Abstract
Background
A recent report from the British Nuffield Council on Bioethics associated ‘emerging biotechnologies’ with a threefold challenge: 1) uncertainty about outcomes, 2) diverse public views on the values and implications attached to biotechnologies and 3) the possibility of creating radical changes regarding societal relations and practices. To address these challenges, leading international institutions stress the need for public involvement activities (PIAs). The objective of this study was to assess the state of PIA reports in the field of biomedical research.
Methods
PIA reports were identified via a systematic literature search. Thematic text analysis was employed for data extraction.
Results
After filtering, 35 public consultation and 11 public participation studies were included in this review. Analysis and synthesis of all 46 PIA studies resulted in 6 distinguishable PIA objectives and 37 corresponding PIA methods. Reports of outcome translation and PIA evaluation were found in 9 and 10 studies respectively (20% and 22%). The paper presents qualitative details.
Discussion
The state of PIAs on biomedical research and innovation is characterized by a broad range of methods and awkward variation in the wording of objectives. Better comparability of PIAs might improve the translation of PIA findings into further policy development. PIA-specific reporting guidelines would help in this regard. The modest level of translation efforts is another pointer to the “deliberation to policy gap”. The results of this review could inform the design of new PIAs and future efforts to improve PIA comparability and outcome translation.
Background
New developments in biomedical research, such as biobank-based research and gene transfer methods, as well as biomedical innovations such as synthetic biology, regenerative medicine, neuroimplants and nanotechnology, particularly attract public attention. In 2009, for example, biobanking was included by the Time Magazine in a list of “10 Ideas Changing the World Right Now” [1]. A recent report from the British Nuffield Council on Bioethics associated emerging biotechnologies with a threefold challenge that demands a more intensive “public discourse ethics”. The three challenges are: 1) “uncertainty” about outcomes; 2) “ambiguity”, meaning disagreement or diverse views and perceptions about the importance, values and implications attached to biotechnologies; and 3) the “transformative potential” to create large-scale, unexpected changes and disrupt existing technologies, relations and practices [2].
Further, specific social, ethical and legal challenges, such as the communication and commercialization of research results, the balancing of individual rights against the collective good, and data protection, to name but a few, require careful consideration and governance. This may be relevant not only to the approval and application of new biomedical technologies, but also to the processes of the underlying research [3]–[5].
To address these challenges, leading international institutions stress the importance of public involvement in biomedical research and innovation [2], [6]–[8]. Public involvement activities (PIAs) are often classified into (3–5) categories with different approaches and objectives, e.g. information/communication, consultation and participation/deliberation [6], [9]–[14].
For this paper, public information is understood as a one-way activity in which scientists provide the public with relevant information on a particular subject “to help them gain knowledge” [12] and ensure that the public can make informed decisions or arrive at an informed opinion. Common methods include websites, information events, and reading material [11]. Public consultation activities seek input from the public in order to consider (informed) public interests and opinions, for instance in policy development or prior to implementing a new technology. This type of PIA can be considered a one-way process too, in that the aim of the PIA ‘initiator’ (a scientific or policy actor) is to receive input from the public, for instance via focus groups, citizen councils or surveys; it is implied that decisions on the issue remain the responsibility of the initiator [9], [11], [12], [14]. Lastly, public participation/deliberation refers to some sort of reciprocal, informed dialogue between science/policy and the public; it differs from public consultation since the public is assigned a more active role not only in the dialogue but also in concomitant decision-making and policy development processes. Typical methods include advisory boards and juries including lay people/public representatives, consensus conferences, and dialogue sessions [2], [6], [11].
Previous studies have assessed and discussed the state of PIAs in health policy [15], [16], bioethics [17], health technology assessment [18], and in the development of research designs for primary health research [19] by using qualitative data analysis, “selective reviews” [15] and narrative review methods.
These previous studies have revealed that A) the majority of PIAs are carried out in the USA, Canada and the UK [15]; B) there is a considerable degree of diversity and lack of specificity regarding PIA objectives and methods [14], [15]; C) there is insufficient translation and evaluation [4], [5]; and D) evidence of the impact of PIAs is limited [13], [16], [20], [21].
The objective of this study was to assess a systematically-derived set of published PIAs in the field of biomedical research and innovation for A) their general characteristics (site, topic, duration and number of participants, year of conduct and year of publication); B) their study aims; C) their use at the planning stage of frameworks or findings from previous PIAs; D) the types of PIA method used; E) the translation of PIA outcomes to policy and practice; and F) measures used for PIA evaluation.
Methods
Database-specific search and selection of studies
We conducted a systematic literature search in the bibliographic databases PubMed, PsychInfo and Scopus from June 14 to July 29, 2013 with an update after three months. We restricted the search to studies published between January 2000 and June 2013 to account for the (most) recent developments in the field of biomedical research, in any language. The search queries combined comprehensive, general search terms representing “public involvement” and “research and innovation” in the field of biotechnology (Table 1). We chose very general and thus unspecific search queries because the indexing of PIAs in electronic databases is not yet well developed. We scanned all the located references for relevance by reading the title, abstract and keywords. Next, we read the full text of potentially relevant articles. We included a publication if it reported directly on the conduct and results of a PIA and if the focus of the PIA was on a topic in biotechnology research and innovation.
Table 1. Database-specific search queries used for the systematic search of publications of PIA reports.
| PubMed |
| (((“biomedical research”[mesh]) OR (((((“Nanomedicine”[Mesh]) OR “Individualized Medicine”[Mesh]) OR “Genetic Therapy”[Mesh]) OR (((((“Biotechnology”[Mesh]) OR “Cell Engineering”[Mesh]) OR “Tissue Banks”[Mesh]) OR “Synthetic Biology”[Mesh]) OR “Regenerative Medicine”[Mesh]))))) AND ((((“Consumer Participation”) OR ((((((“public engagement”) OR “public participation”) OR “public involvement”) OR “public deliberation”) OR “public consultation”) OR “Consumer Participation”[Mesh])))) |
| PsychInfo |
| exp Involvement/or exp Community Involvement/or public involvement.mp. or exp Public Opinion/or public consultation.mp. or public engagement.mp. and exp Genetics/or exp Bioethics/or exp Informed Consent/or biomedical research.mp. or biotechnology.mp. or exp Biotechnology/ |
| Scopus |
| TITLE-ABS-KEY(biotechnology) OR TITLE-ABS-KEY(“biomedical research”) AND TITLE-ABS-KEY(“public involvement”) OR TITLE-ABS-KEY(“public engagement”) OR TITLE-ABS-KEY(“public participation”) OR TITLE-ABS-KEY(“public consultation”) |
Data extraction and synthesis
We used a thematic text analysis approach for data extraction [22]. Data extraction used an assessment matrix listing core aspects of PIAs grouped under four main categories: A) general characteristics, B) PIA concept, C) background/theoretical foundation, and D) methods/procedures (Information S1). The core aspects were identified by a literature review (snowball sampling) of PIA-specific guidelines, frameworks, handbooks, manuals and studies [7], [9], [11], [12], [23]–[26].
All included studies were read in full and assessed independently by three researchers (JL read and assessed all, IH read and assessed 13 and TH read and assessed 33). According to thematic text analysis practice, all relevant text passages related to the core aspects were extracted. A code/keyword was assigned to each extracted text passage to allow for subsequent qualitative and quantitative analysis.
A sample of five studies was assessed by three authors (JL, TH, IH) to pilot-test the construct validity of the assessment matrix and the consistency of the extraction procedure. Results were compared and unclear or divergent coding was resolved after discussion with the fourth author (DS). The consistency of further extractions and codings was discussed in ongoing meetings and discussions between all four researchers.
Based on international categorization schemes, we broadly distinguished the included PIAs either as a public consultation or a public participation/deliberation activity [6], [7], [9], [11], [12], [24], [25], [27]. Because of the many different and sometimes inconsistent wordings we did not directly relate our coding to the self-classifications of included PIA studies (if there were any self-classifications). Instead, we based our classification on the objectives and methods described in the respective papers. We classified a PIA (including methods such as focus groups, surveys, questionnaires etc.) as a ‘public consultation’ if it aimed at gathering input from the public without actively including them in further decision-making or deliberation processes. We classified a PIA as ‘public participation/deliberation’ if it assigned the involved public a more active role in further decision-making or deliberation with decision makers, or offered other means to influence further policy development.
Results
General Information
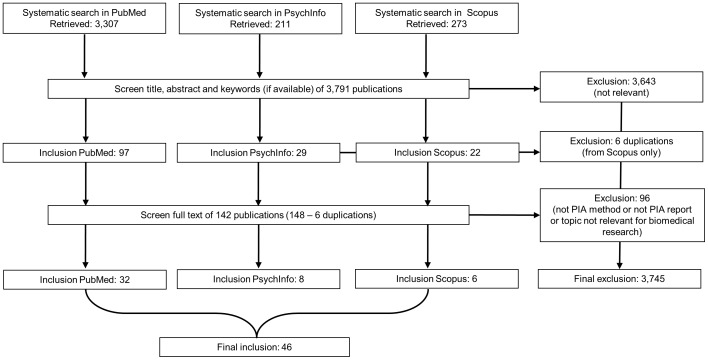
Of 3,791 publications identified via the comprehensive search, we finally included 46 PIA studies in our study (Fig. 1). Four studies discussed two similar PIAs but with distinct thematic foci [28]–[31].
Figure 1. Selection of publications.
Our studies were conducted in 15 different countries with 64% (n = 30) of studies being conducted in the USA (n = 18), Canada (n = 8) or Australia (n = 4). Those studies that provided a date (n = 39; 85%) were carried out between 1999 and 2011 and were published between 2002 and 2013 in 28 different journals (Information S2).
Genetic research was mentioned 20 times as the topic of the PIA (including genomics, pharmacogenomics and personalized genomics), and biobank research was mentioned 18 times (including biobanked specimens, DNA databases, genetic databases and DNA banks). The other topics were biomedical research in general (n = 5), research with bloodspot samples (n = 3), synthetic biology (n = 1), nanotechnology (n = 1), and human cloning research (n = 1). 28% of studies (n = 13) mentioned more than one topic.
Regarding the number of PIA participants, 35% of studies (n = 16) had fewer than 100 participants, 30% (n = 14) had 100–500 participants, and 35% (n = 16) had more than 500 participants.
24% of studies (n = 11) did not specify a duration. Of those that did (n = 35), 67% (n = 31) took longer than a day and 9% (n = 4) took one day or less (Table 2). 63% (n = 22) of those that reported a duration mentioned this only implicitly or used more general wordings such as “two separate weekends” [32] or “34 groups were involved in 50 discussions” [33].
Table 2. General information about the selected studies ordered by PIA category (public consultation and public participation/deliberation) and year of publication (descending); Ca./i., Circa/implicit, respective information was not reported clearly, i.e. reported with some ambiguity; N.S., not specified in PIA report; min, minute(s); h, hours.
| Reference | Country | Topic(s) | Duration | Participants | Conduct | Publication |
| Public Consultation | ||||||
| [39] | USA | Genetic research, biobanking | n.s. | 1,041 (2 phases) | 2009, 2011 | 2013 |
| [40] | Canada | Biomedical research | n.s. | 2,604 | 2010 | 2013 |
| [41] | USA | Biomedical research | 25 min (by 117 participants) | 117 | 2009 | 2013 |
| [42] | USA | Biobank-based research | 16 h (8 groups by 2 hours) | 45 | n.s. | 2013 |
| [43] | USA | Genomic research | Ca. 48 min (by 91 participants) | 91 | n.s. | 2013 |
| [44] | Jordan | Biobanking | 3 months (i) | 3,196 | 2011 | 2013 |
| [45] | USA | Biomedical research with newborn blood samples | 9 months (i) | 3,855 | 2010 | 2012 |
| [46] | Canada | Health-related research with biobanked specimens | 2 months (i) | 330 | 2010 | 2012 |
| [47] | USA | Genetics/genomics research | 2 h (by 8 groups) | 199 | 2009, 2010 | 2012 |
| [48] | Austria | Synthetic biology, biotechnology, nanotechnology | n.s. | 49 | 2008 | 2012 |
| [49] | USA | Newborn blood sample research, genetic testing | 6 months | 128 | 2010 | 2012 |
| [50] | Australia | Biobanking | n.s. | 1,000 | n.s. | 2011 |
| [51] | Sweden | Biobanking | 7 min (by 23 interviews) | 926 | 2007–2008 | 2010 |
| [52] | Saudia Arabia | Medical research, tissue research | 9 months (i) | 528 | 2006–2008 | 2010 |
| [53] | Egypt | Blood samples, genetic research | 7 months (i) | 600 | 2007 | 2010 |
| [54] | UK | Genomic science | 3 phases (Jul–Sept; Mar–Apr; Aug–Sept); 5–9 min (films); n.s. (interviews) | 4,595 (3 phases) | 2003–2004 | 2010 |
| [55] | Finland | Biobanking | n.s. | 1,195 | 2007 | 2009 |
| [56] | USA | Pediatric samples, biobanks | 30 min (by 1186 participant interviews) | 1,186 | 2002–2003 | 2009 |
| [57] | Canada | Genetic database | n.s. | Ca. 4,017 (5 phases) | 2001, 2003 | 2009 |
| [58] | Japan | Pharmacogenomics research, DNA bank, Genomic markers | 2 months (i) | 550 | 2008–2009 | 2009 |
| [31] | USA | Biobanking | 2 h (by 15 groups) | 60 | 2007–2008 | 2009 |
| [59] | USA | Personal genomics | n.s. | 396 | 2009, 2010 | 2008 |
| [60] | Malawi | Biomedical research studies/Health research studies | n.s. | 108 | n.s. | 2008 |
| [28] | USA | Genomic biobanking | 2 h (by 15 groups) | Ca. 60 | 2007 | 2008 |
| [36] | Scotland | DNA database | n.s. | Ca. 17 | 2003–2004 | 2008 |
| [61] | USA | Biomedical research | 3 months (i) | 900 | 2003 | 2007 |
| [62] | UK | Human cloning (research) | 3 phases: 4 months, 5 months, 100 months (i) | Ca. 2,960 | 2003 | 2007 |
| [63] | Canada | Genomics research and biobanking | n.s. | 11 | n.s. | 2007 |
| [64] | USA | Genetics research | 2 h (by 63 participants) | 63 | n.s. | 2005 |
| [33] | Kenya | Biomedical research | 1–2 h (by 34 groups) | Ca. 270 | 2001–2002 | 2005 |
| [65] | USA | Genetics | 8 months | 215 | 2001–2002 | 2005 |
| [66] | USA | Genetic research | 2 h (by 9 groups) | 91 | 2002 | 2004 |
| [67] | Singapore | Genetic research | 2 h (by 12 groups) | 98 | 2002 | 2004 |
| [68] | n.s. | Disease/genetic susceptibility research | 1 h (by 37 participants) | 37 | n.s. | 2003 |
| [69] | Germany | Pre/postnatal genetic testing | n.s. | 2,076 | 2001 | 2002 |
| Public Participation/Deliberation | ||||||
| [70] | Kenya | Genetic/genomic research | 11 months (i) | 63 | 2009–2010 | 2013 |
| [71] | Canada | Research with bloodspot samples from newborn screening | n.s. | 60 | 2009 | 2012 |
| [30] | Australia | Biobanking, health policy for biobanking | 4 days | 16 | 2008 | 2012 |
| [32] | Canada | Biobanking, institutional biobanking policy | 4 days (2 weekends) | 25 | 2009 | 2012 |
| [72] | USA | Genetic variation and happlotype mapping | 2 h+1.5 h+1 day | More than 250 (3 phases) | 2003–2004 | 2012 |
| [29] | Australia | Biobanking | 4 days | 17 | 2008 | 2011 |
| [73] | Canada | Health Technology Assessment | 2.5 days | 420 (survey); 16 (jury) | n.s. | 2008 |
| [74] | Canada | Biobanking | 2 weekends | 21 | 2007 | 2008 |
| [75] | Australia | Clinical genetics services | n.s. | Ca. 400 | 2005 | 2008 |
| [76] | USA | Personalized-medicine research project, biobanking | n.s. | 144 (4 phases) | 2001 (i) | 2008 |
| [77] | USA | Genetic research and technology | 6 months (i) | 63 | 1999 | 2003 |
Study objectives
In the 46 PIAs we found 66 different (wordings for) objectives, such as “assess the public's perceptions”, “offer collective responses that should be understood as tailored policy input” or “determining the feasibility of conducting a citizens [sic] jury”. These different wordings were grouped under 6 distinguishable PIA objectives (see Table 3).
Table 3. PIA Objectives and Methods.
| Objectives | Text Examples | Count (n) | Methods related to the PIA objectives | Count (n) |
| a) Inform/educate (prior to consultation or participation/deliberation) | The forum had four broad objectives: (i) Inform a representative sample of citizens of the competing interests and perspectives on biobanking, […] | 2 | Long film | 1 |
| Short film | 6 | |||
| Informational/introductory presentation | 10 | |||
| Literature (booklet, pamphlet, etc.) | 11 | |||
| Showcards | 2 | |||
| The overall goal of this component of the consultation and communication plan was to educate residents of the 19 Zip code region about the PMRP through talks to community groups and media prior to enrollment, and then through regular newsletters after study participation. | Workbook (to guide group discussion) | 1 | ||
| Presentation of newspaper article | 1 | |||
| Vignettes (Description of current studies/standardized scenarios) | 2 | |||
| Media release (to announce project) | 1 | |||
| Blog (online) | 1 | |||
| b) Consult the public to gather attitudes, opinions, preferences, etc. either | To assess the general attitudes towards genetic research and participation in biobanks in the Long Island/Queens area of New York, and what factors would predict a positive view of such research, participants from the NSLIJ hospital system were surveyed. | 44 | c.1) …without further discussion: | |
| • as a means to use this input for further policy development/decision-making without subsequent active engagement of the consulted public | Questionnaire | 11 | ||
| • or in advance of/as part of a public engagement activity | Questionnaire guide | 1 | ||
| Written Survey | 16 | |||
| Telephone survey | 3 | |||
| Online survey | 3 | |||
| Phone interview | 3 | |||
| Face to face interview | 11 | |||
| Email questions | 1 | |||
| Ranking of research scenarios (by participants) | 3 | |||
| Discrete choice experiment | 1 | |||
| To assess the public's perception of biobank research and the relative importance they place on concerns for privacy and confidentiality, when compared with other key variables when considering participation in biobank research. | Interactive discussion game | 1 | ||
| We explored Canadian values regarding storage and use of NBS samples for various purposes and the forms of parental choice for anonymous research with NBS samples. | c.2)…with further discussion: | |||
| Focus Group | 18 | |||
| Group deliberation/dialogue | 5 | |||
| Small group discussion/deliberation | 6 | |||
| Large group discussion/deliberation | 4 | |||
| Feedback Session | 1 | |||
| Panel interaction | 1 | |||
| Citizen jury (covers c2 and d1) | 1 | |||
| Moderator guide (guiding focus groups) | 3 | |||
| Ratification process (by group, of recommendations/resolutions) | 1 | |||
| c) Engage the public actively in policy development/decision-making processes | […] because the purpose of the research was to inform policy development within NBS and an overview of all of the categories provides guidance on these issues. | 11 | d.1) Disseminating/translating PIA outcomes: | |
| Dissemination via print and electronic media | 4 | |||
| Recommendation report (written) | 4 | |||
| In a significant refinement of methods, we focus on providing public input to institutional practice and governance of biobanks using a tailored workbook structure to guide participants' discussion. | One-day conference | 1 | ||
| Group recommendations (spoken) | 2 | |||
| Community project oversight, project advisory | 3 | |||
| The results of this deliberation offer collective responses that should be understood as tailored policy input, rather than public opinion measurement. | Meetings to discuss results | 2 | ||
| d.2) Considering PIA outcomes in policy: | ||||
| Decision-maker response/feedback (to deliberant's recommendations) | 1 | |||
| d) Investigate impact of PIA on participants | In a real-world experiment, this study on synthetic biology investigated the effect of information uptake and deliberation on opinion certainty and opinion valence in natural groups. | 4 | See c.1) and c.2) for methods | |
| As part of our deliberative engagement, we surveyed the participants both before and after the engagement intervention to determine whether there were attitudinal changes. | ||||
| The purpose of this paper is to explore whether members of the public recall TSUS and whether they use the study to interpret current biomedical research. | ||||
| e) Describe PIA method | In response to this gap in the literature (and to address the deliberative norms of transparency and publicity), the purpose of our paper is to […] describe the processes by which we translated these outputs to policy. | 2 | n.a. | |
| The purpose of this paper is to illustrate a novel method for developing meaningful public input on ethically contentious issues in institutional biobanking policy. | ||||
| f) Test PIA method | The main goals of the PEGV Project were to test a community engagement model, […] | 6 | See a)–d) for methods (the respective PIA method was tested by running the individual methods described above, for instance by handing out reading material to participants) | |
| […] 2 determining the feasibility of conducting a citizens [sic] jury to elicit the views of the public on priorities for HTA; |
62% of objectives (n = 42) related to public consultation, i.e. to a broad understanding of ‘attitudes research’, for example, “to assess attitudes”, “to inquire perceptions [sic]” or “to query about preferences [sic]”.
16% of objectives (n = 11) related to public participation/deliberation, for instance by offering “collective responses that should be understood as tailored policy input, rather than public opinion measurement”.
3% of 68 objectives (n = 2) related to public information. Since our analysis did not include pure public information activities (that is, activities with no further means of public consultation or participation), these objectives were ‘only’ sub-objectives within two PIAs considered as public participation/deliberation (see the Methods section).
The remaining objectives related to a broad understanding of and contribution to PIA research, i.e. describing a PIA method (n = 2), investigating the impact of the PIA on participants (n = 3), and testing a PIA method (n = 6). Overall, 20% (n = 9) of all PIAs reported objectives related to PIA research (two PIAs reported more than one research objective).
PIA categories and PIA methods
We rated 76% (n = 35) of the studies as public consultations, and 24% (n = 11) as public participations/deliberations. In total, we found 146 wordings for the methods applied in the 46 PIAs. However, these wordings often described similar methods and therefore were grouped according to the 6 PIA objectives (see Table 3). Within these 6 PIA objectives, we distinguished 37 individual methods, such as short video (n = 6), literature (n = 11), written survey (n = 16), and citizen jury (n = 1).
‘Public information’ contained many more methods (n = 36) than corresponding objectives (n = 2). This is due to the fact that several PIAs (n = 23) reported the use of informational material such as literature or introductory presentations without also explicitly stating the objective of providing information prior to consultation or participation/deliberation.
References to previous PIA reports, previous PIA research, or PIA guidelines
93% (n = 43) of all studies referred to other PIA reports or PIA research. 42% (n = 18) of all studies explicitly used these references as the methodological framework for their own PIA or to validate their choice of PIA methods. Three studies did not refer to any methodology.
Furthermore, no study reported the explicit or implicit use of public involvement guidelines that describe different PIA objectives and methods (such as “Planning guide for public engagement and outreach in nanotechnology” [7]).
Evaluation and translation of findings
22% of all studies (n = 10) reported some evaluation measures, which related to A) participants' satisfaction during participation (n = 6) or attitude changes (n = 2) and/or B) effectiveness and feasibility of the PIA method (n = 4); two PIAs reported both A) and B).
One of the 35 public consultation studies (3%) and 8 of the 11 public participation/deliberation studies (73%) reported some sort of translational efforts to either disseminate study findings and recommendations or communicate them directly to decision-makers in policy and practice. Translational efforts included A) a final conference and (spoken) presentation of recommendations (n = 3); B) the writing and dissemination of a policy-oriented final report (n = 4); C) community guidance during and after the PIA (n = 3); D) dissemination via print and electronic media (n = 4); and E) dialogue-oriented meetings between researchers and politicians to discuss results (n = 2).
Discussion
This study assessed a systematically-derived sample of 46 peer-reviewed journal articles reporting on public involvement activities (PIAs) in the field of biomedical research and innovation. The 35 public consultation and 11 public deliberation/participation activities used a broad range of different wordings for PIA objectives and corresponding methods. Our categorization reduced this range to 6 different PIA objectives and 37 corresponding PIA methods. These findings confirm previous reviews of PIAs in other areas such as health policy, concluding that public involvement as a tool for governance and democratic decision-making is defined and practiced in many ways [2], [10], [15], [34].
Regarding the clustered PIA objectives in particular, it emerged that a range of significant, classical objectives such as building public trust, empowering individuals to actively engage in political debate, and legitimizing policy were not explicitly stated as objectives in the respective PIA reports [35]. Rather, these objectives were addressed in the study's background sections in a more general sense, e.g. “In the UK, development of DNA databases […] is set against a perceived ‘public crisis in trust’ in both medical and scientific spheres, which, it is argued, has set up ‘a new mood for dialogue’ within the science community […] as a way to boost confidence and reinstate trust” [36]. The lack of objectives related to public trust, empowerment, legitimacy, etc. may partly be explained by the difficulty in linking such ‘thick normative concepts’ like trust to one particular PIA method. In scientific papers, however, the ‘primary’ objectives are directly linked to a specific method. One core task in peer-reviewing scientific papers is to check whether the stated objectives match the methods employed. For instance, the objective of gathering the public's views may be achieved via a focus group, and the objective of policy translation may be achieved via a public hearing based on the focus group findings; but the overarching aim of increasing public trust in a certain biomedical research process does not straightforwardly suggest how to achieve this. Despite these difficulties it might be important to report explicitly what the underlying normative rationale for performing a certain PIA was. This information might support better interpretation of PIA reports by their readers.
Though there is relatively established terminology for PIA categories and objectives [2], [11], [12] as well as guidelines for designing PIAs [6], [7], [9], [24], [25], [27], neither was considered in any of the 46 papers included in this review. While a proliferation of methods should not be seen problematic in itself, easier comparability of PIAs for biomedical research and innovation might improve the translation of PIA findings into further policy development. The development of reporting standards and best practice examples for PIA objectives and methods could be a first step in this direction. The EQUATOR network (www.equator-network.org) might be a good partner for starting the development of such reporting standards.
While research into the quality of PIAs on biomedical issues is important to further strengthen the validity and practical relevance of the public involvement approach, it is currently not widespread. Only 10 (22%) of the identified PIAs reported on evaluation measures such as participants' satisfaction with their participation, changes in attitudes as a result of participation, and the effectiveness and feasibility of PIA methods.
Only 9 (20%) of the 46 PIA studies reported on translation of their findings, despite the importance attributed to this aspect of PIAs [2], [4], [30] The rather modest translation efforts found in this analysis confirm the “deliberation to policy gap” described in other fields of public involvement [4]. Recent reports on public involvement in patient and health services suggest that the translation of findings of PIAs initiated by academic institutions/departments is often limited to publication in peer-reviewed journals, since this is often seen as the main means of research translation [37]. As the PIAs in this review were solely initiated by academic departments, this may partly explain the low use (or at least low reporting) of translation methods. In this sense it is also suggested that “the relationship between the hosts of a public engagement and institutional bodies who actually make policy on the issues under consideration clearly has an impact on the mandate of the public forum to influence policy” [4]. Hence, a potential translation challenge may arise when an (academic) PIA initiator lacks a strong relationship with the (political) body to which results should be translated. Such potential ‘distance’, i.e. divergent nature of work, methods, thematic priorities etc. point to a further aspect that could be considered important for the translation of findings: for PIAs to be relevant for policy development in a particular area, the PIA's topic and outcomes need to account for institutional/policy contexts, rather than only for the ‘research context’ [16], [38]. This means for instance that PIA findings need to be accompanied by practical recommendations on issues that can actually be addressed at a political level. Overall, translation of PIA results is important not only to answer questions for policy, but also questions that improve public understanding of biomedical research (for example regarding data protection in biobanks), i.e. questions of the “public good” [2].
Regarding the analysis of the aspect of translation, we stress the limitation that our analysis only assessed whether translation efforts were reported or not. It might be that translation of PIA findings is often practiced but seldom reported in scientific journals. This may be true especially for public consultation activities, which are not necessarily limited to merely gathering public opinion. However, if the findings of public consultation activities are in fact used to influence policy, future PIA reports could address this more explicitly. In order to improve insight into challenges for translation of consultation and participation/deliberation activities, further research could assess the importance policy and decision-makers actually place on PIA findings. In this sense, it could also be assessed whether there are sufficient ‘contact points’ in policy or practice for communicating PIA findings.
Despite the fact that we employed narrow as well as general search terms for the broad field of biomedical research and innovation, most of the identified PIAs dealt with genetics or biobanking. The fact that biobanking and genetics (potentially) concern more of the public than other emerging biotechnologies such as gene therapy, nanotechnology, neuroimplants, tissue engineering and synthetic biology may partly explain this gap. With recent calls for more public involvement in the field of biomedical research, more PIAs on issues beyond genetics and biobanking are to be expected. The design of new PIAs on relevant issues in specific disciplines of biomedical research could be informed by the results of this review.
We highlight that our categorizations and classifications of text passages from the included PIA reports involved interpretation. We addressed this challenge by having at least two researchers analyzing each text independently, discussing differences and finally agreeing classifications among all four authors. Also, PIAs conducted in disciplines other than biomedical research as well as PIAs published in formats other than peer-reviewed scientific journals may suggest different reporting practices, for instance regarding details on ‘direct’ objectives (to consult the public) and more indirect ones (to increase public trust).
Further consideration of existing PIA guidelines and the development of PIA-specific reporting guidelines could improve the comparability of PIAs, streamline their reporting and thus enforce the still lacking (or underreported) translation of findings.
Supporting Information
PRISMA Checklist.
(PDF)
Assessment matrix. The matrix used to analyze selected studies is divided according to the core aspects of Public Involvement Activities identified by a literature review.
(PDF)
Overview of journals that published selected PIA reports. Sorted according to (1) the number of studies selected from the respective journal and (2) alphabetical order.
(PDF)
Electronic search strategy.
(PDF)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This project was funded by intramural funds of Hannover Medical School and by the German Research Foundation (DFG) via the grant “Cluster of Excellence 62, REBIRTH - From Regenerative Biology to Reconstructive Therapy” (http://www.dfg.de/foerderung/programme/listen/projektdetails/index.jsp?id=24102914). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Time Magazine (2009) 10 Ideas Changing the World Right Now. Time Magazine. New York: Time Inc. [Google Scholar]
- 2.Nuffield Council on Bioethics (2012) Emerging biotechnologies: technology, choice and the public good. Nuffield Council on Bioethics. 978-1-904384-27-4 978-1-904384-27-4.
- 3. O'Doherty KC, Burgess MM (2009) Engaging the public on biobanks: outcomes of the BC biobank deliberation. Public Health Genomics 12:203–215. [DOI] [PubMed] [Google Scholar]
- 4. O'Doherty KC, Hawkins A (2010) Structuring public engagement for effective input in policy development on human tissue biobanking. Public Health Genomics 13:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avard D, Bucci L, Burgess M, Kaye J, Heeney C, et al. (2009) Public Health Genomics (PHG) and Public Participation: Points to Consider. Journal of Public Deliberation 5.. [Google Scholar]
- 6.Organization for Economic Cooperation and Development (2001) Citizens as Partners. Information, Consultation and Public Participation in Policy-Making.
- 7.Organization for Economic Cooperation and Development (2012) Planning Guide for Public Engagement and Outreach in Nanotechnology.
- 8.European Commission (2012) Biobanks for Europe. A Challenge for Governance.
- 9.Involve (2012) Making a Difference: A guide to evaluating public participation in central government.
- 10.RAND Europe (2010) Involving the public in healthcare policy. An update of the research evidence and proposed evaluation framework.
- 11.European Institute for Public Participation (2009) Public Participation in Europe. An international perspective.
- 12.International Association for Public Participation (2007) IAP2 Spectrum of Public Participation.
- 13. Abelson J, Forest PG, Eyles J, Smith P, Martin E, et al. (2003) Deliberations about deliberative methods: issues in the design and evaluation of public participation processes. Soc Sci Med 57:239–251. [DOI] [PubMed] [Google Scholar]
- 14. Rowe G, Frewer LJ (2005) A Typology of Public Engagement Mechanisms. Science, Technology & Human Values 30:251–290. [Google Scholar]
- 15. Abelson J, Blacksher E, Li K, Boesveld S, Goold S (2013) Public Deliberation in Health Policy and Bioethics: Mapping an emerging, interdisciplinary field. Journal of Public Deliberation 9. [Google Scholar]
- 16.Conklin A, Morris Z, Nolte E (2012) What is the evidence base for public involvement in health-care policy?: results of a systematic scoping review. Health Expect. [DOI] [PMC free article] [PubMed]
- 17. De Vries R, Stanczyk AE, Ryan KA, Kim SY (2011) A framework for assessing the quality of democratic deliberation: enhancing deliberation as a tool for bioethics. J Empir Res Hum Res Ethics 6:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moran R, Davidson P (2011) An uneven spread: a review of public involvement in the National Institute of Health Research's Health Technology Assessment program. Int J Technol Assess Health Care 27:343–347. [DOI] [PubMed] [Google Scholar]
- 19. Boote J, Baird W, Beecroft C (2010) Public involvement at the design stage of primary health research: a narrative review of case examples. Health Policy 95:10–23. [DOI] [PubMed] [Google Scholar]
- 20. Rowe G, Frewer LJ (2004) Evaluating Public-Participation Exercises: A Research Agenda. Science, Technology & Human Values 29:512–556. [Google Scholar]
- 21. Avard D, Grégoire G, Jean M (2008) Involving the Public in Public Health Genomics. A review of guidelines and policy statements. GenEdit 6:1–9. [Google Scholar]
- 22.Dixon-Woods M, Agarwal S, Jones D, Young B, Sutton A (2005) Synthesising qualitative and quantitative evidence: a review of possible methods. [DOI] [PubMed]
- 23. Rowe G, Frewer LJ (2000) Public Participation Methods: A Framework for Evaluation. Science, Technology & Human Values 25:3–29. [Google Scholar]
- 24.Nanz P, Fritsche M (2012) Handbuch Bürgerbeteiligung. Verfahren und Akteure, Chancen und Grenzen.
- 25.Organization for Economic Cooperation and Development (2005) Evaluating Public Participation in Policy Making.
- 26.Canadian Environmental Assessment Agency (2008) Public Participation Guide. A guide for meaningful public participation in environmental assessments under the Canadian Environmental Assessment Act. Ottawa.
- 27.Canadian Environmental Assessment Agency (2008) Public Participation Guide. A guide for meaningful public participation in environmental assessments under the Canadian Environmental Assessment Act.
- 28. Murphy J, Scott J, Kaufman D, Geller G, LeRoy L, et al. (2008) Public expectations for return of results from large-cohort genetic research. Am J Bioeth 8:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Molster C, Maxwell S, Youngs L, Kyne G, Hope F, et al. (2013) Blueprint for a deliberative public forum on biobanking policy: were theoretical principles achievable in practice? Health Expect 16:211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Molster C, Maxwell S, Youngs L, Potts A, Kyne G, et al. (2012) An Australian approach to the policy translation of deliberated citizen perspectives on biobanking. Public Health Genomics 15:82–91. [DOI] [PubMed] [Google Scholar]
- 31. Murphy J, Scott J, Kaufman D, Geller G, LeRoy L, et al. (2009) Public perspectives on informed consent for biobanking. Am J Public Health 99:2128–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O'Doherty KC, Hawkins AK, Burgess MM (2012) Involving citizens in the ethics of biobank research: informing institutional policy through structured public deliberation. Soc Sci Med 75:1604–1611. [DOI] [PubMed] [Google Scholar]
- 33. Molyneux CS, Wassenaar DR, Peshu N, Marsh K (2005) ‘Even if they ask you to stand by a tree all day, you will have to do it (laughter)…!’: community voices on the notion and practice of informed consent for biomedical research in developing countries. Soc Sci Med 61:443–454. [DOI] [PubMed] [Google Scholar]
- 34.Gradinger F, Britten N, Wyatt K, Froggatt K, Gibson A, et al. (2013) Values associated with public involvement in health and social care research: a narrative review. Health Expect. [DOI] [PMC free article] [PubMed]
- 35. Goodin RE, Dryzek JS (2006) Deliberative Impacts: The Macro-Political Uptake of Mini-Publics. Politics & Society 34:219–244. [Google Scholar]
- 36. Haddow G, Cunningham-Burley S, Bruce A, Parry S (2008) Generation Scotland: consulting publics and specialists at an early stage in a genetic database's development. Critical Public Health 18:139–149. [Google Scholar]
- 37.Shippee ND, Domecq Garces JP, Prutsky Lopez GJ, Wang Z, Elraiyah TA, et al. (2013) Patient and service user engagement in research: a systematic review and synthesized framework. Health Expect. [DOI] [PMC free article] [PubMed]
- 38. Abelson J, Forest PG, Eyles J, Casebeer A, Martin E, et al. (2007) Examining the role of context in the implementation of a deliberative public participation experiment: results from a Canadian comparative study. Soc Sci Med 64:2115–2128. [DOI] [PubMed] [Google Scholar]
- 39. Kerath SM, Klein G, Kern M, Shapira I, Witthuhn J, et al. (2013) Beliefs and attitudes towards participating in genetic research - a population based cross-sectional study. BMC Public Health 13:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miller FA, Mentzakis E, Axler R, Lehoux P, French M, et al. (2013) Do canadian researchers and the lay public prioritize biomedical research outcomes equally? A choice experiment. Acad Med 88:519–526. [DOI] [PubMed] [Google Scholar]
- 41. Ulrich A, Thompson B, Livaudais JC, Espinoza N, Cordova A, et al. (2013) Issues in biomedical research: what do Hispanics think? Am J Health Behav 37:80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Halverson CM, Ross LF (2012) Attitudes of African-American parents about biobank participation and return of results for themselves and their children. J Med Ethics 38:561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Isler MR, Sutton K, Cadigan RJ, Corbie-Smith G (2013) Community perceptions of genomic research: implications for addressing health disparities. N C Med J 74:470–476. [PubMed] [Google Scholar]
- 44. Ahram M, Othman A, Shahrouri M (2013) Public support and consent preference for biomedical research and biobanking in Jordan. Eur J Hum Genet 21:567–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Botkin JR, Rothwell E, Anderson R, Stark L, Goldenberg A, et al. (2012) Public attitudes regarding the use of residual newborn screening specimens for research. Pediatrics 129:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pullman D, Etchegary H, Gallagher K, Hodgkinson K, Keough M, et al. (2011) Personal privacy, public benefits, and biobanks: A conjoint analysis of policy priorities and public perceptions. Genet Med. [DOI] [PubMed]
- 47. O'Daniel JM, Rosanbalm KD, Boles L, Tindall GM, Livingston TM, et al. (2012) Enhancing geneticists' perspectives of the public through community engagement. Genet Med 14:243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kronberger N, Holtz P, Wagner W (2012) Consequences of media information uptake and deliberation: focus groups' symbolic coping with synthetic biology. Public Underst Sci 21:174–187. [DOI] [PubMed] [Google Scholar]
- 49. Rothwell E, Anderson R, Goldenberg A, Lewis MH, Stark L, et al. (2012) Assessing public attitudes on the retention and use of residual newborn screening blood samples: a focus group study. Soc Sci Med 74:1305–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nicol D, Critchley C (2012) Benefit sharing and biobanking in Australia. Public Underst Sci 21:534–555. [DOI] [PubMed] [Google Scholar]
- 51. Melas PA, Sjoholm LK, Forsner T, Edhborg M, Juth N, et al. (2010) Examining the public refusal to consent to DNA biobanking: empirical data from a Swedish population-based study. J Med Ethics 36:93–98. [DOI] [PubMed] [Google Scholar]
- 52. Al-Qadire MM, Hammami MM, Abdulhameed HM, Al Gaai EA (2010) Saudi views on consenting for research on medical records and leftover tissue samples. BMC Med Ethics 11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Abou-Zeid A, Silverman H, Shehata M, Shams M, Elshabrawy M, et al. (2010) Collection, storage and use of blood samples for future research: views of Egyptian patients expressed in a cross-sectional survey. J Med Ethics 36:539–547. [DOI] [PubMed] [Google Scholar]
- 54. Sturgis P, Brunton-Smith I, Fife-Schaw C (2010) Public attitudes to genomic science: an experiment in information provision. Public Underst Sci 19:166–180. [DOI] [PubMed] [Google Scholar]
- 55. Tupasela A, Sihvo S, Snell K, Jallinoja P, Aro AR, et al. (2010) Attitudes towards biomedical use of tissue sample collections, consent, and biobanks among Finns. Scand J Public Health 38:46–52. [DOI] [PubMed] [Google Scholar]
- 56. Goldenberg AJ, Hull SC, Botkin JR, Wilfond BS (2009) Pediatric biobanks: approaching informed consent for continuing research after children grow up. J Pediatr 155:578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Godard B, Ozdemir V, Fortin M, Egalite N (2010) Ethnocultural community leaders' views and perceptions on biobanks and population specific genomic research: a qualitative research study. Public Underst Sci 19:469–485. [DOI] [PubMed] [Google Scholar]
- 58. Kobayashi E, Sakurada T, Ueda S, Satoh N (2011) Public involvement in pharmacogenomics research: a national survey on patients' attitudes towards pharmacogenomics research and the willingness to donate DNA samples to a DNA bank in Japan. Cell Tissue Bank 12:71–80. [DOI] [PubMed] [Google Scholar]
- 59. Gollust SE, Gordon ES, Zayac C, Griffin G, Christman MF, et al. (2012) Motivations and perceptions of early adopters of personalized genomics: perspectives from research participants. Public Health Genomics 15:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mfutso-Bengo J, Masiye F, Molyneux M, Ndebele P, Chilungo A (2008) Why do people refuse to take part in biomedical research studies? Evidence from a resource-poor area. Malawi Med J 20:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Katz RV, Green BL, Kressin NR, Claudio C, Wang MQ, et al. (2007) Willingness of minorities to participate in biomedical studies: confirmatory findings from a follow-up study using the Tuskegee Legacy Project Questionnaire. J Natl Med Assoc 99:1052–1060. [PMC free article] [PubMed] [Google Scholar]
- 62. Shepherd R, Barnett J, Cooper H, Coyle A, Moran-Ellis J, et al. (2007) Towards an understanding of British public attitudes concerning human cloning. Soc Sci Med 65:377–392. [DOI] [PubMed] [Google Scholar]
- 63. Godard B, Marshall J, Laberge C (2007) Community engagement in genetic research: results of the first public consultation for the Quebec CARTaGENE project. Community Genet 10:147–158. [DOI] [PubMed] [Google Scholar]
- 64. Roberts LW, Warner TD, Geppert CM, Rogers M, Green Hammond KA (2005) Employees' perspectives on ethically important aspects of genetic research participation: a pilot study. Compr Psychiatry 46:27–33. [DOI] [PubMed] [Google Scholar]
- 65. Bates BR, Harris TM (2004) The Tuskegee Study of Untreated Syphilis and public perceptions of biomedical research: a focus group study. J Natl Med Assoc 96:1051–1064. [PMC free article] [PubMed] [Google Scholar]
- 66. Bates BR, Lynch JA, Bevan JL, Condit CM (2005) Warranted concerns, warranted outlooks: a focus group study of public understandings of genetic research. Soc Sci Med 60:331–344. [DOI] [PubMed] [Google Scholar]
- 67. Wong ML, Chia KS, Wee S, Chia SE, Lee J, et al. (2004) Concerns over participation in genetic research among Malay-Muslims, Chinese and Indians in Singapore: a focus group study. Community Genet 7:44–54. [DOI] [PubMed] [Google Scholar]
- 68. Bernhardt BA, Tambor ES, Fraser G, Wissow LS, Geller G (2003) Parents' and children's attitudes toward the enrollment of minors in genetic susceptibility research: implications for informed consent. Am J Med Genet A 116a:315–323. [DOI] [PubMed] [Google Scholar]
- 69. Berth H, Dinkel A, Balck F (2002) Health through gene tests? Acceptance of and concerns about genetic testing in a German representative sample. Zeitschrift für Gesundheitspsychologie 10:97–107. [Google Scholar]
- 70. Marsh V, Kombe F, Fitzpatrick R, Molyneux S, Parker M (2013) Managing misaligned paternity findings in research including sickle cell disease screening in Kenya: ‘consulting communities’ to inform policy. Soc Sci Med 96:192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bombard Y, Miller FA, Hayeems RZ, Carroll JC, Avard D, et al. (2012) Citizens' values regarding research with stored samples from newborn screening in Canada. Pediatrics 129:239–247. [DOI] [PubMed] [Google Scholar]
- 72. Terry SF, Christensen KD, Metosky S, Rudofsky G, Deignan KP, et al. (2012) Community engagement about genetic variation research. Popul Health Manag 15:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Menon D, Stafinski T (2008) Engaging the public in priority-setting for health technology assessment: findings from a citizens' jury. Health Expect 11:282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Secko DM, Preto N, Niemeyer S, Burgess MM (2009) Informed consent in biobank research: a deliberative approach to the debate. Soc Sci Med 68:781–789. [DOI] [PubMed] [Google Scholar]
- 75. Nisselle A, Forbes R, Bankier A, Hughes E, Aitken M (2008) Consumer contribution to the delivery of genetic health services. Am J Med Genet A 146a:2266–2274. [DOI] [PubMed] [Google Scholar]
- 76. McCarty CA, Chapman-Stone D, Derfus T, Giampietro PF, Fost N (2008) Community consultation and communication for a population-based DNA biobank: the Marshfield clinic personalized medicine research project. Am J Med Genet A 146A:3026–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schulz A, Caldwell C, Foster S (2003) “What are they going to do with the information?” Latino/Latina and African American perspectives on the Human Genome Project. Health Educ Behav 30:151–169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
(PDF)
Assessment matrix. The matrix used to analyze selected studies is divided according to the core aspects of Public Involvement Activities identified by a literature review.
(PDF)
Overview of journals that published selected PIA reports. Sorted according to (1) the number of studies selected from the respective journal and (2) alphabetical order.
(PDF)
Electronic search strategy.
(PDF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.