Abstract
SecA is an essential protein possessing ATPase activity in bacterial protein translocation for which Rose Bengal (RB) is the first reported sub-micromolar inhibitor in ATPase activity and protein translocation. Here, we examined the mechanisms of inhibition on various forms of SecA ATPase by conventional enzymatic assays, and by monitoring the SecA-dependent channel activity in the semi-physiological system in cells. We build on the previous observation that SecA with liposomes form active protein-conducting channels in the oocytes. Such ion channel activity is enhanced by purified Escherichia coli SecYEG-SecDF•YajC liposome complexes. Inhibition by RB could be monitored, providing correlation of in vitro activity and intact cell functionality. In this work, we found the intrinsic SecA ATPase is inhibited by RB competitively at low ATP concentration, and non-competitively at high ATP concentrations while the Translocation ATPase with precursors and SecYEG is inhibited non-competitively by RB. The Inhibition by RB on SecA channel activity in the oocytes with exogenous ATP-Mg2+, mimicking translocation ATPase activity, is also non-competitive. The non-competitive inhibition on channel activity has also been observed with SecA from other bacteria which otherwise would be difficult to examine without the cognate precursors and membranes.
Keywords: SecA ATPase, Rose Bengal inhibition, Proteo-liposomes, channel activity, Oocytes
1. Introduction
SecA is an essential protein with ATPase activity in bacterial protein translocation. SecA exists in soluble and membrane-bound forms in bacterial cells [1] and has three levels of ATPase activity: the soluble SecA possesses low intrinsic ATPase, which is stimulated by lipids (Membrane/lipid ATPase), and further stimulated by protein precursors and SecYEG (Translocation ATPase) [2]. The translocation ATPase correlates with the process of protein translocation [3], though the excessive ATP hydrolysis may not correspond directly to the translocation of precursors [4,5,6]. It is generally viewed that in the translocation of precursors across the membranes, the SecYEG complex serves as the essential core of translocation channel and SecA as a sewing machine to hydrolyze ATP pushing precursors through the core channel [6,7]. The other membrane SecDF•YajC complex stabilizes the SecYEG complex during protein translocation [8,9,10]. Purified soluble SecA can insert into membranes, and become an integrated membrane protein [11,12,13]. In addition, there are evidences that SecA alone can integrate into lipids and form a ring-like pore structures [14] that could serve as protein-conducting channels for protein translocation and ion channel activity in the absence of SecYEG [15,16]. SecA from various bacteria with liposomes can also elicit channel activity [15,17]. The SecA-alone channels are less efficient and loss specificity which can be restored by reconstitution with SecYEG/SecDF-YajC [15,17,18]. We hypothesize that there are two SecA-dependent protein translocation pathways: in addition to the one at the high-affinity binding sites with SecYEG, there is another at the low-affinity sites with lipids without SecYEG [13,17,18]. Thus, in our working models [13], one protomer of SecA is still the indispensable enzymatic ATPase of the channel which is formed by a second asymmetric SecA protomer as the core of SecA-alone protein-conducting channel [13]; SecYEG and SecDF•YajC gain efficiency and signal peptide specificity. In this view, the SecA-SecYEG is the high-efficient protein-conducting channels, while SecA-alone channels are less efficient, yet exist [15,17].
SecA serves as an ATPase to drive protein translocation across membranes. SecA possesses low intrinsic ATPase activity that is increased upon interaction with lipids, and the translocation ATPase is further enhanced by protein precursors and SecYEG [2,19]. Such conformational changes of SecA in the membranes alter its sensitivities and the various ATPase activity of SecA in response to SecA inhibitors, sodium azide [20] and Rose Bengal [21]. Inhibitors for SecA and protein translocation have been the topics of extensive studies [reviews [22,23]. Azide has been found to be an effective inhibitor for SecA-dependent translocation, and the mutation of azide-resistant mutant resides in SecA [20]. However, azide does not affect SecA intrinsic ATPase activity [20]. Other SecA inhibitors have been reported [24,25,26]. Rose Bengal (RB) is the first reported sub-micromolar inhibitor in ATPase activities and protein translocation [21].
Here, we further examined the mechanism of inhibition of various forms of SecA activities by RB. We determined the ATPase activities in solution. We also determined the ion channel activities in the oocytes used liposomes reconstituted with various purified bacterial SecA homologs, and with other Sec protein complexes (SecYEG and SecDF•YajC).
2. Material and Methods
2.1. Bacterial strains, medium, and chemicals
Escherichia coli K-12 strain MC4100 and BA13 (MC4100 secA13(am) supF(ts)) [27] from D. Oliver were used in this study. Luria-Bertani (LB) liquid and solid (1.5% agar) media with 0.2% glucose were used for bacterial growth. Rose Bengal and Erythrocin B were from Sigma-Aldrich Corp (St. Louis, MO). All other chemicals are reagent grade from commercial sources.
2.2. Preparations of various SecA proteins, proOmpA, and membrane vesicles
The N-terminal catalytic domain of SecA from E. coli (EcN68) was over-expressed from pIMBB28 obtained from A. Economou [4]. E. coli SecA from BL21(λDE3)/pT7-SecA was purified as described [1,11]. SecA homologous from other bacteria were purified similarly from BL21.19 as described previously [11,28,29]. Preparations for various reagents were described previously: purified proOmpA precursors [30], membrane complexes of SecYEG and SecDF•YajC [17], and urea-washed SecA-depleted BA13 membrane vesicles [21,31]. Protein concentration was determined by A280/260 ratio or Bradford assay as described [31].
2.3. In vitro ATPase activity assay
ATPase activity assays were performed as described previously [2] with minor modifications as described previously [21]. For intrinsic ATPase assays, 5 μg EcSecA was used unless otherwise specified, and for membrane ATPase, 1.5 μg EcSe4cA, 3 μg urea-washed E. coli BA13 membrane. For translocation ATPase assay, reaction mixtures contained 0.5 μg EcSecA, 1 μg proOmpA in addition to membranes. All reactions were carried out at 40°C.
2.4. Liposomes preparation and reconstitution of proteo-liposomes
Liposomes from E. coli total lipids extracts (Avanti Polar Lipid, Inc) were prepared in TAK buffer containing Tris-HCl 50 mM pH 7.6, 20 mM NH4Cl and 25 mM KCl as described [15]. SecA-liposomes were prepared by mixing with purified SecA by vortex and incubated at 4 °C. SecYEG-SecDF•YajC reconstitution with liposomes was as described [17].
2.5. Oocytes preparation, Injection and voltage clamp measurement
Oocytes were obtained from live frog Xenopus laevis (Xenopus Express, Inc) and injected with sample mixtures as described previously [32]. The voltage clamp adapted from an electrophysiological method was used to measure the opening of protein conducting channels as described previously [15,16,32]. Briefly, the 50 nl sample mixtures were injected into dark pole site of oocytes. The effective concentration of each component was based on the average volume of 500 nl oocytes. The ion current was recorded after three hours of incubation at 23 °C. Unless otherwise noted, the amount for each component is 120 ng liposomes, 120 ng SecA, 14 ng proOmpA, 2 mM ATP, and 1 mM Mg++ and where indicated, 0.47 ng of SecYEG and 0.53 ng of SecDF•YajC.
3. Results and Discussion
3.1. Kinetic of RB Inhibition of EcSecA ATPase activities
Previously, we screened a series of compounds including fluorescein analogs using a truncated form of SecA EcN68 which has higher intrinsic activity and is more sensitive to inhibitors [21,24,25,26]. We have found Rose Bengal (RB) to be an effective SecA inhibitor at sub-micromolar for SecA-mediated translocation ATPase and protein translocation [21]. The IC50 for RB inhibition on the SecA ATPase activity varies depending on the SecA interactions with membranes and other ligands [21]. As a way to gain some understanding of the binding site of these inhibitors, we determined their kinetic parameters of EcSecA ATPase as a function of ATP and RB concentrations. Data were fit by nonlinear regression analysis to determine the apparent Michaelis-Menten constants (Table 1). The sigmoid Michaelis-Menten plot of intrinsic ATPase indicates that there may be two inhibition sites. Double reciprocal plots clearly demonstrate two inhibitory mechanisms in different concentration ranges of the substrate ATP (Figure 1A; see also Supplement Fig. 1A for wider ATP concentrations). At low ATP concentrations (below 0.6 mM), RB acts as a competitive inhibitor against the intrinsic ATPase with an apparent Ki of 22.44 ± 3.33 μM, increased Km and about constant Vmax (Table 1). Such results suggest that the inhibitor is capable of binding to the ATP binding site. At high ATP concentrations (above 1 mM), RB acts as a non-competitive inhibitor with an apparent Ki of 57.11 ± 3.37 μM, constant Km, and decreased Vmax (Table 1).
Table 1.
Apparent Michaelis-Menten constants for the three forms of ATPase of EcSecA in the presence of RB. Experimental conditions were as Figure 1. The values of Km and Vmax were determined by nonlinear regression analysis by Prism 5 (GraphPad Software, La Jolla, CA).
| RB concentration | |||||
|
|
|||||
| 0 μM | 20 μM | 40 μM | |||
|
|
|||||
| Intrinsic | High [ATP] Low [ATP] |
Vmax†
Km(mM) Vmax† Km(mM) |
7.37 1.68±0.21 3.07±0.16 0.14 |
6.08 1.68±0.21 3.07±0.16 0.25 |
3.99 1.68±0.21 3.07±0.16 0.46 |
|
| |||||
| 0 μM | 2 μM | 4 μM | |||
|
|
|||||
| Membrane |
Vmax†
Km(mM) |
26.95 0.31±0.03 |
13.61 0.31±0.03 |
8.18 0.31±0.03 |
|
|
| |||||
| 0 μM | 0.75 μM | 1 μM | |||
|
|
|||||
| Translocation |
Vmax†
Km(mM) |
57.27 0.18±0.01 |
23.42 0.18±0.01 |
15.06 0.18±0.01 |
|
Unit: molePi·moleSecA−1min−1
Figure 1.
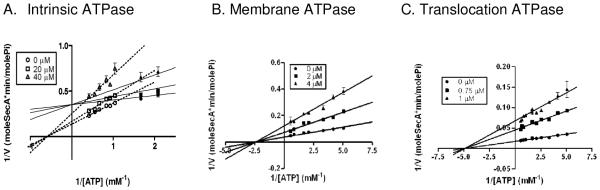
Lineweaver-Burk plots of the inhibitory mechanisms of RB with three forms of ATPase of EcSecA. The assays were carried out as described in Materials and Methods per 50 μL reaction with (A) 5 μg of EcSecA, (B) 1.5 μg of EcSecA and 3 μg of BA13 membrane, and (C) 0.5 μg of EcSecA, 3 μg of BA13 membrane, and 1 μg proOmpA, in the presence of various concentrations of RB and ATP (A) 0.15-2.4 mM; (B) and (C): 0.2-1.8 mM. (A): Competitive inhibition at low ATP concentrations (<0.6 mM, filled symbols), and non-competitive fashion at high ATP concentrations (>1 mM, open symbols) of the intrinsic ATPase by RB; (B): Mostly non-competitive inhibition of the membrane ATPase by RB; and (C): Non-competitive inhibition of the translocation ATPase by RB.
3.2. Mechanisms of Inhibition of intrinsic ATPase: competitive at low ATP and non-competitive at high ATP
Similarly, the inhibition of RB analog, Erythrocin B (EB) on the intrinsic ATPase activity of SecA also shows differential mechanisms at various ATP concentrations (Supplement Fig. 1B). Thus, at low ATP concentrations, EB inhibits the SecA ATPase competitively with Ki of 4.06 ± 0.32 μM, while at high ATP concentration, it inhibits SecA ATPase non-competitively with Ki of 34.29 ± 8.08 μM. These data indicate that RB and EB affect the intrinsic SecA ATPase in a similar manner. The inhibitory mechanism of RB against the intrinsic SecA ATPase from Gram-positive Bacillus subtilis (BsSecA) also shows the two-site inhibition depending on the concentration of ATP as observed in EcSecA (Supplement Figure 1C). This observation indicates that the inhibitory mechanisms of RB against the intrinsic ATPase of BsSecA and EcSecA are similar. Thus the dual competitive and non-competitive inhibitions at various ATP concentrations appear to be a common phenomenon for intrinsic SecA ATPase.
One possible interpretation of these findings is that RB and EB affect two non-identical nucleotide-binding sites on SecA. Two nucleotide-binding sites (NBDs) have been identified in SecA through sequence alignment and biochemical studies [4,8,33,34,35]. NBDI binds to ATP with high affinity and is considered as the catalytic site. However, less is known about the low affinity site (NBDII) and there is no direct evidence of binding of ATP. In this study, kinetics analysis suggests that RB and EB apparently prefer to bind to the high-affinity site; therefore, competitive inhibition is observed at low ATP concentrations. It is also interesting to note that the high-affinity site has a lower Vmax while the low-affinity site has a 2.5 times higher Vmax. A possible interpretation of the results is that high concentration of ATP may be a signal for increasing the ATPase activity of SecA, and NBDII may serve as the sensor. At high ATP concentrations, RB and EB inhibit the activity arisen from the low-affinity site non-competitively. Some fluorescein-related pseudo ATP analogs have been shown to be useful probes for biochemical study of the two ATP binding sites of P-type ATPase [36,37,38]. RB and EB inhibit the two ATP binding sites differently; therefore, this result suggests that these fluorescein analogs may also be a useful tool for unraveling the functional significance of the two nucleotide-binding sites of SecA.
3.2. Non-competitive inhibition of translocation ATPase
The intrinsic ATPase of soluble SecA is low, and the interactions with lipid/membrane increase the membrane ATPase [2]. The inhibition of the SecA membrane ATPase by RB exhibits a “mixed-mechanism.” It has both competitive and non-competitive inhibition characters but more toward the later, resulting in an average Ki of 1.42 ± 0.22 μM, slightly increased Km, and decreased Vmax (Figure 1B and Table 1). On the other hand, RB inhibits the translocation ATPase of SecA only non-competitively in a broad range of ATP concentrations with Ki of 0.43 ± 0.02 μM, constant Km, and decreased Vmax (Figure 1C and Table 1). It is likely that binding to the membrane and precursor proteins dramatically changes the conformation of EcSecA and causes the alteration of inhibition profiles. Since SecA functions in the membranes, the inhibition by RB in the cells is probably non-competitive in regard to ATP.
3.3. Kinetics of inhibition of SecA channel activities in single oocyte cells
In addition to the ATPase activity, we examined SecA function by its ability to elicit ion channel activity in the oocytes [Lin 2006]. As described previously [15], we were able to detect increased outward currents with E. coli SecA by injecting oocytes with proteo-liposomes together with essential factors such as ATP-Mg, and precursor proOmpA, SecA homologs from other bacteria [15]; As shown previously, no activity was observed without SecA, liposomes or injected ATP [15]. Moreover, the soluble SecA2 homologs from some Gram-positive bacteria (e.g. BaSecA2 or SaSecA2) that did not form pore ring-structures with liposomes and non-functional SecA have no channel activities, indicating some specificity of channel formation. We further explore it as a way to examine inhibition in the semi-physiological oocyte system. It should be noted that this assay detects only the signal-peptide-dependent opening of the SecA ion-channel activity with some specificity, not necessarily protein translocation [15,32].
As shown above, the intrinsic ATPase activity of soluble SecA was competitively inhibited by RB and EB at low concentrations of ATP, but non-competitively inhibited at high concentrations. However, SecA membrane ATPase (Fig.1B) and translocation ATPase were mostly non-competitively inhibited (Fig. 1C) by RB. The channel activity on injected EcSecA-liposomes in the oocytes was also non-competitively inhibited (Fig. 2A). Because there is no suitable biochemical assays for protein translocation or translocation ATPase for SecAs from different bacteria that require cognate precursors and membranes [18], we used the oocyte system to measure the kinetics of ATPase of various bacterial SecAs and found that RB also showed non-competitive inhibition with ATP for the liposome channel activity of EcSecA (Fig. 2A), PaSecA (Psuedomonas aeruginosa SecA, Fig. 2B), BsSecA (Fig. 2C) and other SecAs (data not shown).
Figure 2.
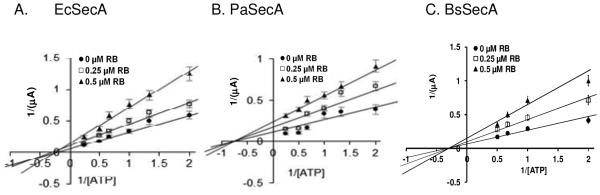
Inhibition kinetics of Rose Bengal in SecA-liposome alone ion channel activity. Non-competitive inhibition of RB on ion channel activity in the oocytes with (A) EcSecA-liposomes, (B) PaSecA-liposomes. (C). BsSecA-liposomes. n =20-30.
3.4. Bacterial protein-conducting channels with SecA-liposomes and SecYEG complexes in oocytes
We previously showed that the SecA-liposomes channels with only SecA protein exhibit lower efficiency and loss of specificity of signal peptides, which were restored with purified SecYEG [15]. Injecting reconstituted SecA-liposomes with purified SecYEG, which by itself has no channel activity, and SecDF•YajC enhanced the channel activity to the same level as native BA13 membranes containing endogeneous SecYEG exhibited in oocytes (data not shown, see ref [15]). The reconstituted complexes exhibited an efficiency and specificity of channel activity, similar to their activity in translocating protein precursors. We have found that the pre-assembled SecA-liposomes with purified SecYEG-DF•YajC, like SecA-liposomes alone, also showed similar non-competitive inhibition pattern by RB on channel activity (Fig. 3). Thus these results validate the in vitro results and the oocyte system, and provide a direct correlation of enzymatic activity between in vitro and in cell conditions.
Figure 3.
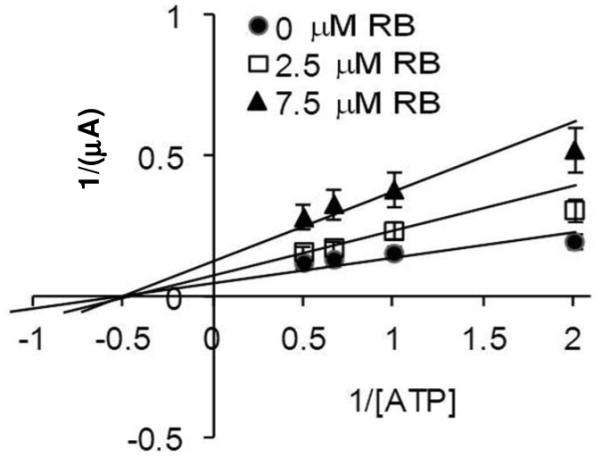
Inhibition kinetics of Rose Bengal with SecA-SecYEG-SecDF•YajC liposome complex. Non-competitive inhibition of RB on the ion channel activity in the oocytes with EcSecA-SecYEG-SecDF•YajC iposomes. n =20-30.
Employing liposomes with SecA homologs provided another means of comparing other bacterial SecA systems in the oocytes. By injecting SecA-liposomes, or reconstituted with SecYEG or SecYEG-SecDF•YajC and various RB concentrations, we determined the IC50 of their sensitivity to RB. Surprisingly, the SecA-only liposomes and the membranes depleted of SecYEG (Re-13 membrane) were about 10 times more sensitive to RB than those with SecYEG (Table 2). Thus the sensitivity of SecA channel activities is greatly influenced by the presence and the properties of SecYEG. The results from various bacterial channel activities were remarkably similar for BaSecA, SaSecA, and PaSecA reconstituted-liposomes (Table 2). Interestingly, the complex of EcSecYEG with various SecA homologs showed intermediate sensitivity to RB as compared to the SecA-liposome and membranes, indicating some interaction of SecA homologs with E. coli heterocomplex of SecYEG for the channel opening activities (Table 2). The addition of SecDF•YajC further elevated the IC50 almost to the same level as seen with wild-type membranes. Previous studies showed that protein translocation of BsSecA- or PaSecA-liposomes cannot be enhanced by the non-cognate E coli SecYEG-SecDF•YajC [18]. As noted above, this assay detects only the signal-peptide-dependent opening of the SecA ion-channel activity, not necessarily protein translocation [15,32].
Table 2.
Rose Bengal IC50 (μM) inhibition of SecA channel activity in oocytes
The IC50 of channel activities for Rose Bengal was determined with liposomes reconstituted with SecAs (120 ng) of E. coli (EcSecA), Bacillus subtilis (BsSecA), S. aureus (SaSecA1), P. aeruginosa (PaSecA) and B. anthraces (BaSecA1). Reconstituted liposomes were injected together with pOmpA and ATP-Mg2+ as described. When indicated, SecYEG at 0.47 ng and SecDF•YajC at 0.53 ng were used for reconstitution, which were the same amount as in SecA-depleted BA13 membranes; Re-13.: Reconstituted membranes after removing SecYEG from B13 membranes. n =20-30.
| SecAs | Lipo | Lipo +SecYEG |
L+SecYEG +SecDF•C |
BA13 memb |
RE-13 memb |
RE-13 memb +SecYEG |
RE-13 memb +SecYEG-DF•C |
|---|---|---|---|---|---|---|---|
| EcSecA | 0.4 ± 0.5 | 0.3 ± 0.1 | 3.8 ± 0.3 | 4.7 ± 0.1 | 0.4 ± 0.1 | 4.2 ± 0.3 | 4.4 ± 0.1 |
| BsSecA | 0.3 ± 0.2 | 3.1 ± 0.2 | 4.5 ± 0.2 | 5.8 ± 0.3 | 0.5 ± 0.5 | 5.0 ± 0.2 | 5.2 ± 0.3 |
| PaSecA | 0.3 ± 0.2 | 1.1 ± 0.2 | 2.0 ± 0.3 | 5.1 ± 0.2 | 0.3 ± 0.5 | 5.1 ± 0.2 | 5.1 ± 0.1 |
| SaSecAl | 0.4 ± 0.1 | 3.1 ± 0.2 | 4.2 ± 0.3 | 6.1 ± 0.3 | 0.5 ± 0.3 | 5.6 ± 0.4 | 5.0 ± 0.3 |
| BaSecAl | 0.3 ± 0.2 | 3.3 ± 0.2 | 4.0 ± 0.3 | 6.1 ± 0.3 | 0.5 ± 0.5 | 5.0 ± 0.2 | 5.3 ± 0.3 |
In conclusion, we found the RB inhibition on the SecA intrinsic ATPase to be competitive at low ATP concentration and non-competitive at high ATP concentration. However, the inhibition on SecA-mediated translocation ATPase activity and ion-channel activity are non-competitive. Moreover, the semi-physiological assays of channel activity in the oocytes allow the assessments of functional activities and inhibitors of SecA homologs from other bacteria that otherwise are not feasible. Another advantage of proteo-liposomes injection is the ability to reconstitute with interacting membrane complexes. The reconstituted SecA-liposomes with SecYEG-SecDF•YajC forms efficient super-complex channels that are as efficient as in the native membranes in the oocytes [17]. The formation of such 7 components complex would have been difficult to accomplish with the traditional cDNA/mRNA methods. The incorporation of SecA-SecYEG-SecDF•YajC complex channels are also more efficient, faster and with higher current than SecA-alone channel [15,17].
Supplementary Material
Highlights.
RB inhibits soluble SecA intrinsic ATPase activity with dual mechanisms
RB inhibits competitively at low ATP and noncompetitively at high ATP
RB inhibits SecA translocation ATPase noncompetitively
RB inhibits SecA-dependent channel activity noncompetitively
Common inhibition mechanisms with other SecA homologs
Acknowledgments
We thank D. Oliver, A. Driessen, A. Economou, F. Duong and E. Lin for plasmids, G. Gadda for helps on data analysis, and J.L. Ingraham and J. Houghton for comments. C. Alexander for technical assistance. This work was supported in parts by the NIH grant GM34788 and AI104168. YHH, and YJH were fellows of Molecular Basis of Disease Program at Georgia State University. The core facility in the Biology department is supported in part by Georgia Research Alliance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cabelli RJ, Dolan KM, Qian LP, Oliver DB. Characterization of membrane-associated and soluble states of SecA protein from wild-type and SecA51(TS) mutant strains of Escherichia coli. J Biol Chem. 1991;266:24420–24427. [PubMed] [Google Scholar]
- 2.Lill R, Dowhan W, Wickner W. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell. 1990;60:271–280. doi: 10.1016/0092-8674(90)90742-w. [DOI] [PubMed] [Google Scholar]
- 3.Driessen AJ, Nouwen N. Protein translocation across the bacterial cytoplasmic membrane. Annual review of biochemistry. 2008;77:643–667. doi: 10.1146/annurev.biochem.77.061606.160747. [DOI] [PubMed] [Google Scholar]
- 4.Karamanou S, Vrontou E, Sianidis G, Baud C, Roos T, Kuhn A, Politou AS, Economou A. A molecular switch in SecA protein couples ATP hydrolysis to protein translocation. Mol Microbiol. 1999;34:1133–1145. doi: 10.1046/j.1365-2958.1999.01686.x. [DOI] [PubMed] [Google Scholar]
- 5.Mao C, Hardy SJ, Randall LL. Maximal efficiency of coupling between ATP hydrolysis and translocation of polypeptides mediated by SecB requires two protomers of SecA. Journal of bacteriology. 2009;191:978–984. doi: 10.1128/JB.01321-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wickner W, Leonard MR. Escherichia coli preprotein translocase. J Biol Chem. 1996;271:29514–29516. doi: 10.1074/jbc.271.47.29514. [DOI] [PubMed] [Google Scholar]
- 7.Manting EH, van Der Does C, Remigy H, Engel A, Driessen AJ. SecYEG assembles into a tetramer to form the active protein translocation channel. EMBO J. 2000;19:852–861. doi: 10.1093/emboj/19.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Economou A, Pogliano JA, Beckwith J, Oliver DB, Wickner W. SecA membrane cycling at SecYEG is driven by distinct ATP binding and hydrolysis events and is regulated by SecD and SecF. Cell. 1995;83:1171–1181. doi: 10.1016/0092-8674(95)90143-4. [DOI] [PubMed] [Google Scholar]
- 9.Matsuyama S, Fujita Y, Mizushima S. SecD is involved in the release of translocated secretory proteins from the cytoplasmic membrane of Escherichia coli. EMBO J. 1993;12:265–270. doi: 10.1002/j.1460-2075.1993.tb05652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pogliano JA, Beckwith J. SecD and SecF facilitate protein export in Escherichia coli. EMBO J. 1994;13:554–561. doi: 10.1002/j.1460-2075.1994.tb06293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Brown T, Tai PC. Identification and characterization of protease-resistant SecA fragments: secA has two membrane-integral forms. J Bacteriol. 1998;180:527–537. doi: 10.1128/jb.180.3.527-537.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramamurthy V, Oliver D. Topology of the integral membrane form of Escherichia coli SecA protein reveals multiple periplasmically exposed regions and modulation by ATP binding. J Biol Chem. 1997;272:23239–23246. doi: 10.1074/jbc.272.37.23239. [DOI] [PubMed] [Google Scholar]
- 13.You Z, Liao M, Zhang H, Yang H, Pan X, Houghton JE, Sui SF, Tai PC. Phospholipids induce conformational changes of SecA to form membrane-specific domains: AFM structures and implication on protein-conducting channels. PloS one. 2013;8:e72560. doi: 10.1371/journal.pone.0072560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang HW, Chen Y, Yang H, Chen X, Duan MX, Tai PC, Sui SF. Ring-like pore structures of SecA: implication for bacterial protein-conducting channels. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4221–4226. doi: 10.1073/pnas.0737415100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh Y.-h., Zhang H, Lin B.-r., Cui N, Na B, Yang H, Jiang C, Sui S.-f., Tai PC. SecA Alone Can Promote Protein Translocation and Ion Channel Activity. J Biol Chem. 2011;286:44702–44709. doi: 10.1074/jbc.M111.300111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin BR, Hsieh YH, Jiang C, Tai PC. Escherichia coli Membranes Depleted of SecYEG Elicit SecA-Dependent Ion-Channel Activity but Lose Signal Peptide Specificity. J Membr Biol. 2012;245:747–757. doi: 10.1007/s00232-012-9477-8. [DOI] [PubMed] [Google Scholar]
- 17.Hsieh YH, Zhang H, Wang H, Yang H, Jiang C, Sui SF, Tai PC. Reconstitution of functionally efficient SecA-dependent protein-conducting channels: transformation of low-affinity SecA-liposome channels to high-affinity SecA-SecYEG-SecDF.YajC channels. Biochem Biophys Res Commun. 2013;431:388–392. doi: 10.1016/j.bbrc.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Hsieh YH, Lin BR, Yu L, Yang H, Jiang C, Sui SF, Tai PC. Specificity of SecYEG for PhoA precursors and SecA homologs on SecA protein-conducting channels. Biochem Biophys Res Commun. 2013;437:212–216. doi: 10.1016/j.bbrc.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Tai PC. ATP is essential for protein translocation into Escherichia coli membrane vesicles. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:4384–4388. doi: 10.1073/pnas.82.13.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliver DB, Cabelli RJ, Dolan KM, Jarosik GP. Azide-resistant mutants of Escherichia coli alter the SecA protein, an azide-sensitive component of the protein export machinery. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:8227–8231. doi: 10.1073/pnas.87.21.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang YJ, Wang H, Gao FB, Li M, Yang H, Wang B, Tai PC. Fluorescein analogues inhibit SecA ATPase: the first sub-micromolar inhibitor of bacterial protein translocation. ChemMedChem. 2012;7:571–577. doi: 10.1002/cmdc.201100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao CVS, De Waelheyns E, Economou A, Anne J. Antibiotic targeting of the bacterial secretory pathway. Biochimica et biophysica acta. 2014;1843:1762–1783. doi: 10.1016/j.bbamcr.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Segers K, Anne J. Traffic jam at the bacterial sec translocase: targeting the SecA nanomotor by small-molecule inhibitors. Chemistry & biology. 2011;18:685–698. doi: 10.1016/j.chembiol.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Chen W, Huang YJ, Gundala SR, Yang H, Li M, Tai PC, Wang B. The first low microM SecA inhibitors. Bioorganic & medicinal chemistry. 2010;18:1617–1625. doi: 10.1016/j.bmc.2009.12.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui J, Jin J, Hsieh YH, Yang H, Ke B, Damera K, Tai PC, Wang B. Design, synthesis and biological evaluation of rose bengal analogues as SecA inhibitors. ChemMedChem. 2013;8:1384–1393. doi: 10.1002/cmdc.201300216. [DOI] [PubMed] [Google Scholar]
- 26.Li M, Huang YJ, Tai PC, Wang B. Discovery of the first SecA inhibitors using structure-based virtual screening. Biochemical and biophysical research communications. 2008;368:839–845. doi: 10.1016/j.bbrc.2008.01.135. [DOI] [PubMed] [Google Scholar]
- 27.Cabelli RJ, Chen L, Tai PC, Oliver DB. SecA protein is required for secretory protein translocation into E. coli membrane vesicles. Cell. 1988;55:683–692. doi: 10.1016/0092-8674(88)90227-9. [DOI] [PubMed] [Google Scholar]
- 28.McNicholas P, Rajapandi T, Oliver D. SecA proteins of Bacillus subtilis and Escherichia coli possess homologous amino-terminal ATP-binding domains regulating integration into the plasma membrane. J Bacteriol. 1995;177:7231–7237. doi: 10.1128/jb.177.24.7231-7237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu L, Yang H, Ho Q, Tai PC. Expression, purification, and characterization of Pseudomonas aeruginosa SecA. Protein Expr Purif. 2006;50:179–184. doi: 10.1016/j.pep.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Xu H, Tai PC. A significant fraction of functional SecA is permanently embedded in the membrane. SecA cycling on and off the membrane is not essential during protein translocation. J Biol Chem. 1996;271:29698–29706. doi: 10.1074/jbc.271.47.29698. [DOI] [PubMed] [Google Scholar]
- 31.Tai PC, Tian G, Xu H, Lian JP, Yu JN. In vitro protein translocation into Escherichia coli inverted membrane vesicles. Methods Cell Biol. 1991;34:167–187. [PubMed] [Google Scholar]
- 32.Lin BR, Gierasch LM, Jiang C, Tai PC. Electrophysiological studies in Xenopus oocytes for the opening of Escherichia coli SecA-dependent protein-conducting channels. J Membr Biol. 2006;214:103–113. doi: 10.1007/s00232-006-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell C, Oliver D. Two distinct ATP-binding domains are needed to promote protein export by Escherichia coli SecA ATPase. Mol. Microbiol. 1993;10:483–497. doi: 10.1111/j.1365-2958.1993.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 34.Nakatogawa H, Mori H, Ito K. Two independent mechanisms down-regulate the intrinsic SecA ATPase activity. J. Biol. Chem. 2000;275:33209–33212. doi: 10.1074/jbc.C000550200. [DOI] [PubMed] [Google Scholar]
- 35.van der Wolk JP, Boorsma A, Knoche M, Schafer HJ, Driessen AJ. The low-affinity ATP binding site of the Escherichia coli SecA dimer is localized at the subunit interface. Biochemistry. 1997;36:14924–14929. doi: 10.1021/bi971766n. [DOI] [PubMed] [Google Scholar]
- 36.Linnertz H, Kost H, Obsil T, Kotyk A, Amler E, Schoner W. Erythrosin 5'-isothiocyanate labels Cys549 as part of the low-affinity ATP binding site of Na+/K+-ATPase. FEBS Lett. 1998;441:103–105. doi: 10.1016/s0014-5793(98)01533-6. [DOI] [PubMed] [Google Scholar]
- 37.Linnertz H, Urbanova P, Obsil T, Herman P, Amler E, Schoner W. Molecular distance measurements reveal an (alpha beta)2 dimeric structure of Na+/K+-ATPase. High affinity ATP binding site and K+-activated phosphatase reside on different alpha-subunits. J Biol Chem. 1998;273:28813–28821. doi: 10.1074/jbc.273.44.28813. [DOI] [PubMed] [Google Scholar]
- 38.Tanfani F, Linnertz H, Obsil T, Krumscheid R, Urbanova P, Jelinek O, Mazzanti L, Bertoli E, Schoner W, Amler E. Effects of fluorescent pseudo-ATP and ATP-metal analogs on secondary structure of Na(+)/K(+)-ATPase. Biochim Biophys Acta. 2000;1457:94–102. doi: 10.1016/s0005-2728(00)00055-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


