Abstract
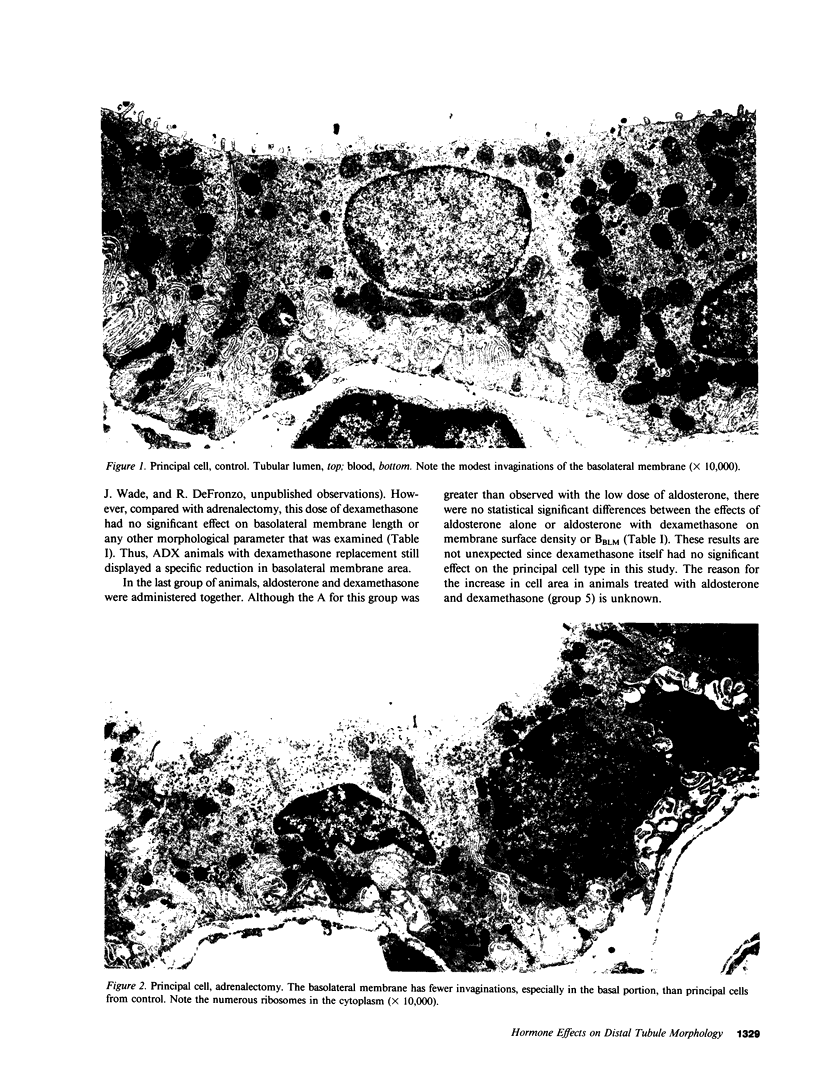
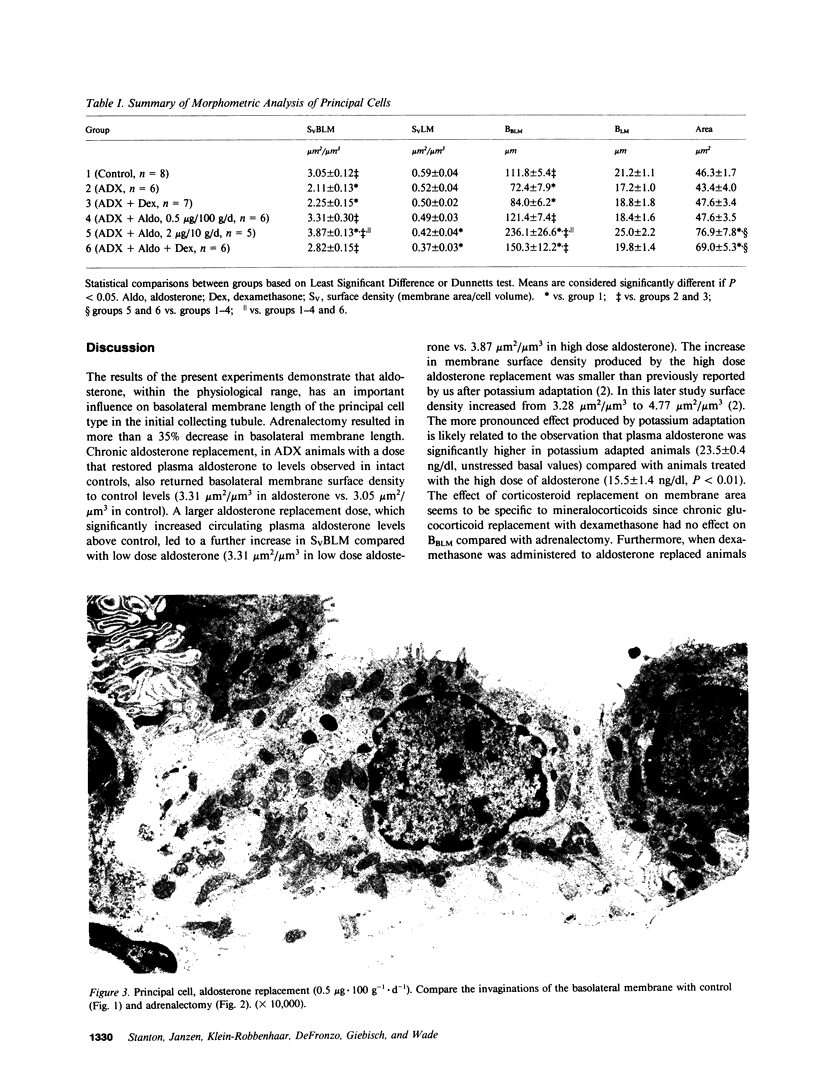
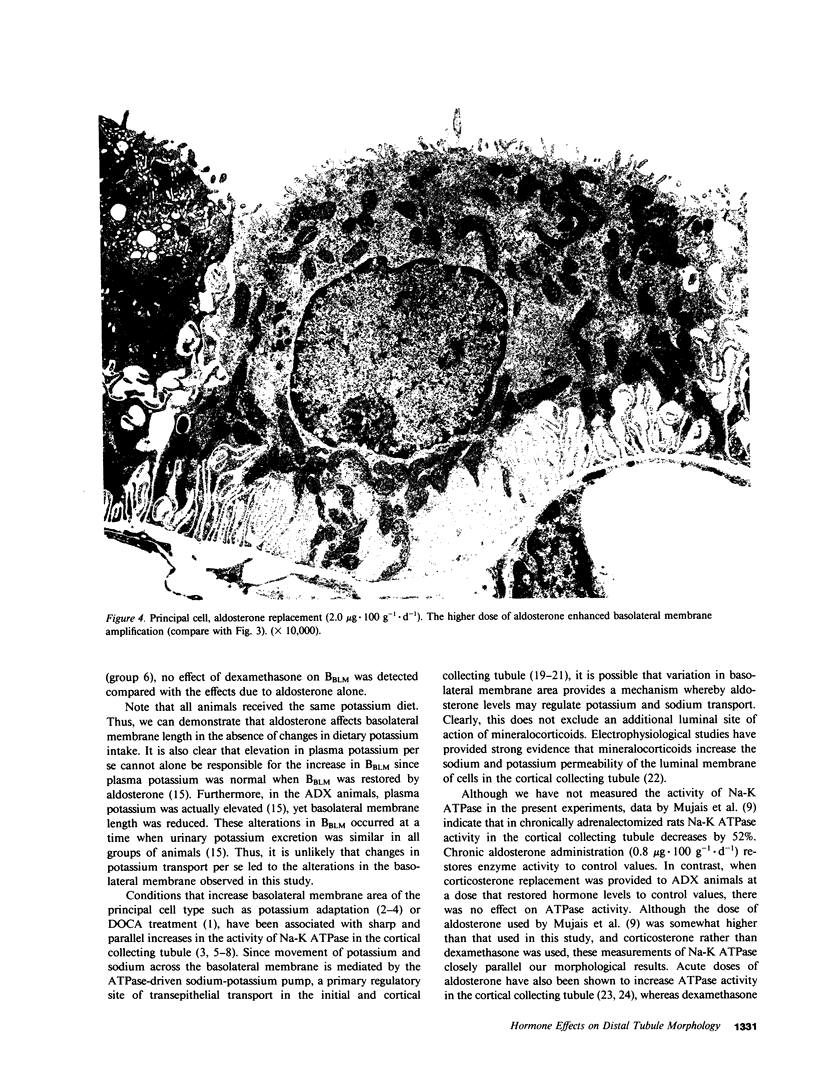
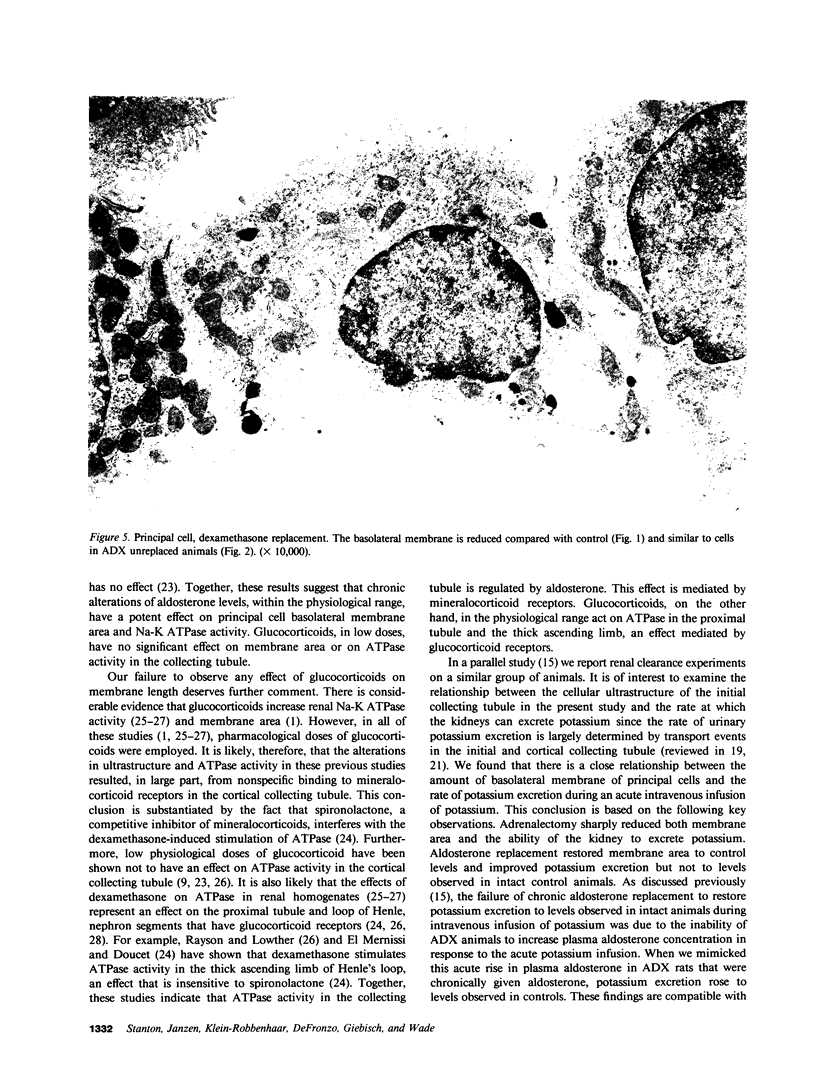
This study examines the effects of adrenalectomy and physiological replacement of mineralocorticoids and glucocorticoids on the cellular ultrastructure of the rat initial collecting tubule (late distal tubule). Animals were adrenalectomized (ADX) and for 10 d received by osmotic minipump either: vehicle, aldosterone (0.5 micrograms X 100 g-1 X d-1), aldosterone (2.0 micrograms X 100 g-1 X d-1), dexamethasone (1.2 micrograms X 100 g-1 X d-1), or aldosterone (0.5 micrograms X 100 g-1 X d-1) with dexamethasone (1.2 micrograms X 100 g-1 X d-1). Radioimmunoassay revealed that the low dose of aldosterone restored plasma aldosterone to control levels. The higher dose of aldosterone increased plasma levels by threefold. Morphometric techniques were used to measure membrane length of individual principal and intercalated cells in each condition. The basolateral membrane length of principal cells decreased by 35% in ADX animals. Low dose aldosterone replacement (0.5 micrograms X 100 g-1 X d-1) in ADX animals maintained membrane length at control values; at a higher level of aldosterone (2.0 micrograms X 100 g-1 X d-1) membrane length increased by 111% compared with control. Dexamethasone treatment, at a level that restored glomerular filtration rate to normal, had no effect on cellular ultrastructure. Combined aldosterone and dexamethasone replacement had no greater effect on basolateral membrane length than aldosterone alone. The length of the luminal membrane of the principal cell type was not affected by ADX or hormone treatment. Intercalated cell membrane length was not affected by ADX or hormone replacement. Thus, chronic aldosterone levels have an important, selective effect on the basolateral membrane of the principal cell. The correlation between these morphological results and the steroid hormone effects on renal electrolyte excretion, reported in the companion paper (15), suggests that basolateral membrane length is an important factor controlling the rate of sodium and potassium transport by the initial collecting tubule.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bia M. J., Tyler K., DeFronzo R. The effect of dexamethasone on renal potassium excretion and acute potassium tolerance. Endocrinology. 1983 Nov;113(5):1690–1696. doi: 10.1210/endo-113-5-1690. [DOI] [PubMed] [Google Scholar]
- Charney A. N., Silva P., Besarab A., Epstein F. H. Separate effects of aldosterone, DOCA, and methylprednisolone on renal Na-K-ATPase,. Am J Physiol. 1974 Aug;227(2):345–350. doi: 10.1152/ajplegacy.1974.227.2.345. [DOI] [PubMed] [Google Scholar]
- Doucet A., Katz A. I. Renal potassium adaptation: Na-K-ATPase activity along the nephron after chronic potassium loading. Am J Physiol. 1980 May;238(5):F380–F386. doi: 10.1152/ajprenal.1980.238.5.F380. [DOI] [PubMed] [Google Scholar]
- Edelman I. S., Marver D. Mediating events in the action of aldosterone. J Steroid Biochem. 1980 Jan;12:219–224. doi: 10.1016/0022-4731(80)90271-x. [DOI] [PubMed] [Google Scholar]
- Edelman I. S. Receptors and effectors in hormone action on the kidney. Am J Physiol. 1981 Oct;241(4):F333–F339. doi: 10.1152/ajprenal.1981.241.4.F333. [DOI] [PubMed] [Google Scholar]
- El Mernissi G., Chabardès D., Doucet A., Hus-Citharel A., Imbert-Teboul M., Le Bouffant F., Montégut M., Siaume S., Morel F. Changes in tubular basolateral membrane markers after chronic DOCA treatment. Am J Physiol. 1983 Jul;245(1):F100–F109. doi: 10.1152/ajprenal.1983.245.1.F100. [DOI] [PubMed] [Google Scholar]
- El Mernissi G., Doucet A. Short-term effects of aldosterone and dexamethasone on Na-K-ATPase along the rabbit nephron. Pflugers Arch. 1983 Oct;399(2):147–151. doi: 10.1007/BF00663911. [DOI] [PubMed] [Google Scholar]
- Ernst S. A., Ellis R. A. The development of surface specialization in the secretory epithelium of the avian salt gland in response to osmotic stress. J Cell Biol. 1969 Feb;40(2):305–321. doi: 10.1083/jcb.40.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M. J., Stanton B. A., Giebisch G. H. Differential acute effects of aldosterone, dexamethasone, and hyperkalemia on distal tubular potassium secretion in the rat kidney. J Clin Invest. 1984 Nov;74(5):1792–1802. doi: 10.1172/JCI111598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte J. G., Black J. A., Forte T. M., Machen T. E., Wolosin J. M. Ultrastructural changes related to functional activity in gastric oxyntic cells. Am J Physiol. 1981 Nov;241(5):G349–G358. doi: 10.1152/ajpgi.1981.241.5.G349. [DOI] [PubMed] [Google Scholar]
- Garg L. C., Knepper M. A., Burg M. B. Mineralocorticoid effects on Na-K-ATPase in individual nephron segments. Am J Physiol. 1981 Jun;240(6):F536–F544. doi: 10.1152/ajprenal.1981.240.6.F536. [DOI] [PubMed] [Google Scholar]
- Geering K., Girardet M., Bron C., Kraehenbühl J. P., Rossier B. C. Hormonal regulation of (Na+,K+)-ATPase biosynthesis in the toad bladder. Effect of aldosterone and 3,5,3'-triiodo-L-thyronine. J Biol Chem. 1982 Sep 10;257(17):10338–10343. [PubMed] [Google Scholar]
- Giebisch G., Stanton B. Potassium transport in the nephron. Annu Rev Physiol. 1979;41:241–256. doi: 10.1146/annurev.ph.41.030179.001325. [DOI] [PubMed] [Google Scholar]
- Gluck S., Cannon C., Al-Awqati Q. Exocytosis regulates urinary acidification in turtle bladder by rapid insertion of H+ pumps into the luminal membrane. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4327–4331. doi: 10.1073/pnas.79.14.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaissling B. Structural aspects of adaptive changes in renal electrolyte excretion. Am J Physiol. 1982 Sep;243(3):F211–F226. doi: 10.1152/ajprenal.1982.243.3.F211. [DOI] [PubMed] [Google Scholar]
- Karnaky K. J., Jr, Ernst S. A., Philpott C. W. Teleost chloride cell. I. Response of pupfish Cyprinodon variegatus gill Na,K-ATPase and chloride cell fine structure to various high salinity environments. J Cell Biol. 1976 Jul;70(1):144–156. doi: 10.1083/jcb.70.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashgarian M., Taylor C. R., Binder H. J., Hayslett J. P. Amplification of cell membrane surface in potassium adaptation. Lab Invest. 1980 Jun;42(6):581–588. [PubMed] [Google Scholar]
- Koeppen B. M., Biagi B. A., Giebisch G. H. Intracellular microelectrode characterization of the rabbit cortical collecting duct. Am J Physiol. 1983 Jan;244(1):F35–F47. doi: 10.1152/ajprenal.1983.244.1.F35. [DOI] [PubMed] [Google Scholar]
- Madsen K. M., Tisher C. C. Cellular response to acute respiratory acidosis in rat medullary collecting duct. Am J Physiol. 1983 Dec;245(6):F670–F679. doi: 10.1152/ajprenal.1983.245.6.F670. [DOI] [PubMed] [Google Scholar]
- Marver D., Kokko J. P. Renal target sites and the mechanism of action of aldosterone. Miner Electrolyte Metab. 1983 Jan-Feb;9(1):1–18. [PubMed] [Google Scholar]
- Mujais S. K., Chekal M. A., Jones W. J., Hayslett J. P., Katz A. I. Regulation of renal Na-K-ATPase in the rat. Role of the natural mineralo- and glucocorticoid hormones. J Clin Invest. 1984 Jan;73(1):13–19. doi: 10.1172/JCI111183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natke E., Jr, Stoner L. C. Na+ transport properties of the peritubular membrane of cortical collecting tubule. Am J Physiol. 1982 Jun;242(6):F664–F671. doi: 10.1152/ajprenal.1982.242.6.F664. [DOI] [PubMed] [Google Scholar]
- O'Neil R. G., Helman S. I. Transport characteristics of renal collecting tubules: influences of DOCA and diet. Am J Physiol. 1977 Dec;233(6):F544–F558. doi: 10.1152/ajprenal.1977.233.6.F544. [DOI] [PubMed] [Google Scholar]
- Petty K. J., Kokko J. P., Marver D. Secondary effect of aldosterone on Na-KATPase activity in the rabbit cortical collecting tubule. J Clin Invest. 1981 Dec;68(6):1514–1521. doi: 10.1172/JCI110405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastegar A., Biemesderfer D., Kashgarian M., Hayslett J. P. Changes in membrane surfaces of collecting duct cells in potassium adaptation. Kidney Int. 1980 Sep;18(3):293–301. doi: 10.1038/ki.1980.139. [DOI] [PubMed] [Google Scholar]
- Rayson B. M., Lowther S. O. Steroid regulation of Na+-K+-ATPase: differential sensitivities along the nephron. Am J Physiol. 1984 May;246(5 Pt 2):F656–F662. doi: 10.1152/ajprenal.1984.246.5.F656. [DOI] [PubMed] [Google Scholar]
- Rodriguez H. J., Sinha S. K., Starling J., Klahr S. Regulation of renal Na+-K+-ATPase in the rat by adrenal steroids. Am J Physiol. 1981 Aug;241(2):F186–F195. doi: 10.1152/ajprenal.1981.241.2.F186. [DOI] [PubMed] [Google Scholar]
- Schwartz G. J., Burg M. B. Mineralocorticoid effects on cation transport by cortical collecting tubules in vitro. Am J Physiol. 1978 Dec;235(6):F576–F585. doi: 10.1152/ajprenal.1978.235.6.F576. [DOI] [PubMed] [Google Scholar]
- Stanton B. A., Biemesderfer D., Wade J. B., Giebisch G. Structural and functional study of the rat distal nephron: effects of potassium adaptation and depletion. Kidney Int. 1981 Jan;19(1):36–48. doi: 10.1038/ki.1981.5. [DOI] [PubMed] [Google Scholar]
- Stanton B. A., Giebisch G. H. Potassium transport by the renal distal tubule: effects of potassium loading. Am J Physiol. 1982 Nov;243(5):F487–F493. doi: 10.1152/ajprenal.1982.243.5.F487. [DOI] [PubMed] [Google Scholar]
- Stanton B., Giebisch G., Klein-Robbenhaar G., Wade J., DeFronzo R. A. Effects of adrenalectomy and chronic adrenal corticosteroid replacement on potassium transport in rat kidney. J Clin Invest. 1985 Apr;75(4):1317–1326. doi: 10.1172/JCI111832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J. B., O'Neil R. G., Pryor J. L., Boulpaep E. L. Modulation of cell membrane area in renal collecting tubules by corticosteroid hormones. J Cell Biol. 1979 May;81(2):439–445. doi: 10.1083/jcb.81.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will P. C., Cortright R. N., Hopfer U. Polyethylene glycols as solvents in implantable osmotic pumps. J Pharm Sci. 1980 Jun;69(6):747–749. doi: 10.1002/jps.2600690644. [DOI] [PubMed] [Google Scholar]
- Wingo C. S., Seldin D. W., Kokko J. P., Jacobson H. R. Dietary modulation of active potassium secretion in the cortical collecting tubule of adrenalectomized rabbits. J Clin Invest. 1982 Sep;70(3):579–586. doi: 10.1172/JCI110650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhall P. B., Tisher C. C. Response of the distal tubule and cortical collecting duct to vasopressin in the rat. J Clin Invest. 1973 Dec;52(12):3095–3108. doi: 10.1172/JCI107509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright F. S., Giebisch G. Renal potassium transport: contributions of individual nephron segments and populations. Am J Physiol. 1978 Dec;235(6):F515–F527. doi: 10.1152/ajprenal.1978.235.6.F515. [DOI] [PubMed] [Google Scholar]