INTRODUCTION
This article reviews canine and feline brain aging. Several key features are discussed and compared, including general aging characteristics and neuropathology. Aging dogs and cats show many similarities in terms of brain changes but also some important differences. Several research groups have been working with aging dogs and cats to test various theories of aging and to develop therapeutics that will be beneficial to both species.
The median life span of dogs varies as a function of breed, with larger breeds typically having shorter life spans than smaller breeds.1–3 For the purposes of this article, several studies that are described have been collected in purpose-bred beagles and additional companion animals and clinical data are shared when available. Beagles have a median life span of 13.9 years, with no significant differences between males and females.4 A young beagle less than 5 years old is similar to humans who are less than 40 years old.3 Middle-aged beagles between 5 and 9 years are similar to humans between 40 and 60 years and beagles more than 9 years old are similar to humans more than 60 years old. However, the larger the breed of dog, the shorter the life span and thus biological age may vary across breeds given a specific age.1
In a laboratory setting and in the veterinary clinic, studies of aging dogs report that some but not all aged dogs are impaired on different measures of learning and memory (see Refs.5–7). Not all old dogs are impaired and not all types of learning and memory are equally affected. Neurobiological changes, as described later, can account for some, but not all, of the clinical signs of cognitive decline in aging dogs.
NEUROBIOLOGY OF AGING IN THE DOG
This article describes several neurobiological changes associated with aging in dogs (Table 1).
Table 1.
Comparison of dog and cat neurologic neurodegenerative features
| Neurobiological Outcome Measures | Canine | Feline | NCL or MPSI | NPC Disease |
|---|---|---|---|---|
| Brain atrophy | Yes | NA | Yes | Yes |
| β-Amyloid | Yes | Yes | Yes | Yes |
| Tau | Yes | Yes | NA | Yes |
| Cerebral amyloid angiopathy | Yes | Yes | NA | NA |
| Infarcts | Yes | NA | NA | NA |
| Vascular disease | Yes | NA | Yes | NA |
| Lipofuscin accumulation | Yes | Yes | Yes | NA |
| Caspase activation | Yes | NA | Yes | Yes |
| DNA fragmentation | Yes | NA | NA | NA |
| Neuron loss: hippocampus | Yes | NA | Yes | Yes |
| Neuron loss: caudate | NA | Yes | Yes | Yes |
| Neuron loss: locus coeruleus | Yes | Yes | NA | NA |
| Neuron loss: Purkinje cerebellar cells | Yes | NA | Yes | Yes |
| White matter degeneration | Yes | NA | Yes | Yes |
| Inflammation | Yes | NA | Yes | Yes |
| Oxidative damage | Yes | NA | Yes | Yes |
| Gliosis | Yes | NA | Yes | Yes |
Abbreviations: MPSI, mucopolysaccharidosis type I; NA, not available; NCL, neuronal ceroid lipofuscinoses; NPC, Niemann-Pick type C.
Brain Atrophy
Old dogs often show marked ventriculomegaly at postmortem examination associated with thinning of the cerebral cortex and the subcortical white matter.8 Magnetic resonance imaging (MRI) studies performed on aged beagle and German shepherd dogs show that cortical atrophy, identified as widened sulci, thinned parenchyma,9 and ventricular dilatation,9–11 progresses with age and is a consistent feature of canine brain aging. Furthermore, MRI studies suggest differential vulnerabilities of specific brain areas to aging. For example, in aging beagle dogs, the prefrontal cortex loses tissue volume at an earlier age (approximately 8–11 years) than does the hippocampus (after 11 years).12 An MRI study by Hasegawa and colleagues13 (2005) suggested that although interthalamic adhesion thickness was smaller in older dogs, those showing age-related cognitive decline and a single dog with GM1 gangliosidosis also had a significant decrease in the thickness.
Periventricular white matter signal abnormalities are frequently seen in MRI of various dog breeds greater than 12 years of age that are evaluated for seizures, vestibular disease, or behavioral abnormalities. These bilaterally symmetric T2-weighted hyperintensities of the internal capsule are suspected to be caused by wallerian degeneration, demyelination, and accompanying gliosis.14
Selective Neuron Loss
Atrophy may result from neuron loss or changes in neuronal density, as reported in normal human brain aging,15,16 although more extensive neuronal loss occurs in Alzheimer disease (AD).17,18 When neurons were counted using unbiased stereological methods within individual subfields of the hippocampus of young (3.4–4.5 years) and old (13.0–15.0 years) dogs, the aged dogs had significantly (~30%) fewer neurons in the hilus of the dentate gyrus.19 A study by Pugliese and colleagues20 (2007) showed that cognitive deficits correlated with loss of Purkinje cells in the cerebellum. More recently, a study by Insua and colleagues21 (2010) examined noradrenergic neurons in the locus coeruleus of aged canines, a group of neurons that are implicated in AD in humans.22,23 Dogs that are cognitively impaired show a significant reduction in noradrenergic neurons. Reduced neurogenesis in the mature brain may also contribute to reduced neuron numbers and age-associated cognitive decline, resulting in slower replacement of dying neurons. In the hippocampus of beagles, a 90% to 95% decline in neurogenesis was measured in aged dogs.24 Similar reductions in neurogenesis in aged dogs have been reported by other investigators.25
Senile Plaques (β-Amyloid) in Dogs
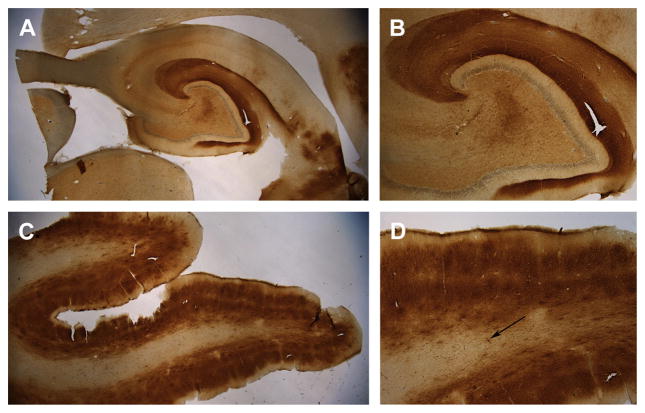
A key feature of canine brain aging was the observation in 1956, by Braumühl, who reported Alzheimer-like senile plaques in aged dogs (reviewed in Ref.26). Senile plaque accumulation in the aged canine brain has been well described.26 Senile plaques are composed of β-amyloid (Aβ) protein, and are one of 2 key types of neurologic pathologies observed in the AD brain.27 The Aβ peptide is produced by the sequential cleavage of the amyloid precursor protein (APP) by beta-secretase and gamma-secretase.28,29 Cleavage by gamma-secretase results in differing lengths of Aβ, with the 42 amino acid form, Aβ1-42 making up most of the insoluble deposits found in the AD brain.30 One of the reasons why the canine brain has been examined extensively for Aβ neurologic disorder is that dogs and humans share an identical amino acid sequence of the protein.31,32 Aβ is thought to be a causative factor for AD in people.33 The observation of brain Aβ first stimulated interest in the use of the dog to model human aging and disease.34 Diffuse plaques are the predominant subtype of Aβ in the aging dog brain (Fig. 1).35–40 Specific brain regions are differentially vulnerable to Aβ.36,41–46 When cortical regions are sampled for Aβ deposition, each region shows a different age of Aβ onset.43 In the dog, Aβ deposition occurs earliest in the prefrontal cortex (see Fig. 1C, D) and later in the temporal cortex, hippocampus (see Fig. 1A, B), and occipital cortex.45
Fig. 1.
Aβ immunostaining (brown) in the brain of a 14-year-old border collie (Martha) with signs of cognitive dysfunction syndrome. Aβ was detected using the 6E10 antibody that binds Aβ 1 to 16. (A) Low-power magnification (1.5×) of extensive Aβ deposition in the hippocampus. (B) Aβ is primarily found in the outer molecular layer of the dentate gyrus, which contains neuron terminals originating from the entorhinal cortex (4×). (C) The prefrontal cortex also shows extensive Aβ deposition that appears most dense in layers II and V of the cortex and is less apparent in the white matter (1.5×). (D) At high magnification (4×), the differential deposition of Aβ in the 6 cortical layers can be seen as well as extensive white matter cerebral amyloid angiopathy (arrow). Sections have been counterstained with cresyl violet (blue).
Beagles that show learning and memory impairments in a laboratory setting with systematic cognitive tests also show higher levels of Aβ plaques than those old dogs without cognitive impairments.47–50 For example, dogs with prefrontal cortex–dependent reversal learning deficits show significantly higher amounts of Aβ in this brain region.48,49 As in laboratory beagles, the extent of Aβ plaques varies as a function of age in companion dogs (including a wide variety of breeds and mixed breeds).50–52 Further, the extent of Aβ plaques correlates with behavior changes and this association remains significant even if age is removed as a covariate.47,50 Case report 1 and Figs. 1 and 2 show a case study of cognitive decline and age-associated Aβ neurologic disorder in a border collie.
Case report 1.
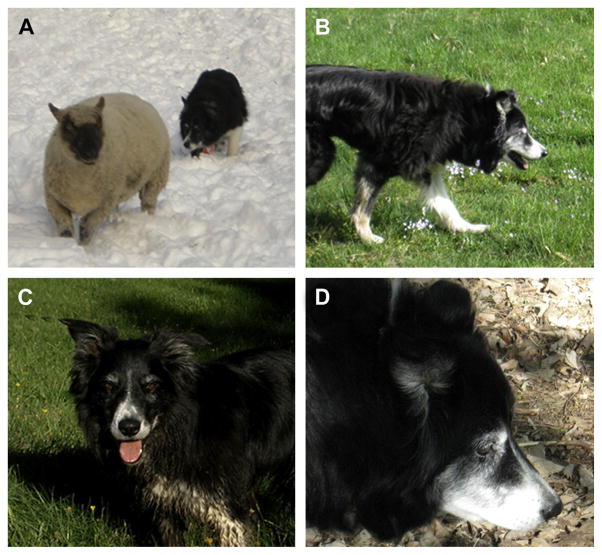
Martha was a female border collie that was a very competent farm dog with what her owner called “lots of sheep savvy.” She was also a fully integrated family member and companion to the family when hiking, skiing, and mountain biking. In addition, she successfully competed in sheep dog trials till the age of 10 years (see Fig. 1A). She and her handler worked their way through the succession of novice-level trials, winning several, and ultimately competing and placing in the highest level, the Open class, which attests to her skill (see Fig. 1B).
By the time Martha was 11 years old she was nearly deaf and slow enough that she could no longer trial. She started to show some confusion with commands at which she was expert. For example, one day her owner took her out in the field and gave her a command to go counter clockwise around the sheep and she went clockwise. Further, when her owner next said “OK, go clockwise” she reversed her direction, when she should have continued the clockwise direction (see Fig. 1C). The next time her owner was working with her, a normally benign ewe turned around to face Martha and stomped her foot. Martha, who would normally have politely stood her ground until the sheep backed down, promptly turned away and left the field. The owner at this time thought, “Well that is proof that Martha is fully retired now.” Not long after the incident with the ewe, the sheep began to act as if Martha did not even exist.
Over the next 2 years, changes in Martha were subtle and included increasing hearing loss and stubbornness. She also stopped having interest in the sheep, and appeared most fixated with another dog (Ida) in the family. Several behaviors with the other dog appeared abnormal. For example, Martha would stare at Ida, the middle dog, whether they were inside or out walking, and then would stalk Ida, despite a lack of interest from Ida in interacting. The owners had to intervene frequently to prevent a snarling episode, although the two dogs never injured each other.
To her last day, Martha’s social skills with human friends persisted. However, she began pacing, panting, and getting stuck under the bedside table at night. During the day when only 1 of the owners was home, Martha was frequently anxious and sometimes clingy, which never used to be the case. She showed signs of disorientation and during walks occasionally lost the younger dogs and her owner although the same daily route was followed. Martha was once found in a road looking up and down as if lost, although she had been walking that direction for years.
Throughout her life Martha had a great appetite and generally excellent health, although she had arthritis in almost every joint in her body and was on high doses of incontinence medication and Rimadyl (a nonsteroidal antiinflammatory drug) for arthritis. For the last year of her life, the owner was unable to get a photograph of Martha looking at her because she was no longer making eye contact (see Fig. 1D).
When Martha was 14 years old, the decision was made to euthanize her and the owner donated her brain to the University of Kentucky to determine whether neurologic disorder was present. Aβ neurologic disorder was extensive in Martha (see Fig. 2).
Fig. 2.
Aging and canine cognitive dysfunction in a border collie (Martha). (A) Martha competing in sheep dog trials when she was young, and (B) still active around the age of 10 years. As she reached 11 years of age she struggled to complete commands issued by her owner (C). At 14 years of age, Martha was generally in good health except for arthritis but would not make eye contact with her owner (D).
The focus in AD pathogenesis has recently shifted from Aβ plaques to considering smaller, soluble forms of Aβ assemblies called Aβ oligomers. Oligomers are highly toxic and impair synaptic function.53 Furthermore, increased oligomer levels are strongly associated with cognitive dysfunction.53–55 A recent study by Pop and colleagues,56 examined the accumulation of oligomeric Aβ in the temporal lobe of canines. This study provided evidence that canines, like humans, experience an increase in toxic oligomers with age.
Vascular Disorders, Cerebrovascular Amyloid Angiopathy
Cerebrovascular amyloid angiopathy (CAA) is the deposition of Aβ in association with the cerebrovasculature (see Fig. 1D). In dogs with CAA, the blood vessels of the brain typically contain the shorter, 40 amino acid–long species of Aβ.57–59 The occipital cortex seems to be particularly vulnerable to CAA in the aged dog brain. Vascular Aβ may compromise the blood-brain barrier, disrupt vessel wall viability,60 and cause microhemorrhages.61
Aged dogs may also show lacunar infarcts of the caudate nucleus and thalamus with most dogs showing no causative metabolic, endocrine, or hypertensive disease.62,63 In a longitudinal study, these lesions were shown to increase in number with advancing age.64 Lacunar infarcts of the caudate nucleus have been induced experimentally in beagle dogs by proximal middle cerebral artery occlusion.65 The cause of naturally occurring lacunar infarcts in dogs remains unidentified.
Tau Neuropathology
Unlike humans but like many other animal species, canines do not develop full-blown neurofibrillary tangles,32,37,39,40 which is the second neuropathologic characteristic of AD. However, aged dogs show the accumulation of several phosphorylated tau epitopes that are consistent with AD in humans and observations in aged cats (described later).66 In a study of cognitively impaired pet dogs that included a variety of breeds, intracellular phosphorylated tau was observed that was similar to the AD brain.67
Oxidative Damage and Mitochondrial Dysfunction
Aging and the production of free radicals can lead to oxidative damage to proteins, lipids, and nucleotides that, in turn, may cause neuronal dysfunction and ultimately neuronal death. In the aging dog, the brain accumulates carbonyl groups, which are a measure of oxidative damage to proteins.68,69 Carbonyl groups are associated with reduced endogenous antioxidant enzyme activity or protein levels, including those of glutamine synthetase and superoxide dismutase (SOD).68,70–72 In addition, increased oxidative damage to proteins can be measured by the end products of lipid peroxidation (oxidative damage to lipids), including 4-hydroxynonenal,50,52,72,73 lipofuscin,50 lipofuscinlike pigments,52,73 or malondialdehyde.68 In addition, oxidative damage to DNA or RNA may be increased in the aged dog brain.5,50
Oxidative damage may also be associated with behavioral decline in dogs. Increased oxidative end products in the aged companion dog brain are correlated with more severe behavioral changes.50,52,69 In laboratory studies of aging beagles, higher protein oxidative damage (3-nitrotyrosine) and lower endogenous antioxidant capacity (SOD and glutathione-S-transferase activities) are associated with poorer prefrontal-dependent and spatial learning.71 Mitochondria are sources of free radicals that damage proteins, lipids, and DNA/RNA.74 In a study of aged beagles, isolated mitochondria showed increased reactive oxygen species production in aged animals relative to young animals.75 Thus, aged dogs show mitochondrial dysfunction and oxidative damage, consistent with humans with age-related neurologic dysfunction.
Correlates of Brain Aging Found in Lysosomal Storage Diseases
Cross-sectional studies of brain aging are negatively influenced by interindividual variations in brain size and structure, as well as by differing rates of atrophy. Longitudinal studies may be limited by repeated access to aged animals and to subject attrition.64 Insight into mechanisms of brain aging can also be obtained through the study of breeding colonies of dogs with naturally occurring hereditary metabolic disorders, including mitochondrial disorders, lysosomal storage diseases, and leukodystrophies, which have clinical, neuropathologic, and biochemical changes similar to those found in old dogs. These disorders allow both cross-sectional and longitudinal studies of disease progression in related animals with short life spans.76,77 For example, more than 40 inherited lysosomal storage diseases (LSDs) have been identified, with many characterized by progressive cognitive decline, memory loss, brain atrophy, loss of myelin, and region-specific loss of neurons as are seen in aging.78,79 Two well-studied diseases are examples of what has been learned by studying affected dogs. The neuronal ceroid lipofuscinoses (NCLs) are characterized by seizures, motor dysfunction, impaired vision, progressive cognitive decline, impaired memory, behavioral problems, brain degeneration, selective neuronal loss that is most severe in the cerebral cortex, white matter atrophy, neuronal accumulation of protein and lipid, and premature death.80,81 Both human patients and affected dogs show various ages of onset, disease course, and neuropathology.80–82 The juvenile-onset form of the disease, known as Batten disease, has naturally occurring analogous disease in English setters, Tibetan terriers, and border collies.83 Biochemical analysis of the brain shows that storage of subunit c of the mitochondrial ATP synthase complex occurs; subunit c is an essential membrane component of the proton channel of the ATP synthase complex, which generates ATP by oxidative phosphorylation.81 Although not fully characterized, the neuronal loss seen in Batten disease is postulated to be caused by mitochondrial dysfunction and energy-linked excitotoxicity.83 Synaptic loss and glial activation also occur in this disease, although the mechanism and contribution of these abnormalities to disease progression are not yet understood.80
The T-maze reversal learning task has been used to evaluate progressive cognitive decline in the dachshund model of late-infantile NCL, which is caused by a mutation in the gene encoding the enzyme tripeptidyl-peptidase 1 (TTP1).84 This model system is particularly interesting because intrathecal administration of TTP1 results in decreased lysosomal storage.85 It is likely that a better understanding of how mitochondrial dysfunction and neuronal loss develop in the NCLs, as well as how they may be reversed with therapy, will shed light on similar mechanisms postulated to occur in brain aging.
Mucopolysaccharidosis type I (MPSI; Hurler syndrome) is another LSD characterized by progressive cognitive decline, including impaired memory and intelligence, in human patients.86 Affected humans and dogs show progressive cortical atrophy, ventricular enlargement, and white matter loss.87 Cardiovascular disease and accumulation of intracellular Aβ are also described in affected patients.88,89 In human patients with MPSI, MRI showed that corpus callosum volume and fractional anisotropy correlated with neuropsychological testing.86 In dogs with MPSI, imaging studies showed corpus callosum volumes to be smaller in affected dogs compared with unaffected dogs; no cognitive studies have yet been performed. Similar to the late-infantile NCL, this disease is amenable to either whole-brain therapy by intrathecal enzyme replacement therapy or to regional gene therapy allowing for the assessment of therapeutic effect.90,91 When treated with intrathecal alpha-iduronidase, the enzyme that is deficient in MPSI, corpus callosum volumes in affected dogs were no longer distinguishable from those in unaffected dogs. These studies indicate the usefulness of quantitative imaging to study brain atrophy over time as well as the effectiveness of therapy in ameliorating the atrophy.
NEUROBIOLOGY OF AGING IN THE CAT
Cats are considered to be old, or senior, starting around the age of 7 to 10 years but consistently after 12 years, depending on the individual animal. Aging cats show several behavioral changes that can be of concern to a pet owner and that are not related to systemic illness or disease.92 Whether there is cognitive decline in aging cats as observed in dogs is still being studied but there are behavioral changes that have been reported clinically.93 There are fewer studies of the aging cat brain compared with aging dogs in the literature, highlighting the significant gaps in knowledge regarding feline brain aging. Several reviews have been written describing feline age-associated neurologic disorder and some of those observations are summarized here.92,93
Neuron Loss and Atrophy
There are several studies suggesting that the caudate nucleus of aging cats is affected by aging, including reduced neuronal numbers and reduction in the density of synapses.94–97 These losses may lead to impairments in motor function.94 The locus coeruleus, a key nucleus responsible for producing the neurotransmitter acetylcholine, which is associated with learning and memory, also shows neuronal losses with age in cats.98
Aβ
Aβ is typically observed in cats more than the age of 10 years,99–103 although there is a report of 7.5-year-old animal showing Aβ disorder.102 Feline Aβ deposition seems to be different from that of dogs and humans in several respects. Unlike human and dog brain, plaques in the cat brain are primarily made up of Aβ1-42 without Aβ1-40 and the peptide is not posttranslationally modified (suggesting a more rapid turnover) in cats compared with dogs and humans.66,99 Further, truncated Aβ (AβpN3) was absent in aged cat brain.104 Blood vessels in aging cat brains are positive for Aβ1-40, the shorter more soluble form of Aβ.66 To our knowledge, there are few reports of the link between behavioral dysfunction and the extent of Aβ disorder as reported in dogs. Although cats that show signs of behavioral dysfunction tend to also have Aβ plaques,100 the severity of behavioral changes does not seem to correlate well with the extent of Aβ disorder.66
Tau Phosphorylation
An interesting feature of the aging cat brain is the detection of phosphorylated tau protein, which is consistent with reports in human AD.105,106 Cats show multiple isoforms of tau protein, as do humans.107 Not all studies consistently observe tau neuropathology in cats101,108,109 and this may be because of methodological challenges. In addition, when using sensitive immunohistochemical methods, phosphorylated tau is not as frequently observed as Aβ plaques, but the epitopes on tau that are phosphorylated overlap with those observed in dogs and humans.66 The morphology of neurons that show an accumulation of intracellular phosphorylated tau suggests a sprouting response, which is also similar to human brain.66 Tau phosphorylation is also more frequently associated with the presence of seizures in aging cats.100
Neuronal Loss in Feline Niemann-Pick Type C Disease and Similarities to AD
Niemann-Pick type C (NPC) disease is another example of how LSDs may contribute to the understanding of neuronal loss, oxidative stress, Aβ deposition, and tau neuropathology in the aging brain. NPC disease is caused by dysfunction of either of 2 proteins, NPC1 or NPC2, which result in lysosomal storage of cholesterol and glycosphingolipids.110 How dysfunction of these proteins results in the dementia and neuronal loss associated with disease has been difficult to determine. A feline model of NPC1 disease exists and has been critical for understanding disease pathogenesis and evaluating therapy.110–115 As disease-modifying therapies are evaluated, clinicians hope to gain insight into the relative contributions of each of the following factors in causing cognitive decline and brain atrophy.
Autophagy, the degradation or recycling of damaged intracellular organelles and aggregated-proteins, is necessary for cellular homeostasis. NPC1 has been implicated in mediating membrane-tethering events between autophagosomes and late endosomes that subsequently fuse with lysosomes to degrade their contents.116 When NPC1 is defective, autophagy is impaired. Because impaired basal autophagy has been found to cause neurodegeneration,117,118 it is postulated that impaired autophagy in NPC1 disease may contribute to the observed neuronal loss as a consequence of the buildup of dysfunctional mitochondria and the accumulation of misfolded proteins, resulting in cell death. Altered autophagosomal-lysosomal function has similarly been implicated in brain aging.119,120
Oxidative stress and the generation of reactive oxygen species have also been proposed as contributing factors for neuronal loss in NPC disease.121 In vitro studies showed increased oxidative stress in cultured neurons and fibroblasts.122 Evaluation of serum from patients with NPC showed decreased fractions of reduced coenzyme Q10 and decreased Trolox-equivalent antioxidant capacity.121 Evaluation of plasma and cerebrospinal fluid from patients and affected cats showed accumulation of cholesterol oxidation products, and, in cats, these oxysterols decreased in response to a disease-modifying therapy.122
In addition, both NPC1 disease and AD are marked by dementia and abnormal cholesterol metabolism; however, they also share abnormalities in Aβ processing and the presence of neurofibrillary tangles.110,123 First, in both affected humans and cats, relative Aβ peptide distributions differ from those found in unaffected individuals, with lower relative levels of Aβ1-37, Aβ1-38 and Aβ1-39 in cats with NPC1 compared with controls.124 Second, neurofibrillary tangles composed of paired helical filaments of phosphorylated microtubular protein accumulate in NPC disease as they do in AD.110 Third, apolipoprotein E4, the isoform associated with increased risk for developing AD, has also been found to be associated with early onset of disease and with increased NPC1 disease severity.123 Other deficits seen in NPC1 disease, including peroxisomal dysfunction,125 abnormal sphingosine metabolism,126 neuroinflammation,127 and induction of apoptosis,128 may also contribute to changes seen in the aging brain.129
CLINICAL IMPLICATIONS OF BRAIN AGING IN DOGS AND CATS
Laboratory-based studies of cognition in beagles suggest that there are age-dependent functional changes related to brain disorders. In the clinic, owners of geriatric dogs frequently report behavioral changes.130–132 Some of these behavioral changes may be linked to systemic illness or other health issues (including sensory decline). When ruled out, a subset of older dogs shows evidence of behavioral changes that are now considered to be signs of canine cognitive dysfunction (CCD). The original reports of CCD were by Ruehl and colleagues133 in the mid 1990s and several reports followed, along with the development of new tools to detect CCD in pet dogs.6,7,93,134,135 CCD has been linked to brain pathology in pet dogs.20,136,137 There are reports that CCD can be reduced in aging dogs through treatment with various pharmaceuticals (eg, Anipryl),134 by dietary intervention using a prescription diet (B/D diet Hills Pet Nutrition134; the same diet used in laboratory studies showing a benefit to cognition and neuropathology138), and to immunotherapy using anti-Aβ approaches.139 In cats, there is less evidence of a form of feline cognitive dysfunction syndrome but evidence is accumulating that a similar phenomenon can occur.66,92,93,100,140
SUMMARY
Aging dogs and cats show features of brain disorders that can be similar to human aging and AD. Neuropathologic changes with age may be linked to signs of cognitive dysfunction both in the laboratory and in a clinic setting. Less is known about cat brain aging and cognition and this represents an area for further study. Neurodegenerative diseases such as LSDs in dogs and cats also show similar features of aging, suggesting some common underlying pathogenic mechanisms and also suggesting pathways that can be modified to promote healthy brain aging.
KEY POINTS.
Brain atrophy, neuron loss, decreased neurogenesis, and oxidative stress but few tau-associated disorders are observed in aging dog brains.
Cerebrovascular pathology can be extensive in canine brain aging.
β-Amyloid protein, associated with Alzheimer disease in humans, is increased with age in the dog brain and is linked to signs of learning and memory impairments.
Lysosomal storage diseases in dogs are associated with similar types of neuropathology as are observed with aging and Alzheimer disease.
Few studies describe the neurobiology of aging in cats but interesting similarities and differences from dogs have been reported.
Feline Niemann-Pick type C disease has several neuropathologic and clinical similarities to Alzheimer disease.
Acknowledgments
The authors are grateful to Virginia Price, DVM, and her stories of Martha and donation of her tissue for research. We thank Judianne Davis-Van Nostrand and Dr William Van Nostrand at Stony Brook University in New York for help with collecting tissue for the study. Ms Katie McCarty collected the Aβ immunostaining data for Martha.
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under award number R01AG031764 (E. Head) and R01NS073661 and P40-02512 (C.H. Vite, Mark Haskins), and the Ara Parseghian Medical Research Foundation (C.H. Vite). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Greer KA, Canterberry SC, Murphy KE. Statistical analysis regarding the effects of height and weight on life span of the domestic dog. Res Vet Sci. 2007;82(2):208–14. doi: 10.1016/j.rvsc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Galis F, Van der Sluijs I, Van Dooren TJ, et al. Do large dogs die young? J Exp Zool B Mol Dev Evol. 2007;308(2):119–26. doi: 10.1002/jez.b.21116. [DOI] [PubMed] [Google Scholar]
- 3.Patronek GJ, Waters DJ, Glickman LT. Comparative longevity of pet dogs and humans: implications for gerontology research. J Gerontol A Biol Sci Med Sci. 1997;52(3):B171–8. doi: 10.1093/gerona/52a.3.b171. [DOI] [PubMed] [Google Scholar]
- 4.Lowseth LA, Gillett NA, Gerlach RF, et al. The effects of aging on hematology and serum chemistry values in the beagle dog. Vet Clin Pathol. 1990;19(1):13–9. doi: 10.1111/j.1939-165x.1990.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 5.Cotman CW, Head E. The canine (dog) model of human aging and disease: dietary, environmental and immunotherapy approaches. J Alzheimers Dis. 2008;15(4):685–707. doi: 10.3233/jad-2008-15413. [DOI] [PubMed] [Google Scholar]
- 6.Landsberg G, Araujo JA. Behavior problems in geriatric pets. Vet Clin North Am Small Anim Pract. 2005;35(3):675–98. doi: 10.1016/j.cvsm.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Landsberg G, Ruehl W. Geriatric behavioral problems. Vet Clin North Am Small Anim Pract. 1997;27(6):1537–59. doi: 10.1016/s0195-5616(97)50138-0. [DOI] [PubMed] [Google Scholar]
- 8.Vandevelde M, Higgins RJ, Oevermann A. Veterinary neuropathology: essentials of theory and practice. West Sussex (United Kingdom): Wiley-Blackwell; 2012. [Google Scholar]
- 9.Su MY, Head E, Brooks WM, et al. MR imaging of anatomic and vascular characteristics in a canine model of human aging. Neurobiol Aging. 1998;19(5):479–85. doi: 10.1016/s0197-4580(98)00081-5. [DOI] [PubMed] [Google Scholar]
- 10.Kimotsuki T, Nagaoka T, Yasuda M, et al. Changes of magnetic resonance imaging on the brain in beagle dogs with aging. J Vet Med Sci. 2005;67(10):961–7. doi: 10.1292/jvms.67.961. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Soriano J, Marin Garcia P, Contreras-Rodriguez J, et al. Age-related changes in the ventricular system of the dog brain. Ann Anat. 2001;183(3):283–91. doi: 10.1016/S0940-9602(01)80236-3. [DOI] [PubMed] [Google Scholar]
- 12.Tapp PD, Siwak CT, Gao FQ, et al. Frontal lobe volume, function, and beta-amyloid pathology in a canine model of aging. J Neurosci. 2004;24(38):8205–13. doi: 10.1523/JNEUROSCI.1339-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasegawa D, Yayoshi N, Fujita Y, et al. Measurement of interthalamic adhesion thickness as a criteria for brain atrophy in dogs with and without cognitive dysfunction (dementia) Vet Radiol Ultrasound. 2005;46(6):452–7. doi: 10.1111/j.1740-8261.2005.00083.x. [DOI] [PubMed] [Google Scholar]
- 14.Grossman RI, Yousem DM. Neuroradiology: the requisites. Philadelphia: Mosby; 2003. [Google Scholar]
- 15.Simic G, Kostovic I, Winblad B, et al. Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer’s disease. J Comp Neurol. 1997;379(4):482–94. doi: 10.1002/(sici)1096-9861(19970324)379:4<482::aid-cne2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 16.West MJ. Regionally specific loss of neurons in the aging human hippocampus. Neurobiol Aging. 1993;14:287–93. doi: 10.1016/0197-4580(93)90113-p. [DOI] [PubMed] [Google Scholar]
- 17.West MJ, Kawas CH, Martin LJ, et al. The CA1 region of the human hippocampus is a hot spot in Alzheimer’s disease. Ann N Y Acad Sci. 2000;908:255–9. doi: 10.1111/j.1749-6632.2000.tb06652.x. [DOI] [PubMed] [Google Scholar]
- 18.Bobinski M, Wegiel J, Tarnawski M, et al. Relationships between regional neuronal loss and neurofibrillary changes in the hippocampal formation and duration and severity of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56(4):414–20. doi: 10.1097/00005072-199704000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Siwak-Tapp CT, Head E, Muggenburg BA, et al. Region specific neuron loss in the aged canine hippocampus is reduced by enrichment. Neurobiol Aging. 2008;29(1):39–50. doi: 10.1016/j.neurobiolaging.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pugliese M, Gangitano C, Ceccariglia S, et al. Canine cognitive dysfunction and the cerebellum: acetylcholinesterase reduction, neuronal and glial changes. Brain Res. 2007;1139:85–94. doi: 10.1016/j.brainres.2006.12.090. [DOI] [PubMed] [Google Scholar]
- 21.Insua D, Suarez ML, Santamarina G, et al. Dogs with canine counterpart of Alzheimer’s disease lose noradrenergic neurons. Neurobiol Aging. 2008;31(4):625–35. doi: 10.1016/j.neurobiolaging.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Dringenberg HC. Alzheimer’s disease: more than a ‘cholinergic disorder’ - evidence that cholinergic-monoaminergic interactions contribute to EEG slowing and dementia. Behav Brain Res. 2000;115(2):235–49. doi: 10.1016/s0166-4328(00)00261-8. [DOI] [PubMed] [Google Scholar]
- 23.Grudzien A, Shaw P, Weintraub S, et al. Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer’s disease. Neurobiol Aging. 2007;28(3):327–35. doi: 10.1016/j.neurobiolaging.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Siwak-Tapp CT, Head E, Muggenburg BA, et al. Neurogenesis decreases with age in the canine hippocampus and correlates with cognitive function. Neurobiol Learn Mem. 2007;88(2):249–59. doi: 10.1016/j.nlm.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pekcec A, Baumgartner W, Bankstahl JP, et al. Effect of aging on neurogenesis in the canine brain. Aging Cell. 2008;7(3):368–74. doi: 10.1111/j.1474-9726.2008.00392.x. [DOI] [PubMed] [Google Scholar]
- 26.Cummings BJ, Head E, Ruehl W, et al. The canine as an animal model of human aging and dementia. Neurobiol Aging. 1996;17(2):259–68. doi: 10.1016/0197-4580(95)02060-8. [DOI] [PubMed] [Google Scholar]
- 27.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41(4):479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 28.Murphy MP, LeVine H., 3rd Alzheimer’s disease and the amyloid-beta peptide. J Alzheimers Dis. 2010;19(1):311–23. doi: 10.3233/JAD-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selkoe DJ. Amyloid beta-protein and the genetics of Alzheimer’s disease. J Biol Chem. 1996;271:18295–8. doi: 10.1074/jbc.271.31.18295. [DOI] [PubMed] [Google Scholar]
- 30.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81(2):741–66. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 31.Johnstone EM, Chaney MO, Norris FH, et al. Conservation of the sequence of the Alzheimer’s disease amyloid peptide in dog, polar bear and five other mammals by cross-species polymerase chain reaction analysis. Brain Res Mol Brain Res. 1991;10(4):299–305. doi: 10.1016/0169-328x(91)90088-f. [DOI] [PubMed] [Google Scholar]
- 32.Selkoe DJ, Bell DS, Podlisny MB, et al. Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer’s disease. Science. 1987;235(4791):873–7. doi: 10.1126/science.3544219. [DOI] [PubMed] [Google Scholar]
- 33.Hardy J. Alzheimer’s disease: the amyloid cascade hypothesis: an update and reappraisal. J Alzheimers Dis. 2006;9(3 Suppl):151–3. doi: 10.3233/jad-2006-9s317. [DOI] [PubMed] [Google Scholar]
- 34.Wisniewski HM, Wegiel J, Morys J, et al. Aged dogs: an animal model to study beta-protein amyloidogenesis. In: Maurer K, Riederer P, Beckman H, editors. Alzheimer’s disease. Epidemiology, neuropathology, neurochemistry and clinics. New York: Springer-Verlag; 1990. pp. 151–67. [Google Scholar]
- 35.Cummings BJ, Su JH, Cotman CW, et al. Beta-amyloid accumulation in aged canine brain: a model of early plaque formation in Alzheimer’s disease. Neurobiol Aging. 1993;14(6):547–60. doi: 10.1016/0197-4580(93)90038-d. [DOI] [PubMed] [Google Scholar]
- 36.Giaccone G, Verga L, Finazzi M, et al. Cerebral preamyloid deposits and congophilic angiopathy in aged dogs. Neurosci Lett. 1990;114:178–83. doi: 10.1016/0304-3940(90)90068-k. [DOI] [PubMed] [Google Scholar]
- 37.Morys J, Narkiewicz O, Maciejewska B, et al. Amyloid deposits and loss of neurones in the claustrum of the aged dog. Neuroreport. 1994;5(14):1825–8. doi: 10.1097/00001756-199409080-00035. [DOI] [PubMed] [Google Scholar]
- 38.Okuda R, Uchida K, Tateyama S, et al. The distribution of amyloid beta precursor protein in canine brain. Acta Neuropathol. 1994;87:161–7. doi: 10.1007/BF00296186. [DOI] [PubMed] [Google Scholar]
- 39.Russell MJ, White R, Patel E, et al. Familial influence on plaque formation in the beagle brain. Neuroreport. 1992;3(12):1093–6. doi: 10.1097/00001756-199212000-00015. [DOI] [PubMed] [Google Scholar]
- 40.Uchida K, Tani Y, Uetsuka K, et al. Immunohistochemical studies on canine cerebral amyloid angiopathy and senile plaques. J Vet Med Sci. 1992;54(4):659–67. doi: 10.1292/jvms.54.659. [DOI] [PubMed] [Google Scholar]
- 41.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 42.Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993;33:403–8. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- 43.Head E, McCleary R, Hahn FF, et al. Region-specific age at onset of beta-amyloid in dogs. Neurobiol Aging. 2000;21(1):89–96. doi: 10.1016/s0197-4580(00)00093-2. [DOI] [PubMed] [Google Scholar]
- 44.Ishihara T, Gondo T, Takahashi M, et al. Immunohistochemical and immunoelectron microscopical characterization of cerebrovascular and senile plaque amyloid in aged dogs’ brains. Brain Res. 1991;548:196–205. doi: 10.1016/0006-8993(91)91122-h. [DOI] [PubMed] [Google Scholar]
- 45.Thal DR, Rub U, Orantes M, et al. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791–800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 46.Wisniewski HM, Johnson AB, Raine CS, et al. Senile plaques and cerebral amyloidosis in aged dogs. Lab Invest. 1970;23:287–96. [PubMed] [Google Scholar]
- 47.Colle MA, Hauw JJ, Crespeau F, et al. Vascular and parenchymal Ab deposition in the aging dog: correlation with behavior. Neurobiol Aging. 2000;21:695–704. doi: 10.1016/s0197-4580(00)00113-5. [DOI] [PubMed] [Google Scholar]
- 48.Cummings BJ, Head E, Afagh AJ, et al. Beta-amyloid accumulation correlates with cognitive dysfunction in the aged canine. Neurobiol Learn Mem. 1996;66(1):11–23. doi: 10.1006/nlme.1996.0039. [DOI] [PubMed] [Google Scholar]
- 49.Head E, Callahan H, Muggenburg BA, et al. Visual-discrimination learning ability and beta-amyloid accumulation in the dog. Neurobiol Aging. 1998;19(5):415–25. doi: 10.1016/s0197-4580(98)00084-0. [DOI] [PubMed] [Google Scholar]
- 50.Rofina JE, van Ederen AM, Toussaint MJ, et al. Cognitive disturbances in old dogs suffering from the canine counterpart of Alzheimer’s disease. Brain Res. 2006;1069(1):216–26. doi: 10.1016/j.brainres.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 51.Rofina J, van Andel I, van Ederen AM, et al. Canine counterpart of senile dementia of the Alzheimer type: amyloid plaques near capillaries but lack of spatial relationship with activated microglia and macrophages. Amyloid. 2003;10(2):86–96. doi: 10.3109/13506120309041730. [DOI] [PubMed] [Google Scholar]
- 52.Rofina JE, Singh K, Skoumalova-Vesela A, et al. Histochemical accumulation of oxidative damage products is associated with Alzheimer-like pathology in the canine. Amyloid. 2004;11(2):90–100. doi: 10.1080/13506120412331285779. [DOI] [PubMed] [Google Scholar]
- 53.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192(1):106–13. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tomic JL, Pensalfini A, Head E, et al. Soluble fibrillar oligomer levels are elevated in Alzheimer’s disease brain and correlate with cognitive dysfunction. Neurobiol Dis. 2009;35(3):352–8. doi: 10.1016/j.nbd.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lacor PN, Buniel MC, Chang L, et al. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci. 2004;24(45):10191–200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pop V, Head E, Berchtold NC, et al. Aβ aggregation profiles and shifts in APP processing favor amyloidogenesis in canines. Neurobiol Aging. 2012;33(1):108–20. doi: 10.1016/j.neurobiolaging.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Attems J. Sporadic cerebral amyloid angiopathy: pathology, clinical implications, and possible pathomechanisms. Acta Neuropathol. 2005;110(4):345–59. doi: 10.1007/s00401-005-1074-9. [DOI] [PubMed] [Google Scholar]
- 58.Attems J, Jellinger KA, Lintner F. Alzheimer’s disease pathology influences severity and topographical distribution of cerebral amyloid angiopathy. Acta Neuropathol. 2005;110(3):222–31. doi: 10.1007/s00401-005-1064-y. [DOI] [PubMed] [Google Scholar]
- 59.Herzig MC, Van Nostrand WE, Jucker M. Mechanism of cerebral beta-amyloid angiopathy: murine and cellular models. Brain Pathol. 2006;16(1):40–54. doi: 10.1111/j.1750-3639.2006.tb00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prior R, D’Urso D, Frank R, et al. Loss of vessel wall viability in cerebral amyloid angiopathy. Neuroreport. 1996;7:562–4. doi: 10.1097/00001756-199601310-00044. [DOI] [PubMed] [Google Scholar]
- 61.Uchida K, Nakayama H, Goto N. Pathological studies on cerebral amyloid angiopathy, senile plaques and amyloid deposition in visceral organs in aged dogs. J Vet Med Sci. 1991;53(6):1037–42. doi: 10.1292/jvms.53.1037. [DOI] [PubMed] [Google Scholar]
- 62.Garosi L, McConnell JF, Platt SR, et al. Clinical and topographic magnetic resonance characteristics of suspected brain infarction in 40 dogs. J Vet Intern Med. 2006;20(2):311–21. doi: 10.1892/0891-6640(2006)20[311:catmrc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 63.Goncalves R, Carrera I, Garosi L, et al. Clinical and topographic magnetic resonance imaging characteristics of suspected thalamic infarcts in 16 dogs. Vet J. 2011;188(1):39–43. doi: 10.1016/j.tvjl.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 64.Su MY, Tapp PD, Vu L, et al. A longitudinal study of brain morphometrics using serial magnetic resonance imaging analysis in a canine model of aging. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(3):389–97. doi: 10.1016/j.pnpbp.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 65.Liu S, Hu WX, Zu QQ, et al. A novel embolic stroke model resembling lacunar infarction following proximal middle cerebral artery occlusion in beagle dogs. J Neurosci Methods. 2012;209(1):90–6. doi: 10.1016/j.jneumeth.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 66.Head E, Moffat K, Das P, et al. Beta-amyloid deposition and tau phosphorylation in clinically characterized aged cats. Neurobiol Aging. 2005;26(5):749–63. doi: 10.1016/j.neurobiolaging.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 67.Yu CH, Song GS, Yhee JY, et al. Histopathological and immunohistochemical comparison of the brain of human patients with Alzheimer’s disease and the brain of aged dogs with cognitive dysfunction. J Comp Pathol. 2011;145(1):45–58. doi: 10.1016/j.jcpa.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 68.Head E, Liu J, Hagen TM, et al. Oxidative damage increases with age in a canine model of human brain aging. J Neurochem. 2002;82:375–81. doi: 10.1046/j.1471-4159.2002.00969.x. [DOI] [PubMed] [Google Scholar]
- 69.Skoumalova A, Rofina J, Schwippelova Z, et al. The role of free radicals in canine counterpart of senile dementia of the Alzheimer type. Exp Gerontol. 2003;38:711–9. doi: 10.1016/s0531-5565(03)00071-8. [DOI] [PubMed] [Google Scholar]
- 70.Kiatipattanasakul W, Nakamura S, Kuroki K, et al. Immunohistochemical detection of anti-oxidative stress enzymes in the dog brain. Neuropathology. 1997;17:307–12. [Google Scholar]
- 71.Opii WO, Joshi G, Head E, et al. Proteomic identification of brain proteins in the canine model of human aging following a long-term treatment with antioxidants and a program of behavioral enrichment: relevance to Alzheimer’s disease. Neurobiol Aging. 2008;29(1):51–70. doi: 10.1016/j.neurobiolaging.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hwang IK, Yoon YS, Yoo KY, et al. Differences in lipid peroxidation and Cu,Zn-superoxide dismutase in the hippocampal CA1 region between adult and aged dogs. J Vet Med Sci. 2008;70(3):273–7. doi: 10.1292/jvms.70.273. [DOI] [PubMed] [Google Scholar]
- 73.Papaioannou N, Tooten PC, van Ederen AM, et al. Immunohistochemical investigation of the brain of aged dogs. I. Detection of neurofibrillary tangles and of 4-hydroxynonenal protein, an oxidative damage product, in senile plaques. Amyloid. 2001;8:11–21. doi: 10.3109/13506120108993810. [DOI] [PubMed] [Google Scholar]
- 74.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994;91:10771–8. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Head E, Nukala VN, Fenoglio KA, et al. Effects of age, dietary, and behavioral enrichment on brain mitochondria in a canine model of human aging. Exp Neurol. 2009;220(1):171–6. doi: 10.1016/j.expneurol.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuruppu DK, Matthews BR. Young-onset dementia. Semin Neurol. 2013;33(4):365–85. doi: 10.1055/s-0033-1359320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Swain GP, Prociuk M, Bagel JH, et al. Adeno-associated virus serotypes 9 and rh10 mediate strong neuronal transduction of the dog brain. Gene Ther. 2014;21(1):28–36. doi: 10.1038/gt.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ellinwood NM, Vite CH, Haskins ME. Gene therapy for lysosomal storage diseases: the lessons and promise of animal models. J Gene Med. 2004;6(5):481–506. doi: 10.1002/jgm.581. [DOI] [PubMed] [Google Scholar]
- 79.Raz N, Rodrigue KM, Haacke EM. Brain aging and its modifiers: insights from in vivo neuromorphometry and susceptibility weighted imaging. Ann N Y Acad Sci. 2007;1097:84–93. doi: 10.1196/annals.1379.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dolisca SB, Mehta M, Pearce DA, et al. Batten disease: clinical aspects, molecular mechanisms, translational science, and future directions. J Child Neurol. 2013;28(9):1074–100. doi: 10.1177/0883073813493665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hofmann SL, Peltonen L. The Neuronal Ceroid Lipofuscinoses. In: Valle D, Beaudet AL, Vogelstein B, et al., editors. OMMBID - The Online Metabolic and Molecular Bases of Inherited Diseases. New York, NY: McGraw-Hill; 2014. [Accessed August 20, 2014]. http://ommbid.mhmedical.com/content.aspx?bookid=474&Sectionid=45374157. [Google Scholar]
- 82.Jolly RD. Comparative biology of the neuronal ceroid-lipofuscinoses (NCL): an overview. Am J Med Genet. 1995;57(2):307–11. doi: 10.1002/ajmg.1320570240. [DOI] [PubMed] [Google Scholar]
- 83.Jolly RD, Brown S, Das AM, et al. Mitochondrial dysfunction in the neuronal ceroid-lipofuscinoses (Batten disease) Neurochem Int. 2002;40(6):565–71. doi: 10.1016/s0197-0186(01)00128-0. [DOI] [PubMed] [Google Scholar]
- 84.Sanders DN, Kanazono S, Wininger FA, et al. A reversal learning task detects cognitive deficits in a Dachshund model of late-infantile neuronal ceroid lipofuscinosis. Genes Brain Behav. 2011;10(7):798–804. doi: 10.1111/j.1601-183X.2011.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vuillemenot BR, Katz ML, Coates JR, et al. Intrathecal tripeptidyl-peptidase 1 reduces lysosomal storage in a canine model of late infantile neuronal ceroid lipofuscinosis. Mol Genet Metab. 2011;104(3):325–37. doi: 10.1016/j.ymgme.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 86.Shapiro E, Guler OE, Rudser K, et al. An exploratory study of brain function and structure in mucopolysaccharidosis type I: long term observations following hematopoietic cell transplantation (HCT) Mol Genet Metab. 2012;107(1–2):116–21. doi: 10.1016/j.ymgme.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vite CH, Nestrasil I, Mlikotic A, et al. Features of brain MRI in dogs with treated and untreated mucopolysaccharidosis type I. Comp Med. 2013;63(2):163–73. [PMC free article] [PubMed] [Google Scholar]
- 88.Pelissier C, Roudier M, Boller F. Factorial validation of the severe impairment battery for patients with Alzheimer’s disease. A pilot study. Dement Geriatr Cogn Disord. 2002;13(2):95–100. doi: 10.1159/000048640. [DOI] [PubMed] [Google Scholar]
- 89.Ferris S, Karantzoulis S, Somogyi M, et al. Rivastigmine in moderately severe-to-severe Alzheimer’s disease: severe impairment battery factor analysis. Alzheimers Res Ther. 2013;5(6):63. doi: 10.1186/alzrt229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dierenfeld AD, McEntee MF, Vogler CA, et al. Replacing the enzyme alpha-L-iduronidase at birth ameliorates symptoms in the brain and periphery of dogs with mucopolysaccharidosis type I. Sci Transl Med. 2010;2(60):60ra89. doi: 10.1126/scitranslmed.3001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen A, Vogler C, McEntee M, et al. Glycosaminoglycan storage in neuroanatomical regions of mucopolysaccharidosis I dogs following intrathecal recombinant human iduronidase. APMIS. 2011;119(8):513–21. doi: 10.1111/j.1600-0463.2011.02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gunn-Moore D, Moffat K, Christie LA, et al. Cognitive dysfunction and the neurobiology of ageing in cats. J Small Anim Pract. 2007;48(10):546–53. doi: 10.1111/j.1748-5827.2007.00386.x. [DOI] [PubMed] [Google Scholar]
- 93.Landsberg GM, Denenberg S, Araujo JA. Cognitive dysfunction in cats: a syndrome we used to dismiss as ‘old age’. J Feline Med Surg. 2010;12(11):837–48. doi: 10.1016/j.jfms.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Levine MS, Lloyd RL, Fisher RS, et al. Sensory, motor and cognitive alterations in aged cats. Neurobiol Aging. 1987;8:253–63. doi: 10.1016/0197-4580(87)90010-8. [DOI] [PubMed] [Google Scholar]
- 95.Levine MS, Adinolfi AM, Fisher RS, et al. Quantitative morphology of medium-sized caudate spiny neurons in aged cats. Neurobiol Aging. 1986;7(4):277–86. doi: 10.1016/0197-4580(86)90008-4. [DOI] [PubMed] [Google Scholar]
- 96.Levine MS, Adinolfi AM, Fisher RS, et al. Ultrastructural alterations in caudate nucleus in aged cats. Brain Res. 1988;440(2):267–79. doi: 10.1016/0006-8993(88)90995-x. [DOI] [PubMed] [Google Scholar]
- 97.Levine MS. Neurophysiological and morphological alterations in caudate neurons in aged cats. Ann N Y Acad Sci. 1988;515:314–28. doi: 10.1111/j.1749-6632.1988.tb33002.x. [DOI] [PubMed] [Google Scholar]
- 98.Zhang JH, Sampogna S, Morales FR, et al. Age-related changes in cholinergic neurons in the laterodorsal and the pedunculo-pontine tegmental nuclei of cats: a combined light and electron microscopic study. Brain Res. 2005;1052(1):47–55. doi: 10.1016/j.brainres.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 99.Cummings BJ, Satou T, Head E, et al. Diffuse plaques contain C-terminal A beta 42 and not A beta 40: evidence from cats and dogs. Neurobiol Aging. 1996;17(4):653–9. doi: 10.1016/0197-4580(96)00062-0. [DOI] [PubMed] [Google Scholar]
- 100.Gunn-Moore DA, McVee J, Bradshaw JM, et al. Ageing changes in cat brains demonstrated by beta-amyloid and AT8-immunoreactive phosphorylated tau deposits. J Feline Med Surg. 2006;8(4):234–42. doi: 10.1016/j.jfms.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakamura S, Nakayama H, Kiatipattanasakul W, et al. Senile plaques in very aged cats. Acta Neuropathol. 1996;91:437–9. doi: 10.1007/s004010050448. [DOI] [PubMed] [Google Scholar]
- 102.Brellou G, Vlemmas I, Lekkas S, et al. Immunohistochemical investigation of amyloid beta-protein (Abeta) in the brain of aged cats. Histol Histopathol. 2005;20(3):725–31. doi: 10.14670/HH-20.725. [DOI] [PubMed] [Google Scholar]
- 103.Takeuchi Y, Uetsuka K, Murayama M, et al. Complementary distributions of amyloid-beta and neprilysin in the brains of dogs and cats. Vet Pathol. 2008;45(4):455–66. doi: 10.1354/vp.45-4-455. [DOI] [PubMed] [Google Scholar]
- 104.Chambers JK, Mutsuga M, Uchida K, et al. Characterization of AbetapN3 deposition in the brains of dogs of various ages and other animal species. Amyloid. 2011;18(2):63–71. doi: 10.3109/13506129.2011.570385. [DOI] [PubMed] [Google Scholar]
- 105.Goedert M, Jakes R, Crowther RA, et al. The abnormal phosphorylation of tau protein at Ser-202 in Alzheimer disease recapitulates phosphorylation during development. Proc Natl Acad Sci U S A. 1993;90:5066–70. doi: 10.1073/pnas.90.11.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16(3):271–84. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- 107.Janke C, Beck M, Stahl T, et al. Phylogenetic diversity of the expression of the microtubule-associated protein tau: implications for neurodegenerative disorders. Brain Res Mol Brain Res. 1999;68:119–28. doi: 10.1016/s0169-328x(99)00079-0. [DOI] [PubMed] [Google Scholar]
- 108.Braak H, Braak E, Strothjohann M. Abnormally phosphorylated tau protein related to the formation of neurofibrillary tangles and neuropil threads in the cerebral cortex of sheep and goat. Neurosci Lett. 1994;171:1–4. doi: 10.1016/0304-3940(94)90589-4. [DOI] [PubMed] [Google Scholar]
- 109.Kuroki K, Uchida K, Kiatipattanasakul W, et al. Immunohistochemical detection of tau proteins in various non-human animal brains. Neuropathology. 1997;17:174–80. [Google Scholar]
- 110.Walkley SU, Suzuki K. Consequences of NPC1 and NPC2 loss of function in mammalian neurons. Biochim Biophys Acta. 2004;1685(1–3):48–62. doi: 10.1016/j.bbalip.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 111.March PA, Thrall MA, Brown DE, et al. GABAergic neuroaxonal dystrophy and other cytopathological alterations in feline Niemann-Pick disease type C. Acta Neuropathol. 1997;94(2):164–72. doi: 10.1007/s004010050689. [DOI] [PubMed] [Google Scholar]
- 112.Zervas M, Somers KL, Thrall MA, et al. Critical role for glycosphingolipids in Niemann-Pick disease type C. Curr Biol. 2001;11(16):1283–7. doi: 10.1016/s0960-9822(01)00396-7. [DOI] [PubMed] [Google Scholar]
- 113.Somers KL, Brown DE, Fulton R, et al. Effects of dietary cholesterol restriction in a feline model of Niemann-Pick type C disease. J Inherit Metab Dis. 2001;24(4):427–36. doi: 10.1023/a:1010588112003. [DOI] [PubMed] [Google Scholar]
- 114.Ward S, O’Donnell P, Fernandez S, et al. 2-Hydroxypropyl-beta-cyclodextrin raises hearing threshold in normal cats and in cats with Niemann-Pick type C disease. Pediatr Res. 2010;68(1):52–6. doi: 10.1203/PDR.0b013e3181df4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ferris S, Cummings J, Christensen D, et al. Effects of donepezil 23 mg on severe impairment battery domains in patients with moderate to severe Alzheimer’s disease: evaluating the impact of baseline severity. Alzheimers Res Ther. 2013;5(1):12. doi: 10.1186/alzrt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sarkar S, Carroll B, Buganim Y, et al. Impaired autophagy in the lipid-storage disorder Niemann-pick type C1 disease. Cell Rep. 2013;5(5):1302–15. doi: 10.1016/j.celrep.2013.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hara T, Nakamura K, Matsui M, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441(7095):885–9. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 118.Keller JN, Dimayuga E, Chen Q, et al. Autophagy, proteasomes, lipofuscin, and oxidative stress in the aging brain. Int J Biochem Cell Biol. 2004;36(12):2376–91. doi: 10.1016/j.biocel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 119.Komatsu M, Waguri S, Chiba T, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441(7095):880–4. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 120.Kiselyov K, Jennigs JJ, Jr, Rbaibi Y, et al. Autophagy, mitochondria and cell death in lysosomal storage diseases. Autophagy. 2007;3(3):259–62. doi: 10.4161/auto.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fu R, Yanjanin NM, Bianconi S, et al. Oxidative stress in Niemann-Pick disease, type C. Mol Genet Metab. 2010;101(2–3):214–8. doi: 10.1016/j.ymgme.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Porter FD, Scherrer DE, Lanier MH, et al. Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann-Pick C1 disease. Sci Transl Med. 2010;2(56):56ra81. doi: 10.1126/scitranslmed.3001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fu R, Yanjanin NM, Elrick MJ, et al. Apolipoprotein E genotype and neurological disease onset in Niemann-Pick disease, type C1. Am J Med Genet A. 2012;158A(11):2775–80. doi: 10.1002/ajmg.a.35395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mattsson N, Olsson M, Gustavsson MK, et al. Amyloid-beta metabolism in Niemann-Pick C disease models and patients. Metab BrainDis. 2012;27(4):573–85. doi: 10.1007/s11011-012-9332-8. [DOI] [PubMed] [Google Scholar]
- 125.Schedin S, Sindelar PJ, Pentchev P, et al. Peroxisomal impairment in Niemann-Pick type C disease. J Biol Chem. 1997;272(10):6245–51. doi: 10.1074/jbc.272.10.6245. [DOI] [PubMed] [Google Scholar]
- 126.Lloyd-Evans E, Morgan AJ, He X, et al. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat Med. 2008;14(11):1247–55. doi: 10.1038/nm.1876. [DOI] [PubMed] [Google Scholar]
- 127.Baudry M, Yao Y, Simmons D, et al. Postnatal development of inflammation in a murine model of Niemann-Pick type C disease: immunohistochemical observations of microglia and astroglia. Exp Neurol. 2003;184(2):887–903. doi: 10.1016/S0014-4886(03)00345-5. [DOI] [PubMed] [Google Scholar]
- 128.Wu YP, Mizukami H, Matsuda J, et al. Apoptosis accompanied by up-regulation of TNF-alpha death pathway genes in the brain of Niemann-Pick type C disease. Mol Genet Metab. 2005;84(1):9–17. doi: 10.1016/j.ymgme.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 129.van Echten-Deckert G, Walter J. Sphingolipids: critical players in Alzheimer’s disease. Prog Lipid Res. 2012;51(4):378–93. doi: 10.1016/j.plipres.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 130.Houpt KA, Beaver B. Behavioral problems of geriatric dogs and cats. Vet Clin North Am Small Anim Pract. 1981;11(4):643–52. doi: 10.1016/s0195-5616(81)50076-3. [DOI] [PubMed] [Google Scholar]
- 131.Mosier JE. Effect of aging on body systems of the dog. Vet Clin North Am Small Anim Pract. 1989;19(1):1–12. doi: 10.1016/s0195-5616(89)50001-9. [DOI] [PubMed] [Google Scholar]
- 132.Chapman BL, Voith VL. Behavioral problems in old dogs: 26 cases (1984–1987) J Am Vet Med Assoc. 1990;196(6):944–6. [PubMed] [Google Scholar]
- 133.Ruehl WW, Bruyette DS, DePaoli A, et al. Canine cognitive dysfunction as a model for human age-related cognitive decline, dementia and Alzheimer’s disease: clinical presentation, cognitive testing, pathology and response to 1-deprenyl therapy. Prog Brain Res. 1995;106:217–25. doi: 10.1016/s0079-6123(08)61218-2. [DOI] [PubMed] [Google Scholar]
- 134.Landsberg GM, Nichol J, Araujo JA. Cognitive dysfunction syndrome: a disease of canine and feline brain aging. Vet Clin North Am Small Anim Pract. 2012;42(4):749–68. vii. doi: 10.1016/j.cvsm.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 135.Bosch MN, Pugliese M, Gimeno-Bayon J, et al. Dogs with cognitive dysfunction syndrome: a natural model of Alzheimer’s disease. Curr Alzheimer Res. 2012;9(3):298–314. doi: 10.2174/156720512800107546. [DOI] [PubMed] [Google Scholar]
- 136.Pugliese M, Geloso MC, Carrasco JL, et al. Canine cognitive deficit correlates with diffuse plaque maturation and S100beta (−) astrocytosis but not with insulin cerebrospinal fluid level. Acta Neuropathol. 2006;111(6):519–28. doi: 10.1007/s00401-006-0052-1. [DOI] [PubMed] [Google Scholar]
- 137.Pugliese M, Mascort J, Mahy N, et al. Diffuse beta-amyloid plaques and hyper-phosphorylated tau are unrelated processes in aged dogs with behavioral deficits. Acta Neuropathol. 2006;112(2):175–83. doi: 10.1007/s00401-006-0087-3. [DOI] [PubMed] [Google Scholar]
- 138.Head E, Cotman CW, Zicker SC, et al. The use of dietary antioxidants and mitochondrial co-factors to promote successful aging. Curr Top Nutraceutical Res. 2005;3(2):85–94. [Google Scholar]
- 139.Bosch MN, Gimeno-Bayon J, Rodriguez MJ, et al. Rapid improvement of canine cognitive dysfunction with immunotherapy designed for Alzheimer’s disease. Curr Alzheimer Res. 2013;10(5):482–93. doi: 10.2174/15672050113109990129. [DOI] [PubMed] [Google Scholar]
- 140.Gunn-Moore DA. Cognitive dysfunction in cats: clinical assessment and management. Top Companion Anim Med. 2011;26(1):17–24. doi: 10.1053/j.tcam.2011.01.005. [DOI] [PubMed] [Google Scholar]