Abstract
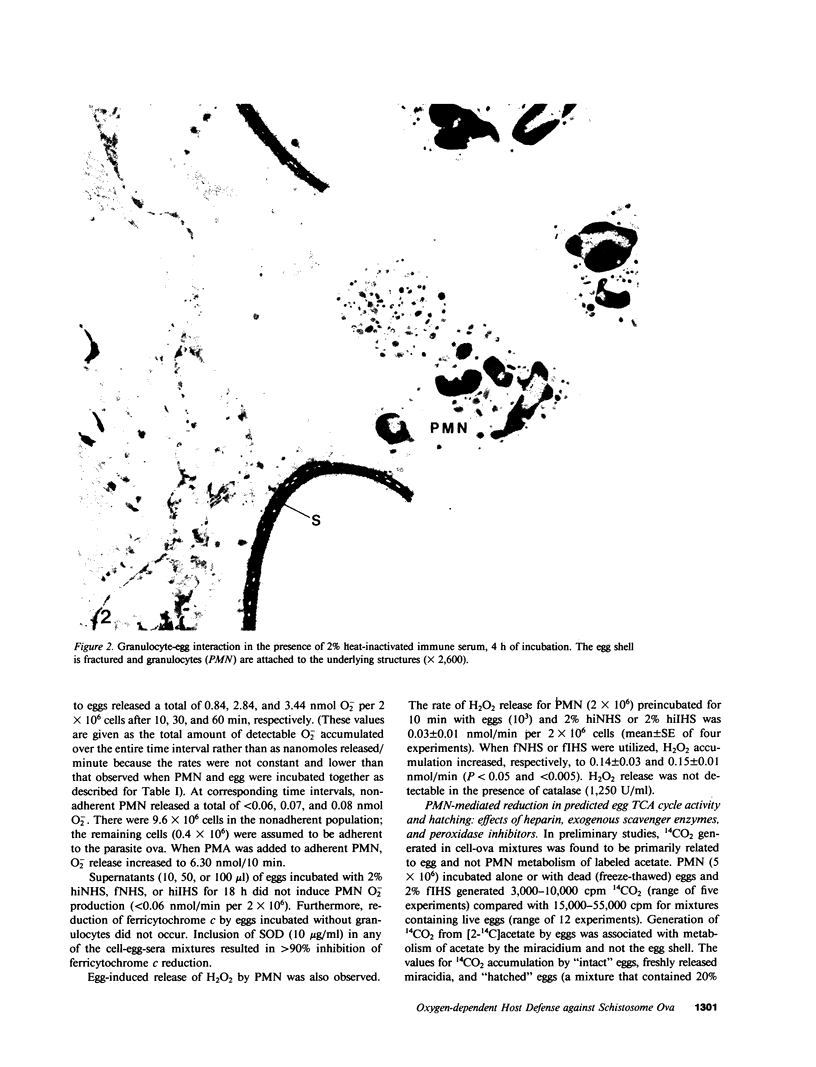
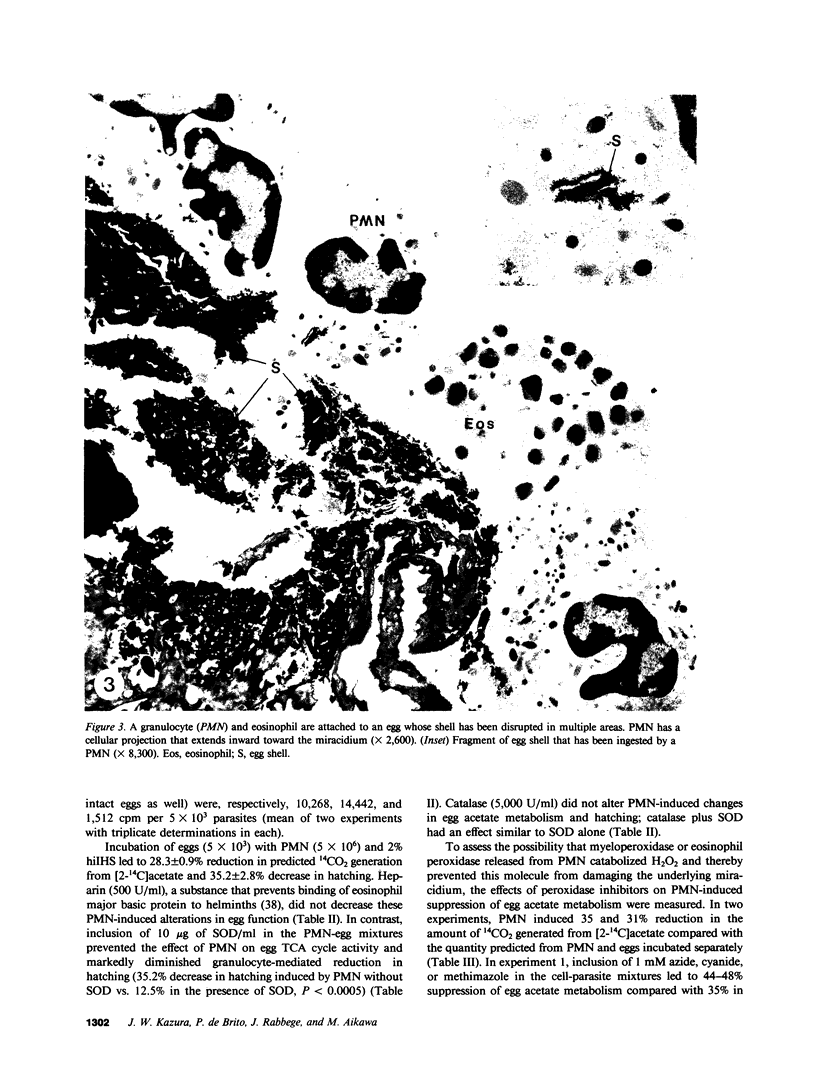
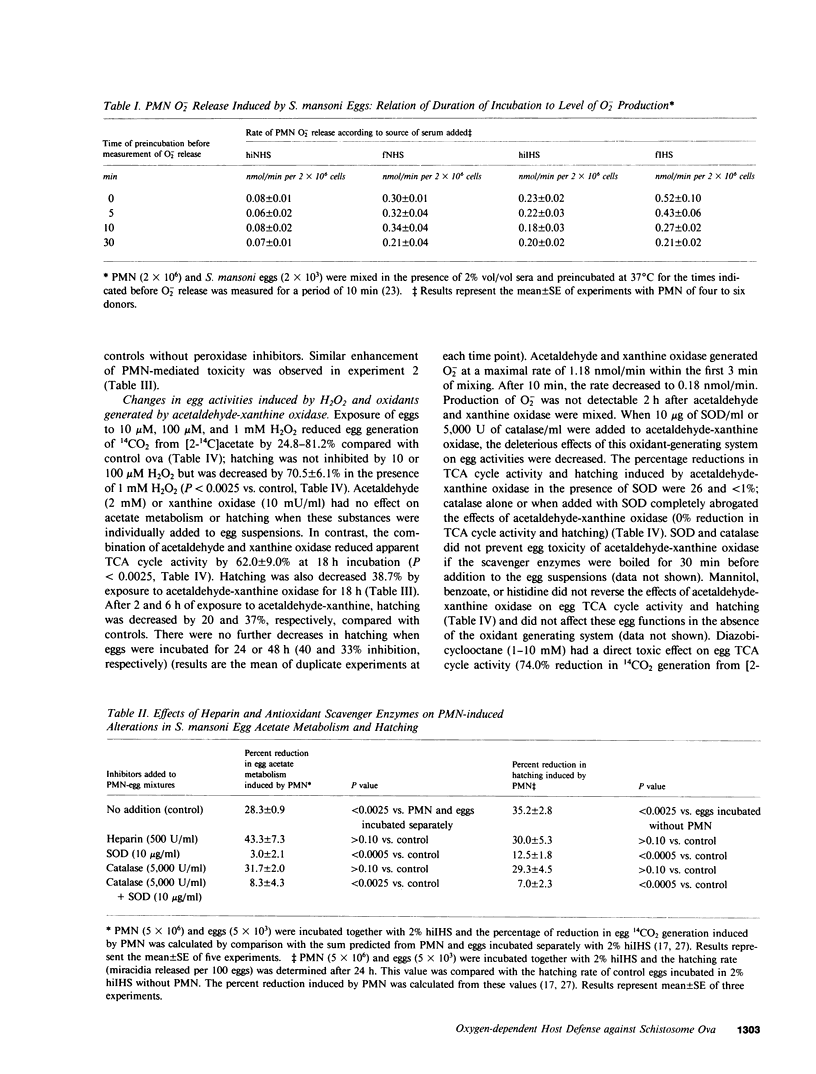
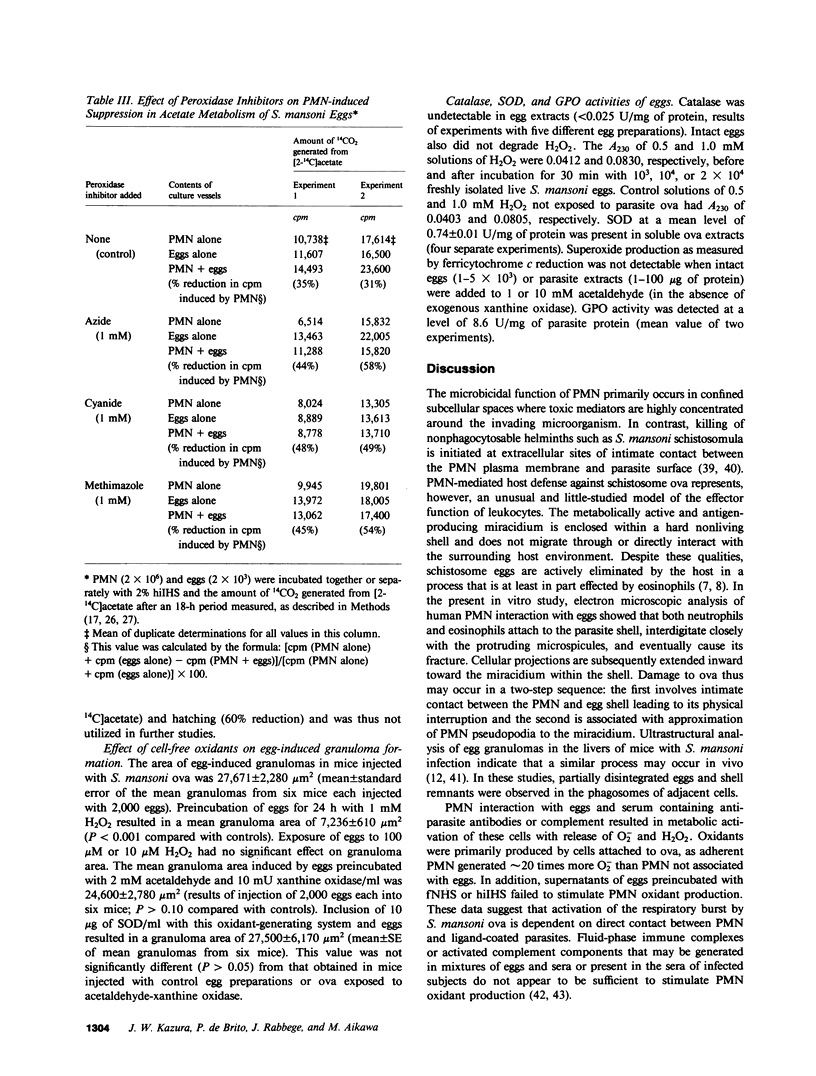
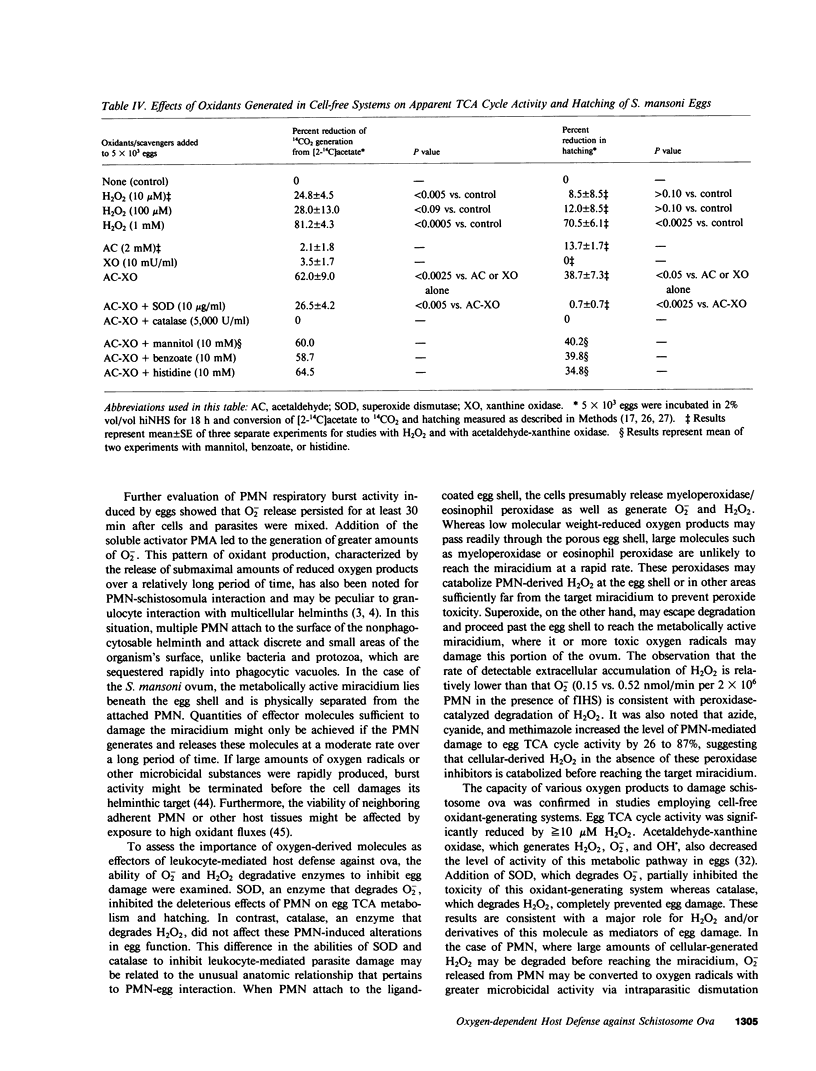
The objectives of this study were to describe the ultrastructure of granulocyte-Schistosoma mansoni egg interaction and to determine the role of reduced oxygen products as effectors of cell-mediated damage to the parasite target. Granulocytes attached to the parasites and closely applied their plasma membranes to the microspicules of the egg shell 30 min after mixing in the presence of immune serum. By 4 h, the egg shell was fractured and granulocyte pseudopodia extended toward the underlying miracidium. Granulocyte attachment to eggs resulted in release of O2- (0.30-0.52 nmol/min per 2 X 10(6) cells) and accumulation of H2O2 (0.14-0.15 nmol/min) in the presence of antibody or complement. Granulocytes reduced egg tricarboxylic-acid cycle activity and hatching by 28.3 +/- 0.9 and 35.2 +/- 2.8%, respectively (cell-egg ratio of 1,000: 1). Exogenous superoxide dismutase (10 micrograms/ml) inhibited granulocyte toxicity for egg metabolic activity (3.0 +/- 2.1% reduction in acetate metabolism vs. 28.3 +/- 0.9% decrease in controls without superoxide dismutase, P less than 0.0005) and hatching (12.5 +/- 1.8% reduction, P less than 0.0005), whereas catalase and heparin had no effect. Inhibitors of myeloperoxidase (1 mM azide, cyanide, and methimazole) augmented granulocyte-mediated toxicity of egg tricarboxylic-acid cycle activity (44-58% reduction in activity vs. 31 and 35% reduction in controls), suggesting that H2O2 released from cells was degraded before reaching the target miracidium. Oxidants generated by acetaldehyde (2 mM)-xanthine oxidase (10 mU/ml) also decreased egg metabolic activity and hatching by 62.0 +/- 9.0 and 38.7 +/- 7.3%, respectively. Egg damage by the cell-free system was partially prevented by superoxide dismutase (26.5 +/- 4.2% reduction in egg tricarboxylic-acid activity) and completely blocked by catalase (0% reduction in activity). These data suggest that granulocyte-mediated toxicity for S. mansoni eggs is dependent on release of O2- or related molecules. These oxygen products, unlike H2O2, may readily reach the target miracidium where they may be converted to H2O2 or other microbicidal effector molecules.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambruso D. R., Johnston R. B., Jr Lactoferrin enhances hydroxyl radical production by human neutrophils, neutrophil particulate fractions, and an enzymatic generating system. J Clin Invest. 1981 Feb;67(2):352–360. doi: 10.1172/JCI110042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar A. R., Smithers S. R., Kay A. B. Killing of schistosomula of Schistosoma mansoni coated with antibody and/or complement by human leukocytes in vitro: requirement for complement in preferential killing by eosinophils. J Immunol. 1979 Feb;122(2):628–637. [PubMed] [Google Scholar]
- BROWNE H. G., THOMAS J. I. A method for isolating pure, viable schistosome eggs from host tissues. J Parasitol. 1963 Jun;49:371–374. [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Bass D. A., Szejda P. Eosinophils versus neutrophils in host defense. Killing of newborn larvae of Trichinella spiralis by human granulocytes in vitro. J Clin Invest. 1979 Nov;64(5):1415–1422. doi: 10.1172/JCI109599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth A. E., Wassom D. L., Gleich G. J., Loegering D. A., David J. R. Damage to schistosomula of Schistosoma mansoni induced directly by eosinophil major basic protein. J Immunol. 1979 Jan;122(1):221–229. [PubMed] [Google Scholar]
- Caulfield J. P., Korman G., Butterworth A. E., Hogan M., David J. R. Partial and complete detachment of neutrophils and eosinophils from schistosomula: evidence for the establishment of continuity between a fused and normal parasite membrane. J Cell Biol. 1980 Jul;86(1):64–76. doi: 10.1083/jcb.86.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Myeloperoxidase--H2O2--halide system: cytotoxic effect on human blood leukocytes. Blood. 1977 Jul;50(1):65–70. [PubMed] [Google Scholar]
- Dean D. A., Wistar R., Murrell K. D. Combined in vitro effects of rat antibody and neutrophilic leukocytes on schistosomula of Schistosoma mansoni. Am J Trop Med Hyg. 1974 May;23(3):420–428. doi: 10.4269/ajtmh.1974.23.420. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Clark R. A., Haudenschild C. C. Damage to Candida albicans hyphae and pseudohyphae by the myeloperoxidase system and oxidative products of neutrophil metabolism in vitro. J Clin Invest. 1980 Nov;66(5):908–917. doi: 10.1172/JCI109958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flohé L., Brand I. Kinetics of glutathione peroxidase. Biochim Biophys Acta. 1969;191(3):541–549. doi: 10.1016/0005-2744(69)90347-7. [DOI] [PubMed] [Google Scholar]
- Glauert A. M., Oliver R. C., Thorne K. J. The interaction of human eosinophils and neutrophils with non-phagocytosable surfaces: a model for studying cell-mediated immunity in schistosomiasis. Parasitology. 1980 Jun;80(3):525–537. doi: 10.1017/s0031182000000986. [DOI] [PubMed] [Google Scholar]
- Goldfine H. Comparative aspects of bacterial lipids. Adv Microb Physiol. 1972;8:1–58. doi: 10.1016/s0065-2911(08)60187-3. [DOI] [PubMed] [Google Scholar]
- Hang L. M., Warren K. S., Boros D. L. Schistosoma mansoni: antigenic secretions and the etiology of egg granulomas in mice. Exp Parasitol. 1974 Apr;35(2):288–298. doi: 10.1016/0014-4894(74)90035-6. [DOI] [PubMed] [Google Scholar]
- Hopkins J., Tudhope G. R. Glutathione peroxidase in human red cells in health and disease. Br J Haematol. 1973 Nov;25(5):563–575. doi: 10.1111/j.1365-2141.1973.tb01768.x. [DOI] [PubMed] [Google Scholar]
- James S. L., Colley D. G. Eosinophil-mediated destruction of Schistosoma mansoni eggs. J Reticuloendothel Soc. 1976 Nov;20(5):359–374. [PubMed] [Google Scholar]
- Jandl R. C., André-Schwartz J., Borges-DuBois L., Kipnes R. S., McMurrich B. J., Babior B. M. Termination of the respiratory burst in human neutrophils. J Clin Invest. 1978 May;61(5):1176–1185. doi: 10.1172/JCI109033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazura J. W., Aikawa M. Host defense mechanisms against Trichinella spiralis infection in the mouse: eosinophil-mediated destruction of newborn larvae in vitro. J Immunol. 1980 Jan;124(1):355–361. [PubMed] [Google Scholar]
- Kazura J. W., Fanning M. M., Blumer J. L., Mahmoud A. A. Role of cell-generated hydrogen peroxide in granulocyte-mediated killing of schistosomula of Schistosoma mansoni in vitro. J Clin Invest. 1981 Jan;67(1):93–102. doi: 10.1172/JCI110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980 Sep;93(3):480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- Kloetzel K. A collagenaselike substance produced by eggs of Schistosoma mansoni. J Parasitol. 1968 Feb;54(1):177–178. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahmoud A. A., Mandel A., Warren K., Webster L. T., Jr Niridazole. II. A potent long-acting suppressant of cellular hypersensitivity. J Immunol. 1975 Jan;114(1 Pt 2):279–283. [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCray P. B., Gibson D. D., Fong K. L., Hornbrook K. R. Effect of glutathione peroxidase activity on lipid peroxidation in biological membranes. Biochim Biophys Acta. 1976 Jun 22;431(3):459–468. [PubMed] [Google Scholar]
- Misra H. P. Generation of superoxide free radical during the autoxidation of thiols. J Biol Chem. 1974 Apr 10;249(7):2151–2155. [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Olds G. R., Mahmoud A. A. Role of host granulomatous response in murine schistosomiasis mansoni. eosinophil-mediated destruction of eggs. J Clin Invest. 1980 Dec;66(6):1191–1199. doi: 10.1172/JCI109970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PELLEGRINO J., OLIVEIRA C. A., FARIA J., CUNHA A. S. New approach to the screening of drugs in experimental schistosomiasis mansoni in mice. Am J Trop Med Hyg. 1962 Mar;11:201–215. doi: 10.4269/ajtmh.1962.11.201. [DOI] [PubMed] [Google Scholar]
- Race G. J., Martin J. H., Moore D. V., Larsh J. E., Jr Scanning and transmission electronmicroscopy of Schistosoma mansoni eggs, cercariae, and adults. Am J Trop Med Hyg. 1971 Nov;20(6):914–924. doi: 10.4269/ajtmh.1971.20.914. [DOI] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Bactericidal activity of a superoxide anion-generating system. A model for the polymorphonuclear leukocyte. J Exp Med. 1979 Jan 1;149(1):27–39. doi: 10.1084/jem.149.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson J. C., Caulfield J. P., David J. R. Schistosoma mansoni: post-transformational surface changes in schistosomula grown in vitro and in mice. Exp Parasitol. 1980 Dec;50(3):369–383. doi: 10.1016/0014-4894(80)90040-5. [DOI] [PubMed] [Google Scholar]
- Santoro F., Prata A., Castro C. N., Capron A. Circulating antigens, immune complexes and C3d levels in human schistosomiasis: relationship with Schistosoma mansoni egg output. Clin Exp Immunol. 1980 Nov;42(2):219–225. [PMC free article] [PubMed] [Google Scholar]
- Santoro F., Prata A., Silva A. E., Capron A. Correlation between circulating antigens detected by the radioimmunoprecipitation-polyethylene glycol assay (RIPEGA) and C1q-binding immune complexes in human schistosomiasis mansoni. Am J Trop Med Hyg. 1981 Sep;30(5):1020–1025. doi: 10.4269/ajtmh.1981.30.1020. [DOI] [PubMed] [Google Scholar]
- Seed J. L., Bennett J. L. Schistosoma mansoni: phenol oxidase's role in eggshell formation. Exp Parasitol. 1980 Jun;49(3):430–441. doi: 10.1016/0014-4894(80)90077-6. [DOI] [PubMed] [Google Scholar]
- Simon R. H., Scoggin C. H., Patterson D. Hydrogen peroxide causes the fatal injury to human fibroblasts exposed to oxygen radicals. J Biol Chem. 1981 Jul 25;256(14):7181–7186. [PubMed] [Google Scholar]
- Smith M. D. The ultrastructural development of the schistosome egg granuloma in mice. Parasitology. 1977 Aug;75(1):119–123. doi: 10.1017/s0031182000048381. [DOI] [PubMed] [Google Scholar]
- Stenger R. J., Warren K. S., Johnson E. A. An ultrastructural study of hepatic granulomas and schistosome egg shells in murine hepatosplenic schistosomiasis mansoni. Exp Mol Pathol. 1967 Aug;7(1):116–132. doi: 10.1016/0014-4800(67)90041-x. [DOI] [PubMed] [Google Scholar]
- Stjernholm R. L., Warren K. S. Schistosoma mansoni: utilization of exogenous metabolites by eggs in vitro. Exp Parasitol. 1974 Oct;36(2):222–232. doi: 10.1016/0014-4894(74)90061-7. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., Douglass K. H., McIntyre P. A. Hydrogen peroxide production and killing of Staphylococcus aureus by human polymorphonuclear leukocytes. Blood. 1977 Mar;49(3):437–444. [PubMed] [Google Scholar]
- Vadas M. A., David J. R., Butterworth A., Pisani N. T., Siongok T. A. A new method for the purification of human eosinophils and neutrophils, and a comparison of the ability of these cells to damage schistosomula of Schistosoma mansoni. J Immunol. 1979 Apr;122(4):1228–1236. [PubMed] [Google Scholar]
- Warren K. S. The immunopathogenesis of schistosomiasis: a multidisciplinary approach. Trans R Soc Trop Med Hyg. 1972;66(3):417–434. doi: 10.1016/0035-9203(72)90273-8. [DOI] [PubMed] [Google Scholar]
- de Brito P. A., Kazura J. W., Mahmoud A. A. Host granulomatous response in schistosomiasis mansoni. Antibody and cell-mediated damage of parasite eggs in vitro. J Clin Invest. 1984 Nov;74(5):1715–1723. doi: 10.1172/JCI111589. [DOI] [PMC free article] [PubMed] [Google Scholar]