Abstract
Advances in the neurosciences have placed the field in the position where it is poised to significantly reduce the burden of nervous system disorders. However, drug discovery, development and translation for nervous system disorders still pose many unique challenges. The key scientific challenges can be summarized as follows: mechanisms of disease, target identification and validation, predictive models, biomarkers for patient stratification and as endpoints for clinical trials, clear regulatory pathways, reliability and reproducibility of published data, and data sharing and collaboration. To accelerate nervous system drug development the Institute of Medicine’s Forum on Neuroscience and Nervous System Disorders has hosted a series of public workshops that brought together representatives of industry, government (including both research funding and regulatory agencies), academia, and patient groups to discuss these challenges and offer potential strategies to improve the translational neuroscience.
Keywords: eTOC Blurb currently missing, DJS: no action required, Cell Press will handle
Introduction
Brain disorders, already common, are increasing in incidence worldwide based on such factors as the epidemiologic transition from infectious to noncommunicable disease and aging of populations in most countries. At the same time, there are clinically significant gaps in current treatments resulting in serious unmet medical need. Despite high and growing prevalence (Chan et al., 2013, Herbert et al., 2013), enormous contributions to disability worldwide (Vos et al., 2012), and substantial economic burden (Bloom et al., 2011; Hurd et al., 2013), there are no disease-altering therapies for neurodegenerative disorders, no treatments for the core symptoms of autism or the disabling cognitive or deficit symptoms of schizophrenia, and large numbers of individuals with epilepsy, depression, brain injury, and posttraumatic stress disorder gain little benefit from current treatments. Despite the substantial commercial opportunities that thus exist, attempts to discover effective, mechanistically new medications to treat nervous system disorders have proven so difficult that many companies have retreated from the field (Paul et al., 2010, Insel, 2012). Compared with other disease areas, failure rates in late stage clinical trials are disproportionately high for neurologic and psychiatric disease based, in part, on the complexity of the human brain, the difficulty of examining it directly in life, and significant evolutionary obstacles to the development of animal models that predict treatment drug efficacy, especially for disorders that affect the cerebral cortex (Hyman, 2012).
Overall, the scientific challenges to translational neuroscience research taken together with the financial risks (see Choi et al. article in this volume; Insel et al., 2012) have discouraged new investment in treatments for brain disorders both in the U.S. and globally. A number of pharmaceutical companies have divested themselves almost entirely from neuroscience research programs; most have decreased their investment relative to other therapeutic areas like oncology and cardiovascular disease (Table 1) (Miller, 2010, Abbott, 2011). In 2013, the FDA approved 27 new molecular entities of which only four had indications for nervous system disorders, and only 2 of these were therapeutic agents with the other 2 being imaging agents (FDA, 2013).
Table 1.
CNS Program Portfolios in Large Pharma: 2009 vs. 2014
| 2009 | 2014 | |
|---|---|---|
| TOTAL PROGRAMS | 267 | 129 |
| ABBOTT/ABBVIE | 17 | 10 |
| ASTRA ZENECA | 21 | 7 |
| BRISTOL MYERS SQUIBB | 12 | 2 |
| GLAXO SMITH KLINE | 40 | 14 |
| JOHNSON & JOHNSON | 18 | 17 |
| LILLY | 16 | 9 |
| MERCK+SCHERING PLOUGH | 32 | 7 |
| NOVARTIS | 14 | 15 |
| PFIZER+WYETH | 46 | 15 |
| ROCHE+GENENTECH | 22 | 21 |
| SANOFI+GENZYME | 29 | 12 |
| 267 | 129 |
Total number of discovery, preclinical and clinical drug development programs addressing neurology or psychiatry disease targets, visible from publicly available sources including SEC filings, investor briefings, and company websites. Reproduced by permission of Cell Press, Choi et al. (2014).
Across all disease areas, drug discovery and development are lengthy, costly, and risky processes. For disease areas, such as neurology and psychiatry where the science is less mature, the likelihood of failure may be so great, and the financial risk to companies so high that even the existence of large populations in need of treatment may not be enough to encourage investment. The average cost of bringing a new drug to market in any disease area has been estimated to be greater than $1 billion. Even when successful, the time from the start of a discovery program to regulatory approval, can take 12 to 15 years (Wegener and Rujescu, 2013). Drugs intended to treat psychiatric or neurologic disorders, on average, have longer mean times to complete clinical trials and longer times to regulatory approval (Tufts CSDD, 2012). Given, in addition, the increased probability of late stage clinical trial failures for central nervous system (CNS) drugs (Bunnage, 2011), it is not surprising that leadership within industry is increasingly investing elsewhere.
The greater risk and cost associated with treatment discovery and development for human brain disorders is directly related to the scientific challenges. In directing investment away from neurology and psychiatry, industry leadership points to several hurdles: (1) target identification and validation have lagged compared with other disease areas such as metabolism, infectious disease, and cancer; (2) significant disillusionment with use of animal models to predict efficacy; and (3) lacking biomarkers for most brain disorders, stratification of populations for clinical trial is difficult and often impossible, and endpoints must often be measured using subjective rating scales. Across all of industry, companies are experimenting with ways of making drug development more successful and efficient. What is critically needed for brain disorders is acceleration of scientific progress: better understandings of basic disease mechanisms and improved ability to translate such discoveries into biomarkers and therapeutics.
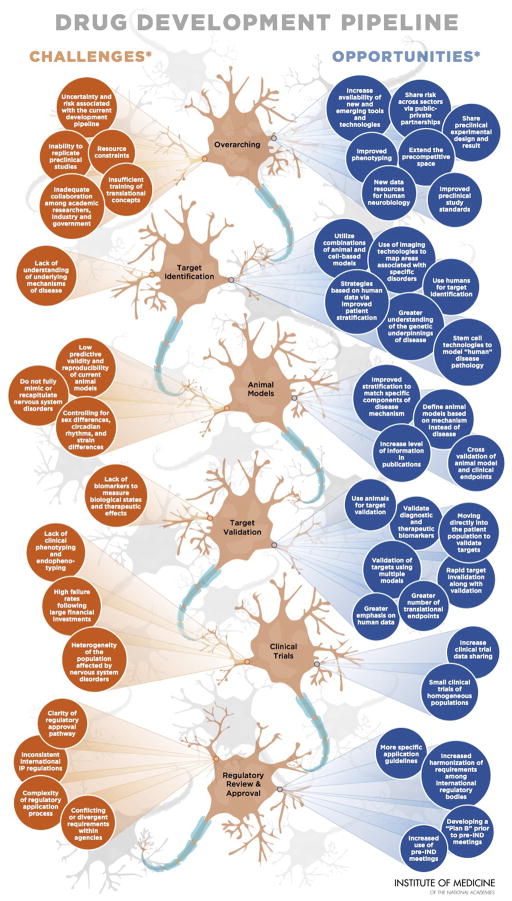
Given this context, the Institute of Medicine’s Forum on Neuroscience and Nervous System Disorders has hosted a series of public workshops that brought together representatives of industry, government (including both research funding and regulatory agencies), academia, and patient groups to discuss these challenges and offer potential strategies to improve the translational neuroscience (IOM, 2007, 2008, 2009a, 2011a, 2011b, 2011c, 2012, 2013a, 2013b, 2013c, 2013d ww.iom.edu/neuroforum). The challenges and opportunities that the IOM has explored are summarized in a recent graphic reproduced in Figure 1 and are briefly discussed below.
Figure 1. Improving and Accelerating Therapeutic Development for Nervous System Disorders.
Although there is a high burden associated with nervous system disorders, development of new therapeutics remains stagnant. Over the last decade, fewer new drugs for nervous system disorders have garnered approval in comparision to other therapeutic areas. Current data suggest that drug development, from the start of a discovery program to regulatory approval, can take an average of 12 to 15 years. This familiar statistic prompts an equally familiar question: Can the therapeutic development timeline be improved and accelerated by addressing challenges and developing opportunities? The challenges and opportunities presented in this graphic are not related and are presented in no certain order. The opportunities listed should not be interpreted as solutions to the challenges identified.
This graphic illustrates common challenges and opportunities suggested by individual participants in workshops hosted by the IOM Forum on Neuroscience and Nervous System Disorders (Accelerating Therapeutic Development for Nervous System Disorders Towards First-in-Human Trials [April 8–9, 2013] and improving Translation of Animal Models for Nervous System Disorders (March 28–29, 2012]). Statements, recommendations, and opinions expressed are those of the individual participants and are not necessarily endorsed or verified by the IOM, and should not be construed as reflecting any group consensus.
IND, investigational new drug; IP, intellectual property. Adapted and reprinted with permission from the Institute of Medicine.
Challenges and Opportunities
The prevalence and burden of brain disorders ensure that leadership within industry, academia, and government does not forget them. As alluded to, however, to spur new investment in this international enterprise advances in science and new approaches to translation are needed. The key scientific challenges can be summarized as follows: mechanisms of disease, target identification and validation, predictive models, biomarkers for patient stratification and as endpoints for clinical trials, clear regulatory pathways, reliability and reproducibility of published data, and data sharing and collaboration.
Mechanisms of Disease
The human brain is arguably the most complex object of biological study, has significant recent evolutionary changes that are poorly modeled in animals, and cannot readily be studied in life. In contrast to other disease areas, such as cancer, surgical procedures that yield relevant tissue can almost never be undertaken, and in any case, most brain disorders are not cell autonomous. Given the large number of cell types in the brain, their diverse synaptic connections, and the complexity of neural circuit structure and function, deep understandings of disease mechanism remain difficult to achieve. Because the pathophysiology of brain disorders is generally poorly understood, it is difficult to identify promising molecular targets and validate them. Without better understanding of disease mechanisms, it is also challenging to construct predictive animal models. Alzheimer’s disease (AD), for which both neuropathology and genetics have provided useful clues to pathogenesis, can serve as an example of the challenges for CNS therapeutics. AD is an area of intense focus in both academia and industry, with a major current effort to generate therapeutics based on the beta amyloid pathway, while investigating other promising mechanisms. These efforts notwithstanding, neither the normal nor possible pathogenic roles of beta amyloid peptides are well understood. Moreover, animal models that overexpress beta amyloid generally lack the key property of human neurodegenerative disorders, cell death. Mechanisms other than beta amyloid are also not well understood and are at an earlier stage.
While there is no substitute for relevant scientific discoveries, the production of better tools to interrogate brain function as well as disease mechanisms should prove generally useful as will the judicious use of large-scale collaborative organization of science. Important tools that are under development include enumeration and characterization of neural cell types, a goal of the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) initiative (The White House, 2013) and characterization of neural connectivity that yields for small and large-scale circuits. Enhanced sharing of data and larger-scale collaboration, as has been increasingly modeled in the genetics community, could help advance basic scientific knowledge about diverse diseases. For example, both genetics and cellular findings have revealed potential commonalities across neurodegenerative diseases that could contribute to development of therapeutics that may address more than one neurodegenerative disease (IOM, 2013b).
Enhanced Target Identification and Validation
Target identification is a critical step in the drug discovery and development pipeline. Across all of medicine, genetics continues to provide important molecular clues to disease pathogenesis, but for brain disorders in which defects in synaptic communication and functional connectivity represent the primary pathology (e.g., epilepsy, mood disorders, posttraumatic stress disorder, schizophrenia), genetics may represent the only feasible path to molecular mechanisms. The genetic causes of rare Mendelian brain disorders, such as Huntington’s disease and familial forms of AD, Parkinson’s disease, and Amyotrophic lateral sclerosis, have been revealed over the past three decades. Insight into polygenic brain disorders, including schizophrenia, bipolar disorder, and late onset forms of Alzheimer’s disease are well under way (Ripke, et al., 2014, Lambert, et al., 2013). These provide the greatest new opportunity to target identification in a generation (IOM, 2007, 2009b, 2011a, 2013b). Among the challenges that exist for putting emerging genetic discovery to work in understandings of disease mechanism and target identification is the fact that almost all risk alleles have limited penetrance. Even rare variants that have arisen more recently in human population history and have been less subject to evolutionary pressure than older common variants, rarely act in Mendelian or near-Mendelian fashion. Thus systems biology approaches, often requiring new cell-type specific molecular information, are likely to prove critical.
Target validation is an iterative process of increasing confidence in a target, which can be conceptualized as continuing through phase 3 clinical trials. In most disease areas, however, apparent efficacy in an animal model of disease represents an important step in validation, and within industry may represent an important ‘go-no go’ decision point prior to reaching the clinic. For CNS disorders, however, the increasing concerns about the predictive validity of many current animal models must be addressed if industry is to re-invest. To put the concern directly, many companies have come to the view that current putative brain disease models and animal-based assays of drug action are likely screening out potentially efficacious drugs, and screening in drugs that will not demonstrate efficacy in clinical trials. There is growing agreement that there are no animal models of psychiatric disorders such as schizophrenia or depression that capture the relevant pathophysiology. It has been argued (see below) that at least in the near term models of specific disease components may be possible if attention is paid to the evolutionary conservation of relevant mechanisms to the human. For example, the longstanding failure to develop analgesic drugs with new mechanisms (e.g., beyond opiates, nonsteroidal anti-inflammatory drugs and some anticonvulsants) has been thought to reflect, in large part, the use of animal based assays of both acute and chronic pain that do not correspond well to the human situation (Mogil, 2009). In psychiatry, antipsychotic and antidepressant drugs were discovered serendipitously when prototype drugs were administered to humans for other indications. Assays developed with these drugs, such as amphetamine-induced hyperlocomotion for antipsychotic drugs and forced swim and tail suspension for antidepressant drugs have, over decades, only detected molecules that share a mechanism with 1950’s prototypes and, with few exceptions, have not advanced efficacy (Hyman 2012). Approaches to produce better predictive models, whether based on cells, animals, or human biology has been a critical area of discussion within the meetings organized by IOM Neuroscience Forum.
Along with the process of target validation, it is critical to establish that therapeutic levels of a drug can be reliably delivered to the brain, that at those levels the drug binds its target, and modifies the disease pathway in the desired directions. Absent such information, a clinical trial cannot test the target validation hypothesis. While this may seem obvious, there are many clinical trials in recent CNS research in which companies have been uncertain as to whether a prior failure truly tested a hypothesis. Lack of attention to these factors, along with failures to publish underlying data, leads to costly and futile repetition of failures. Such concerns are now being addressed by some funding agencies, including the NIH (http://www.nimh.nih.gov/about/director/2014/a-new-approach-to-clinical-trials.shtml)
Beyond drug properties, validated biomarkers are critical to confirming nervous system targets (IOM, 2007). For example, many failed clinical trials meant to test the beta amyloid hypothesis were undercut by the inclusion of patients with dementia caused by Lewy body disease rather than Alzheimer’s disease (Jack Jr. and Holtzman, 2013), and by the inclusion of a significant number of patients in some of these trials with amyloid imaging signals below predetermined thresholds for amyloid positivity (Vellas, 2013).
From the point of view of cost and efficiency, the ability to convincingly invalidate targets is nearly as important as validation (IOM, 2011a). For targets that are well along in the validation process, first-in-human trials might be used to generate validation data, a concept that will be explored further on, might be another approach for accelerating the drug development pipeline (IOM, 2013a).
Animal and Non-Animal Models of Disease Mechanisms
The failure of animal models to predict accurately the efficacy of drugs with new mechanisms for nervous system disorders has been a central problem in drug development (IOM, 2013c). Using animals to study nervous system disorders can be especially difficult at least in part due to differences between animals and humans in cell types, transmitter function and anatomy. Given the heterogeneity of common human diseases and evolutionary difference between humans and laboratory animals, it is highly unlikely that any single model, tool or technique could provide a complete picture of a disease. Reliance on single models as efficacy gates or on a suite of models that is too narrow has likely contributed to failures in CNS drug development. Developing and integrating new approaches that utilize combinations of animal and non-animal models of disease mechanisms, along with new tools, technologies and techniques, might illuminate the underlying biological mechanisms of diseases and improve target identification, validation and therapeutic development (IOM, 2013a, 2013c).
Animal models may be valuable at capturing a particular aspect of a disease or studying the function of a specific molecular target, but animal models cannot be expected to recapitulate the full mechanism or symptomatology of a human disease. Therefore, animal models are better thought of more narrowly, for example, as models of a particular disease mechanism and not a complete model of a disease (IOM, 2010a, 2013c). Doing so will help ensure that an animal model serves as one of many important tools to study and validate disease mechanisms and targets, and that results derived from animal model studies are not over enthusiastically interpreted, and publicized, as evidence that the investigational agent will provide therapeutic benefit in the human disease.
Although animal models can reasonably assist in the prioritization of compounds for a validated target, they are not always as useful in prioritizing compounds aimed at novel targets. The absence of an animal model for recurring mood disorders (e.g., bipolar disorder) is one such example (Banasar et al., 2011, Manji et al., 2001, Savitz et al., 2013). Animal models do not always accurately predict dose, efficacy, and research priority. Some of these failures relate to the lack of understanding of the mechanisms for disease; how can successful animal models be created based on unknown mechanisms? Another explanation for these failures might not be poor animal models but rather researchers prematurely moving forward with answers from models without systemically validating the data across multiple animal and non-animal disease models. In addition, translation of behaviors is especially problematic and presents a challenge in deciphering results; this challenge can be a significant barrier to developing drugs for nervous system disorders with a behavioral component (e.g., depression or anxiety).
Emerging tools and technologies (e.g., iPSCs, humanized animal models, computational neuroscience) provide important opportunities to further elucidate mechanisms of diseases and assist in identifying and validating drug targets (IOM, 2013a). For example, stem cell technologies can be used to model “human” disease pathology at the cellular level and in some cases may be a faster and better alternative to animal models. Humanized animal models – developed by engrafting human tissue stem cells into mice - may help improve understanding of nervous system disorders and identify mechanisms of disease. In addition, it is important to take into account potential sex differences in animal models, which have been shown to impact data reproducibility and their utility (Clayton and Collins, 2014, IOM, 2010). Computational neuroscience in conjunction with neuroimaging might aid in understanding the underlying neurobiological mechanisms of diseases; imaging technologies might be helpful in this regard as well (IOM, 2013a, Manji et al., 2014). However, it is important to note that, like animal models, these tools and technologies do not fully mimic or recapitulate human diseases and disorders.
An initial focus on human phenotypes rather than animal models might also provide an opportunity to better inform the drug discovery process (IOM, 2013a). A specific issue addressed by workshop (IOM, 2013a) was under what circumstances it would be both ethical and practical Further, to ensure the humane care and use of animals, numerous laws, policies, and regulations are in place governing the use of animals in research, and certain animal regulations have implications specific to neuroscience research. However, there is minimal harmonization of these rules between different countries, which can inherently create challenges for the international research enterprise (IOM, 2012).
Clinical Trials
For many mental and neurological disorders, determining the prospects of a drug requires clinical testing, which is difficult and expensive and beleaguered by such challenges as patient heterogeneity, a lack of biomarkers, subjective and insensitive rating scales, enrolling patients at the earliest stage in their disease progression and lengthy trial durations. Success rates for new drugs in Phase II clinical trials have fallen to less than 20 percent, whereas the number of preclinical drugs needed to yield one approved drug has more than doubled (Arrowsmith, 2011). Unlike in other fields, detailed clinical phenotyping and endotyping are not always present, even though failures of clinical trials are almost always predictable due to the known heterogeneity of the patient population. This heterogeneity necessitates larger, more complex, and thus more expensive clinical trials. Alternatively, precision medicine and improved patient stratification may alleviate the need for large trials.
The paucity of suitable biomarkers might be another cause of the slowdown in developing therapeutics (IOM, 2007). Biomarkers can enhance target validation and determination of efficacy, but the process and resources necessary to develop candidate biomarkers into reliable tools are too great for academic researchers. On the other hand, industry does invest significantly in the development of biomarkers; however, the challenge has been that the development and validation of such markers frequently lags behind the development of the therapeutic molecule. Biomarker development might not be considered a priority by academic, government, and industry research programs despite the potential for biomarkers to propel drug discovery forward. The critical importance of target engagement biomarkers and/or pharmacodynamic biomarkers, to demonstrate functional modulation of the targeted pathway in the appropriate compartment in humans, has been convincingly demonstrated by a landmark study in which a retrospective analysis of Pfizer early development programs was performed (Morgan, 2012). The major findings from this study were not only that available target engagement and pharmacodynamics biomarkers provided confidence that the intended therapeutic mechanism of action was adequately tested in the clinical experiment, but also in the cases where such markers were available and shown to be sensitive to drug treatment, there was a higher probability of achieving a positive proof-of-concept in the clinic. More recently, a similar retrospective analysis of early development programs at AstraZeneca has corroborated these findings and more specifically shown that projects with efficacy biomarkers available at the start of their clinical testing had a greater prospect of advancing to the next level of testing (Cook, 2014).
The lengthy time for conducting clinical trials, especially preventative trials, is another particular challenge for mental and neurological disorders [see Choi et al. article in this volume]. For example, Alzheimer’s disease is a progressive disease, with the degenerative condition developing decades prior to the onset of clinical symptoms. Balancing the resources and commitment with the risks is a significant hurdle to investments in preventative clinical trials. Together these challenges to conducting clinical trails, coupled with a low success rate, are in part hindering translation of preclinical compounds into the clinical space. What is the impact of a lack of phenotyping and endotyping on the identification and validation of targets? How could the development of biomarkers be accelerated? How might extending the patent life of therapeutics increase the attractiveness for conducting lengthy clinical trials?
There is also a need for better patient stratification due to the high heterogeneity of patients with a nervous system disorder [IOM, 2007, 2013a]. Through clinical phenotyping and identification of common genetic variants, small clinical trials of homogeneous populations could be useful to establish proof of concept and further understand disease pathogenesis (Fishman, 2013; Leaf, 2013). In particular, populations, such as those with rare disease mutations, could be used as human “knockout” experiments to highlight shared and divergent mechanisms of disease.
Recognizing the daunting challenge of both biomarker discovery and validation and therapeutic development for central nervous system disorders a number of public private partnerships have been established that bridge organizational and international boundaries to drive progress via collaborative efforts. This is no better exemplified by the Alzheimer’s Disease Neuroimaging Initiative (ADNI (http://www.adni-info.org)), a longitudinal study started in 2005 to discover and validate fluid and imaging biomarkers in AD progression. The success of ADNI is undisputed, as evidenced by its catalyst to form ADNI 2 to study rate of disease progression, and the data derived from ADNI forms the basis for our current diagnosis of AD in its earliest form. Coordinated efforts in therapeutic development include the Dominantly Inherited Alzheimer’s Network (DIAN (http://dian-info.org)), an international collaboration funded by the National Institute on Aging to study rare forms of AD caused by gene mutations, and the European Platform for Proof-of-Concept for Prevention in Alzheimer’s Disease (EPOC-AD), an effort catalyzed by the European Innovative Medicines Initiative (IMI) to establish registries of AD patient cohorts for clinical trials and to implement adaptive trial design principles to accelerate proof-of-concept clinical studies.
Pharmaceutical companies, academic researchers, and government agencies compile large quantities of clinical research data that, if shared more widely both within and across sectors, could improve public health, enhance patient safety, and spur drug development (IOM, 2013d). Clinical trials, ongoing and completed, offer a wealth of information and opportunities. Through the incorporation of new biomarkers, trials may identify surrogate markers and shed light on future analysis (Manji et al, 2014; IOM, 2013d). Sharing of data from clinical trials, either successful or failed, could also be used to develop new hypotheses. However, much of the data generated by clinical trials are not public or shared beyond the data holder, and significant barriers to sharing these data exist. Therefore, a framework is required to ensure the responsible sharing of clinical trial data (IOM, 2013d); such a framework is currently being developed by the IOM (IOM, 2014).
Streamlined and Updated Regulatory Approval Pathways
Current regulatory processes may at times slow the drug development pipeline. Training and increased clarity around requirements for obtaining approval of an IND application - including safety pharmacology, extensive toxicity testing, and testing for absorption, distribution, metabolism, and excretion - might facilitate easier and faster regulatory approvals. The validation of surrogate markers, especially those that can be used in lengthy prevention trials, is also needed. Accelerated and conditional approval pathways could provide another strong mechanism for accelerating the drug development pipeline; however, increased knowledge about and willingness to use these pathways for nervous system disorders drugs are needed (see Choi et al, Neuron 2014). Further, the heterogeneity and complexity of the pathophysiology of many neuroscience disorders suggest that a single drug is not likely to be as effective for any given disorder. Rather, multipronged approaches to treatments, whether combinations of drugs, behavioral and psychotherapies, or devices, may be required to effectively treat some disorders. However, at this stage there is limited research exploring these options. For the limited areas where there is clinical data, the regulatory mechanisms for these types of combination therapies are not well developed (IOM, 2013a) and reimbursement policy is unclear.
Reliability and Reproducibility of Published Data
Reproducibility remains a critical issue for translation. The ability to rely on published data and process those data from one lab to another is critical for the successful translation of discovery research. The issues of reproducibility in basic and translational research are spilling over to the development of drugs. On the one hand, industry has the capability for rapid validation and determination of efficacy; however, the need to rely on the quality of preclinical data can hinder efforts (Prinz et al., 2011). Combined, these factors mean that the development of one drug takes longer and, in the end, requires a greater financial commitment. As previously mentioned, these challenges have led some companies to move away from drug development in areas that are particularly difficult. Even a broad look at the issue makes the complexity apparent: is the problem rooted in basic research outcomes and reporting, the translation from preclinical to clinical research, the design and execution of clinical trials, or all the above? How can decisions regarding when to go into clinical trials be made when the scientific validity and reproducibility of preclinical data are in question? How can the translation of studies and development of drugs be improved if the reproducibility and reliability of the foundational studies are low?
Current research paradigms might need to change, particularly for preclinical studies (IOM, 2013a, 2013c). It might be beneficial for preclinical studies to have rigorous standards, similar to clinical trials, to ensure sound research design and credible statistical analyses. This in turn could improve the reproducibility of preclinical studies, which is a major challenge for the field and a stumbling block for successful translation and movement along the drug development pipeline. One mechanism that might enhance reproducibility is the development of mechanisms to share preclinical experimental designs, data, and results in an accessible repository.
Multiple journals are actively working to develop and promote mechanisms for increasing the reproducibility and transparency of research. For example, Nature and the Nature research journals are introducing measures to ensure that methodological details are reported in full by providing more space for methods. The editors have also indicated that authors will be encouraged to include raw data in submissions and that statistics will be more closely examined. In a similar effort, Science will be adding members to their board of reviewing editors who have the statistical knowledge to carefully review and scrutinize data analysis methods. In addition, Science is adopting recommendations to increase transparency, including asking authors to indicate if there was a pre-experimental plan for data handling, if sample size estimations were conducted, whether samples were treated randomly, and if the experimenter was blinded. Many of these changes correspond to recommendations from a workshop hosted by the National Institute of Neurological Disorders and Stroke (Landis et al., 2012). Mechanisms for promoting best practices for preclinical studies include reporting of sample-size estimations, randomization, treatment blinding, and the handling of data.
The National Institutes of Health (NIH) is also tackling issues of reproducibility (Collins and Tabak, 2014). NIH is currently developing training modules on enhancing reproducibility and increased transparency. Mandatory training for NIH intramural postdoctoral fellows will include responsible conduct of research with an emphasis on good experimental design. In addition, several NIH institutes are developing and testing mechanisms to more systematically evaluate grant applications. NIH, working with publishers and industry, is also considering other ways to increase the transparency of published data and to improve reproducibility.
Lastly, another important consideration that has come up for much discussion concerns the question of publication bias, or more specifically the over-representation of positive data in the published literature and corresponding under-representation of negative data (IOM 2013a, 2013c, 2013d). The current incentive schemes for publication do not adequately provide for the consistent publication of negative findings, which can frequently be lost to the field, and also can result in unnecessary duplication of efforts across laboratories. In this regard there is currently a dichotomy between publication of clinical and preclinical research as it is commonplace to find publication of clinical study outcomes irrespective of whether the primary endpoints of the study were met. Moreover, clinical study protocols include pre-specified endpoints with statistical calculations of anticipated effect sizes and associated power calculations, and a pre-stated plan for data handling and reporting. Such rigor is consistently lacking in preclinical research reporting and undoubtedly contributes to the bias towards publication of positive findings. Can we achieve the right balance for reporting of both positive and negative findings in preclinical research, and ensure that the latter is valued and incentivized?
Collaboration and Data Sharing
Improving and accelerating the drug development pipeline for nervous system disorders can only occur if the scientific community (academia, industry, and government) comes together in the precompetitive space to discuss the challenges and opportunities to move the field forward as a whole. The field has finally reached the point where success cannot be achieved independently. The organizational challenges in the drug development pipeline are daunting. One of the largest challenges is that the majority of the field is working competitively on the same few targets. Grant review, especially in times when funding is tight, does not reward high-risk research. As a result, most research focuses on a select number of target and strategies. For example, 75% of all protein research is performed on the 10% of proteins that were identified prior to the mapping of the genome. Although the human genome encodes for over 500 kinases, of the over 20,000 papers published in 2009 on protein kinases, 65% focused on 50 proteins that were researched back in the 1990s (Edwards et al., 2011).
Innovation has also been hampered, in part, because of the absence of strong collaborations and cross training with disciplines outside the neurosciences such as engineering, chemistry, physics, and mathematics (IOM, 2008, 2010b, 2012). The ability to develop the tools and technologies needed to explore an organ as inaccessible as the brain is likely hindered by the division of fields, which has led to an absence of new ways to think about fundamental questions. For the field to make breakthrough findings, stronger collaborations will be needed across disciplines that are not traditionally viewed as being part of the neuroscience community, for example, system engineering, computational mathematics, and computer science (IOM, 2008, 2013a).
There is a need to “de-risk” research throughout the drug discovery process through sharing of risk and collaboration across sectors (IOM, 2007, 2009a, 2009b, 2011a, 2011b, 2013a, 2013d). Resources could be saved, attracting companies to reinvest in nervous system disorder drug discovery. As discovery research advances and becomes increasingly resource intensive and challenging, researchers could gain confidence and increase the probability of success through greater collaboration. No one group will be able to solve the challenges associated with developing therapeutics; therefore, incentives, both financial and in career development pathways, are needed to ensure effective collaborations take place, including the rapid sharing of positive and negative data.
Conclusions
Given the challenges surrounding current therapeutic development practices for nervous system disorders, a focus on the ability to predict outcomes and determine which steps are or are not informative in the development pipeline would likely be helpful. It is important to recognize the limitations related to de-risking research, target identification and validation, the regulatory process, clinical trials, and reproducibility. Although clinical outcomes cannot be predicted and additional research is needed, new and emerging strategies, tools, and technologies are being developed that could potentially improve the drug development process. Strengthened research on potentially common disease mechanisms and the use of animal and non-animal models of mechanism will also advance early stage drug research and development. Further, patient stratification, target identification based on human data, systematic evaluations of failed clinical trials, open data sharing among researchers, a centralized data base for preclinical trials, increased collaborative efforts and decreased replication, and improved guidance on regulatory issues are just a few potential solutions. Novel approaches and infrastructure changes to the current drug development pipeline might improve the efficacy of research and support a more efficient process. Although there are several bottlenecks in the current pipeline, there are many opportunities to facilitate drug discovery, development, translation for nervous system disorders through changes in methodological approaches, shifts in current processes, and modifications to the infrastructural components in drug development.
Acknowledgments
The authors would like to thank Dennis Choi for his thoughtful comments on the draft manuscript. The responsibility for the content of this article rests with the authors and does not necessarily represent the views of the Institute of Medicine, its committees, or its convening activities. Similarly, the views expressed at Institute of Medicine workshops and in the workshop summaries are those of individual participants and do not necessarily represent the views of the Institute of Medicine.
Footnotes
B. Altevogt, J. Dunlop, and D. Pankevich have no personal conflicts. J. Dunlop is an employee at AstraZeneca. S. Hyman serves on the science advisory boards for Novartis, AstraZeneca, and Fidelity Biosciences and the board of directors for Q-State. F. Gage is Founder and on the Scientific Advisory Board of Stem Cells Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott A. Novartis to shut brain research facility. Nature. 2011;480:161–162. doi: 10.1038/480161a. [DOI] [PubMed] [Google Scholar]
- Arrowsmith J. Trial watch: Phase 11 failures: 2008–2010. Nat Rev Drug Discov. 2011;10:328–329. doi: 10.1038/nrd3439. [DOI] [PubMed] [Google Scholar]
- Bloom DE, Cafiero ET, Jané-Llopis E, Abrahams-Gessel S, Bloom LR, Fathima S, Feigl AB, Gaziano T, Mowafi M, Pandya A, et al. The Global Economic Burden of Noncommunicable Diseases. Geneva: World Economic Forum; 2011. [Google Scholar]
- Bunnage ME. Getting pharmaceutical R&D back on target. Nat Chem Biol. 2011;7:335–339. doi: 10.1038/nchembio.581. [DOI] [PubMed] [Google Scholar]
- Chan KY, Wang W, Wu JJ, Liu L, Theodoratou E, Car J, Middleton L, Russ TC, Deary IJ, Campbell H, Wang W, Rudan I. Global Health Epidemiology Reference Group (GHERG) Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990–2010: a systematic review and analysis. Lancet. 2013;381:2016–2023. doi: 10.1016/S0140-6736(13)60221-4. [DOI] [PubMed] [Google Scholar]
- Choi Dennis W, Armitage Robert, Brady Linda S, Coetzee Timothy, Fisher William, Hyman Steven, Pande Atul, Paul Steven, Potter William, Roin Benjamin, Sherer Todd. Medicines for the Mind: Policy-Based “Pull” Incentives for Creating Breakthrough CNS Drugs. Neuron. 2014;84 doi: 10.1016/j.neuron.2014.10.027. this issue. [DOI] [PubMed] [Google Scholar]
- Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D, Brown D, Alexander R, March R, Morgan P, Satterthwaite E, Pangalos MN. Lessons learned from the fate of AstraZeneca’s drug pipeline: a five dimensional framework. Nat Rev Drug Discov. 2014;13:419–431. doi: 10.1038/nrd4309. [DOI] [PubMed] [Google Scholar]
- Edwards AM, Isserlin R, Bader GD, Frye SV, Willson TM, Yu FH. Too many roads not taken. Nature. 2011;470:163–165. doi: 10.1038/470163a. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Howard RJ, Kupfer DJ, Malloch G, Marston HM, Nutt DJ, Robbins TW, Stahl SM, Tricklebank MD, Williams JH. Drug research: A plan for mental illness. Nature. 2012;483:269. doi: 10.1038/483269a. [DOI] [PubMed] [Google Scholar]
- Herbert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368:1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE. Revolution stalled. Sci Transl Med. 2012;4:155cm11. doi: 10.1126/scitranslmed.3003142. [DOI] [PubMed] [Google Scholar]
- Insel TR, Gogtay N. National Institute of Mental Health clinical trials: new opportunities, new expectations. JAMA Psychiatry. 2014;71:745–746. doi: 10.1001/jamapsychiatry.2014.426. [DOI] [PubMed] [Google Scholar]
- Insel TR, Voon V, Nye JS, Altevogt BM, Bullmore ET, Goodwin GM, Howard RJ, Kupfer DJ, Malloch G, Marston HM, et al. Innovative solutions to novel drug development in mental health. Neurosci Behavr Rev. 2013;37:2438–2444. doi: 10.1016/j.neubiorev.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. Next-Generation Treatments for Mental Disorders. Sci Trans Med. 2012;4:1–9. doi: 10.1126/scitranslmed.3004873. [DOI] [PubMed] [Google Scholar]
- IOM (Institute of Medicine) Discussion framework for clinical trial data sharing: Guiding principles, elements, and activities. Washington, DC: The National Academies Press; 2014. [PubMed] [Google Scholar]
- IOM. Improving and Accelerating Therapeutic Development for Nervous System Disorders - Workshop Summary. Washington, DC: The National Academies Press; 2013a. [PubMed] [Google Scholar]
- IOM. Neurodegeneration: Exploring Commonalities Across Diseases - Workshop Summary. Washington, DC: The National Academies Press; 2013b. [PubMed] [Google Scholar]
- IOM. Improving the Utility and Translation of Animal Models for Nervous System Disorders - Workshop Summary. Washington, DC: The National Academies Press; 2013c. [PubMed] [Google Scholar]
- IOM. Sharing Clinical Research Data - Workshop Summary. Washington, DC: The National Academies Press; 2013d. [PubMed] [Google Scholar]
- IOM. International Animal Research Regulations: Impact on Neuroscience Research: Workshop Summary. Washington, DC: The National Academies Press; 2012. [PubMed] [Google Scholar]
- IOM. Glutamate-related biomarkers in drug development for disorders of the nervous system: Workshop summary. Washington, DC: The National Academies Press; 2011a. [PubMed] [Google Scholar]
- IOM. Future opportunities to leverage the Alzheimer’s Disease neuroimaging Initiative: A Workshop Summary. Washington, DC: The National Academies Press; 2011b. [PubMed] [Google Scholar]
- IOM. Sex differences and implications for translational neuroscience research: A workshop summary. Washington, DC: The National Academies Press; 2011c. [PubMed] [Google Scholar]
- IOM. Transforming clinical research in the United States: Challenges and opportunities: Workshop summary. Washington, DC: The National Academies Press; 2010a. [PubMed] [Google Scholar]
- IOM. Building a national framework for the establishment of regulatory science in drug development: Workshop summary. Washington, DC: The National Academies Press; 2010b. [PubMed] [Google Scholar]
- IOM. Venture philanthropy strategies to support translational research: Workshop summary. Washington, DC: The National Academies Press; 2009a. [PubMed] [Google Scholar]
- IOM. Breakthrough business models: Drug development for rare and neglected diseases and individualized therapies: Workshop summary. Washington, DC: The National Academies Press; 2009b. [PubMed] [Google Scholar]
- IOM. From Molecules to Minds: Challenges for the 21st Century. Workshop Summary. Washington, DC: The National Academies Press; 2008. [PubMed] [Google Scholar]
- IOM. Neuroscience biomarkers and biosignatures: converging technologies, emerging partnerships. Washington, DC: The National Academies Press; 2007. [PubMed] [Google Scholar]
- Jack C, Jr, Holtzman DM. Biomarker Modeling of Alzheimer’s Disease. Neuron. 2013;80:1347–1358. doi: 10.1016/j.neuron.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, Jun G, DeStefano AL, Bis JC, Beecham GW, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Insel TW, Narayan VA. Harnessing the informatics revolution for neuroscience drug R&D. Nat Rev Drug Discov. 2014;13:561–562. doi: 10.1038/nrd4395. [DOI] [PubMed] [Google Scholar]
- Miller G. Is Pharma Running Out of Brainy Ideas? Science. 2010;329:502–504. doi: 10.1126/science.329.5991.502. [DOI] [PubMed] [Google Scholar]
- Mogil JS. Animal models of pain: progress and challenges. Nat Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, Schacht AL. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov. 2010;9:203–214. doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- Prinz F, Schlange T, Asadullah K. Believe it or not: how much can we rely on published data on potential drug targets? Nat Rev Drug Discov. 2011;10:712. doi: 10.1038/nrd3439-c1. [DOI] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufts Center for the Study of Drug Development. [accessed August 15, 2014];Drugs to treat CNS diseases take 35% longer to develop than other drugs. 2012 http://csdd.tufts.edu/news/complete_story/pr_ir_marapr_2012.
- U.S. Food and Drug Administration. [accessed August 15, 2014];New Molecular Entity Approvals for 2013. 2013 http://www.fda.gov/drugs/developmentapprovalprocess/druginnovation/ucm381263.htm.
- Vellas B, Carrillo MC, Sampaio C, Brashear HR, Siemers E, Hampel H, Schneider LS, Weiner M, Doody R, Khachaturian Z. Designing drug trials for Alzheimer’s disease: What we have learned from the release of the phase III antibody trials: A report from the EU/US/CTAD Task Force. Alzheimers Dement. 2013;9:438–444. doi: 10.1016/j.jalz.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990—2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener G, Rujescu D. The current development of CNS drug research. Int J Neuropsychoph. 2013;16:1687–1693. doi: 10.1017/S1461145713000345. [DOI] [PubMed] [Google Scholar]
- The White House. [accessed September 10, 2014];BRAIN Initiative. 2013 http://www.whitehouse.gov/share/brain-initiative.