Abstract
Objective
The immune system has been implicated as one mechanism underlying the benefits of acupuncture therapy. Evidence suggests that acupuncture can ameliorate symptoms of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS), but the association between clinical response and the immune system has not been investigated.
Design/Setting
We investigated 12 CP/CPPS patients participating in a prospective randomized clinical trial comparing acupuncture versus sham acupuncture for effects on cellular immunity. Blood samples were taken before the first needling and after the last of 20 treatment sessions (week 10). Patients also completed questionnaires examining their CP/CPPS symptoms and mood status at the baseline and end of study visits.
Results
At the end of study 8 of 12 participants (67%) were classified as treatment responders, 4 participants each from the acupuncture and sham groups. The acupuncture group averaged a 5% increase in natural killer cell levels compared to corresponding sham (-13%; p=0.03). Similarly, patients randomized to acupuncture reported a reduction in other white blood cell parameters examined, supporting the possibility that immunity might be important in the pathophysiology of CP/CPPS.
Conclusions
The specific effect of acupuncture on CP/CPPS remains unclear. Further research is warranted to examine the mechanisms by which acupuncture therapy may improve clinical symptoms in patients with CP/CPPS.
Keywords: Chronic prostatitis/chronic pelvic pain syndrome, acupuncture, immune system, Traditional Chinese Medicine, neuroendocrine system
Introduction
Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is common afflicting approximately 2% to 10% of men younger than 50 years of age1 resulting in significant economic loss and reduced quality of life2. CP/CPPS is characterized by perineal, lower abdominal, penile and ejaculatory pains, frequently accompanied by urinary symptoms and/or voiding dysfunction3. Increasing evidence suggests that acupuncture could benefit men with CP/CPPS4, 5. Our group recently showed that acupuncture was a promising treatment for patients with CP/CPPS 6, with nearly twice as many CP/CPPS patients responding to acupuncture treatment than to sham acupuncture.
CP/CPPS has been associated with increased auto-reactive T cells 7, 8. In a recent animal study, Quick and colleagues demonstrated that transfer of T cells, and more specifically CD4+ T cells, could mediate development of pelvic pain in mice8. Based on these studies, we investigated the possibility that acupuncture may affect the immune system in patients with CP/CPPS and whether these changes might correlate with clinical response to treatment.
Methods
Study setting and procedures
This was part of a larger, randomised controlled clinical trial that has been published6, 9. Briefly, participants were men aged ≥20 years with a National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) total score ≥15 (scale 0-43) and symptoms for ≥3 within the preceding 6 months. Exclusion criteria included: bacterial prostatitis, urinary tract infection within 1 year or any consensus CP/CPPS exclusion criterion. The protocol was approved by the Joint School of Pharmaceutical Sciences, University of Science Malaysia-Penang Hospital Committee on Clinical Studies and the University of Washington Institutional Review Board.
Acupuncture and sham acupuncture were administered by three acupuncturists (minimum 2,800 hours training in China each) at 4 acupuncture points: CV1-Huiyin, CV4-Guan Yuan, SP6-Sanyinjiao and SP9-Yinlingquan, bilaterally without any needle stimulation (Supplementary Table 1 & Supplementary Figure 1) inserted to varying depth between 40-60mm. Shallow needling 0.5cm away from the acupuncture points was employed in the sham acupuncture arm which has been previously validated as a reliable sham procedure9. Each needle placement lasted for 30 minutes, twice weekly for 10 weeks, with subjects in supine position in the afternoon between 2pm – 4pm to reduce the influence of circadian rhythm on lymphocyte subsets 10. Participants did not use any medications or supplemental therapies known to affect immune function during the study.
Biochemical & flow cytometry analyses
White blood cell counts were calculated using an automated haemoanalyser (Beckman Coulter Electronics, Krefeld, Germany). Cellular immunity was evaluated by flow cytometry (FASCalibur flow cytometer, BD Biosciences, CA, US). In addition, plasma cortisol and opioid levels were measured. To reduce inter-assay variance, all assays were completed within the same batch.
Outcomes
Treatment efficacy was assessed by having participants complete the Chinese version of the NIH-CPSI that has been validated for use in our population11 and a questionnaire evaluating mood using a 10-point Likert scale immediately after the first, 10th and 20th (final) visit. Responders to treatment were defined as a 6-point decrease in the NIH-CPSI total score from baseline to week 10, based upon consensus of expert opinion and published recommendations6, 12.
Statistical analyses
We tested the hypothesis that acupuncture treatment would result in a decrease in T-cells compared to sham acupuncture. Because this is a paired comparison, we estimated that 6 participants in each group would provide statistical power of 80% to detect a 15.0% difference in the CD4+ T cell count based on a previously published study13. Descriptive analyses compared the study groups for each demographic and clinical variable. Biochemical data are presented in two forms (1) absolute levels and (2) changes in levels from baseline, calculated by subtracting the absolute level at the end of the study from baseline, divided by baseline values. Analyses were performed using PASW Statistics for Windows (IBM SPSS, Version 20.0, Armonk, NY). The significance level was set at p <0.05, 2-sided.
Results
Participants
A total of 12 participants were recruited into the study. Participants’ mean age was 42.9 ± 10.6 years (mean ± 1 SD), with CP/CPPS symptoms for an average of 10.2 ± 6.8 months and mean NIH-CPSI total score of 25.8 ± 5.8 (range 15 – 43) at study entry. Treatment groups were similar in demographic and clinical variables. At the end of study 8 of 12 participants (67%) were classified as treatment responders, 4 participants each from the acupuncture and sham groups.
Immunological parameters
Baseline cellular immununological variables were similar and within normal limits in both groups. No significant difference in change of absolute white cell counts were noted, although changes were greater in the acupuncture than sham acupuncture group. Similarly, no significant differences between the groups were noted in the mean absolute leukocyte counts.
To minimize inter-individual differences between participants, data were also analysed as percentage of change from baseline. Compared to sham acupuncture, patients in the acupuncture group showed an increase in NK lymphocyte sub-populations (p=0.03). In contrast, no significant changes were found in the other lymphocyte subpopulations. Similarly, total white cell values decreased more in the acupuncture group than sham acupuncture group, but none of these changes reached statistical significance (Table 1). Plasma cortisol, leucineencephalin and beta-endorphin levels were unaffected by treatment. Comparison of treatment responders to non-responders did not show any significant difference when analysed as absolute count or percentage change from baseline.
Table 1.
Percentage change in immunological parameters measurement among the participants
| Test | Acupuncture (N = 6) | Sham Acupuncture (N = 6) | p-value |
|---|---|---|---|
| Leukocytes (%) | −19.81 (26.53) | −9.58 (18.50) | 0.46 |
| Neutrophil (%) | −28.26 (33.19) | −11.65 (25.24) | 0.35 |
| Lymphocyte (%) | −2.35 (26.29) | 2.44 (21.28) | 0.74 |
| Monocyte (%) | −4.08 (35.89) | −3.40 (29.55) | 0.97 |
| Eosinophil (%) | 41.46 (86.69) | 4.88 (54.45) | 0.41 |
| Basinophil (%) | −17.25 (19.53) | −7.43 (45.07) | 0.64 |
| CD3+ (%) | 6.48 (7.30) | 2.00 (1.08) | 0.17 |
| CD4+ (%) | 11.51 (9.64) | 3.16 (5.61) | 0.10 |
| CD8+ (%) | 0.13 (13.77) | 2.79 (4.19) | 0.65 |
| CD19+ (%) | 2.64 (16.99) | 1.37 (24.42) | 0.92 |
| Natural Killer Cells (%) | 5.83 (8.86) | −13.51 (15.39) | 0.03 |
Subjective ratings
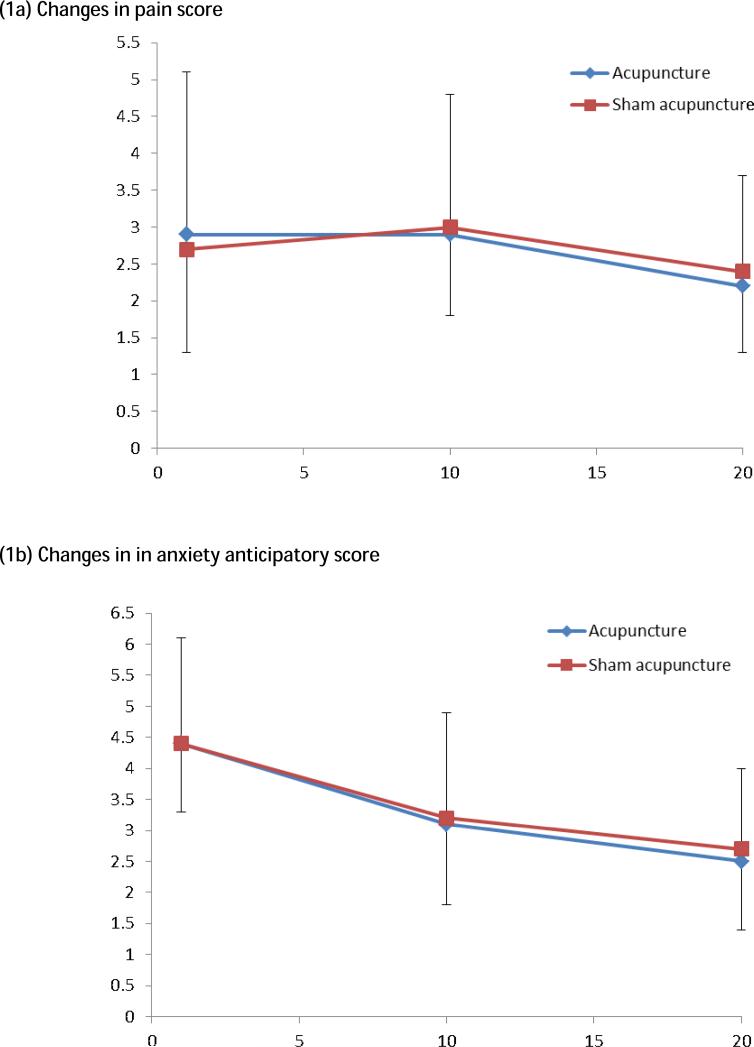
Mood ratings at the end of each session showed an average trend to be lower as the number of weeks progressed. There was no significant difference between mood ratings between the treatment groups (Figure 1).
Figure 1.
Changes in participant subjective scores over the 20 treatment sessions.
Discussion
In this study, CP/CPPS patients were randomized to acupuncture or corresponding sham acupuncture. The data indicated that acupuncture could modulate and increase the levels of NK cells in our population. NK cells serve a major role in the innate and adaptive response against infections and tumours through production of various cytokines14, 15. High NK cell levels have been speculated to play a protective role in preventing CP/CPPS 16 and other chronic inflammatory conditions, such as arthritis and multiple sclerosis17. It is possible that NK cells might act by producing T-helper cytokines that have been associated with disease remission18.
Emotional disturbances has been shown to cause immune impairment, possibly through effects on nervous and endocrine systems19. To overcome this problem, we used penetrating sham needling as a sham control9. To further ensure that differences in anxiety/stress and pain during acupuncture or sham did not affect the immune system we asked participants to rate their pain and anxiety experiences throughout the study. We found no differences in reported pain or anxiety experience between the treatment groups, further supporting our previous findings that the penetrating sham treatment was a valid method of blinding9.
This study had several strengths. Because leukocyte counts have circadian rhythms, we ensured all blood samples were obtained in the afternoon between 2-4 PM20. The control group received sham acupuncture, that has been previously validated as a reliable and valid method of blinding participants9. As such, we could objectively examine effects of acupuncture, since the immune system have been shown to affected by psychological factors through the central nervous and endocrine systems21, 22. We also had a population that accepts both traditional Chinese and Western medical approaches.
This study has important limitations. We had no healthy sample controls who did not receive treatment. We had a small sample size of only 12 participants due to the difficulty in getting patients to comply with the strict treatment and sampling protocol. Therefore, our study may be underpowered to detect a true difference that may have existed in this cohort. Because NK cell functions are not disease-specific, our findings support the need to evaluate other immune receptors and cytokines to clarify the effect of acupuncture. We also recognize the possibility that acupuncture-related changes in NK cells might occur but that such changes might not lead to improvement in patients’ symptoms. Nevertheless, our rigid protocol allowed us to adequately control many variables and inherent issues with acupuncture studies to examine possible mechanisms underlying the potential efficacy of acupuncture therapy in this difficult population. Participants in the sham group were also not inert to treatment with an equal number of participants responding to treatment as in the acupuncture group. These observations suggest that the sham procedure is a treatment and that future studies should include sham acupuncture and, perhaps, untreated controls to elucidate the precise mechanisms of action of acupuncture. We would recommend such studies should consider recruiting a larger cohort with a sample size of at least 60 in each arm, assuming that there is a 10 point difference between cohorts.
Conclusion
In summary, we found that acupuncture treatment may play a role in immunomodulation of CP/CPPS patients by augmenting NK cells levels. Further studies are warranted to examine the possible mechanisms of acupuncture on patients with CP/CPPS and other chronic pain conditions.
Supplementary Material
Highlights.
We compared immune functions in patients treated with acupuncture or sham.
Levels of natural killer cells were significantly higher in acupuncture patients
Changes in other leukocyte parameters were also higher in the acupuncture group.
These findings provide ‘proof-of-concept’ supporting future studies for acupuncture
Acknowledgements
Role and source of funding
The funding organization had no input into the design and conduct of the study; in the collection, analysis and interpretation or review; or in the approval of the manuscript.
Funding
This work was supported in part by NIH grants DK065266 and DK38955, National Institutes of Health, Bethesda, Maryland, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial registration: ClinicalTrials.gov number, NCT00260637)
Conflict of interest statement
The authors have no conflict of interest.
References
- 1.Krieger JN, Lee SWH, Jeon J, Cheah PY, Liong ML, Riley DE. Epidemiology of prostatitis. International journal of antimicrobial agents. 2008;31:85–90. doi: 10.1016/j.ijantimicag.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SWH, Cheah PY, Liong ML, Yuen KH, Schaeffer AJ, Propert K, et al. Demographic and Clinical Characteristics of Chronic Prostatitis: Prospective Comparison of the University of Sciences Malaysia Cohort With the United States National Institutes of Health Cohort. The Journal of urology. 2007;177:153–8. doi: 10.1016/j.juro.2006.08.098. [DOI] [PubMed] [Google Scholar]
- 3.Krieger J, Nyberg JL, Nickel J. NIH consensus definition and classification of prostatitis. JAMA. 1999;282:236–7. doi: 10.1001/jama.282.3.236. [DOI] [PubMed] [Google Scholar]
- 4.Lee S-H, Lee B-C. Electroacupuncture Relieves Pain in Men With Chronic Prostatitis/Chronic Pelvic Pain Syndrome: Three-arm Randomized Trial. Urology. 2009;73:1036–41. doi: 10.1016/j.urology.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 5.Lee S-H, Lee B-C. Use of Acupuncture as a Treatment Method for Chronic Prostatitis/Chronic Pelvic Pain Syndromes. Curr Urol Rep. 2011;12:288–96. doi: 10.1007/s11934-011-0186-0. [DOI] [PubMed] [Google Scholar]
- 6.Lee SWH, Liong ML, Yuen KH, Leong WS, Chee C, Cheah PY, et al. Acupuncture versus Sham Acupuncture for Chronic Prostatitis/Chronic Pelvic Pain. The American journal of medicine. 2008;121:79.e1–.e7. doi: 10.1016/j.amjmed.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.John H, Maake C, Barghorn A, Zbinden R, Hauri D, Joller-Jemelka HI. Immunological alterations in the ejaculate of chronic prostatitis patients: clues for autoimmunity. Andrologia. 2003;35:294–9. [PubMed] [Google Scholar]
- 8.Quick ML, Wong L, Mukherjee S, Done JD, Schaeffer AJ, Thumbikat P. Th1-Th17 Cells Contribute to the Development of Uropathogenic <italic>Escherichia coli</italic>-Induced Chronic Pelvic Pain. PLoS ONE. 2013;8:e60987. doi: 10.1371/journal.pone.0060987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SWH, Liong ML, Yuen KH, Leong WS, Khan NK, Krieger JN. Validation of a sham acupuncture procedure in a randomised, controlled clinical trial of chronic pelvic pain treatment. Acupuncture in Medicine. 2011;29:40–6. doi: 10.1136/aim.2010.003137. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki S, Toyabe S, Moroda T, Tada T, Tsukahara A, Iiai T, et al. Circadian rhythm of leucocytes and lymphocyte subsets and its possible correlation with the function of the autonomic nervous system. Clinical & Experimental Immunology. 1997;110:500–8. doi: 10.1046/j.1365-2249.1997.4411460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheah P, Liong M, Yuen K, Lee S, Yang J, Teh C, et al. Reliability and validity of the National Institutes of Health: Chronic Prostatitis Symptom Index in a Malaysian population. World J Urol. 2006;24:79–87. doi: 10.1007/s00345-005-0037-z. [DOI] [PubMed] [Google Scholar]
- 12.Alexander RB, Propert KJ, Schaeffer AJ, Landis JR, Nickel JC, O'Leary MP, et al. Ciprofloxacin or Tamsulosin in Men with Chronic Prostatitis/Chronic Pelvic Pain SyndromeA Randomized, Double-Blind Trial. Annals of Internal Medicine. 2004;141:581–9. doi: 10.7326/0003-4819-141-8-200410190-00005. [DOI] [PubMed] [Google Scholar]
- 13.Jong M, Hwang S, Chen F. Effects of electro-acupuncture on serum cytokine level and peripheral blood lymphocyte subpopulation at immune-related and non-immune-related points. Acupunct Electrother Res. 2006;31:45–9. doi: 10.3727/036012906815844337. [DOI] [PubMed] [Google Scholar]
- 14.Zamai L, Ponti C, Mirandola P, Gobbi G, Papa S, Galeotti L, et al. NK Cells and Cancer. The Journal of Immunology. 2007;178:4011–6. doi: 10.4049/jimmunol.178.7.4011. [DOI] [PubMed] [Google Scholar]
- 15.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: Function and Regulation by Innate Cytokines. Annual Review of Immunology. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 16.Vykhovanets EV, Resnick MI, Marengo SR. The healthy rat prostate contains high levels of natural killer-like cells and unique subsets of CD4+ helper-inducer T cells: Implications for prostatitis. J Urol. 2005;173:1004–10. doi: 10.1097/01.ju.0000149130.06055.f2. [DOI] [PubMed] [Google Scholar]
- 17.Koch J, Steinle A, Watzl C, Mandelboim O. Activating natural cytotoxicity receptors of natural killer cells in cancer and infection. Trends in Immunology. 34:182–91. doi: 10.1016/j.it.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi Y, Takahashi H, Satoh T, Okazaki Y, Mizuki N, Takahashi K, et al. Natural killer cells control a T-helper 1 response in patients with Behcet's disease. Arthritis Research & Therapy. 2010;12:R80. doi: 10.1186/ar3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mori H, Nishijo K, Kawamura H, Abo T. Unique immunomodulation by electro-acupuncture in humans possibly via stimulation of the autonomic nervous system. Neuroscience Letters. 2002;320:21–4. doi: 10.1016/s0304-3940(02)00012-5. [DOI] [PubMed] [Google Scholar]
- 20.Huang W, Taylor A, Howie J, Robinson N. Is the Diurnal Profile of Salivary Cortisol Concentration a Useful Marker for Measuring Reported Stress in Acupuncture Research? A Randomized Controlled Pilot Study. J Altern Complement Med. 2012;18:242–50. doi: 10.1089/acm.2010.0325. [DOI] [PubMed] [Google Scholar]
- 21.Ader R, Cohen N, Felten D. Psychoneuroimmunology: interactions between the nervous system and the immune system. The Lancet. 1995;345:99–103. doi: 10.1016/s0140-6736(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 22.Butts CL, Sternberg EM. Neuroendocrine factors alter host defense by modulating immune function. Cellular Immunology. 2008;252:7–15. doi: 10.1016/j.cellimm.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.