Abstract
Differential neural activation at encoding can predict which stimuli will be subsequently remembered or forgotten, and memory deficits are pronounced in schizophrenia. We used event-related fMRI to investigate subsequent memory effects for visual fractals in patients with schizophrenia (n=26) and healthy controls (n=28). Participants incidentally encoded the fractals during an oddball task and 10 minutes later they made old/new recognition memory judgments on 30 target fractals and 30 foil fractals. We found evidence for subsequent memory (SM, subsequently remembered > subsequently forgotten) effects on regional brain activation in both groups but with distinct patterns. Region of interest analyses in controls demonstrated SM activation in both medial temporal lobe (MTL) and fusiform cortex (FF), whereas patients showed SM effects only in the FF. There were no significant between group differences in MTL activation; however, patients demonstrated greater FF activation than controls. Notably, greater FF activation during successful encoding was associated with more severe negative symptoms. Exploratory whole brain analyses in patients demonstrated SM activation in the occipital pole, lateral occipital cortex, left inferior temporal gyrus, and fusiform cortex; whereas in controls there was no significant activation that survived correction for multiple comparisons. Our findings suggest that patients, particularly those with prominent negative symptoms, may activate FF as a compensatory strategy to promote successful encoding, with relatively less reliance on MTL recruitment.
Keywords: Recognition memory, Magnetic resonance imaging, Visual object memory
1. Introduction
Memory dysfunction is central in schizophrenia, remaining stable over time and not accounted for by education, gender or medication status (Censits et al., 1997; Sideman et al., 1998; Aleman et al., 1999). Patients often suffer from specifically impaired episodic memory whereby memory for past events is compromised (Gold et al., 1992; Heinrichs and Zakzanis, 1998; Aleman et al., 1999; Cirillo and Seidman, 2003). During encoding of past events, differential neural activation occurs for stimuli that will be subsequently remembered compared to forgotten. This subsequent memory (SM) effect characterizes the neural activity that supports successful encoding (Brewer et al., 1998; Wagner et al., 1998; Paller and Wagner, 2002; Reber et al., 2002). SM has consistently been associated with activation in five brain regions: the medial temporal lobe, fusiform cortex, left inferior frontal cortex, premotor cortex, and the posterior parietal cortex (Kim, 2011). Activation in these regions differs according to the way the stimuli are encoded, either incidentally or intentionally, and the stimuli type, visual or verbal.
Encoding of visual compared to verbal stimuli, elicits the greatest SM activation in the medial temporal lobe and fusiform cortex, among these five main regions (Kim, 2011). Thus, the network required for SM can be divided into functional components including content processing and storage regions (Otten and Rugg, 2001a; Rugg et al., 2002; Kim, 2011). Content processing regions, such as the fusiform cortex, transform sensory input into internal representations that are interpreted downstream (Kirchhoff et al., 2002; Paller and Wagner, 2002). Storage regions, namely the medial temporal lobe, bind content representations into stable memory traces for retrieval during a subsequent encounter (Squire et al., 2004). Little work has assessed whether patients with schizophrenia demonstrate the same neural patterns related to subsequent memory as healthy subjects (Bonner-Jackson et al., 2008; Ragland et al., 2012).
We used event-related functional magnetic resonance imaging (fMRI) to investigate SM for incidentally encoded patterned visual stimuli – fractals – in patients with schizophrenia. Schizophrenia has been associated with functional impairment of the medial temporal lobe, evidenced by deficient patterns of activation during memory encoding and retrieval (Heckers, 2001; Preston et al., 2005; Ragland et al., 2009). It is less clear whether schizophrenia relates to functional impairment of the fusiform cortex, particularly when patients attempt to encode complex patterned visual stimuli such as fractals. Prior evidence suggesting impairment of the fusiform cortex relates primarily to deficits in facial processing (Gur et al., 2002; Quintana et al., 2003; Johnston et al., 2005). However, some results suggest that activation in the fusiform is preserved in patients (Yoon, D’Esposito and Carter, 2006).
We aimed to investigate patterns of SM activation in patients with schizophrenia compared to healthy controls. We hypothesized that patients would demonstrate deficient medial temporal lobe activation, reflecting a compromised ability to bind visual representations into stable memory traces, but intact SM activation in the fusiform cortex, reflecting the ability to encode visual fractals. Greater effort for encoding by patients would result in greater fusiform activation. In that case, patients would demonstrate compensatory strategies for successful encoding by over-activating the fusiform relative to healthy controls. Given prior evidence that memory impairment in schizophrenia is associated with negative symptoms (Aleman et al., 1999), we assessed the relationship between SM activation abnormalities and negative symptom severity, in comparison to positive symptom severity.
2. Methods
2.1. Participants
The sample included 26 patients with schizophrenia (15 males) and 28 healthy controls (15 males), drawn from a larger sample that also included 36 family members of patients, resulting in 90 total participants. All participants were right-handed volunteers at the University of Pennsylvania Schizophrenia Research Center. Participants underwent standard assessment, including medical, neurological, psychiatric, neurocognitive, and laboratory tests. Psychiatric evaluation included clinical interviews, a structured interview (SCID-P, First et al., 1996), and collateral history from family, caregivers and records. Trained investigators administered symptom scales. Patients had a DSM-IV diagnosis of schizophrenia (N = 22) or schizoaffective disorder (N = 4), determined by consensus conference based on all available information and all were on an average of 287.2 mg of antipsychotic medication (in chlorpromazine equivalent units), predominantly on second generation antipsychotics (N = 19) and some on first generation antipsychotics (N = 7). Patients were clinically stable outpatients at the time of study and had been on medication for an average of 40 months. Symptoms were assessed with the Scale for Assessment of Negative Symptoms (SANS, Andreasen, 1984a, a verage total SANS score M = 1.1, SD = 0.6). Symptoms were also assessed with the Scale for the Assessment of Positive Symptoms (SAPS, Andreasen, 1984b, a verage total SAPS score M = 0.6, SD = 0.5). Patients had no history of other disorders or events affecting brain function (i.e., current or history of substance abuse). After a complete description of the study, written informed consent was obtained from the participants. Healthy controls were similarly screened and had no Axis I disorder and no first-degree relative with such a disorder.
2.2. Procedure
During scanning, participants completed 3 tasks including an oddball task, a recognition memory task; and in between those tasks, participants completed an unrelated perceptual integration task. The perceptual integration task assessed the ability to perceive biologic motion using moving dot stimuli; results of this task will be presented elsewhere. In the 9-minute oddball task, participants were instructed to respond to targets (green circle, 15% of trials) and refrain from responding to standard stimuli (red circle, 70% of trials). Without prior mention in the instructions, novel fractals would appear infrequently (15% of trials). Each novel fractal was a unique image and thereby distracting relative to the standard and target stimuli. Participants were not asked to remember the novel fractals; therefore the fractals were incidentally encoded. Incidental-encoding engages activation in the middle temporal lobe (Stark and Okado, 2003; Henson, 2005). Approximately ten minutes after the oddball task, participants completed a 6-minute recognition memory task that included 30 target fractals (used as novel stimuli in the oddball task) and 30 foil fractals. Stimuli in both the oddball and recognition memory tasks were presented in a random order on the screen for 1 second with a variable inter-stimulus interval ranging from 2–18 seconds. Participants were asked to discriminate between target and foils and responded with a button press to make old/new recognition memory judgments. Responses and reaction times were recorded.
After the scan, participants completed a web-based adaptation of a computerized neurocognitive battery (CNB) that included a shape memory task, consisting of 10 targets and 10 foils selected evenly from each type of shape (Glahn et al., 1997; Gur et al., 2001, 2010, 2012; Irani et al., 2012). Each stimulus was a blue two-dimensional shape (a triangle, square, pentagon, hexagon or octagon with varying degrees of shading) within a three-dimensional figure. Stimuli were presented for 1 second each on a computer screen and participants were asked to memorize the stimuli. Immediately after the study phase, participants made old/new recognition memory judgments, responses and reaction times were recorded. Participants received no feedback on whether or not their responses were correct.
2.3. Image Acquisition
Functional blood-oxygen-level-dependent (BOLD) data was acquired on a 3-T Siemens Tim Trio scanner using a quadrature head coil. Structural images were acquired axially using a magnetization prepared rapid acquisition gradient-echo (MPRAGE), T1- weighted sequence (repetition time/ echo time (TR/TE) = 1630/3.87 ms, field of view (FOV) = 240 × 180 mm, matrix = 256 × 192, slice thickness/gap = 1/0 mm) with a voxel resolution of 0.9375 × 0.9375 × 1.00 mm. This sequence was used for spatial normalization and for anatomic overlays of functional data. Functional images (178 images for the oddball task and 126 images for the recognition memory task) were acquired axially using a 40-slice gradient-echo (GE) echo-planar imaging (EPI) sequence (TR/TE = 3000/30 ms, FOV = 240 × 240 mm, matrix = 64 × 64, slice thickness/gap = 3/0 mm) with a nominal voxel resolution of 3.00 × 3.00 × 3.00 mm.
2.4. Image Processing
The fMRI data from the incidental- encoding (oddball) task was preprocessed and analyzed using FEAT (FMRI Expert Analysis Tool v 5.0.2.1), part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). The functional images were slice-time corrected, motion-corrected to the median image with tri-linear interpolation, high-pass filtered (100 s), spatially smoothed (5 mm full width half maximum (FWHM) Gaussian isotropic kernel), and grand mean scaled. A brain extraction tool was used to remove non-brain areas from the high-resolution structural image (Smith, 2002). The functional images were coregistered to the structural image and transformed by trilinear interpolation into standard anatomical space (Jenkinson and Smith, 2001; Jenkinson et al., 2002) using the T1 Montreal Neurological Institute (MNI) template.
2.5. Statistical Analysis
Subject-level time-series statistical analysis was carried out by using FILM (FMRIB’s Improved Linear Model) with local autocorrelation correction (Woolrich et al., 2001). Condition events were modeled with a canonical (double-gamma) hemodynamic response function and its temporal derivative. Event types modeled included: subsequently remembered fractals and subsequently forgotten fractals. The design matrix also included 6 motion parameters derived from motion correction, to reduce residual motion effects. Groups did not differ significantly in head motion (t = 0.70, df = 21, p = 0.49). The primary subsequent memory contrast of interest compared activation at encoding of correctly remembered novel visual fractals versus those that were forgotten (SM, subsequently remembered > subsequently forgotten).
After preprocessing, whole brain statistical analysis was completed for each individual in subject space, and resulting contrast maps were spatially normalized as mentioned above. Group –level random effects analyses were performed in FSL, using FMRIB’s Local Analysis of Mixed Effects (FLAME 1) (Beckmann et al., 2003; Woolrich et al., 2004) during estimation of statistical significance. Within-group analyses were accomplished by entering whole brain contrasts into one-sample t-tests. Between-group analyses used two-sample t-tests.
We selected the medial temporal lobe, consisting primarily of the hippocampus and amygdala, and the fusiform cortex as a priori regions of interest (ROI). Bilateral masks for these ROIs were drawn based on coordinates of peak activation from an SM activation meta-analysis of 74 fMRI studies (Kim, 2001). We drew 12 mm spheres around the coordinates, after converting from Talairach to MNI space and averaging the slightly asymmetric coordinates from the left and right peaks, thus producing symmetric bilateral masks (MNI coordinates: right peak, 20 -9 -20; left peak -20 9 -20). In addition, separate group level covariate analyses were performed in the MTL and FF ROIs for the SM contrast and performance on a shape memory test administered during the CNB. We tested the correlation between negative symptom severity and performance on CNB shape memory.
For all ROI analyses, significance thresholds were based on spatial extent, applying a minimum height threshold z > = 1.65 and a cluster p < 0.05. The cluster-size cutoff (102 voxels in the MTL ROI; 104 voxels in the FF ROI) was determined using Monte Carlo simulation (AlphaSim, D.B. Ward, http://afni.nimh.nih.gov/pub/dist/doc/program_help/AlphaSim.html) within the relevant mask (Forman et al., 1995; Xiong et al., 1995).
We also completed exploratory whole brain analyses to investigate regions, beyond our a prior ROIs, that may be involved in the SM effect. In addition, separate group level covariate analyses were performed in the whole brain to assess the relationship between the SM contrast and clinical symptoms in patients, with an a priori focus on negative symptoms (SANS). We calculated a total score and a score for each of the subscales for the SANS. For all whole brain analyses, significance thresholds were based on spatial extent, applying a minimum height threshold z > = 1.65 and a cluster p < 0.05.
3. Results
3.1. Behavioral Performance
During the in-scanner memory task, healthy controls and patients remembered a similar number of targets (controls: M = 15.2, SD = 4.8; patients: M = 13.9, SD = 6.2; t (24) = 0.90, p = 0.37). However, controls were faster than patients when remembering targets (controls: M = 1015.45 ms, SD = 151.24; patients: M = 1163.62 ms, SD = 213.08; (t (24) = 3.8, p < 0.0001). There was a significant between group difference for true negatives (controls M = 23.2, SD = 4.52; patients M = 20.07, SD = 6.81; t (26) = 2.07, p = 0.05).
3.2. Task Activation: Region of Interest (ROI) Analyses
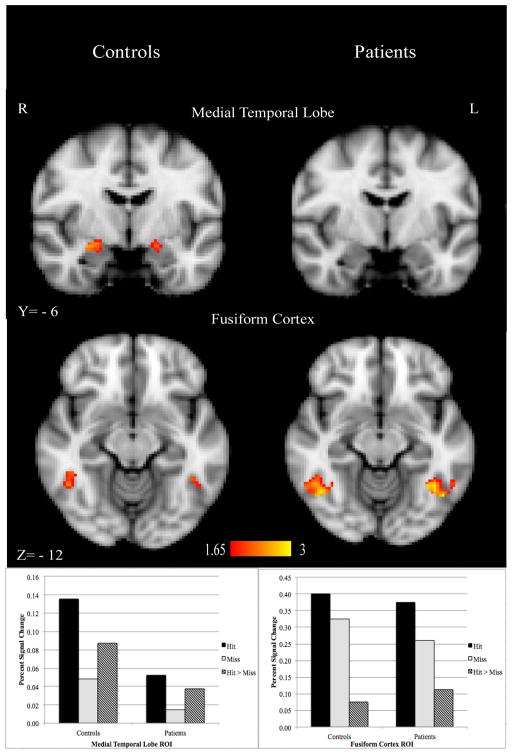
ROI analyses in the medial temporal lobe (MTL) and fusiform gyrus (FF) revealed a different SM activation pattern (subsequently remembered > subsequently forgotten) between groups. Healthy controls activated both the MTL and FF more for subsequently remembered fractals; patients showed SM effects only in FF, and the SM effect in FF was significantly greater in patients than controls (Figure 1).
Figure 1.
Region of interest (ROI) analysis in the medial temporal lobe (MTL) and fusiform cortex (FF) in controls and patients with schizophrenia. Small volume cluster corrected (z > 1.65, cluster p < 0.05) as determined by Monte Carlo simulation (AlphaSim); overlaid on the MNI template brain. Subsequent memory (Hit > Miss) activation in both the MTL and FF was significant in controls; however, only activation in the FF was significant for patients.
3.3. Task Activation: Exploratory Whole Brain Analyses
In exploratory whole brain analysis, patients demonstrated SM activation in the occipital pole, temporal occipital fusiform cortex, lateral occipital cortex, and left inferior temporal gyrus (Figure 2). In contrast, healthy controls did not demonstrate significant whole brain activation that survived correction for multiple comparisons. Group differences in the SM contrast were not significant after multiple comparisons correction.
Figure 2.
Voxel-wise whole brain clusters with significant subsequent memory activation in the occipital pole, lateral occipital cortex, left inferior temporal gyrus, and fusiform cortex in patients with schizophrenia (cluster corrected p < 0.05, voxel height threshold z > 1.65, overlaid on the MNI template brain). This whole brain analysis yielded no significant clusters in controls. Patients demonstrated greater activation in the fusiform than controls.
3.4. Correlation with Clinical Symptoms and Cognitive Performance
In the patient group, there was a positive correlation between negative symptoms and SM activation in the FF ROI. That is, greater activation in the FF ROI for items that were successfully encoded was associated with more severe negative symptoms.
During the out of scanner CNB shape memory task, patients remembered an average of 7.82 (SD = 1.62, 78%) shapes and there was no between group difference (controls: 8.43 (SD = 1.72, 84%); t (26) = 1.24, p = 0.22). There was a positive correlation between performance (i.e., total correct) on CNB shape memory task and SM activation in the FF ROI in patients. That is, the better a patient’s memory for shapes on an independent task, the more activation in the FF ROI during successful encoding of visual fractals. This correlation was not evident in controls, nor was it evident for any other memory task (i.e., word or face memory). There was no significant correlation between CNB shape memory and negative symptoms (r = − 0.05, n.s.).
Lastly, we extracted percent signal change from the MTL and FF ROIs and correlated the neural activity with medication status (in chlorpromazine equivalence units). Neither correlation yielded a significant result; therefore we believe that antipsychotic medication did not drive our subsequent memory effect.
4. Discussion
The subsequent memory (SM) effect - differential neural activation for stimuli that will be successfully remembered compared to forgotten - characterizes the neural activity that supports successful encoding (Brewer et al., 1998; Wagner et al., 1998; Paller and Wagner, 2002; Reber et al., 2002). While robust SM effects have been demonstrated in healthy populations, less is known about whether patients with schizophrenia demonstrate these patterns of neural activation. We used event-related fMRI to investigate patterns of SM activation in patients and examined how these patterns differ from healthy controls. We found that during incidental- encoding of visual fractals, healthy controls showed SM effects in both the medial temporal lobe and fusiform cortex. However, as hypothesized, patients did not show medial temporal lobe activation, albeit without a significant group difference. Also as hypothesized, the SM effect in the fusiform was intact in patients, and in fact was significantly greater in patients compared to healthy controls. It appears that in order to successfully encode the stimuli, patients had to over-activate the fusiform, perhaps serving a compensatory function in the context of deficient medial temporal lobe activation. Our data, which suggests that patients have to activate regions to a greater degree than controls to achieve the same level of performance, is consistent with the well-documented inefficiency hypothesis (Ragland et al., 1998; Callicott et al., 1998; 2003; Manoach et al., 1999; Manoach, 2003; Potkin et al., 2009). Moreover, a compensatory interpretation of fusiform activity is supported by the observation that SM over-activation in the fusiform was positively correlated with severity of negative symptoms. Furthermore, fusiform activation was correlated with performance on an independent shape memory test, only in the patient group.
During encoding, the medial temporal lobe, which is an integral region involved in memory, creates new associations and durable memory traces (Schacter and Wagner, 1999; Achim and Lepage, 2005; Henson, 2005). We found that healthy controls activated the medial temporal lobe during encoding more for visual fractals that were subsequently remembered compared to those that were forgotten, however, there was no significant medial temporal lobe SM effect in patients. This suggests deficient medial temporal lobe activation in patients. Schizophrenia has been associated with medial temporal lobe abnormalities, both structural and functional, including abnormal glutamate receptor expression (Beneyto et al., 2007), decreased volumes (Hirayasu et al., 1998; Gur et al., 2000), reduced activation (Achim and Lepage, 2005) and hyper-activation (Holt et al., 2006; Marwick and Hall, 2008). Our results are consistent with a meta-analysis reporting significantly reduced patient activation of the medial temporal lobe, namely in the right hippocampus, during encoding (Achim and Lepage, 2005). The right hippocampus was also the area where our SM effect was the strongest in controls.
Patients may have shown less medial temporal lobe activation due to stimuli type (Gur et al., 1994). Prior studies investigating SM effect in patients with schizophrenia have, to our knowledge, only used verbal stimuli (Bonner-Jackson et al., 2008) with the exception of Ragland et al. 2012 that used objects. However, our study differs in that we used visual fractals in an incidental- learning paradigm. The novelty-encoding hypothesis suggests that encoding is biased toward processing novel, compared to familiar, stimuli (Tulving et al., 1996). Participants are likely to have higher familiarity with the verbal material but less so for pictorial material used in SM experiments, therefore this hypothesis would predict greater SM activation in the medial temporal lobe during encoding of visual relative to verbal stimuli (Kim, 2011). Our data support the novelty-encoding hypothesis, as this is what we found in controls, further promoting the idea that patients demonstrate deficit in medial temporal lobe activation, albeit without a significant group difference.
Related to the fusiform, we found that, as hypothesized, patients activated only the fusiform cortex during encoding of visual fractals that they would later remember. This fusiform activation was significantly greater in patients compared to controls. The fusiform cortex is actively involved in visuo-perceptual processing, critical for the successful encoding and interpretation of visual stimuli (Kanwisher et al., 2001). Schizophrenia has been associated with abnormalities in the fusiform cortex, both structural and functional, ranging from decreased volume (Onitsuka et al., 2003; Nestor et al., 2007) to decreased activation (Gur et al., 2002; Quintana et al., 2003). However, prior findings of reduced fusiform activation in schizophrenia were from studies of facial processing. Our data suggest that patients can robustly recruit fusiform in the service of memory encoding, and indeed do so more than controls. Perhaps certain components of visual processing, likely those subserved by the ventral stream, remain intact in patients, and these are recruited to compensate for dysfunction in medial temporal lobe regions (Merigan and Maunsell, 1993; Butler et al., 2001, 2008; Doniger et al., 2002; Ungerleider and Pasternak, 2004; Foxe et al., 2005).
We suggest that during successful encoding of visual fractals, patients over activated the fusiform in an attempt to compensate for deficient medial temporal lobe activation. Given that negative symptoms impair memory performance, it is likely that patients with the most severe symptom impairment would rely more upon compensatory neural engagement (Aleman et al., 1999). We observed a positive correlation between fusiform activation and negative symptoms suggesting that the more negative symptoms a patient reported, the more fusiform activated during successful encoding. The indications of compensatory fusiform activation extended beyond negative symptomatology and were also evident in the positive correlation observed in patients between fusiform activation and performance on an independent shape memory task. The ability to engage the fusiform was associated with successful memory of geometric shapes that were challenging to remember (Gur et al., 2012; Collier et al., 2014). Neither of these correlations were significant in the control group. Taken together, the more sick a patient, the more they engaged the fusiform, likely in effort to compensate for inefficient medial temporal lobe activation; and the more a patient compensated, the better they performed on an independent shape memory task. In our sample, negative symptoms and shape memory were not correlated and our data suggest that the fusiform can independently influence these two variables. Future studies can test the proposed compensatory mechanism of the fusiform and directly compare each of these variables.
Patients likely experience deficits in episodic memory because of inefficient recruitment of strategies that support successful encoding (Achim and Lepage, 2005). Orienting patients toward specific encoding strategies such as making abstract/concrete judgments, living/non-living judgments for words, shallow/deep-encoding judgments, may mitigate these deficits (Ragland et al., 2003; Weiss et al., 2003; Bonner-Jackson et al., 2008; Ranganath et al., 2008). When provided with an encoding strategy, patients recruit the same brain regions (inferior frontal gyrus, left superior parietal lobule and left postcentral gyrus) that controls utilize automatically, suggesting that the neural networks engaged during episodic memory in controls are also engaged in patients (Bonner-Jackson et al., 2008). Identifying additional strategies that ameliorate episodic memory deficits in patients is a ripe area of future research.
Behaviorally, memory for target stimuli did not differ between healthy controls and patient. With similar behavioral performance across groups, any difference in neural activation is less likely attributable to task performance or task difficulty and more likely due to differences in the cognitive strategies employed to perform on the task. We used an -encoding task where encoded stimuli were task- irrelevant (novel fractal distractors in the oddball task), so that subjects successfully focusing on the oddball task might have little encoding of the fractals. This presents a limitation in comparing our results to those of other subsequent memory studies which have used explicit encoding tasks and could contribute to the relatively weak subsequent memory effect found in controls. It is possible that the increased SM in patients could reflect in part a failure to suppress task-irrelevant processing during the oddball task. However, there were no group differences in activation or task performance in the visual oddball task (Collier et al., 2014), rendering this interpretation unlikely. Nonetheless, future studies comparing incidental and explicit encoding SM paradigms in schizophrenia will be of interest.
Memory performance is among the strongest predictors of functional outcomes and memory is also among the areas of greatest cognitive dysfunction in schizophrenia (Green, 1996; Heinrichs and Zakzanis, 1998; Miley et al., 2005). Despite remembering about as many targets as healthy controls, patients employed a distinct pattern of brain activity during successful encoding of visual fractals. A potential interpretation of our findings is that while patients were able to process the content of the visual fractals, their MTL was less efficient in binding the representations together to form a stable memory trace and therefore they had to over-activate the fusiform to successfully remember. Our findings illuminate the neural signature of subsequent memory and provide evidence for compensatory neural strategies in patients with schizophrenia.
Acknowledgments
The first author acknowledges support from a NIH Ruth Kirschstein National Research Service Postdoctoral Fellowship (NIH T32 MH019112) and P50 MH064045. We thank Ryan Hopson and Kosha Ruparel for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achim AM, Lepage M. Episodic memory-related activation in schizophrenia: meta-analysis. The British Journal of Psychiatry. 2005;187 (6):500–509. doi: 10.1192/bjp.187.6.500. [DOI] [PubMed] [Google Scholar]
- Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. American Journal of Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Scale for the assessment of negative symptoms (SANS) University of Iowa; Iowa City: 1984a. [Google Scholar]
- Andreasen NC. Scale for the assessment of positive symptoms (SAPS) University of Iowa; Iowa City: 1984b. [Google Scholar]
- Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007;32 (9):1888–1902. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- Bonner-Jackson A, Yodkovik N, Csernansky JG, Barch DM. Episodic memory in schizophrenia: The influence of strategy use on behavior and brain activation. Psychiatry Research: Neuroimaging. 2008;164 (1):1–15. doi: 10.1016/j.pscychresns.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC. Dysfunction of early-stage visual processing in schizophrenia. American Journal of Psychiatry. 2001;158 (7):1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- Butler PD, Silverstein SM, Dakin SC. Visual perception and its impairment in schizophrenia. Biological Psychiatry. 2008;64 (1):40–47. doi: 10.1016/j.biopsych.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. American Journal of Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Ramsey NF, Tallent K, Bertolino A, Knable MB, Coppola R, Goldberg T, van Gelderen P, Mattay VS, Frank JA, Moonen CTW, Weinberger DR. Functional magnetic resonance imaging brain mapping in psychiatry: methodological issues illustrated in a study of working memory in schizophrenia. Neuropsychopharmacology. 1998;18 (3):186–196. doi: 10.1016/S0893-133X(97)00096-1. [DOI] [PubMed] [Google Scholar]
- Censits DM, Ragland JD, Gur RC, Gur RE. Neuropsychological evidence supporting a neurodevelopmental model of schizophrenia: A longitudinal study. Schizophrenia Research. 1997;24:289–29. doi: 10.1016/s0920-9964(96)00091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier AK, Wolf DH, Valdez JN, Turetsky BI, Elliott MA, Gur RE, Gur RC. Comparison of auditory and visual oddball fMRI in schizophrenia. Schizophrenia Research. 2014;158 (1–3):183–188. doi: 10.1016/j.schres.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo MA, Seidman LJ. Verbal declarative memory dysfunction in schizophrenia: from clinical assessment to genetics and brain mechanisms. Neuropsychology Review. 2003;13 (2):43–77. doi: 10.1023/a:1023870821631. [DOI] [PubMed] [Google Scholar]
- Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Archives of General Psychiatry. 2002;59 (11):1011–1020. doi: 10.1001/archpsyc.59.11.1011. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders, patient edition (SCID-P), version 2. Biometics Research; New York: 1996. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster_size threshold. Magnetic Resonance in Medicine. 1995;33 (5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Murray MM, Javitt DC. Filling-in in schizophrenia: a high-density electrical mapping and source-analysis investigation of illusory contour processing. Cerebral Cortex. 2005;15 (12):1914–1927. doi: 10.1093/cercor/bhi069. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Gur RC, Ragland JD, Censits DM, Gur RE. Reliability, performance characteristics, construct validity, and an initial clinical application of the visual object learning task. Neuropsychology. 1997;11 (4):602–612. doi: 10.1037//0894-4105.11.4.602. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? American Journal of Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recognition. Vision Research. 2001;41 (10):1409–1422. doi: 10.1016/s0042-6989(01)00073-6. [DOI] [PubMed] [Google Scholar]
- Gold JM, Randolph C, Carpenter CJ, Goldberg TE, Weinberger DR. Forms of memory failure in schizophrenia. Journal of Abnormal Psychology. 1992;101 (3):487. doi: 10.1037//0021-843x.101.3.487. [DOI] [PubMed] [Google Scholar]
- Gur RE, McGrath C, Chan RM, Schroeder L, Turner T, Turetsky BI, Kohler C, Alsop D, Maldjian J, Ragland JD, Gur RC. An fMRI study of facial emotion processing in patients with schizophrenia. American Journal of Psychiatry. 2002;159 (12):1992–1999. doi: 10.1176/appi.ajp.159.12.1992. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Turner TH, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning: I. methodology and validation in healthy people. Neuropsychopharmacology. 2001;25:766–776. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, Loughead J, Connolly JJ, Qiu H, Mentch FD, Abou-Sleiman PM, Hakonarson H, Gur RE. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. 2012;26 (2):251–265. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Turetsky BI, Cowell PE, Finkelman C, Maany V, Grossman RI, Arnold SE, Bilker WB, Gur RC. Temporolimbic volume reductions in schizophrenia. Archives of General Psychiatry. 2000;57 (8):769–775. doi: 10.1001/archpsyc.57.8.769. [DOI] [PubMed] [Google Scholar]
- Hirayasu Y, Shenton ME, Salisbury DF, Dickey CC, Fischer IA, Mazzoni P, Kisler T, Arakaki H, Kwon JS, Anderson Je, Yurgelun-Todd D, Tohen M, McCarley RW. Lower left temporal lobe MRI volumes in patients with first- episode schizophrenia compared with psychotic patients with first-episode affective disorder and normal subjects. American Journal of Psychiatry. 1998;155 (10):1384–1391. doi: 10.1176/ajp.155.10.1384. [DOI] [PubMed] [Google Scholar]
- Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11:520–528. doi: 10.1002/hipo.1068. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Henson R. A mini-review of fMRI studies of human medial temporal lobe activity associated with recognition memory. Quarterly Journal of Experimental Psychology. 2005;58B (3/4):340–360. doi: 10.1080/02724990444000113. [DOI] [PubMed] [Google Scholar]
- Herrmann MJ, Ellgring H, Fallgatter AJ. Early-stage face processing dysfunction in patients with schizophrenia. American Journal of Psychiatry. 2004;161 (5):915–917. doi: 10.1176/appi.ajp.161.5.915. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Kunkel L, Weiss AP, Goff DC, Wright CI, Shin LM, Rauch SL, Hootnick J, Heckers S. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophrenia Research. 2006;82 (2):153–162. doi: 10.1016/j.schres.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Irani F, Brensinger CM, Richard J, Calkins ME, Moberg PJ, Bilker W, Gur RE, Gur RC. Computerized neurocognitive test performance in schizophrenia: a lifespan analysis. American Journal of Geriatric Psychiatry. 2012;20 (1):41–52. doi: 10.1097/JGP.0b013e3182051a7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimization method for robust affine registration of brain images. Medical Image Analysis. 2001;5 (2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17 (2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johnston PJ, Stojanov W, Devir H, Schall U. Functional MRI of facial emotion recognition deficits in schizophrenia and their electrophysiological correlates. European Journal of Neuroscience. 2005;22 (5):1221–1232. doi: 10.1111/j.1460-9568.2005.04294.x. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Downing P, Epstein R, Kourtzi Z. Functional neuroimaging of human visual recognition. In: Cabeza R, Kingstone A, editors. The Handbook on Functional Neuroimaging. MIT Press; Cambridge: 2001. pp. 109–152. [Google Scholar]
- Kerns JG, Nuechterlein KH, Braver TS, Barch DM. Executive functioning component mechanisms and schizophrenia. Biological Psychiatry. 2008;64 (1):26–33. doi: 10.1016/j.biopsych.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Kim H. Neural activity that predicts subsequent memory and forgetting: A meta-analysis of 74 fMRI studies. Neuroimage. 2011;54:2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. Journal of Neuroscience. 2000;20:6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PG, Onitsuka T, Gurrera RJ, Niznikiewicz M, Frumin M, Shenton ME, McCarley RW. Dissociable contributions of MRI volume reductions of superior temporal and fusiform gyri to symptoms and neuropsychology in schizophrenia. Schizophrenia Research. 2007;91 (1):103–106. doi: 10.1016/j.schres.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophrenia Research. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Press DZ, Thangaraj V, Searl DC, Halpern E, Saper CB, Waranch S. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biological Psychiatry. 1999;45:1128–1137. doi: 10.1016/s0006-3223(98)00318-7. [DOI] [PubMed] [Google Scholar]
- Marwick K, Hall J. Social cognition in schizophrenia: a review of face processing. British Medical Bulletin. 2008;88 (1):43–58. doi: 10.1093/bmb/ldn035. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JH. How parallel are the primate visual pathways? Annual Review of Neuroscience. 1993;16 (1):369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- Milev P, Ho BC, Arndt S, Andreasen NC. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: A longitudinal first- episode study with 7-year follow-up. American Journal of Psychiatry. 2005;162:495–506. doi: 10.1176/appi.ajp.162.3.495. [DOI] [PubMed] [Google Scholar]
- Onitsuka T, Shenton ME, Kasai K, Nestor PG, Toner SK, Kikinis R, Jolesz FA, McCarley RW. Fusiform gyrus volume reduction and facial recognition in chronic schizophrenia. Archives of General Psychiatry. 2003;60 (4):349–355. doi: 10.1001/archpsyc.60.4.349. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Henson RN, Rugg MD. Depth of processing effects on neural correlates of memory encoding: relationship between findings from across- and within-task comparisons. Brain. 2001;124 (2):399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- Paller K, Wagner A. Observing the transformation of experience into memory. Trends in Cognitive Science. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Turner JA, Brown GG, McCarthy G, Greve DN, Glover GH, Manoach DS, Belger A, Diaz M, Wible CG, Ford JM, Mathalon DH, Gollub R, Lauriello J, O’Leary D, van Erp TGM, Toga AW, Preda A, Lim KO. Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophrenia Bulletin. 2009;35 (1):19–31. doi: 10.1093/schbul/sbn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AR, Shohamy D, Tamminga CA, Wagner AD. Hippocampal function, declarative memory, and schizophrenia: Anatomic and functional neuroimaging considerations. Current Neurology and Neuroscience Report. 2005;5:249–256. doi: 10.1007/s11910-005-0067-3. [DOI] [PubMed] [Google Scholar]
- Quintana J, Wong T, Ortiz-Portillo E, Marder SR, Mazziotta JC. Right lateral fusiform gyrus dysfunction during facial information processing in schizophrenia. Biological Psychiatry. 2003;53 (12):1099–1112. doi: 10.1016/s0006-3223(02)01784-5. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Blumenfeld RS, Ramsay IS, Yonelinas A, Yoon J, Solomon M, Carter CS, Ranganath C. Neural correlates of relational and item-specific encoding during working and long-term memory in schizophrenia. NeuroImage. 2012;59 (2):1719–1726. doi: 10.1016/j.neuroimage.2011.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Glahn DC, Censits DM, Smith RJ, Lazarev MG, Alavi A, Gur RE. Frontotemporal cerebral blood flow change during executive and declarative memory tasks in schizophrenia: a positron emission tomography study. Neuropsychology. 1998;12 (3):399–413. doi: 10.1037//0894-4105.12.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Laird AR, Ranganath C, Blumenfeld RS, Gonzales SM, Glahn DC. Prefrontal activation deficits during episodic memory in schizophrenia. The American Journal of Psychiatry. 2009;166 (8):863–874. doi: 10.1176/appi.ajp.2009.08091307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Moelter ST, McGrath C, Hill SK, Gur RE, Bilker WB, Sigel SJ, Gur RC. Levels-of-processing effect on word recognition in schizophrenia. Biological Psychiatry. 2003;54 (11):1154–1161. doi: 10.1016/s0006-3223(03)00235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Minzenberg M, Ragland JD. The cognitive neuroscience of memory function and dysfunction in schizophrenia. Biological Psychiatry. 2008;19:417–427. doi: 10.1016/j.biopsych.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber PJ, Siwiec RM, Gitleman DR, Parrish TB, Mesulam MM, Paller KA. Neural correlates of successful encoding identified using functional magnetic resonance imaging. Journal of Neuroscience. 2002;22:9541–9548. doi: 10.1523/JNEUROSCI.22-21-09541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg M, Otten L, Henson R. The neural basis of episodic memory: evidence from functional neuroimaging. Philos Philosophical Transactions of the Royal Society B: Biological Sciences. 2002;357:1097–1110. doi: 10.1098/rstb.2002.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Stone WS, Jones R, Harrison RH, Mirsky AF. Comparative effects of schizophrenia and temporal lobe epilepsy on memory. Journal of the International Neuropsychological Society. 1998;4:342–352. [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire L, Stark C, Clark R. The medial temporal lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Stark CEL, Okado Y. Making memories without trying: Medial temporal lobe activity associated with incidental memory formation during recognition. Journal of Neuroscience. 2003;23:6748–6753. doi: 10.1523/JNEUROSCI.23-17-06748.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3- Dimensional Proportional System: An Approach to Cerebral Imaging. New York: 1988. [Google Scholar]
- Tulving E, Markowitsch HJ, Craik FI, Habib R, Houle S. Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cerebral Cortex. 1996;6 (1):71–79. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- Ungerleider L, Pasternak T. Ventral and dorsal cortical processing streams. In: Chalupa LM, Werner JS, editors. Visual Neurosciences. MIT Press; Cambridge: 2004. pp. 541–563. [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Schacter DL, Goff CD, Rauch SL, Alpert NM, Fischman AJ, Heckers S. Impaired hippocampal recruitment during normal modulation of memory performance in schizophrenia. Biological Psychiatry. 2003;53 (1):48–55. doi: 10.1016/s0006-3223(02)01541-x. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Becmann CF, Jenkinson SM. Multilevel linear modeling for fMRI group analysis using Bayesian inference. Neuroimage. 2004;21 (4):1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of fMRI data. Neuroimage. 2001;14 (6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Xiong J, Gao JH, Lancaster JL, Fox PT. Clustered pixels analysis for functional MRI activation studies of the human brain. Human Brain Mapping. 1995;3 (4):287–301. [Google Scholar]
- Yoon JH, D'Esposito M, Carter CS. Preserved function of the fusiform face area in schizophrenia as revealed by fMRI. Psychiatry Research: Neuroimaging. 2006;148 (2):205–216. doi: 10.1016/j.pscychresns.2006.06.002. [DOI] [PubMed] [Google Scholar]