lineage cells (arrowhead). (E–F) Immunofluorescence detection of GFP (green), revealing the eyaA3-Gal4 expression pattern. At 25 °C (E), eyaA3-Gal4 drives expression of UAS-GFP at high levels in cyst cells (arrow) but not in CySCs (arrowheads). At 29 °C (F), eyaA3-Gal4 drives expression in both CySCs and cyst cells. (G–I) Immunofluorescence detection of Chinmo (green) and Tj (red) in adult testes. Hubs marked by dashed line. In control testes (G), Chinmo is detected in CySCs and their immediate daughters (arrows), which are identified as Tj+ nuclei near the hub, as well as in cyst cells (arrowheads). After chinmo-RNAi is expressed only in cyst cells (eyaA3-Gal4 at 25 °C) (H), Chinmo remains detectable in CySCs and their immediate daughters (arrows) but not in older cyst cells (arrowheads). After chinmo-RNAi is expressed in both CySCs and cyst cells (eyaA3-Gal4 at 29 °C) (I), Chinmo is no longer detected in CySCs (arrows) or cyst cells (arrowheads). (J–L) Immunofluorescence detection of cytoplasmic GFP (green), revealing the c587-Gal4 expression pattern. c587-Gal4 drives expression of UAS-GFP in the cytoplasm of early CySC lineage cells in control testes (J) and in somatic cells in the germarium in control ovaries (L). Older follicle cells in control ovaries (L) and follicle-like cells in chinmoST testes (K) lack c587-Gal4 activity. DAPI marks nuclei (blue in A–E, J–L and white in G–I). Scale bars = 20 μm.
Figure S3. (Related to Figure 7) chinmoST regulates dsx transcription rather than female-specific splicing of mRNAs in the canonical sex determination pathway, and dsx-RNAi partially phenocopies the chinmo mutant phenotype.
(A) RT-PCR detection of male and female spliced forms of sxl, tra or dsx mRNA shows that the female spliced forms are not ectopically expressed in control, chinmoST or chinmoKG testes. Actin-5C is used as a control. (B) Immunofluorescence detection of DsxM (green) in control ovaries. DsxM is not detectable in follicle cells (arrowhead) or any other cells in ovarioles. The green staining outside the ovarioles reflects non-specific staining of the ovarian sheath. (C–D) Immunofluorescence detection of GFP(green) in control ovaries to reflect the transcription of dsx-Gal4 (two different lines). dsx-Gal4-1 is expressed in escort cells (arrow) but not in follicle cells (arrowhead). dsx-Gal4-2 is absent from control ovaries, including follicle cells (arrowhead). (E–F) Immunofluorescence detection of DsxM (green) in adult testes before and after induction of dsx-RNAi (line 1) in the CySC lineage. Before RNAi induction (E), DsxM is expressed in the nuclei of hub cells (asterisk) and CySCs lineage cells (arrow) (n=18 testes). After RNAi induction (F), DsxM levels are low or absent in most CySCs and cyst cells (arrow) but remain high in hub cells (n=25 testes). (G) Composite bar graph showing the percentage of testes with normal, mild, or severe phenotypes after expression of dsx-RNAi (line 1) in the CySC lineage in adult testes for the number of days indicated (genotype: c587-Gal4; UAS-dsx-RNAi-1; tub-Gal80ts). In this experiment, morphologically wild type testes were scored as normal; testes with somatic cell aggregates and/or over-proliferating early germ cells at the apex, but not away from the apex, were scored as having a mild phenotype; and testes with over-proliferating early germ cells throughout the entire testis were scored as having a severe phenotype (see Fig. 6J). Before RNAi induction (0 d at 29 °C), all testes look normal. After RNAi induction in CySC lineage cells, testes display a range of mild to severe phenotypes. (H–J) Immunofluorescence detection of FasIII and 1B1 (green) and DAPI (blue) in adult testes before and after induction of dsx-RNAi (line 2) in the CySC lineage (genotype: UAS-dsx-RNAi-2; eyaA3-Gal4; tub-Gal80ts). Before RNAi induction (H), testes look normal (100% of testes, n =35). After RNAi induction (29 °C for 2 weeks) (I, J), germ cells overproliferate and arrest at early spermatogonial stages (72% of testes, n = 39). In addition, these testes contain some aggregates of FasIII+ cells at the periphery, which resemble the follicle-like cells in chinmo-RNAi testes (J, arrows). Vasa marks germ cells (red) and DAPI marks nuclei (blue). Scale bars = 20 μm.
Table S1 (Related to Figure 2): Phenotype characterization for combinations of chinmo mutant alleles
Table S2 (Related to Figure 2): The chinmoST phenotype can be rescued by Chinmo overexpression in the CySC lineage
Table S3a (Related to Figure 5): Chinmo knockdown in CySCs and cyst cells (CC), but not in late cyst cells alone, induces the follicle cell-like phenotype[1]
Table S3b (Related to Figure 5): Chinmo knock down in CySCs and cyst cells induces the follicle cell-like phenotype with time
Table S4 (Related to Figure 6): Follicle-like cells arise from the CySC lineage
Acknowledgments
We thank Helen Salz and Amir Oryan for helpful discussions; Margaret de Cuevas, Leah Greenspan, David Zarkower and Geraldine Seydoux for comments on the manuscript; Yijie Li for help with dissections; Nick Sokol, Brian Oliver, Gyunghee Lee, Denise Montell and Xiaobo Wang for flies or antisera. This work was funded by the NIH (HD040307, HD052937, EM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- Amoyel M, Sanny J, Burel M, Bach EA. Hedgehog is required for CySC self-renewal but does not contribute to the GSC niche in the Drosophila testis. Development. 2013;140:56–65. doi: 10.1242/dev.086413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304:1331–1334. doi: 10.1126/science.1097676. [DOI] [PubMed] [Google Scholar]
- Brennan MD, Werner AJ, Goralski TJ, Mahowald AP. The follicle cells are a major site of vitellogenin synthesis in Drosophila melanogaster. Dev Biol. 1982;89:225–236. doi: 10.1016/0012-1606(82)90309-8. [DOI] [PubMed] [Google Scholar]
- Burtis KC, Coschigano KT, Baker BS, Wensink PC. The doublesex proteins of Drosophila melanogaster bind directly to a sex-specific yolk protein gene enhancer. EMBO. 1991;10:2577–2582. doi: 10.1002/j.1460-2075.1991.tb07798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Chen SC, Prasad M, He L, Wang X, Choesmel-Cadamuro V, Sawyer JK, Danuser G, Montell DJ. Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell. 2014;157:1146–1159. doi: 10.1016/j.cell.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Jang AC, Lin CH, Montell DJ. Castor is required for Hedgehog-dependent cell-fate specification and follicle stem cell maintenance in Drosophila oogenesis. Proc Natl Acad Sci U S A. 2013;110:E1734–1742. doi: 10.1073/pnas.1300725110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coschigano KT, Wensink PC. Sex-specific transcriptional regulation by the male and female doublesex proteins of Drosophila. Genes & Development. 1993;7:42–54. doi: 10.1101/gad.7.1.42. [DOI] [PubMed] [Google Scholar]
- de Cuevas M, Lilly M, Spradling AC. Germline cyst formation in Drosophila. Annu Rev Genet. 1997 doi: 10.1146/annurev.genet.31.1.405. [DOI] [PubMed] [Google Scholar]
- Flaherty MS, Salis P, Evans CJ, Ekas LA, Marouf A, Zavadil J, Banerjee U, Bach EA. chinmo is a functional effector of the JAK/STAT pathway that regulates eye development, tumor formation, and stem cell self-renewal in Drosophila. Dev Cell. 2010;18:556–568. doi: 10.1016/j.devcel.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller M, Spradling A. Male and Female Drosophila Germline Stem Cells: Two Versions of Immortality. Science. 2007:316. doi: 10.1126/science.1140861. [DOI] [PubMed] [Google Scholar]
- Gönczy P, Dinardo S. The germ line regulates somatic cyst cell proliferation and fate during Drosophila spermatogenesis. Development. 1996;122:2437–2447. doi: 10.1242/dev.122.8.2437. [DOI] [PubMed] [Google Scholar]
- Hanson JA, Ambaye AB. Adult Testicular Granulosa Cell Tumor- A Review of the Literature for Clinicopathologic Predictors of Malignancy. Arch Pathol Lab Med. 2011:135. doi: 10.5858/2009-0512-RSR.1. [DOI] [PubMed] [Google Scholar]
- Hempel LU, Oliver B. Sex-specific DoublesexM expression in subsets of Drosophila somatic gonad cells. BMC Dev Biol. 2007;7:113. doi: 10.1186/1471-213X-7-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hime GR, Brill J, Fuller M. Assembly of ring canals in the male germ line from structural components of the contractile ring. Journal of Cell Science. 1996;109:2779–2788. doi: 10.1242/jcs.109.12.2779. [DOI] [PubMed] [Google Scholar]
- Issigonis M, Matunis E. The Drosophila BCL6 homolog Ken and Barbie promotes somatic stem cell self-renewal in the testis niche. Dev Biol. 2012;368:181–192. doi: 10.1016/j.ydbio.2012.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SM, Blochlinger K. cut interacts with Notch and Protein kinase A to regulate egg chamber formation and to maintain germline cyst integrity during Drosophila oogenesis. Dev Biol. 1997;124:3663–3672. doi: 10.1242/dev.124.18.3663. [DOI] [PubMed] [Google Scholar]
- Jinks TM, Polydorides A, Calhoun G, Schedl P. The JAK/STAT Signaling Pathway Is Required for the Initial Choice of Sexual Identity in Drosophila melanogaster. Molecular. 2000;5:581–587. doi: 10.1016/s1097-2765(00)80451-7. [DOI] [PubMed] [Google Scholar]
- Kopp A. Dmrt genes in the development and evolution of sexual dimorphism. Trends in genetics : TIG. 2012;28:175–184. doi: 10.1016/j.tig.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherman JL, Dinardo S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell stem cell. 2008;3:44–54. doi: 10.1016/j.stem.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Spradling AC. The Drosophilafusome, a germline-speci c organelle, contains membrane skeletal proteins and functions in cyst formation. Development. 1994;120:947–956. doi: 10.1242/dev.120.4.947. [DOI] [PubMed] [Google Scholar]
- Logan S, Garabedian MJ, Wensink PC. Yolk protein exp ovary DNA regions that regulate the ovarian transcriptional specificity of Drosophila yolk protein genes. Genes & Development. 1989;3:1453–1461. doi: 10.1101/gad.3.9.1453. [DOI] [PubMed] [Google Scholar]
- Mahowald AP, Kambysellis MP. Oogenesis. In: Ashburner M, Wright TRF, editors. The genetics and biology of Drosophila. New York: Academic Press; 1980. pp. 141–224. [Google Scholar]
- Margolis J, Spradling AC. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development. 1995;131:3797–3807. doi: 10.1242/dev.121.11.3797. [DOI] [PubMed] [Google Scholar]
- Masuyama H, Matsuda M. Dmrt1 mutation causes a male-to-female sex reversal after the sex determination by Dmy in the medaka. Chromosome Res. 2012;20:163–176. doi: 10.1007/s10577-011-9264-x. [DOI] [PubMed] [Google Scholar]
- Matson CK, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature. 2011;476:101–104. doi: 10.1038/nature10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson CK, Zarkower D. Sex and the singular DM domain: insights into sexual regulation, evolution and plasticity. Nature reviews Genetics. 2012;13:163–174. doi: 10.1038/nrg3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matunis E, Stine RR, de Cuevas M. Recent advances in Drosophila male germline stem cell biology. Spermatogenesis. 2012;2:137–144. doi: 10.4161/spmg.21763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matunis E, Tran J, Gonczy P, Caldwell K, DiNardo S. punt and schnurri regulate a somatically derived signal that restricts proliferation of committed progenitors in the germline. Development. 1997;124:4383–4391. doi: 10.1242/dev.124.21.4383. [DOI] [PubMed] [Google Scholar]
- McKearin DM, Spradling AC. bag-of-marbles: a Drosophila gene required to initiate both male and female gametogenesis. Genes & Development. 1990;4:2242–2251. doi: 10.1101/gad.4.12b.2242. [DOI] [PubMed] [Google Scholar]
- Michel M, Kupinski AP, Raabe I, Bokel C. Hh signalling is essential for somatic stem cell maintenance in the Drosophila testis niche. Development. 2012;139:2663–2669. doi: 10.1242/dev.075242. [DOI] [PubMed] [Google Scholar]
- Nakada D, Oguro H, Levi BP, Ryan N, Kitano A, Saitoh Y, Takeichi M, Wendt GR, Morrison SJ. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature. 2014;505:555–558. doi: 10.1038/nature12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinett C, Vaughan A, Knapp J, Baker BS. Sex and the Single Cell. II. There Is a Time and Place for Sex. PlOS Biology. 2010:8. doi: 10.1371/journal.pbio.1000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata K, Takase M, Nakamura M. The Dmrt1 expression in sex-reversed gonads of amphibians. General and Comparative Endocrinology. 2002;127:232–241. doi: 10.1016/s0016-6480(02)00039-4. [DOI] [PubMed] [Google Scholar]
- Stine RR, Matunis EL. Stem cell competition: finding balance in the niche. Trends in cell biology. 2013;23:357–364. doi: 10.1016/j.tcb.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran J, Brenner TJ, DiNardo S. Somatic control over the germline stem cell lineage during Drosophila spermatogenesis. Nature. 2000:407. doi: 10.1038/35037613. [DOI] [PubMed] [Google Scholar]
- Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI, et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139:1130–1142. doi: 10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Wawersik M, Milutinovich A, Casper AL, Matunis E, Williams B, Van Doren M. Somatic control of germline sexual development is mediated by the JAK/STAT pathway. Nature. 2005;436:563–567. doi: 10.1038/nature03849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth C, Jimenez E, Van Doren M. Development of sexual dimorphism in the Drosophila testis. Spermatogenesis. 2012;2:129–136. doi: 10.4161/spmg.21780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, McBride D, Nandi S, McQueen HA, McGrew MJ, Hocking PM, Lewis PD, Sang HM, Clinton M. Somatic sex identity is cell autonomous in the chicken. Nature. 2010;464:237–242. doi: 10.1038/nature08852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Lin S, Kao CF, Awasaki T, Chiang AS, Lee T. Gradients of the Drosophila Chinmo BTB-zinc finger protein govern neuronal temporal identity. Cell. 2006;127:409–422. doi: 10.1016/j.cell.2006.08.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. (Related to Figure 1 and 2) Reduction of Chinmo causes somatic cells in adult testes to be gradually replaced by cells resembling ovarian follicle cells.
(A–C) Immunofluorescence detection of β-galactosidase (green) to visualize M5-4, a male-specific escargot enhancer trap, and Vasa (red) to visualize germ cells. In control testes (A), M5-4 is expressed in hub cells (yellow arrow), GSCs and early differentiated germ cells (yellow arrowheads), but not in CySC lineage cells (white arrowhead) or later differentiated germ cells (white arrow). After RNAi-mediated depletion of chinmo from the CySC lineage (c587-Gal4; UAS-chinmo-RNAi) (B), hub cells and germ cells continue to express M5-4, suggesting that they maintain characteristics of their male identity. Germ cells away from the testis apex fail to downregulate M5-4 (yellow arrowhead), indicating that they do not differentiate properly. M5-4 is absent from ovaries (C). (D) Immunofluorescence detection of FasIII (green at cell periphery) to visualize follicle-like cells in testes, and Vasa (red) to visualize germ cells. At 2–3 weeks of age, germ cells in chinmoST testes degenerate; only somatic cells remain at the testis apex. (E–F) Immunofluorescence detection of Chinmo (red) in adult testes containing chinmo1 or control clones verifies the specificity of anti-Chinmo antisera in adult testes. Hubs marked by dashed line. Chinmo levels are decreased in a chinmo1 germ cell clone (white arrowhead) and somatic clones (white arrows), identified by the absence of GFP (green), compared to neighboring GFP-positive heterozygous germ cells (one indicated, open arrowhead) and somatic cells (yellow arrow) (E). Wild-type control somatic clones and neighboring cells (white and yellow arrows, respectively) (F) have similar levels of Chinmo. DAPI marks nuclei (blue). Scale bars = 20 μm.
Figure S2. (Related to Figure 5) Chinmo is required autonomously in adult CySC lineage cells to prevent their transformation into female soma.
(A–B) Immunofluorescence detection of nuclear-localized UAS-GFP (green) in adult testes reveals that c587-Gal4 (A) and tj-Gal4 (B) are expressed in early CySC lineage cells but not in hub cells (asterisk) or germ cells (red). (C–D) Immunofluorescence detection of Chinmo (green) in adult testes verifies the efficacy of chinmo RNAi in the CySC lineage (genotype: c587-Gal4; UAS-chinmo RNAi/tub-Gal80ts). Before expression of chinmo RNAi (C), Chinmo is detected in hub cells (asterisk), CySC lineage cells (arrowheads), and germ cells (red). After 6 days of chinmo-RNAi expression in CySC lineage cells (D), Chinmo is no longer detected in CySC lineage cells (arrowhead). (E–F) Immunofluorescence detection of GFP (green), revealing the eyaA3-Gal4 expression pattern. At 25 °C (E), eyaA3-Gal4 drives expression of UAS-GFP at high levels in cyst cells (arrow) but not in CySCs (arrowheads). At 29 °C (F), eyaA3-Gal4 drives expression in both CySCs and cyst cells. (G–I) Immunofluorescence detection of Chinmo (green) and Tj (red) in adult testes. Hubs marked by dashed line. In control testes (G), Chinmo is detected in CySCs and their immediate daughters (arrows), which are identified as Tj+ nuclei near the hub, as well as in cyst cells (arrowheads). After chinmo-RNAi is expressed only in cyst cells (eyaA3-Gal4 at 25 °C) (H), Chinmo remains detectable in CySCs and their immediate daughters (arrows) but not in older cyst cells (arrowheads). After chinmo-RNAi is expressed in both CySCs and cyst cells (eyaA3-Gal4 at 29 °C) (I), Chinmo is no longer detected in CySCs (arrows) or cyst cells (arrowheads). (J–L) Immunofluorescence detection of cytoplasmic GFP (green), revealing the c587-Gal4 expression pattern. c587-Gal4 drives expression of UAS-GFP in the cytoplasm of early CySC lineage cells in control testes (J) and in somatic cells in the germarium in control ovaries (L). Older follicle cells in control ovaries (L) and follicle-like cells in chinmoST testes (K) lack c587-Gal4 activity. DAPI marks nuclei (blue in A–E, J–L and white in G–I). Scale bars = 20 μm.
Figure S3. (Related to Figure 7) chinmoST regulates dsx transcription rather than female-specific splicing of mRNAs in the canonical sex determination pathway, and dsx-RNAi partially phenocopies the chinmo mutant phenotype.
(A) RT-PCR detection of male and female spliced forms of sxl, tra or dsx mRNA shows that the female spliced forms are not ectopically expressed in control, chinmoST or chinmoKG testes. Actin-5C is used as a control. (B) Immunofluorescence detection of DsxM (green) in control ovaries. DsxM is not detectable in follicle cells (arrowhead) or any other cells in ovarioles. The green staining outside the ovarioles reflects non-specific staining of the ovarian sheath. (C–D) Immunofluorescence detection of GFP(green) in control ovaries to reflect the transcription of dsx-Gal4 (two different lines). dsx-Gal4-1 is expressed in escort cells (arrow) but not in follicle cells (arrowhead). dsx-Gal4-2 is absent from control ovaries, including follicle cells (arrowhead). (E–F) Immunofluorescence detection of DsxM (green) in adult testes before and after induction of dsx-RNAi (line 1) in the CySC lineage. Before RNAi induction (E), DsxM is expressed in the nuclei of hub cells (asterisk) and CySCs lineage cells (arrow) (n=18 testes). After RNAi induction (F), DsxM levels are low or absent in most CySCs and cyst cells (arrow) but remain high in hub cells (n=25 testes). (G) Composite bar graph showing the percentage of testes with normal, mild, or severe phenotypes after expression of dsx-RNAi (line 1) in the CySC lineage in adult testes for the number of days indicated (genotype: c587-Gal4; UAS-dsx-RNAi-1; tub-Gal80ts). In this experiment, morphologically wild type testes were scored as normal; testes with somatic cell aggregates and/or over-proliferating early germ cells at the apex, but not away from the apex, were scored as having a mild phenotype; and testes with over-proliferating early germ cells throughout the entire testis were scored as having a severe phenotype (see Fig. 6J). Before RNAi induction (0 d at 29 °C), all testes look normal. After RNAi induction in CySC lineage cells, testes display a range of mild to severe phenotypes. (H–J) Immunofluorescence detection of FasIII and 1B1 (green) and DAPI (blue) in adult testes before and after induction of dsx-RNAi (line 2) in the CySC lineage (genotype: UAS-dsx-RNAi-2; eyaA3-Gal4; tub-Gal80ts). Before RNAi induction (H), testes look normal (100% of testes, n =35). After RNAi induction (29 °C for 2 weeks) (I, J), germ cells overproliferate and arrest at early spermatogonial stages (72% of testes, n = 39). In addition, these testes contain some aggregates of FasIII+ cells at the periphery, which resemble the follicle-like cells in chinmo-RNAi testes (J, arrows). Vasa marks germ cells (red) and DAPI marks nuclei (blue). Scale bars = 20 μm.
Table S1 (Related to Figure 2): Phenotype characterization for combinations of chinmo mutant alleles
Table S2 (Related to Figure 2): The chinmoST phenotype can be rescued by Chinmo overexpression in the CySC lineage
Table S3a (Related to Figure 5): Chinmo knockdown in CySCs and cyst cells (CC), but not in late cyst cells alone, induces the follicle cell-like phenotype[1]
Table S3b (Related to Figure 5): Chinmo knock down in CySCs and cyst cells induces the follicle cell-like phenotype with time
Table S4 (Related to Figure 6): Follicle-like cells arise from the CySC lineage