Abstract
Purpose
Maternal lead exposure is associated with poor birth outcomes in populations with moderate to high blood levels. However, no studies have looked at exposure levels commonly experienced by US women.
Methods
We evaluated the relationship between maternal red blood cell (RBC) lead levels in mid-pregnancy and birth outcomes in 949 mother-child pairs in a pre-birth cohort. We used multiple linear regression and logistic regression, adjusted for potential confounders including maternal age, race, pre-pregnancy BMI and smoking, to relate maternal lead to infant birth size and risk for preterm birth (<37 weeks).
Results
Mean RBC lead level was 1.2 μg/dL (Range: 0.0, 5.0). Mean (SD) birthweight was 3505 (520) grams, birthweight for gestational age z-score 0.22 (0.93), and length of gestation 39.5 (1.7) weeks. Mothers in the highest vs. lowest lead quartile did not have higher odds (OR 1.85, 95% CI: 0.79, 4.34) of preterm delivery; after stratifying by child sex there was an association among males (OR: 5.51, 95% CI: 1.21, 25.15) but not females (OR 0.82, 95% CI: 0.24, 2.85). Maternal RBC lead was not associated with any continuous outcomes in combined or sex-stratified analyses.
Conclusions
Maternal lead exposure, even at very low levels, may adversely affect some child birth outcomes, particularly preterm birth among males.
Keywords: Pregnancy, lead, preterm birth, birthweight
Introduction
Lead is a ubiquitous environmental toxicant. As studies have accumulated demonstrating the adverse effects of childhood lead exposure on neurodevelopment, recent attention has turned to the effects of prenatal exposure (1,2). Pregnancy is an especially vulnerable time, not only because of the unique sensitivity of the developing fetus to exogenous insults, but also because lead previously stored in bones can mobilize with maternal calcium stores and become an endogenous source of exposure (3-5). Lead readily crosses the placenta and has been measured in fetal brains as early as the first trimester (6).
Higher lead levels during pregnancy have been associated with adverse effects for a range of outcomes, including risks for gestational hypertension, preeclampsia, poor fetal growth and impaired neurodevelopment (7-12). However, few studies have examined exposure levels within the range commonly experienced by US women. US guidelines recommend follow-up for pregnant women with a whole blood lead level ≥ 5 ug/dL. About 1% of US women of childbearing age (15-44 years) exceed this threshold (6). Mean lead level among US women is 0.6 ug/dL, and little is known about the effects of prenatal exposure at this level (6,10,13,14).
Our study aimed to determine associations of prenatal lead exposure, at levels commonly experienced by US women, with fetal growth and birth outcomes.
Methods
Study Subjects
Study subjects were mother-child pairs in Project Viva, a prospective pre-birth cohort designed to study prenatal risk factors on pregnancy and child health outcomes. We recruited women at their first prenatal visit from Harvard Vanguard Medical Associates, a large group practice in eastern Massachusetts. Eligibility criteria included English speaking, singleton pregnancy, and < 22 weeks gestation. Recruitment procedures have been described in detail elsewhere (15). All women provided written informed consent and the research was approved by the Institutional Review Board at Harvard Pilgrim Health Care.
We recruited 64% of those eligible between 1999 and 2002; 2,128 gave birth to a live infant and were enrolled in the cohort. We collected blood samples from 1,614 women (76%) at a mean of 27.9 weeks gestation. Because of funding limitations, we analyzed samples from 950 women for lead. After assay, we excluded from analysis one participant with a red blood cell (RBC) level substantially higher than the rest of the cohort (9.8 ug/dL), as our intention was to study the effect of very low exposure. Overall, participants included were similar in baseline characteristics to those excluded (data not shown). However, participants in this analysis had babies who weighed on average 79 grams more and had a mean birthweight for gestational age z-score of 0.09 units higher, compared to those not included. Participants in this study were also more likely to be Caucasian (75% vs. 60%).
Measurement of Lead
We collected blood in vacutainer tubes containing ethylenediaminetetraacetic acid and put the samples on ice immediately. Within 24 hours we centrifuged the blood and stored separate aliquots of plasma and erythrocytes. We stored the erythrocytes at −80°C until assay.
Samples were analyzed for lead concentrations at the Trace Metals Laboratory at Harvard School of Public Health in Boston, MA. To measure lead in RBCs, samples were weighed and digested for 24 hours in 2ml of concentrated nitric acid (HNO3) and 1ml of 30% hydrogen peroxide (H2O2) per 1g of RBCs. Samples were subsequently diluted to 10ml with deionized water. Lead concentrations in RBCs were measured using a dynamic reaction cell-inductively coupled plasma mass spectrometer (Elan DRC II, Perkin Elmer, Norwalk, CT).
Quality control measures included analysis of initial and continuous calibration verification standards (National Institute of Standards and Technology Standard Reference Material for trace elements in water [NIST SRM 1643e]), 1ppb lead standard, procedural blanks, QC standard, (National Institute of Standard and Technology Standard Reference Material (NIST SRM) 1643d-trace elements in water [NIST SRM955b-lead in blood]). Results given were the average of five replicate measurements. The limit of detection for this procedure is 0.2ng/ml in RBCs. Recovery of the analysis of QC standard by this procedure is 90-110% with <5% precision.
Ascertainment of Birth Outcomes
Women reported last menstrual period (LMP) at enrollment. We obtained delivery date from medical records. We calculated gestational age by subtracting LMP date from delivery date, or by ultrasound (9.6% of participants) where an ultrasound was available and differed from LMP by more than 10 days (16). We defined preterm birth as birth before 37 weeks. We obtained birthweight from hospital medical records, and calculated birthweight for gestational age z-scores using a US national reference (17). At the hospital, research assistants measured infant birth length and head circumference for 541 and 596 infants, respectively.
Covariates
We examined covariates that could be associated with maternal lead level or birth outcomes. Through self-administered questionnaires and interviews we collected maternal demographics including age, income, race, country of birth, marital status, and education level; and pregnancy health information including smoking status, diet, and pre-pregnancy weight and height. We collected parity from medical records. We also considered vitamin D intake, fish intake, iron intake, and anemia status as covariates, but did not include them in the final model because they did not change results appreciably.
We calculated gestational weight gain by subtracting reported pre-pregnancy weight from the last weight recorded before delivery in the medical record. We calculated pre-pregnancy body mass index (BMI, kg/m2) from self-reported pre-pregnancy weight and height. We calculated gestational age at the time of blood draw by subtracting LMP date or ultrasound date from date of blood draw.
Statistical Analyses
We assigned women to quartile of lead exposure based on their continuous blood lead levels, with the lowest quartile serving as the reference group. To test for trend across quartiles of exposure in our linear regression models we assigned the median value to each quartile of maternal RBC lead and included it as a continuous exposure.
We conducted multivariable-adjusted linear regression models to examine the associations between maternal blood lead level and gestational age, birthweight, birthweight for gestational age z-score, birth length and head circumference. We also conducted multivariable-adjusted logistic regression to examine the association between maternal blood lead level and odds of preterm birth. We assessed effect modification by child sex through stratification (18). We also performed sensitivity analyses including only women with RBC lead levels <3 ug/dL, and defining preterm birth as <35 weeks. Results were similar.
We adjusted for covariates identified in the literature and those found to confound the relationship between lead and our outcomes. Using this approach, we identified and adjusted for gestational weight gain, pre-pregnancy BMI, race, country of birth, second trimester calcium intake, parity, smoking in pregnancy and age, and child sex. We also adjusted for weight of the blood sample and gestational age at maternal blood draw. Models for birthweight, head circumference, and length were further adjusted for gestational age. Birth length models also adjusted for maternal height.
We used a chained equations approach to multiply impute values for missing covariates and missing birth length and head circumference (19-22). We generated 50 imputed datasets and all model results are generated by appropriately combining results (19). To avoid incorrect imputations, we used all 2,128 participants in the imputation process, but included only subjects with a valid lead measurement (n=949) in the analysis (20). The characteristics of the imputed sample were nearly identical to those with complete data (Table 1). We performed data analyses using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
Table 1.
Maternal and Child Characteristics for 949 Project Viva Participants
| Complete Data | Imputed Data | ||||||
|---|---|---|---|---|---|---|---|
| Total | Total | Quartile of Lead | |||||
| N | Mean (SD) or N (%) | 1st | 2nd | 3rd | 4th | ||
| Mean (SD) or % | |||||||
| Maternal Characteristics | |||||||
| RBC Lead Level (ug/dL) | 829 | 1.22 (0.59) | 1.22 (0.62) | 0.65 (0.15) Med= 0.68 | 0.96 (0.09) Med=0.95 | 1.27 (0.12) Med=1.27 | 2.02 (0.60) Med=1.85 |
| Recreated WB Lead Level (ug/dL)1 | 742 | 0.41 (0.20) | 0.42 (0.21) | 0.22 (0.05) | 0.33 (0.04) | 0.43 (0.05) | 0.68 (0.21) |
| Gestational Age at Lead Measurement (weeks) | 943 | 27.9 (1.4) | 27.9 (1.4) | 27.8 (1.4) | 27.8 (1.4) | 27.9 (1.5) | 28 (1.5) |
| Hematocrit (%) | 854 | 34.0 (2.4) | 34.0 (2.5) | 34.0 (2.7) | 34.2 (2.7) | 34.0 (2.6) | 33.9 (2.6) |
| Pre-Pregnancy BMI (kg/m2) | 945 | 24.6 (5.1) | 24.6 (5.1) | 24.5 (5.2) | 24.3 (5.1) | 24.7 (5.5) | 24.8 (5.4) |
| GWG (kgs) | 938 | 15.6 (5.3) | 15.6 (5.4) | 15.6 (5.3) | 15.8 (5.6) | 15.6 (6.1) | 15.2 (5.5) |
| Age (years) | 949 | 32.4 (4.9) | 32.4 (4.9) | 31.3 (5.3) | 32.0 (5.1) | 32.8 (5.1) | 33.3 (5.0) |
| Total energy-adjusted 2nd trimester Calcium intake (mg) | 946 | 1437 (385) | 1436 (385) | 1521 (415) | 1422 (376) | 1402 (402) | 1400 (412) |
| Income <= $70k | 901 | 334 (37.1%) | 37.9% | 32.9% | 38.7% | 40.9% | 39.2% |
| Smoked in Pregnancy | 947 | 111 (11.7%) | 11.7% | 10.7% | 10.2% | 11.4% | 14.7% |
| Caucasian | 946 | 709 (75.0%) | 75.0% | 81.5% | 80.4% | 73.2% | 64.8% |
| Non-US Born | 937 | 175 (18.7%) | 18.6% | 10.8% | 14.6% | 21.9% | 27.2% |
| Nulliparious | 949 | 470 (49.5%) | 49.5% | 53.1% | 46.4% | 47.5% | 51.0% |
| Child Characteristics | |||||||
| Male | 949 | 475 (50.1%) | 50.1% | 47.4% | 53.2% | 48.3% | 51.3% |
| Preterm (<37 weeks) | 949 | 64 (6.7%) | 6.7% | 5.1% | 5.3% | 7.2% | 9.5% |
| Males | 475 | 35 (7.4%) | 7.4% | 2.9% | 5.5% | 8.7% | 12.1% |
| Females | 474 | 29 (6.1%) | 6.1% | 7.0% | 5.1% | 5.7% | 6.6% |
| Gestational Age (weeks) | 949 | 39.5 (1.7) | 39.5 (1.7) | 39.6 (1.7) | 39.7 (1.6) | 39.5 (1.9) | 39.4 (1.8) |
| Birthweight (grams) | 949 | 3505 (520) | 3505 (520) | 3527 (567) | 3538 (548) | 3502 (535) | 3452 (549) |
| Head circumference (cm) | 596 | 34.1 (1.3) | 34.0 (1.5) | 34.1 (1.6) | 34.1 (1.5) | 34.0 (1.5) | 34.0 (1.6) |
| Birth length (cm) | 541 | 49.9 (2.1) | 49.7 (2.4) | 49.8 (2.5) | 49.9 (2.3) | 49.7 (2.7) | 49.5 (2.5) |
| Birthweight for gestational age z-score | 949 | 0.22 (0.93) | 0.22 (0.93) | 0.26 (1.00) | 0.25 (1.02) | 0.25 (0.92) | 0.13 (0.99) |
RBC Lead Level * Hematocrit. RBC Lead level is approximately 3 times the whole blood level.
Results
Sample Characteristics
75.0% of women were white with 37.9% reporting an annual household income < $70,000. The average age of women was 32.4 years and 49.5% were nulliparious. Average gestational age was 39.5 weeks with 6.7% of the deliveries preterm. Mean birthweight was 3505 grams and mean birthweight for gestational age z-score was 0.22. Mean head circumference and birth length were 34.0 and 49.7 centimeters, respectively.
RBC Lead Level
Mean maternal RBC blood lead level was 1.2 ug/dL (SD: 0.6, range: 0.0 to 5.0), roughly equivalent to a whole blood level of 0.4 ug/dL, based on the estimate that red cell concentrations are roughly three-times greater than whole blood levels (23,24). Lead levels in our sample were slightly below the US national average for women, 0.6 ug/dL in whole blood (6).
Gestational Age and Preterm Birth
We did not see evidence that lead quartile was associated with gestational age. However, in adjusted sex-stratified analyses, we observed a suggestive trend (p for trend = 0.08) of decreasing gestational age across lead quartile among males, with an average decrease of 0.43 weeks (95% CI: -0.92, 0.06) comparing mothers in the highest to lowest quartile. There was no evidence of an association among females (Table 2).
Table 2.
Associations of maternal prenatal lead level with gestation length and infant size at birth among 949 mother-child pairs in Project Viva
| Linear Regression: Beta estimates (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Outcome | Model | Quartile 1 (ref) | Quartile 2 | Quartile 3 | Quartile 4 | P for trend |
| Gestational Age (wks) | Crude1 | 0.00 | 0.08 (−0.24, 0.40) | −0.11 (−0.43, 0.21) | −0.16 (−0.48, 0.16) | 0.18 |
| Adjusted2 | 0.00 | 0.04 (−0.28, 0.36) | −0.14 (−0.47, 0.18) | −0.17 (−0.51, 0.16) | 0.20 | |
| Birthweight3 (grams) | Crude1 | 0 | 12 (−90, 113) | −27 (−128, 75) | −77 (−178, 24) | 0.08 |
| Adjusted2 | 0 | −17 (−98, 63) | −15 (−95, 64) | −47 (−128, 35) | 0.27 | |
| Birthweight for gestational age Z-Score | Crude1 | 0.00 | −0.01 (−0.20, 0.17) | −0.01 (−0.19, 0.17) | −0.13 (−0.31, 0.05) | 0.14 |
| Adjusted2 | 0.00 | −0.03 (−0.21, 0.14) | −0.02 (−0.19, 0.15) | −0.11 (−0.28, 0.07) | 0.25 | |
| Head Circumference3 (cm) | Crude1 | 0.00 | 0.01 (−0.27, 0.28) | −0.11 (−0.39, 0.17) | −0.15 (−0.43, 0.14) | 0.23 |
| Adjusted2 | 0.00 | −0.06 (−0.29, 0.17) | −0.08 (−0.31, 0.15) | −0.08 (−0.33, 0.16) | 0.56 | |
| Birth Length3,4 (cm) | Crude1 | 0.00 | 0.13 (−0.32, 0.57) | −0.05 (−0.51, 0.41) | −0.31 (−0.76, 0.14) | 0.09 |
| Adjusted2 | 0.00 | 0.03 (−0.33, 0.40) | 0.02 (−0.36, 0.39) | −0.15 (−0.54, 0.23) | 0.37 | |
| Stratified Results: Males only | ||||||
| Gestational Age (wks) | Crude1 | 0.00 | −0.06 (−0.53, 0.40) | −0.18 (−0.66, 0.31) | −0.39 (−0.87, 0.08) | 0.08 |
| Adjusted2 | 0.00 | −0.11 (−0.58, 0.35) | −0.19 (−0.68, 0.30) | −0.43 (−0.92, 0.06) | 0.08 | |
| Birthweight3 (grams) | Crude1 | 0 | −45 (−193, 104) | −67 (−222, 87) | −151 (−301, −1) | 0.04 |
| Adjusted2 | 0 | −33 (−149, 83) | −11 (−132, 109) | −44 (−165, 77) | 0.57 | |
| Birthweight for gestational age Z-Score | Crude1 | 0.00 | −0.07 (−0.33, 0.18) | −0.05 (−0.32, 0.21) | −0.20 (−0.46, 0.06) | 0.15 |
| Adjusted2 | 0.00 | −0.07 (−0.32, 0.17) | 0.00 (−0.25, 0.26) | −0.11 (−0.37, 0.15) | 0.48 | |
| Head Circumference3 (cm) | Crude1 | 0.00 | −0.20 (−0.59, 0.20) | −0.28 (−0.70, 0.15) | −0.40 (−0.83, 0.02) | 0.07 |
| Adjusted2 | 0.00 | −0.12 (−0.45, 0.21) | −0.13 (−0.48, 0.23) | −0.15 (−0.53, 0.22) | 0.50 | |
| Birth Length4 (cm) | Crude1 | 0.00 | 0.03 (−0.62, 0.68) | 0.04 (−0.66, 0.74) | −0.49 (−1.16, 0.19) | 0.11 |
| Adjusted2 | 0.00 | 0.11 (−0.43, 0.65) | 0.24 (−0.32, 0.80) | −0.07 (−0.65, 0.51) | 0.73 | |
| Stratified Results: Females only | ||||||
| Gestational Age (wks) | Crude1 | 0.00 | 0.22 (−0.22, 0.66) | −0.06 (−0.49, 0.38) | 0.07 (−0.36, 0.51) | 0.97 |
| Adjusted2 | 0.00 | 0.15 (−0.30, 0.59) | −0.10 (−0.55, 0.35) | 0.03 (−0.42, 0.49) | 0.91 | |
| Birthweight (grams) | Crude1 | 0 | 54 (−84, 191) | 8 (−125, 142) | −13 (−148, 122) | 0.65 |
| Adjusted2 | 0 | −1 (−114, 112) | −22 (−130, 86) | −42 (−157, 72) | 0.41 | |
| Birthweight for gestational age Z-Score | Crude1 | 0.00 | 0.05 (−0.21, 0.31) | 0.03 (−0.21, 0.28) | −0.06 (−0.31, 0.19) | 0.55 |
| Adjusted2 | 0.00 | 0.00 (−0.25, 0.26) | −0.05 (−0.29, 0.19) | −0.10 (−0.35, 0.16) | 0.39 | |
| Head Circumference3 (cm) | Crude1 | 0.00 | 0.13 (−0.25, 0.50) | 0.03 (−0.34, 0.40) | 0.05 (−0.31, 0.41) | 0.96 |
| Adjusted2 | 0.00 | 0.01 (−0.31, 0.34) | −0.03 (−0.35, 0.29) | −0.02 (−0.34, 0.30) | 0.86 | |
| Birth Length4 (cm) | Crude1 | 0.00 | 0.14 (−0.47, 0.75) | −0.15 (−0.74, 0.44) | −0.18 (−0.79, 0.42) | 0.40 |
| Adjusted2 | 0.00 | −0.01 (−0.52, 0.51) | −0.18 (−0.68, 0.32) | −0.18 (−0.72, 0.36) | 0.44 | |
Adjusted for sample weight of blood
Model 1 + additionally adjusted for gestational age at blood draw, gestational weight gain, pre-pregnancy BMI, maternal race/ethnicity, maternal country of birth, maternal 2nd trimester calcium intake, smoking in pregnancy, parity, maternal age and child sex.
Birthweight, head circumference additionally adjusted for gestational age at birth.
Length model additionally adjusted for maternal height and gestational age at birth.
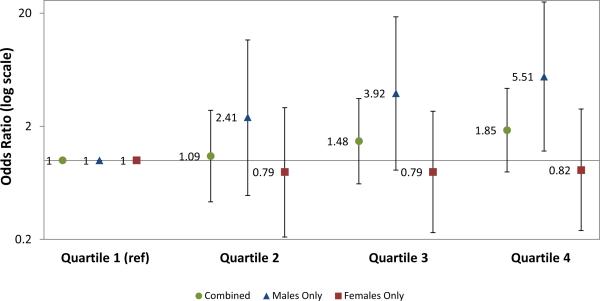
Among males only, we also observed an increase in odds of preterm birth, with mothers in the highest quartile having 5.5 times the odds of giving birth prematurely, compared to those in the lowest quartile (OR: 5.51, 95% CI: 1.21, 25.15). There was no increased risk among females (OR 0.82, 95% CI: 0.24, 2.85) (Figure).
Figure 1.
Adjusted odds of preterm birth (<37 weeks) by quartile of maternal prenatal lead level, among all infants and stratified by infant sex. Data from 949 mother-child pairs in Project Viva. Adjusted for sample weight of blood, gestational age at blood draw, gestational weight gain, pre-pregnancy BMI, maternal race, maternal country of birth, 2nd trimester calcium intake, smoking in pregnancy, parity, maternal age and child sex.
Infant size at birth
We observed decreasing birthweight across quartiles of maternal lead, with an adjusted difference of -47 grams (95% CI: -128, 35) in the highest vs. lowest quartile, with similar estimates in sex-stratified analyses (Table 2). However, confidence limits were wide and the trend across quartiles was not significant (p for trend= 0.27). We did not find associations of lead level with birthweight for gestational age z-score, head circumference or birth length in combined or sex-stratified analyses (Table 2).
Discussion
There are limited data on lead exposure in populations with whole blood lead levels <1 ug/dL. Studies of preterm birth in populations with blood lead levels <10 ug/dL have generally been null with associations only found when looking at higher levels (25,26). Some studies have shown inverse relationships between maternal lead and birth size, while others report no association (10,27-29). Most studies of head circumference and birth length are null among mothers with low exposure, but again results vary (27,28).
With respect to preterm birth, most studies of low exposure report null results, though studies with higher levels are significant (10). This inconsistency with our finding could be explained by differences in quartile levels, as prior studies would likely have categorized our entire population as low exposure. Our result was also driven by effect modification with child sex, which to our knowledge no other study has examined. While this sex-specific association could be due to chance, intrauterine growth, including placental growth, differs by sex (30-32). Lead is transferred from mother to child through the placenta (6), and it is plausible that differences in the growth and development of this organ could differentially affect how males and females respond to lead and nutritional exposures. Other recent studies have also suggested fetal sex differences in sensitivity to hyperglycemia, percentage of body fat, and programming for hypertension (33-35).
Though not significant, our birthweight estimate is fairly consistent with a large study of over 40,000 women (10). They restricted analyses to whole blood levels < 10 ug/dL (mean: 2.2 ug/dL) and found that changes in birthweight varied across the distribution, with the largest effect size at the lowest exposures. For example, a whole blood lead level of 1 vs. 0 ug/dL was associated with 25 gram decrease in birthweight, while a change from 10 to 9 ug/dL was associated with a 4 gram decrease. The exposure contrast of 1 vs. 0 ug/dL roughly reflects our highest to lowest quartile comparison, and our finding of a 47 gram decrease is similar in effect size.
Our study has several strengths including prospective design, large sample size and detailed covariate information. Many other study populations have been drawn from registries or other reporting where a disproportionate number of women with high levels are sampled, and thus have limited generalizability to the US. Our sample provided the opportunity to study lead at levels experienced by most pregnant women in the US.
Our study has several limitations, including a fairly narrow exposure distribution. However, this gave us the opportunity to study the association of very low level exposure, and this is the only study to our knowledge that exclusively looks at very low exposure. We do not have information on housing age or other sources of lead exposure. Our results are likely not generalizable to populations with different exposure ranges, durations and intensities, or to lower income and racial/ethnic minority populations who may have different sources of lead exposure. Although this is the only study to our knowledge that has measured maternal lead in RBCs we believe it is an accurate biomarker as 99% of whole blood lead is found in RBCs, and thus RBC lead is very highly correlated with whole blood concentrations (24,36). Lastly, our sample size was quite large, but may not have been large enough to detect statistical differences.
Our results suggest maternal lead exposure, even at very low levels, may adversely affect some child birth outcomes, particularly preterm birth among males. Additional studies of low lead exposure and differential effects by child sex are warranted.
Acknowledgements
We are greatly indebted to Project Viva mothers and children for their participation.
Funding
This project was funded by grants from the National Institutes of Health (K24 HD069408, R01 ES016314, R01 HD 034568), the March of Dimes Foundation, and support from the Harvard Pilgrim Health Care Foundation. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
List of abbreviations and acronyms
- RBC
Red Blood Cell
- BMI
Body Mass Index
- CDC
Centers for Disease Control and Prevention
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None of the authors has any conflict of interest to report.
References
- 1.Bellinger DC. Very low lead exposures and children's neurodevelopment. Curr Opin Pediatr. 2008 Apr;20(2):172–177. doi: 10.1097/MOP.0b013e3282f4f97b. [DOI] [PubMed] [Google Scholar]
- 2.Tellez-Rojo MM, Bellinger DC, Arroyo-Quiroz C, Lamadrid-Figueroa H, Mercado-Garcia A, Schnaas-Arrieta L, et al. Longitudinal associations between blood lead concentrations lower than 10 microg/dL and neurobehavioral development in environmentally exposed children in Mexico City. Pediatrics. 2006 Aug;118(2):e323–30. doi: 10.1542/peds.2005-3123. [DOI] [PubMed] [Google Scholar]
- 3.Gulson BL, Mizon KJ, Korsch MJ, Palmer JM, Donnelly JB. Mobilization of lead from human bone tissue during pregnancy and lactation--a summary of long-term research. Sci Total Environ. 2003 Feb 15;303(1-2):79–104. doi: 10.1016/s0048-9697(02)00355-8. [DOI] [PubMed] [Google Scholar]
- 4.Manton WI, Angle CR, Stanek KL, Kuntzelman D, Reese YR, Kuehnemann TJ. Release of lead from bone in pregnancy and lactation. Environ Res. 2003 Jun;92(2):139–151. doi: 10.1016/s0013-9351(03)00020-3. [DOI] [PubMed] [Google Scholar]
- 5.Rothenberg SJ, Khan F, Manalo M, Jiang J, Cuellar R, Reyes S, et al. Maternal bone lead contribution to blood lead during and after pregnancy. Environ Res. 2000 Jan;82(1):81–90. doi: 10.1006/enrs.1999.4007. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention [11/11, 2012];CDC Guidelines for the Identification and Management of LEad Exposure in Pregnant and Lactating Women. 2010 Available at: htto://stacks.cdc.gov/view/cdc/7133.
- 7.Al-Saleh I, Nester M, Mashhour A, Moncari L, Shinwari N, Mohamed G, et al. Prenatal and postnatal lead exposure and early cognitive development: longitudinal study in Saudi Arabia. J Environ Pathol Toxicol Oncol. 2009;28(4):283–302. doi: 10.1615/jenvironpatholtoxicoloncol.v28.i4.40. [DOI] [PubMed] [Google Scholar]
- 8.Jelliffe-Pawlowski LL, Miles SQ, Courtney JG, Materna B, Charlton V. Effect of magnitude and timing of maternal pregnancy blood lead (Pb) levels on birth outcomes. J Perinatol. 2006 Mar;26(3):154–162. doi: 10.1038/sj.jp.7211453. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy DA, Woodland C, Koren G. Lead exposure, gestational hypertension and pre eclampsia: a systematic review of cause and effect. J Obstet Gynaecol. 2012 Aug;32(6):512–517. doi: 10.3109/01443615.2012.693987. [DOI] [PubMed] [Google Scholar]
- 10.Zhu M, Fitzgerald EF, Gelberg KH, Lin S, Druschel CM. Maternal low-level lead exposure and fetal growth. Environ Health Perspect. 2010 Oct;118(10):1471–1475. doi: 10.1289/ehp.0901561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellinger D, Leviton A, Waternaux C, Needleman H, Rabinowitz M. Longitudinal analyses of prenatal and postnatal lead exposure and early cognitive development. N Engl J Med. 1987 Apr 23;316(17):1037–1043. doi: 10.1056/NEJM198704233161701. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez-Avila M, Peterson KE, Gonzalez-Cossio T, Sanin LH, Aro A, Schnaas L, et al. Effect of maternal bone lead on length and head circumference of newborns and 1-month-old infants. Arch Environ Health. 2002 Sep-Oct;57(5):482–488. doi: 10.1080/00039890209601441. [DOI] [PubMed] [Google Scholar]
- 13.Bellinger D, Leviton A, Rabinowitz M, Allred E, Needleman H, Schoenbaum S. Weight gain and maturity in fetuses exposed to low levels of lead. Environ Res. 1991 Apr;54(2):151–158. doi: 10.1016/s0013-9351(05)80097-0. [DOI] [PubMed] [Google Scholar]
- 14.Jones L, Parker J, Mendola P. NCHS data brief no 52. National Center for Health Statistics; Hyattsville, MD: 2010. [10/19/2012]. Blood lead and mercury levels in pregnant women in the United States, 2003-2008. http://www.cdc.gov/nchs/databriefs/db52.htm. [PubMed] [Google Scholar]
- 15.Oken E, Baccarelli A, Gold D, Kleinman K, Litonjua A, De Meo D, et al. Cohort Profile: Project Viva. International Journal of Epidemiology. 2014 doi: 10.1093/ije/dyu008. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, Lieberman ES, Kleinman KP, Lipshultz SE. Maternal age and other predictors of newborn blood pressure. J Pediatr. 2004 Feb;144(2):240–245. doi: 10.1016/j.jpeds.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 17.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003 Jul 8;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellinger DC. Effect modification in epidemiologic studies of low-level neurotoxicant exposures and health outcomes. Neurotoxicol Teratol. 2000 Jan-Feb;22(1):133–140. doi: 10.1016/s0892-0362(99)00053-7. [DOI] [PubMed] [Google Scholar]
- 19.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Wiley; 1987. [Google Scholar]
- 20.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011 Feb 20;30(4):377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 21.van Buuren S, Ouddshoom DGM. Flexible multivariate imputation by MICE. : Leidon: TNO Preventie e Gezonheid, TNO/PG 99.054. 1999.
- 22.Little R, Rubin D. Statistical analysis with missing data. Wiley; Hoboken, NJ: 2002. [Google Scholar]
- 23.Wright RO, Hu H, Maher TJ, Amarasiriwardena C, Chaiyakul P, Woolf AD, et al. Effect of iron deficiency anemia on lead distribution after intravenous dosing in rats. Toxicol Ind Health. 1998 Jul-Aug;14(4):547–551. doi: 10.1177/074823379801400405. [DOI] [PubMed] [Google Scholar]
- 24.Leggett RW. An age-specific kinetic model of lead metabolism in humans. Environ Health Perspect. 1993 Dec;101(7):598–616. doi: 10.1289/ehp.93101598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cantonwine D, Hu H, Sanchez BN, Lamadrid-Figueroa H, Smith D, Ettinger AS, et al. Critical windows of fetal lead exposure: adverse impacts on length of gestation and risk of premature delivery. J Occup Environ Med. 2010 Nov;52(11):1106–1111. doi: 10.1097/JOM.0b013e3181f86fee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu M, Fitzgerald EF, Gelberg KH. Exposure sources and reasons for testing among women with low blood lead levels. Int J Environ Health Res. 2011 Aug;21(4):286–293. doi: 10.1080/09603123.2010.550035. [DOI] [PubMed] [Google Scholar]
- 27.Gundacker C, Frohlich S, Graf-Rohrmeister K, Eibenberger B, Jessenig V, Gicic D, et al. Perinatal lead and mercury exposure in Austria. Sci Total Environ. 2010 Nov 1;408(23):5744–5749. doi: 10.1016/j.scitotenv.2010.07.079. [DOI] [PubMed] [Google Scholar]
- 28.Schell LM, Denham M, Stark AD, Parsons PJ, Schulte EE. Growth of infants' length, weight, head and arm circumferences in relation to low levels of blood lead measured serially. Am J Hum Biol. 2009 Mar-Apr;21(2):180–187. doi: 10.1002/ajhb.20842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sowers M, Jannausch M, Scholl T, Li W, Kemp FW, Bogden JD. Blood lead concentrations and pregnancy outcomes. Arch Environ Health. 2002 Sep-Oct;57(5):489–495. doi: 10.1080/00039890209601442. [DOI] [PubMed] [Google Scholar]
- 30.Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJ. Boys live dangerously in the womb. Am J Hum Biol. 2010 May-Jun;22(3):330–335. doi: 10.1002/ajhb.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Zegher F, Devlieger H, Eeckels R. Fetal growth: boys before girls. Horm Res. 1999;51(5):258–259. doi: 10.1159/000023382. [DOI] [PubMed] [Google Scholar]
- 32.Forsen T, Eriksson JG, Tuomilehto J, Osmond C, Barker DJ. Growth in utero and during childhood among women who develop coronary heart disease: longitudinal study. BMJ. 1999 Nov 27;319(7222):1403–1407. doi: 10.1136/bmj.319.7222.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Regnault N, Gillman MW, Rifas-Shiman SL, Eggleston E, Oken E. Sex-specific associations of gestational glucose tolerance with childhood body composition. Diabetes Care. 2013 Oct;36(10):3045–3053. doi: 10.2337/dc13-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hawkes CP, Hourihane JO, Kenny LC, Irvine AD, Kiely M, Murray DM. Gender- and gestational age-specific body fat percentage at birth. Pediatrics. 2011 Sep;128(3):e645–51. doi: 10.1542/peds.2010-3856. [DOI] [PubMed] [Google Scholar]
- 35.van Abeelen AF, de Rooij SR, Osmond C, Painter RC, Veenendaal MV, Bossuyt PM, et al. The sex-specific effects of famine on the association between placental size and later hypertension. Placenta. 2011 Sep;32(9):694–698. doi: 10.1016/j.placenta.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 36.Lamadrid-Figueroa H, Tellez-Rojo MM, Hernandez-Cadena L, Mercado-Garcia A, Smith D, Solano-Gonzalez M, et al. Biological markers of fetal lead exposure at each stage of pregnancy. J Toxicol Environ Health A. 2006 Oct;69(19):1781–1796. doi: 10.1080/15287390600630195. [DOI] [PubMed] [Google Scholar]