Abstract
A small library of amino acid ester prodrugs of 6-β-naltrexol (NTXOL, 1) was prepared in order to investigate the candidacy of these prodrugs for microneedle-enhanced transdermal delivery. Six amino acid ester prodrugs were synthesized (6a-f). 6b, 6d, and 6e were stable enough at skin pH (pH 5.0) to move forward to studies in 50% human plasma. The lead compound (6e) exhibited the most rapid bioconversion to NTXOL in human plasma (t½ = 2.2 ± 0.1 h).
Keywords: Prodrugs, Amino acid esters, Microneedles, Stability, Transdermal
A recent surge in research interest has focused on the design of amino acid prodrugs as delivery systems. The strategy has been applied to oral1, intraocular2, intransal3 and intravenous4 therapeutic agents which exhibit suboptimal physiochemical properties that limit drugability. Generally, the goal of amino acid prodrug design is to target nutrient transporters at various biological barriers; however, at skin-relevant pH 5.0, the free amine of an amino acid promoiety can also enhance aqueous solubility. Indeed, amino acid promoieties have already been explored as solubility-enhancing agents in the design of injectable metronidazole prodrugs.5,6 In passive transdermal delivery systems, where intact stratum corneum (SC) limits the permeability of hydrophilic molecules, ionizable amine promoieties would be expected to diminish molecular permeability of a prodrug. However, the use of microneedles to create skin microchannels followed by the application of a drug-containing transdermal formulation allows for delivery of hydrophilic species.7 This method is referred to in this article as microneedle-enhanced transdermal delivery (MN) and is more specifically known as the “poke and patch” technique. Therefore, our goal in this study was to synthesize amino acid prodrugs of NTXOL (2) and to investigate their stability properties in buffers and in human plasma (HP), in order to predict MN candidacy. The field of MN is relatively new8, and to our knowledge, there are currently few examples of amino acid prodrugs in the literature which are intended for MN.9
MN is a technique in which small microchannels are created in the skin with micrometer-sized needles. SC is bypassed in this way, and as a consequence of this, MN has expanded the pool of molecules that can be delivered by the percutaneous route. Improved skin transport of hydrophilic or charged compounds,10-12 and molecules with large molecular weights in the kilodalton range13 has been observed utilizing MN. For instance, transdermal delivery of large proteins14 and hydrophilic compounds such as calcein15 have been enhanced by MN. Previously, work in our labs demonstrated that the FDA-approved opioid antagonist naltrexone (NTX, 1) and its active metabolite, 6-β-naltrexol (NTXOL, 2) could not be delivered in therapeutic levels by passive transdermal delivery techniques.16 Accordingly, attempts were first made to manipulate the physiochemical properties of NTX by the prodrug approach. Straight-chain and branched-chain alkyl esters and carbonates of NTX were prepared, but these prodrugs achieved suboptimal skin diffusion for therapeutic delivery in man.17-21 Therefore, it was envisaged that a switch to MN, and the use of more hydrophilic amino acid prodrugs of 2, might be an appropriate strategy. NTXOL is attractive for this research, because it is thought to be instrumental in the therapeutic effects of orally dosed NTX. Also, 2 has an aliphatic hydroxyl group, which is not present in the NTX molecule that is more suitable for the design of ester prodrugs. We postulated that identification of a lead NTXOL prodrug for further MN studies could eventually be advantageous to improve the therapeutic outcomes of patients during protracted alcohol and/or opioid cessation efforts, since current forms of NTX therapy are associated with adverse events that lead to noncompliance.22 In this respect, we have recently demonstrated that pegylated prodrugs of NTX, while more soluble in 0.3 M acetate buffer than NTX, unfortunately exhibit problematic viscosity properties that limit microchannel transport when compared to NTX itself.23
Amino acid ester prodrug design has been approached in this study as an alternative strategy to improve molecular hydrophilicity while simultaneously avoiding the creation of a viscous and oily prodrug material. Herein we describe the synthesis and stability of amino acid ester prodrugs of 2. Among the compounds mentioned, 6e is the established lead compound for further MN developmental work.
It is known that amino acid ester prodrugs can vary widely in their stability properties in various hydrolytic media (enzymatic and non-enzymatic), depending on molecular factors and on the pH of the hydrolysis media. Skin pH is around 5.0 on average. Thus, we set the criterion that no produg could degrade more than ten percent within the intended forty-eight hour time course of future skin diffusion studies that would be conducted in 0.3 M acetate buffer vehicle (i.e. t90 ≥ 48 hours at pH 5.0). Also, since a prodrug must hydrolyze in vivo to release parent drug, rapid hydrolysis at pH 7.4, with or without enzyme catalysis, was established as an important physiochemical parameter. Prodrugs exhibiting a combination of these features were considered to be appropriate molecules for further drug development.
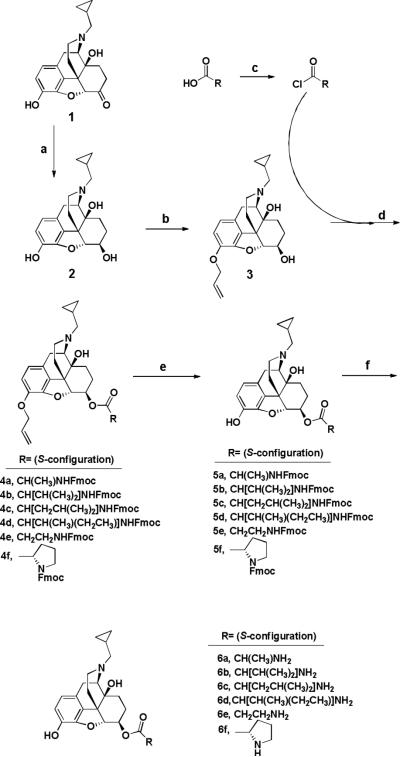
Amino acid esters of 2 were prepared as depicted in Scheme 1. NTXOL (2) was afforded in 85-92.7% yield (depending on scale) from NTX using the synthetic methodology of de Costa et al.24 3-O-allyl-NTXOL (3) was prepared by treating 2 with allyl bromide under reflux in acetone in the presence of potassium carbonate. Excess allyl bromide was washed out with hexanes. For each coupling reaction, 3 and DMAP were dissolved at room temperature in DCM. The appropriate Fmoc-protected amino acid promoiety was converted to an activated acid chloride in a separate flask by sonicating it in DCM with thionyl chloride under an argon stream, as described by Sureshbabu et al.,25 and the residual HCl that had not been blown off in the argon stream was quenched with DMAP base. The cocktail containing 3 and DMAP was then added drop-wise to the acid chloride solution over the course of two minutes at 0°C, and the reaction mixture was warmed to room temperature and left to react for four hours.
Scheme 1.
Synthesis of amino acid ester prodrugs of 6-β-naltrexol: (a) formamidinesulfinic acid (4.0 eq), aqueous NaOH (0.53 M), 80°C, 1.5 h;24 (b) allyl bromide (1.1 eq), K2CO3 (4 eq), acetone, reflux, 5.5 h; (c) Fmoc-protected amino acids (2.0 eq), SOCl2 (1.99 eq), sonicate 30 min, DMAP (2.0 eq), argon stream; (d) DMAP (2.0 eq), DCM, 0°C - rt, 4 h, argon atmosphere; (e) PMHS (3.0 eq), 2M ZnCl2 in Et2O (52 drops via syringe/100 mg 2), (PPh3)4Pd (5 mol%), THF, rt, 24 h, argon atmosphere; (f) Octanethiol (10 eq), DBU (25 mol%), THF, rt, 24 h, argon atmosphere.
In every case, the reactions were worked up with DI water and brine. Final purification was achieved by chromatography over silica that had been pretreated with 1% TEA in hexanes. Isolated precursors (4a-f) were used without further purification. Estimated isolation yields were 75 - 90%.
Deallylation was performed utilizing the methods of Chandrasekhar et al.26 with modifications. The fully protected precursor was dissolved in THF and treated with tetrakis(triphenylphosphine)palladium ((PPh3)4Pd) and polymethylhydrosiloxane (PMHS). Zinc chloride (2M solution in diethyl ether) was added with rapid stirring, and the reaction was run for 24 h. The THF was evaporated under an argon stream, and the gummy residue was reconstituted in DCM and worked up with a 1% sodium bicarbonate solution and brine. The combined organic fractions were concentrated and chromatographed as before to afford the deallylated prodrug product in estimated yields ranging from 50 to 85%. Compounds 5a-f were advanced to the next step without further purification.
Fmoc removal was accomplished by 24h treatment with DBU base (25 mol%) and octanethiol (10 eq.) in dry THF, as described by Sheppeck et al.27 In the case of 6e, the final prodrug was isolated by precipitation and trituration from diethyl ether. The -amino acid ester prodrugs were recovered via aqueous workup and column chromatography, utilizing silica deactivated with 1% TEA in hexanes, and the final prodrugs were eluted with copious column washes using ethyl acetate. 6a and 6f required acetone and TEA to be eluted from silica, and hydrolytic degradation of these prodrugs resulted. The range of yields at this stage was ~20% (6a) to 80% (6d). The final prodrugs were characterized by 1H NMR and 13C NMR spectrometry, and ESI-MS analysis.32 Final yields of prodrugs are reported along with the spectral data. Despite some degradation during purification, compounds 6a and 6f were obtained in sufficient purity for stability comparison to the other prodrugs.
For prodrugs 6a-f, stability studies were conducted in donor vehicle (0.3M acetate buffer, pH 5.0). For prodrugs 6b and 6e, stability studies were also conducted in receiver solution (25mM HEPES-buffered Hanks’ balanced salt solution, pH 7.4). These donor and receiver solutions have been used routinely in our skin diffusion studies. Reactions were initiated by charging a 10 mL volume of hydrolysis media thermostated at 32±0.5 °C (pH 5.0 samples) and 37±0.5 °C (pH 7.4 samples) with approximately 1 mg of prodrug followed by vortex stirring for one minute. The suspension was then filtered through a 0.45μm nylon syringe filter (Acrodisc® Premium 25mm Syringe Filter). Aliquots were withdrawn over a period of approximately 3 half-lives. Samples were then diluted with ACN-water 70:30 (v/v) for HPLC analysis. The HPLC system consisted of a Waters 717 plus autosampler, a Waters 600 quaternary pump, and a Waters 2487 dual wavelength absorbance detector with Waters Empower™ software. A Brownlee (Wellesley, MA, USA) C-8 reversed phase Spheri-5 μm column (220×4.6 mm) with a C-8 reversed phase guard column of the same type (15×3.2 mm) by Perkin Elmer® was used with the UV detector set at a wavelength of 215nm or 278nm. The mobile phase consisted of 70:30 (v/v) ACN:(0.1% TFA with 0.065% 1-octane sulfonic acid sodium salt, adjusted to pH 3.0 with TEA aqueous phase). Samples were run at a flow rate of 1.5 ml/min with a run time of 4 min. The amino acid ester prodrugs showed pseudo-first-order kinetic behavior upon hydrolysis. Apparent pseudo-first-order hydrolysis rate constants (Kapp) were estimated from the slope of the log-transformed amount of prodrug remaining in the medium. All stability studies were carried out in duplicate (n=2) except for the pH 7.4 stability study of 6d. Compound 6d was highly insoluble in pH 7.4 buffer, and had to be assayed a different way. At this time, it was also prudent to develop a method for human plasma (HP) stability studies using gradient mobile phase methodology, so the pH 7.4 buffer stability of 6d was assayed alongside the HP hydrolysis studies as described below (n=3).
A stock solution of 1.6 mM 6d was prepared in DMSO. A stock internal standard (IS) solution of salicylamide (0.55 mM) in methanol containing 0.04% heptafluorobuyric acid ion pairing agent (HFBA) was also prepared. An eight-point calibration curve was generated from working solutions of 6d and the IS solution using the same sample workup as described below. Reactions were initiated by adding 40.0 μL of the prodrug stock solution to 760.0 μL of a pre-warmed (37±0.5 °C) solution of 50% PBS (initial reaction concentration of 82 μM). More concentrated reactions were impossible due to extensive precipitation of 6d at higher levels. Sampling over the course of time was done by the following method. 50.0 μL aliquots of the reaction mixture were removed at pre-determined time points. 50.0 μL of internal standard solution and 50.0 μL of blank 0.04% HFBA in methanol were added to the reaction mixture aliquots in low volume inserts, and the solutions were vortexed. Samples were immediately injected onto a 4 μm Waters Phenyl column (3.9 x 150 mm) attached to a 4 μm Nova-pak® C18 3.9 x 20 mm guard column. The mobile phase consisted of an aqueous solution of 0.04% (HFBA) (Solvent A) and 0.04% HFBA in methanol (Solvent B). 6d was eluted with a gradient program at 0.3 mL/min.
HP stability studies (50% HP in 50% PBS buffer) of 6b, 6d and 6e were conducted as follows. Stock solutions of the prodrugs were prepared in DMSO. The stock concentrations were 2.3 mM (6b), 1.6 mM (6d), and 7.2 mM (6e). It was not possible to perform the reactions at the same concentrations, because 6b and 6d exhibited the tendency to precipitate at 7.2 mM concentration. Reactions were initiated by adding 40.0 μL of the prodrug stock solution to 760.0 μL of a pre-warmed (37±0.5 °C) solution of 50% HP in 50% PBS buffer (pH 7.4). Sampling over the course of time was carried out using the following method. 50.0 μL aliquots of a reaction mixture were removed at various time points and placed into 1000 μL Eppendorf tubes. 50.0 μL of internal standard solution was added to the samples, and 300.0 μL of ice cold methanol containing 0.04% HFBA was charged to the tubes to precipitate proteins. The mixtures were vortexed for 30 seconds and subsequently centrifuged for 10 minutes at 10,000 rpm. Supernatants were removed into individual culture tubes and dried under N2 gas in a bath maintained at 37±0.5 °C. Residues were reconstituted in 150.0 μL methanol containing 0.04% HFBA by vortexing for 30 seconds. Samples were immediately transferred to low-volume inserts and injected onto the guard column/Waters Phenyl column described above for HPLC analysis. Gradient programs and flow rates varied between 6b, 6d and 6e in the plasma stability studies. This was necessary to avoid matrix peak interference with the analytes. Data were analyzed with an eight-point standard curve in each case. The system consisted of an Agilent 1100 series HPLC instrument equipped with a G1322A Degasser, a G1311A Quat Pump, a G1313A autosampler, and a photodiode array detector set at 280 nm.
6b, 6d and 6e showed pseudo-first-order kinetic hydrolysis behavior in plasma, and 6d also hydrolyzed similarly in 50% PBS buffer. Apparent pseudo-first-order hydrolysis rate constants (Kapp) were calculated from the slope of log transformed AUCprodrug/AUCIS as a function of time using the standard curves. All plasma stability studies, and the pH 7.4 buffer stability study of 6d, were carried out in triplicate (n=3). Table 1 summarizes the stability data which are reported as the mean ± SD.
Table 1.
In vitro stability of prodrugs 6a-f in buffers and in 50% human plasma (HP).
| Prodrug | t90 (pH 5.0) | t1/2 (pH 7.4) | t1/2 (50% HP) |
|---|---|---|---|
| 6a | 9.9 ± 0.5 h | no data | no data |
| 6b | 5.2 ± 0.2 d | 0.48 ± 0.03 d | 0.76 ± 0.03 d |
| 6c | 0.9 ± 0.0 d | no data | no data |
| 6d | 14 d | 0.9 ± 0.0 d | 1.3 ± 0.1 d |
| 6e | 5.6 ± 0.5 d | 0.5 ± 0.2 d | 2.2 ± 0.1 h |
| 6f | 5.2 ± 0.7 h | no data | no data |
It can be seen in Table 1 that 6a, 6c, and 6f were not suitable for MN based on our minimum stability criterion; t90 ≥ 48 hours at pH 5.0 and 32 °C. The latter cutoff was established for our studies, because it becomes difficult to estimate the extent of bioconversion in the viable skin following a diffusion experiment if a prodrug extensively degrades during the experiment. Also, high levels of hydrolysis before skin transport do not allow a true estimation of drug flux. Prodrug moieties with bulky side chains enhanced stability in this series of prodrugs, with 6e also showing similar stability. Overall, the best MN candidates were determined to be 6b, 6d and 6e based on the pH 5.0 stability criteria. Prodrugs that did not meet these minimum criteria were not pursued in further stability tests (Table 1).
Adequate stability at pH 5.0 was not enough to establish our prodrugs as appropriate MN candidates. In a prodrug design, rapid degradation under physiological conditions is desired, because the prodrug itself is just a delivery system for the parent drug. As such, an ideal prodrug for the MN paradigm should also exhibit rapid hydrolysis at pH 7.4 (enzyme-assisted in plasma or in a chemical buffer). This is true because viable skin bioconversion at pH 7.4 below the SC layer is expected to greatly enhance skin transport of the parent drug based on previous studies.17-21,28-31 In Table 1, it is obvious that significant hydrolysis rate enhancements of approximately one order of magnitude or more were observed at pH 7.4 as compared to pH 5.0; however, these rates were not rapid enough to ensure enhanced skin transport of 2. Therefore, we examined the hydrolysis behaviors of 6b, 6d, and 6e in 50% HP to see if further rate enhancements could be expected in vivo. Interestingly, 6b and 6d had significantly increased half-lives in plasma compared to those observed in pH 7.4 buffers which was likely to be due to protein binding. In contrast, the half-life of 6e was reduced by approximately 5.4-fold in HP compared to pH 7.4 PBS buffer. Therefore, 6e was the lead compound of this series of prodrugs. Also, the tendency of 6b and 6d to precipitate at pH 7.4 in PBS buffer is not a desirable physiochemical property.
Although, 6e was the established lead compound, its plasma half-life is still fairly long for an ideal MN candidate, and it would be necessary to perform skin diffusion and disposition studies to determine if faster rates of bioconversion could be achieved in viable skin tissue. Alternatively, hydrolysis of the prodrug in vivo may occur by numerous routes, including hepatic metabolism, following systemic delivery. Overall, 6e appears to be a suitable prodrug for future MN studies that will utilize a “poke and partch” drug delivery paradigm.
Acknowledgment
This work was supported by NIH Grant R01 DA13425.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beauchamp LM, Orr GF, de Miranda P, Burnette T, Krenitsky TA. Antiviral Chem. Chemother. 1992;3:157. [Google Scholar]
- 2.Anand BS, Katragadda S, Nashed YE, Mitra A. Curr. Eye Res. 2004;29:153. doi: 10.1080/02713680490504614. [DOI] [PubMed] [Google Scholar]
- 3.Yang C, Mitra AK. J. Pharm. Sci. 2001;90:340. doi: 10.1002/1520-6017(200103)90:3<340::aid-jps9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 4.Altomare C, Trapani G, Latrofa A, Serra M, Sanna E, Biggio G, Liso G. Eur. J. Pharm. Sci. 2003;20:17. doi: 10.1016/s0928-0987(03)00161-1. [DOI] [PubMed] [Google Scholar]
- 5.Bundgaard H, Larsen C, Arnold E. International Journal of Pharmaceutics. 1984;18:79. [Google Scholar]
- 6.Bundgaard H, Larsen C, Thorbek P. Int. J. Pharm. 1984;18:67. [Google Scholar]
- 7.Arora A, Prausnitz MR, Mitragotri S. Int. J. Pharm. 2008;364:227. doi: 10.1016/j.ijpharm.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henry S, McAllister DV, Allen MG, Prausnitz MR. J. Pharm. Sci. 1998;87:922. doi: 10.1021/js980042+. [DOI] [PubMed] [Google Scholar]
- 9.Stinchcomb AL. 2012 U.S. Patent Application EP2473475 A1.
- 10.Wermeling DP, Banks SL, Hudson DA, Gill HS, Gupta J, Prausnitz MR, Stichcomb AL. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2058. doi: 10.1073/pnas.0710355105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banks SL, Pinninti RR, Gill HS, Crooks PA, Prausnitz MR, Stinchcomb AL. Pharm. Res. 2008;25:1964. doi: 10.1007/s11095-008-9578-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banks SL, Pinninti RR, Gill HS, Paudel KS, Crooks PA, Brogden NK, Prausnitz MR, Stinchcomb AL. J. Pharm. Sci. 2010;99:3072. doi: 10.1002/jps.22083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verbaan FJ, Bal SM, Van d. B. D. J., Groenink WHH, Verpoorten H, Luettge R, Bouwstra JA. J. Controlled Release. 2007;117:238. doi: 10.1016/j.jconrel.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 14.McAllister DV, Wang PM, Davis SP, Park J.-h., Canatella PJ, Allen MG, Prausnitz MR. Proc. Natl. Acad. Sci. U. S. A. 2003;100:13755. doi: 10.1073/pnas.2331316100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry S, McAllister DV, Allen MG, Prausnitz MR. J. Pharm. Sci. 1998;87:922. doi: 10.1021/js980042+. [DOI] [PubMed] [Google Scholar]
- 16.Paudel KS, Nalluri BN, Hammell DC, Valiveti S, Kiptoo P, Hamad MO, Crooks PA, Stinchcomb AL. J. Pharm. Sci. 2005;94:1965. doi: 10.1002/jps.20398. [DOI] [PubMed] [Google Scholar]
- 17.Stinchcomb AL, Swaan PW, Ekabo O, Harris KK, Browe J, Hammell DC, Cooperman TA, Pearsall MJ. Pharm. Sci. 2002;91:2571. doi: 10.1002/jps.10239. [DOI] [PubMed] [Google Scholar]
- 18.Vaddi HK, Hamad MO, Chen J, Banks SL, Crooks PA, Stinchcomb AL. Pharm. Res. 2005;22:758. doi: 10.1007/s11095-005-2592-9. [DOI] [PubMed] [Google Scholar]
- 19.Valiveti S, Hammell DC, Paudel KS, Hamad MO, Crooks PA, Stinchcomb AL. J. Controlled Release. 2005;102:509. doi: 10.1016/j.jconrel.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Pillai O, Hamad MO, Crooks PA, Stinchcomb AL. Pharm. Res. 2004;21:1146. doi: 10.1023/b:pham.0000033000.03652.73. [DOI] [PubMed] [Google Scholar]
- 21.Hammell DC, Stolarczyk EI, Klausner M, Hamad MO, Crooks PA, Stinchcomb AL. J. Pharm. Sci. 2005;94:828. doi: 10.1002/jps.20238. [DOI] [PubMed] [Google Scholar]
- 22.Milewski M, Brogden NK, Stinchcomb AL. Expert Opin. Drug Delivery. 2010;7:617. doi: 10.1517/17425241003663228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milewski M, Yerramreddy TR, Ghosh P, Crooks PA, Stinchcomb AL. J. Controlled Release. 2010;146:37. doi: 10.1016/j.jconrel.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Costa BR, Iadarola MJ, Rothman RB, Berman KF, George C, Newman AH, Mahboubi A, Jacobson AE, Rice KC. J. Med. Chem. 1992;35:2826. doi: 10.1021/jm00093a016. [DOI] [PubMed] [Google Scholar]
- 25.Sureshbabu VV, Hemantha HP. ARKIVOC (Gainesville, FL, U. S.) 2008:243. [Google Scholar]
- 26.Chandrasekhar S, Raji RC, Jagadeeshwar RR. Tetrahedron. 2001;57:3435. [Google Scholar]
- 27.Sheppeck JE, Kar H, Hong H. Tetrahedron Lett. 2000;41:5329. [Google Scholar]
- 28.Hamad MO, Kiptoo PK, Stinchcomb AL, Crooks PA. Bioorg. Med. Chem. 2006;14:7051. doi: 10.1016/j.bmc.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Hammell DC, Hamad M, Vaddi HK, Crooks PA, Stinchcomb AL. J. Controlled Release. 2004;97:283. doi: 10.1016/j.jconrel.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 30.Kiptoo PK, Hamad MO, Crooks PA, Stinchcomb AL. J Control Release. 2006;113:137. doi: 10.1016/j.jconrel.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Kiptoo PK, Paudel KS, Hammell DC, Hamad MO, Crooks PA, Stinchcomb AL. Eur J Pharm Sci. 2008;33:371. doi: 10.1016/j.ejps.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.(2): 1HNMR spectral data were identical to de Costa et al; 21 Yield: 92.7%: (3): 1H NMR (CDCl3) 300 MHz:_δ 6.71 (d, J = 10 Hz, 1H), 6.56 (d, J = 10 Hz, 1H), 6.11-6.02 (m, 1H), 5.39 (d, J = 10 Hz, 1H), 5.26 (d, J = 10 Hz, 1H), 4.70-4.60 (m, 2H), 4.50 (d, J = 10 Hz, 1H), 3.65-3.55 (m, 1H), 3.52 (d, J = 5 Hz, 1H), 3.10 (d, J = 5 Hz, 1H), 3.03 (d, J = 15 Hz, 1H), 2.68-2.52 (m, 2H), 2.36 (d, J = 5 Hz, 2H), 2.29-2.22 (m, 1H), ), 2.18-2.11 (m, 2H), 2.04-1.94 (m, 1H), 1.68-1.59 (m, 1H), 1.52-1.47 (m, 1H), 1.41-1.34 (m, 2H), 0.88 -0.80 (m, 1H), 0.56-0.51 (m, 2H), 0.15-0.11 (m, 2H) ppm; Yield: 93.2%: (6b): 1H NMR 500 MHz (CDCl3): 7.72-7.63 (m, <1H), 7.60-7.53 (m, <1H), 7.50-7.44 (m, <1H), 6.71 (d, J = 10 Hz, 1H), 6.59 (d, J = 10 Hz, 1H), 4.95-4.82 (m, 1H), 4.53 (d, J = 10 Hz, 1H), 3.56 (d, J = 5 Hz, 1H), 3.11 (d, J = 5 Hz, 1H), 3.03 (d, J =15 Hz, 1H), 2.69-2.55 (m, 2H), 2.39 (d, J = 5 Hz, 2H), 2.31-2.21 (m, 1H), 2.20-2.10 (m, 2H), 2.10-2.01 (m, 1H), 1.76-1.65 (m, 1H), 1.64-1.57 (m, 1H), 1.53-1.42 (m, 2H), 1.33-1.19 (m, 2H), 1.04 (d, J = 5 Hz, ½ H), 1.00 (d, J = 5 Hz, 3H), 0.97 (d, J = 5 Hz, ½ H) 0.93 (d, J = 5 Hz, 3 H), 0.89-0.79 (m, 3H), 0.58-0.48 (m, 2H), 0.19-0.09 (m, 2H) ppm; 13C NMR (CDCl3) 300 MHz: δ 175.48, 141.98, 141.01, 132.14, 130.82, 128.69, 123.01, 119.58, 118.70, 92.34, 70.09, 62.41, 59.42, 58.77, 48.30, 44.16, 31.98, 31.01, 29.59, 24.31, 22.85, 19.00, 17.96, 9.77, 4.30 ppm; MS (ESI) m/z: 443.9 (MH+); Yield: 36.2%: (6c): 1H NMR 500 MHz (CDCl3): δ 7.70-7.65 (d, J = 10 Hz, 1H), 6.59 (d, J = 10 Hz, 1H), 4.91-4.83 (m, 1H), 4.54 (d, J = 5 Hz, 1H), 3.68 (t, J =15 Hz, 1H), 3.11 (d, J = 5 Hz, 1H), 3.03 (d, J = 20 Hz, 1H), 2.70-2.56 (m, 2H), 2.39 (d, J = 5 Hz, 2H), 2.30-2.22 (m, 1H), 2.21-2.12 (m, 1H), 2.12-2.02 (m, 1H), 1.87-1.75 (m, 1H), 1.71-1.62 (m, 2H), 1.62-1.58 (m, 1H), 1.58-1.52 (m, 1H), 1.52-1.44 (m, 3H), 0.92 (t, 7H, overlap with cyclopropyl methine), 0.59-0.50 (m, 2H), 0.19-0.12 (m, 2H) ppm; 13C NMR (CDCl3) 300 MHz: δ 176.91, 142.00, 141.15, 130.87, 119.66, 118.75, 92.37, 76.52, 70.19, 62.55, 59.50, 52.31, 48.40, 44.22, 43.92, 31.08, 29.66, 25.04, 24.21, 23.01, 22.93, 22.63, 9.84, 4.34 ppm; MS (ESI) m/z: 457.8 (MH+); Yield: 38.4%: (6d): 1H NMR 500 MHz CDCl3: 7.70-7.65 (m, <1H), 7.58-7.53 (m, <1H), 7.50-7.44 (m, <1H), 6.70 (d, J =10 Hz, 1H), 6.58 (d, J =10 Hz, 1H), 4.85-4.94 (m, 1H), 4.52 (d, J = 5 Hz, 1H), 3.67 (d, J = 5 Hz, 1H), 3.10 (d, J = 5 Hz, 1H), 3.02 (d, J =15 Hz, 1H), 2.69-2.55 (m, 2H), 2.39 (d, J = 5 Hz, 2H), 2.31-2.21 (m, 1H), 2.2-2.12 (m, 1H), 2.12-2.02 (m, 1H), 1.96-1.85 (m, 1H), 1.70-1.57 (m, 2H), 1.53-1.44 (m, 2H), 1.44-1.36 (m, 1H), 1.30-1.19 (m, 2H), 0.95-0.89 (m, 7H), 0.89-0.79 (m, 2H), 0.58-0.50 (m, 2H), 0.19-0.09 (m, 2H) ppm; 13C NMR (CDCl3) 300 MHz: δ 175.58, 142.01, 140.98, 132.29, 130.83, 128.54, 123.11, 119.58, 118.66, 92.29, 70.12, 62.46, 59.43, 57.60, 48.33, 44.16, 39.24, 30.99, 29.67, 25.86, 24.34, 22.88, 15.56, 12.33, 9.81, 4.28 ppm; MS (ESI) m/z: 457.9 (MH+); Yield: 40%: (6e): 1H NMR 500 MHz (d6-DMSO): δ 6.59 (d, J =10 Hz, 1H), 6.53 (d, J =10 Hz, 1H), 4.87 (s, broad), 4.45 (m, 2H), 3.64-3.57 (m, 1H), 3.03 (d, J = 5 Hz, 1H), 2.96 (d, J = 20 Hz 1H), 2.76 (t, J = 15 Hz, 2H), 2.62-2.53 (td, 2H), 2.40 (t, J =15 Hz, 2H), 2.36-2.27 (m, 2H), 2.23-2.12 (m, 1H), 1.85-1.72 (m, 2H), 1.70-1.57 (m, 2H), 1.48 (d, J = 10 Hz, 1H), 1.33-1.19 (m, 3H), 0.87-0.79 (m, 1H), 0.42-0.51 (m, 2H), 0.16-0.06 (m, 2H) ppm; 13C NMR (d6-DMSO) 300 MHz: δ 171.49, 165.90, 141.62, 140.31, 131.11, 123.21, 118.63, 117.05, 90.43, 75.09, 69.33, 61.45, 58.33, 53.9, 47.30, 43.44, 37.93, 30.23, 29.28, 23.26, 22.15, 9.31, 3.78 ppm; MS (ESI) m/z: 415.9 (MH+); Yield: 32.2%.