Abstract
The effect of neonatal exposure to diethylstilbestrol (DES), a potent synthetic estrogen, was examined to evaluate whether the CD-1 (estrogen insensitive, outbred) and C57 (estrogen sensitive, inbred) mouse strains differ in their response to estrogen disruption of male ExG differentiation. CD-1 and C57BL/6 litters were injected with sesame oil or DES (200 ng/g/5μl in sesame oil vehicle) every other day from birth to day 10. Animals were sacrificed at the following time points: birth, 5, 10 and 60 days postnatal. Neonatally DES-treated mice from both strains had many ExG abnormalities that included the following: (a) severe truncation of the prepuce and glans penis, (b) an abnormal urethral meatus, (c) ventral tethering of the penis, (d) reduced os penis length and glans width, (e) impaired differentiation of cartilage, (f) absence of urethral flaps, and (g) impaired differentiation of erectile bodies. Adverse effects of DES correlated with the expression of estrogen receptors within the affected tissues. While the effects of DES were similar in the more estrogen-sensitive C57BL/6 mice versus the less estrogen-sensitive CD-1 mice, the severity of DES effects was consistently greater in C57BL/6 mice. We suggest that many of the effects of DES, including the induction of hypospadias, are due to impaired growth and tissue fusion events during development.
Keywords: Neonatal, diethylstilbestrol, urethra, prepuce, hypospadias, mouse, penis
INTRODUCTION
Differences amongst mouse strains in response to estrogen was demonstrated decades ago when Gardner and Argyris identified variability in response of vaginal mucosa to estrogen in inbred mouse strains (Gardner and Argyris, 1957). Since then evidence has demonstrated strain-specific variability in response to estrogen for a variety of endpoints in both female and male mice (Spearow et al., 1999; Spearow and Barkley, 1999; Spearow et al., 2001). Given the essential role of external genitalia (ExG) in reproduction and the known teratogenesis of estrogen on ExG development (Baskin et al., 2001b; Goyal et al., 2007), in the accompanying paper we examined strain-specific differences on ExG development following prenatal treatment with diethylstilbestrol (DES). These studies demonstrated an enhanced incidence and severity of male ExG malformations in inbred C57BL/6 versus outbred CD-1 mice (Mahawong et al., 2014). Given that the neonatal mouse model best represents the developmental stages of in utero human estrogenic exposure (Ma, 2009), in this paper we extend our analysis of strain differences in estrogenic susceptibility to mice treated neonatally with DES.
Outbred CD-1 and inbred C57Bl6 strains are the most commonly used mouse models for investigating the effects of estrogens on development. CD-1 is popular because of its vigor, ease of breeding, and large litter size even though CD-1 mice are less sensitive to estrogen than inbred mouse strains (Spearow et al., 1999). Another commonly used mouse strain is C57BL/6, which represents the genetic background of many mutant mouse models, including steroid hormone receptor knockout mice. C57BL/6 mice are particularly sensitive to estrogens (Spearow et al., 1999). Thus, use of CD-1 mice may under-estimate the effects of estrogen, and use of C57BL/6 mice over-estimate the effects estrogen.
Whereas the presence (males) or relative absence (females) of androgens plays a central role in sex differentiation of the ExG (Rodriguez et al., 2012), exogenous estrogenic compounds can derail ExG development by impairing differentiation and eliciting abnormal morphogenetic patterns including induction of hypospadias in mice and rats (Cunha et al., 2014; Mahawong et al., 2014; Goyal et al., 2007; Vorherr et al., 1979). Exogenous estrogens have been shown to perturb patterning of the penile urethral meatus, to reduce overall size of the external genitalia, to impair bone and cartilage differentiation and growth, to induce abnormal differentiation of fat cells within cavernous spaces, to impair smooth muscle differentiation and to induce hypospadias (Goyal et al., 2005; Goyal et al., 2007; Blaschko et al., 2013; Rodriguez et al., 2012; Cunha et al., 2014).
The field of mouse hypospadias has suffered for many years due to uncritical evaluation of developmental defects and resultant ambiguity in the literature. For example, many studies report “hypospadias” as an open urethral/preputial groove observed at the end of gestation in mice treated prenatally with estrogens (Kim et al., 2004). The tacit (but unproven) assumption is that such estrogen-induced malformations observed in late gestation fetuses are irreversible and will result in enduring malformations in adulthood. In this regard, to date there are 22 reports of murine “hypospadias” based upon an open urethral/preputial groove in late gestation mice without verification that the observed embryonic malformations persist as adult hypospadias (Cunha et al., 2014). Such embryonic malformations may represent retardation of development capable of resolving to normality with time as discussed previously (Cunha et al., 2014; Mahawong et al., 2014). It is for this reason that we have proposed objective criteria of mouse hypospadias and emphasize that to avoid ambiguity, the best time to diagnose mouse hypospadias is at puberty or better yet in adulthood when penile development is complete and malformations (if present) are irreversible and enduring (Cunha et al., 2014).
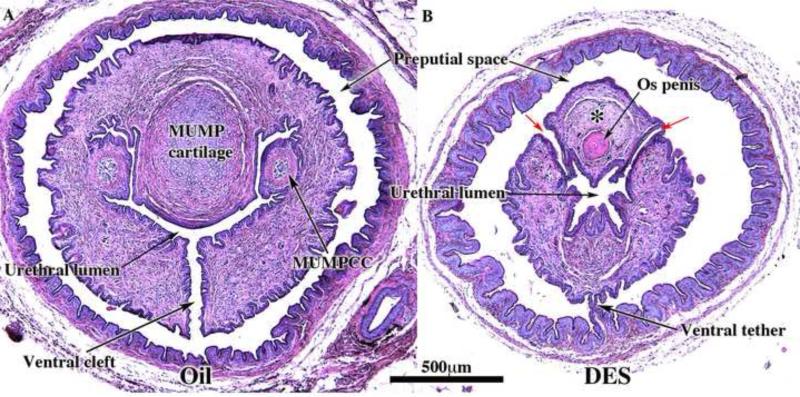
Hypospadias in male mice involves two structures: (a) the prepuce and (b) the penile urethra. Preputial hypospadias is an abnormality in the form of the external prepuce and typically is manifest as exaggerated clefting of the external prepuce and generalized preputial hypoplasia (Mahawong et al., 2014). [It should be recognized that the mouse has two prepuces, external and internal (Blaschko et al., 2013)]. Fundamentally, mouse penile hypospadias is an abnormality in the urethral meatus either in shape, position or both that departs significantly from normal morphology. The mouse urethral meatus is formed by the fusion of the male urogenital mating protuberance (MUMP) with the MUMP ridge (Weiss et al., 2012; Yang et al., 2010; Blaschko et al., 2013; Rodriguez et al., 2011). Accordingly, we define mouse penile hypospadias as an abnormality in the morphological patterning of the elements that form the urethral meatus (the MUMP and MUMP ridge) (Blaschko et al., 2013; Mahawong et al., 2014; Cunha et al., 2014), which can be readily detected in end-on photographs, scanning electron micrographs (SEMs) or histologic sections (Blaschko et al., 2013; Mahawong et al., 2014). Proximal to the urethral meatus are two structures, the urethral flaps and the os penis (Rodriguez et al., 2011), which accordingly are rarely observed in transverse sections containing an open ventral urethral cleft as described previously [see Figure 2 in (Mahawong et al., 2014)]. Thus, “exposed urethral flaps” and “exposed os penis” are additional objective criteria of mouse hypospadias usually associated with an elongated ventral cleft in the MUMP ridge (Mahawong et al., 2014).
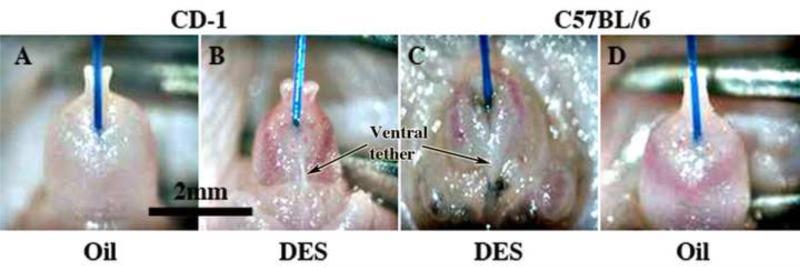
Figure 2.
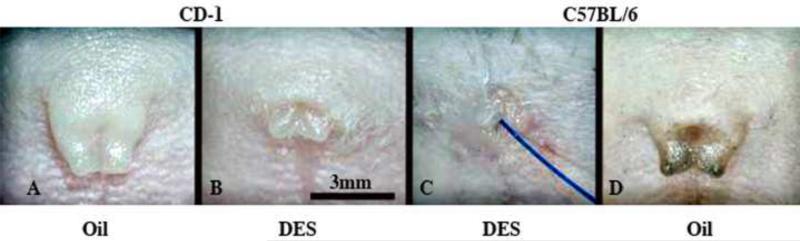
Wholemount photos of external prepuces of adult male neonatally oil- and DES-treated CD-1 and C57BL/6 mice. Note in (A & D) that the external prepuce is distally clefted in neonatally oil-treated male mice, and that neonatal DES-treatment (B & C) elicits profound hypoplasia and distortion of the external prepuce. The effect of neonatal DES is more severe in C57BL/6 mice (C) in which the prepuce is almost completely obliterated. Blue suture threaded from the bladder to the exterior indicates the external preputial orifice in (C).
Based upon morphology of human hypospadias, the ideal expectation for an animal model of hypospadias is an abnormal urethral meatus at “mid-shaft”. We have recently questioned whether such an abnormality is possible in mice given that the mechanism of penile development is different in mouse versus human (Mahawong et al., 2014). The human penile urethra forms as a result of midline fusion of the edges of the urethral groove (Yamada et al., 2003). In contrast, formation of most of the mouse urethra has been suggested to occur via extension and canalization of the embryonic urethral plate (Seifert et al., 2008), even though the mouse urethral meatus appears to develop via fusion of the elements (MUMP and MUMP ridge) constituting the urethral opening (Blaschko et al., 2013; Mahawong et al., 2014; Rodriguez et al., 2011). Whereas the developing human genital tubercle (penile rudiment) always has a free surface exposed to amniotic fluid (Baskin, 2000; Jirasek, 1971), the mouse genital tubercle is over-grown by the developing external prepuce (Perriton et al., 2002; Petiot et al., 2005; Baskin et al., 2004), and consequently the epidermal surface of the mouse penis is represented from birth onward by the solid preputial epithelial lamina, the precursor of the inner preputial epithelium and penile surface epithelium. In fact, the mouse penile surface epithelium is not manifest until puberty (days 24-30 postnatal) when penile development is complete (Mahawong et al., 2014). Thus, for several weeks before development of the mouse penile surface, the mouse penile urethra (with the exception of the meatal region) is fully formed and appropriately incorporated into the substance of the penis. Thus, fusion events may only be involved in development of the mouse penile urethral meatus.
Hypospadias of the human penile shaft involves the absence/defect of 3 elements: (a) ventral skin, (b) epithelium of the ventral urethral wall, and (c) associated stroma and the corpus spongiosum forming the stroma of the ventral urethral wall (See Fig. 15 of the accompanying paper) (Mahawong et al., 2014). Even though hypospadias of the mouse penile shaft may be unlikely as discussed above, it is imperative to recognize estrogen-induced defects in the mouse penis that are homologous or related to the 3 defective elements constituting human mid-shaft hypospadias. Accordingly, in neonatally DES-treated mice we have observed defects in penile skin, urethral epithelium and the corpus cavernosum urethrae, the mouse homologue to the human corpus spongiosum.
The purpose of this paper is to explore teratogenic strain differences in ExG in neonatally DES-treated mice including hypospadias, to summarize differences in prenatal (accompanying paper) versus neonatal DES-treatment, and to provide evidence for the developmental basis of estrogen-induced ExG malformations. It is worth noting that exposure to exogenous estrogens such as DES during the perinatal period elicits a variety of abnormalities in both male and female reproductive tracts and mammary gland (Bern and Talamantes, 1981; Mori et al., 1979; McLachlan et al., 1975; McLachlan et al., 1980). Our results suggest that developmental defects elicited by DES are profoundly different for prenatal versus neonatal DES treatment and that estrogen-induced teratogenesis is similar but consistently more severe in C57BL/6 versus CD-1 mice.
MATERIALS AND METHODS
Animal and housing
The University of California, San Francisco (UCSF) Institutional Animal Care and Use Committee approved all animal protocols. Adult wild-type CD-1 and C57BL/6 mice (Charles River Breeding Laboratories, Wilmington, MA, USA) were housed in polycarbonate cages (20×25×47 cm3) with laboratory-grade pellet bedding in the UCSF Pathogen Specific Barrier housing facility. The mice were given Purina lab diet and tap water ad libitum. They were acclimated to 20° to 23°C and 40% to 50% humidity on a schedule of 14 hours light and 10 hours dark. After mating within the same strains, pregnant female mice were separated from the males and were monitored until delivery.
Interventions
Ten CD-1 and 14 C57BL/6 litters were used. Neonates from the same litter were injected with the same agent to prevent cross-contamination. Neonates were weighed and injected intra-peritoneally every other day at postnatal days 1, 3, 5, 7, and 9 with DES (200 ng/gbw in ~5μl in sesame oil vehicle) as described previously (Rodriguez et al., 2012). As controls, neonates of five CD-1 and seven C57BL/6 litters were injected with sesame oil (5μl) on the same schedule. Separate 50 μl Hamilton syringes with a 28-gauge needle were used for each agent.
Specimen preparation and analysis
On postnatal days 5, 10 and 60, males from each litter were sacrificed. ExG were photographed under a dissection microscope using a digital camera to identify surface features, dissected, and then fixed in 10% buffered formalin. Samples were paraffin embedded and serially sectioned (transversely and longitudinally) at 7μm for histological staining with hematoxylin and eosin.
Metrics of pertinent key morphological features were obtained using direct microscopic measurement of transverse or longitudinal sections or by counting the number of serial transverse sections containing the object of interest. Organ width was measured at mid-glans. Our morphological analysis of the male ExG focused on the external prepuce and the distal aspect of the penis, which is the distinctive area of this organ. This paper is based upon analysis of 56 CD-1 and 70 C57BL/6 mice.
Scanning electron microscopy
Surface details were elucidated using scanning electron microscopy (SEM). ExG specimens for this purpose were dissected and fixed in 2% glutaraldehyde in 0.1M sodium cacodylate buffer at pH 7.2 for 6 hours. The specimens were then fixed in 2% osmium tetraoxide for 2 hours, dehydrated in serial alcohol solutions and critical point dried in a Tousimis AutoSamdri 815 Critical Point Dryer (Tousimis, Rockville, MD). The samples were then mounted on a stub with carbon tape, and images were obtained using a Hitachi TM-1000 Scanning Electron Microscope (Hitachi High Technologies America, Inc. Pleasanton, CA).
Optical projection tomography
Dissected mouse ExG from 5-day-old mice were fixed in 4% paraformaldehyde (PFA) for 2h, and then stored in 70% methanol. When ready for staining, samples were bleached overnight in a H2O2 solution composed of 15 parts of 30% H2O2 and 17 parts DMSO in 100% methanol. Samples were then incubated in acetone at room temperature (RT) for 3h, moved sequentially to 100% methanol, and frozen at -80oC for 1h and sequentially thawed 5 times. Following washing in Tris-buffered saline containing 1% Triton X-100 (TBST), samples were incubated in antibody block (TBST plus 10% goat serum) overnight at RT on a rocker. After blocking, the primary antibody (mouse anti-E-cadherin diluted 1:100, BD Transduction Laboratories, San Jose, CA) was applied for 48-72 at RT with rocking. Samples were washed for two days in a 5-10ml volume of TBST with media changes every 2h (min 5 changes per day). The secondary antibody, Alexa Fluor 555 goat anti-mouse (Life Technologies, Foster City, CA), was diluted in TBST and applied for 48-72h at RT before finally washing in TBST for 3 days as previously described. Finally, samples were post-fixed in 4% PFA for 2h at 4oC, and moved to test tubes containing 1% PFA for storage until ready for imaging. All solutions, except for methanol, were filtered through a 0.2um filter. Imaging was performed by the Histology and Light Microscopy Core at the Gladstone Institute, UCSF, which then provided the computer files and Volocity software for analysis.
Immunohistochemical Analysis
Immunohistochemistry was carried out on penises of 10-day untreated mice. Penile specimens were formalin fixed, paraffin embedded and serially sectioned at 7μm. Immunohistochemistry was carried out as previously described (Rodriguez et al., 2012) utilizing anti-ERα (Dako, Carpinteria, CA, USA). Signal detection was achieved using the Vector ABC System (Vector Laboratories, Foster City, CA, USA) followed by exposure to diaminobenzidine (Sigma®). Sections exposed to all steps except the application of the primary antibodies were used as negative controls.
Statistics
Statistical analysis to compare disruption endpoints between strains was done using Student's t tests. A p<0.05 was considered significant. Data are expressed as mean ± SE.
RESULTS
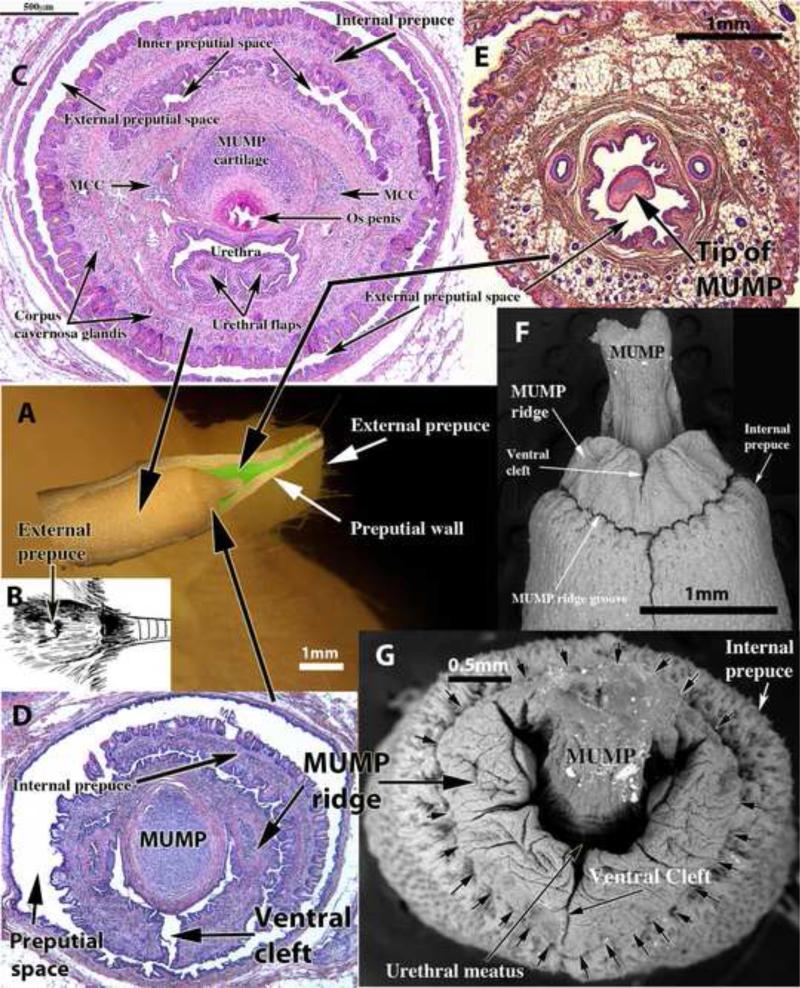
Figure 1 illustrates the normal anatomy of adult male mouse ExG. Superimposed on the side view of the mouse perineum (Fig. 1A) and positioned precisely is a dissected penis and preputial wall. The superimposed images (Fig. 1A) of the external prepuce and penis are at the same magnification and demonstrate the fact that at rest (flaccid condition) the penis is an “internal organ” deeply placed within the external preputial space (Figs. 1A, 1C, 1D & 3) defined by the thick preputial wall (Figs. 1E & 13A1 and 13D1 and see also Figure 6 in the accompanying paper). The elevation in the perineum (Figs. 1A, 1B, 2A & 2D) is the external prepuce, which is also displayed as a side view image (Fig. 1A). The positions of transverse sections (Figs. 1C-E) are demarcated by the large black arrows. Figure 1E shows the tip of the penis (actually the tip of the MUMP) within the external preputial space and the thick wall of the external prepuce containing numerous hair follicles and adipose tissue. More proximally, figure 1D shows the MUMP as well as the open ventral cleft in the MUMP ridge, which is also seen in the SEMs (Figs. 1F, 1G & 7A). The penis is freely mobile within the external preputial space being attached to the inner preputial epithelium only in the depth of the preputial space where the inner preputial epithelium reflects onto the surface of the penis (Fig. 3) as described previously (Cunha et al., 2014; Rodriguez et al., 2011). The Y-shaped urethral meatus (Figs. 1D, 1G & 7A) is composed of the male urogenital mating protuberance (MUMP) fused to the MUMP ridge, which in turn is circumscribed by the internal prepuce (Fig. 1C, 1D, 1F, 1G, & 7A) (Blaschko et al., 2013). The urethral meatus forms as a result of closure of the ventral cleft in the MUMP ridge (Fig. 1D, 1F, 1G, 7A, 13A2 & 13D2). Figure 1C, a transverse section of the penis of a neonatally oil-treated adult mouse, illustrates the arrangement of internal structures in the penile shaft. The proximal aspect of the MUMP cartilage dorsally overlaps the os penis. The urethra with its urethral flaps lie ventral to the MUMP cartilage and os penis. There are 3 erectile bodies within the glans: the circumferential corpus cavernosum glandis, the MUMP corpora cavernosa, and the corpus cavernosum urethrae (Figs. 1C, 4A, 8A-D & 9A).
Figure 1.
Illustrations of normal (untreated/oil-treated) adult mouse penile anatomy. (A) is a side view of the adult mouse external prepuce with a dissected preputial wall and penis superimposed. Both are of the same magnification, are positioned precisely and indicate that the flaccid penis is an “internal organ” positioned deep to the tip of the external prepuce. (B) is a drawing of the adult mouse perineum indicating that the elevation in the perineum is the external prepuce. (C-E) are transverse histologic sections taken at the positions indicated by the large black arrows. In (D) note the open ventral cleft in the MUMP ridge. In (C-E) it is evident that the penis resides within the external preputial space. In (F), a SEM (ventral view), note the ventral cleft in the MUMP ridge. In (G), an end-on SEM, note the Y-shaped urethral meatus formed by the MUMP fused to the MUMP ridge, which is ventrally clefted.
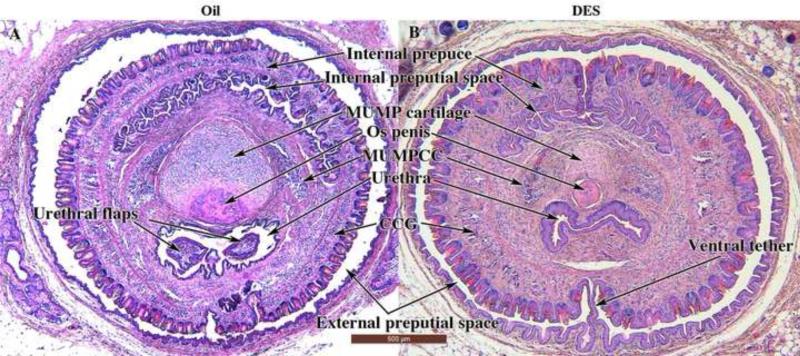
Figure 3.
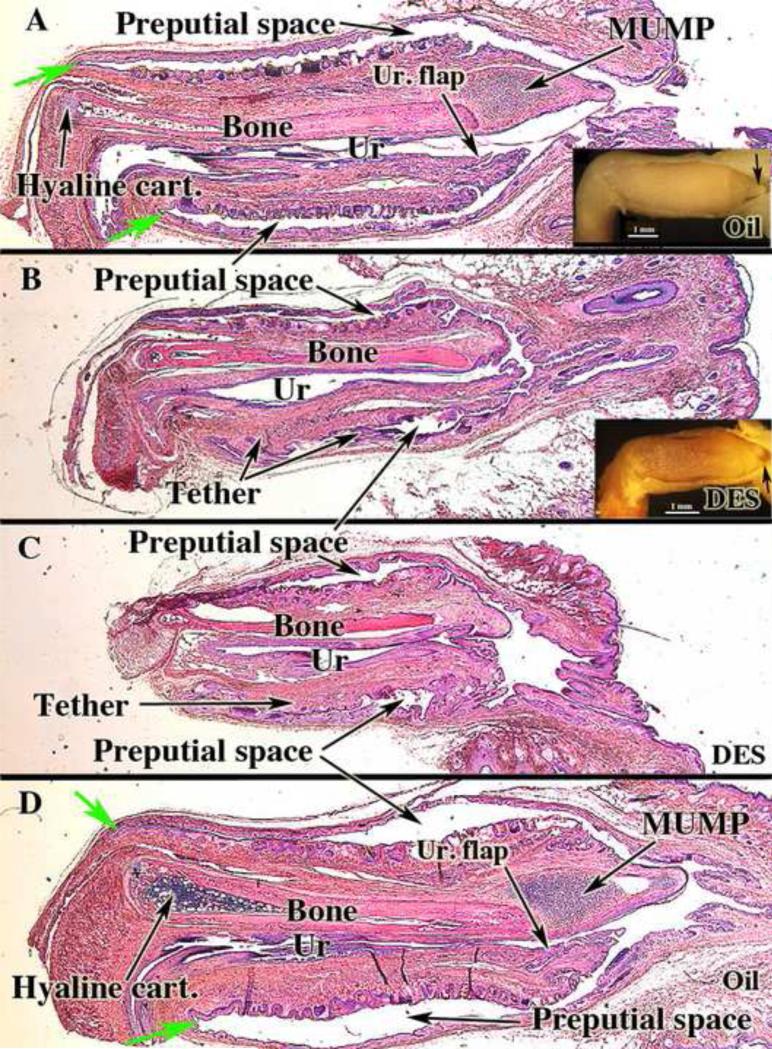
Mid-sagittal sections of external genitalia of adult neonatally oil- and DES-treated CD-1 and C57BL/6 male mice. Note reduction in penis length and bone length and absence of the MUMP in both of the DES-treated specimens (B & C). The ventral tether can be seen in the DES-treated specimens (B & C). Note also in (A & B) the reflection of inner preputial epithelium onto the penis surface (green arrows). Ur = urethra; Ur. flap = urethral flap; Hyaline cart. = Hyaline cartilage. Wholemount insets (A & B) demonstrated the presence (A, arrow) of the MUMP in the oil-treated specimen and its absence (B, arrow) in the neonatally DES-treated specimen.
Figure 13.
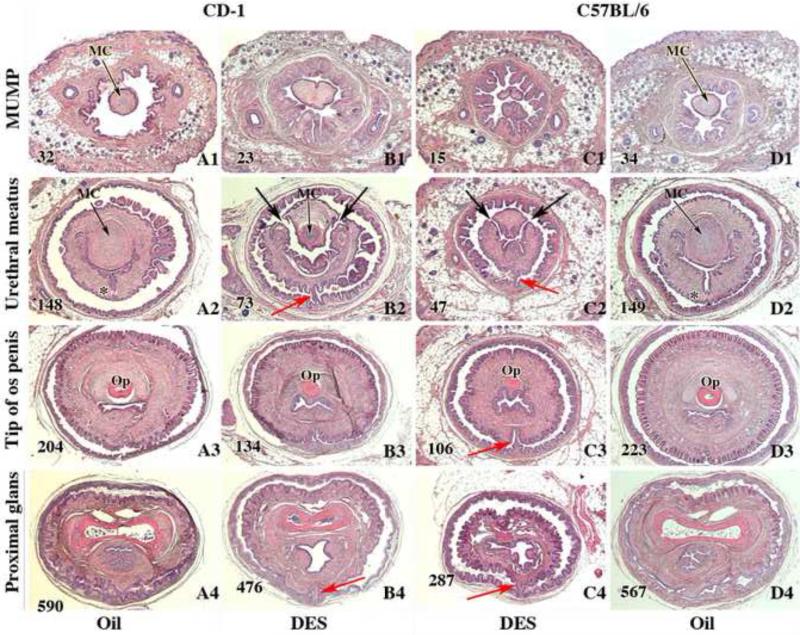
Transverse sections of external genitalia of neonatally oil- and DES-treated adult male CD-1 and C57BL/6 mice displayed in 4 areas: distal MUMP (A1, B1, C1 & D1), near the level of closure of the ventral cleft (A2, B2, C2 & D2), near the tip of the eosinophilic os penis (Op) (A3, B3, C3, & D3), and near the proximal aspect of the os penis (A4, B4. C4, & D4). Note bluish staining of the MUMP cartilage (MC) in the oil-treated specimens (A1, D1, A2 and D2), the hypoplastic MUMP cartilage (MC) in the DES-treated CD-1 mouse (B2), and the absence of MUMP cartilage in DES-treated specimens (B1, C1 & C2). Note closure of the ventral cleft in the MUMP ridge with midline stromal confluence across the midline (*) in A2 and D2 (oil-treated) and open dorsal clefts between the MUMP and the MUMP ridge in the DES-treated specimens (large black arrows in B2 & C2). Red arrows denote ventral tethering in DES-treated specimens (B2, B4, C2 to C-4). Section numbers are placed on each image to denote the number of sections from the distal tip of the MUMP and verify overall truncation of the penis in DES-treated specimens.
Figure 6.
Wholemount photos of penises of adult neonatally oil- and DES-treated CD-1 and C57BL/6 mice. Blue sutures have been passed from the bladder to the exterior to denote the urethral meatus. Comparing (A, B & D) note that the length of the MUMP is reduced by DES treatment (B) and additionally penile morphology is profoundly distorted (C) with DES effects more severe in C57BL/6 versus CD-1 mice. Note ventral tether.
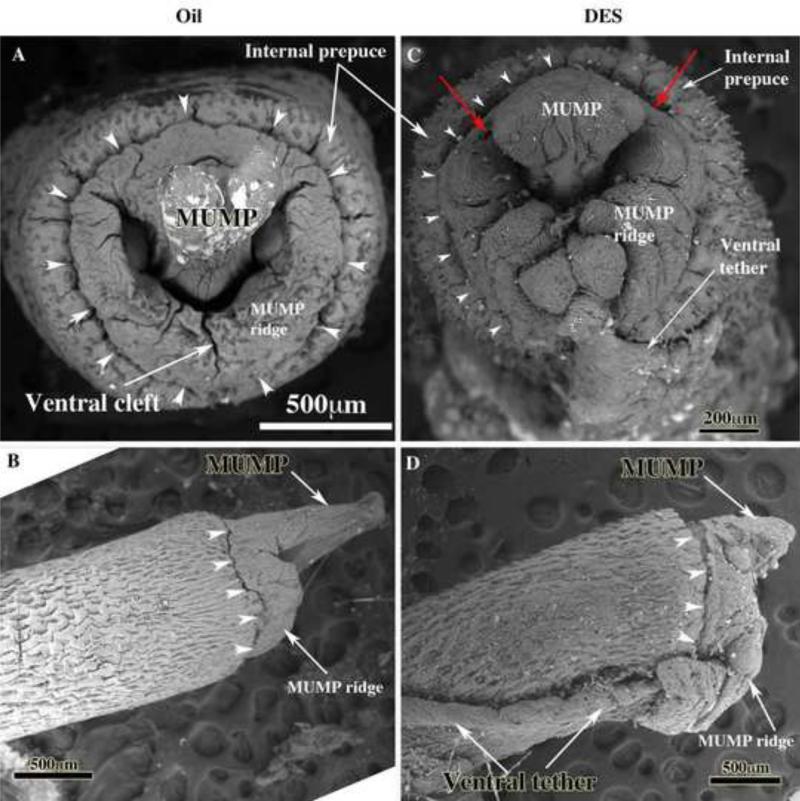
Figure 7.
Scanning electron micrographs (end-on [A & C) and lateral views [B & D]) of penises of adult neonatally oil- and DES-treated CD-1 mice. Normal penile morphology is depicted in (A & B) in which the Y-shaped urethral meatus (A) is formed by the fusion of the MUMP with the MUMP ridge that displays a ventral cleft and other less prominent clefts. Arrowheads indicate the MUMP ridge groove in all images. In adult neonatally DES-treated CD-1 mice the urethral meatus is severely distorted (C), the MUMP is truncated (D) and a ventral tether is present (C & D) that connects the ventral surface of the penis to the inner wall of the prepuce. Red arrows indicate dorsal clefts between the MUMP and MUMP ridge in the DES-treated specimen.
Figure 4.
Transverse sections of the penis of adult neonatally oil- and DES-treated CD-1 mice depicting the arrangement of internal structures. In both cases the sections are located near the distal tip of the os penis. Note the absence of urethral flaps in the neonatally DES-treated specimen. Erectile bodies are labeled MUMPCC= MUMP corpus cavernosa and CCG = corpus cavernosum glandis.
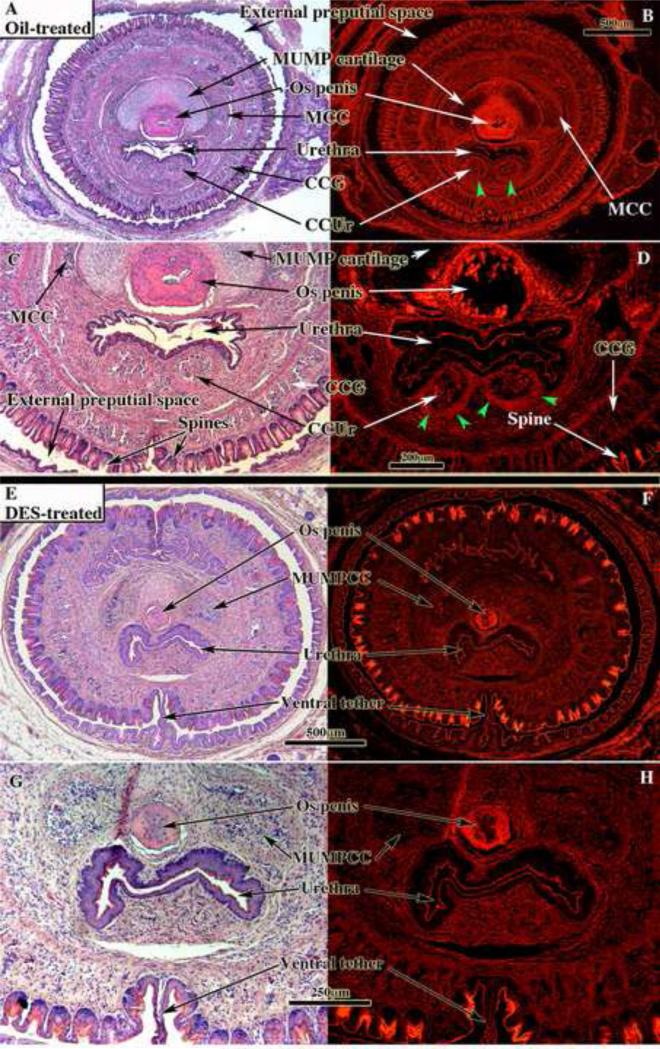
Figure 8.
Penile sections of oil-treated (A-D) and neonatally DES treated (E-H) adult mice stained with H&E photographed with standard white light (left images) or fluorescence (right images), which highlights the well-defined smooth muscle sheathes (green arrowheads in [B & D]) surrounding the corpus cavernosum urethrae (CCUr). Note that demarcation of the erectile bodies is distinct in the oil-treated specimens (A-D), but indistinct in the DES-treated specimens (E-H). Urethral flaps are not seen in the oil-treated (A-D) specimens, as the sections are proximal to the urethral flaps. MUMPCC = MUMP corpus cavernosa, CCG = corpus cavernosum glandis.
Figure 9.
Penile sections of oil-treated (A) and neonatally DES treated (B) adult mice stained with H&E. In both cases the sections are located in a comparable region distally where the urethral lumen is confluent with the preputial space. In the neonatally oil-treated specimen (A) note ventral cleft in the MUMP ridge, well-differentiated MUMP cartilage, and well-demarcated MUMP corpus cavernosa (MUMPCC). Note the smooth muscle “capsules” surrounding the MUMP corpora cavernosa. In the neonatally DES-treated specimen note the absence of the ventral cleft in the MUMP ridge, the ventral tether and bilateral dorsal clefts between the MUMP ridge and the MUMP, which contains poorly differentiation MUMP cartilage (*).
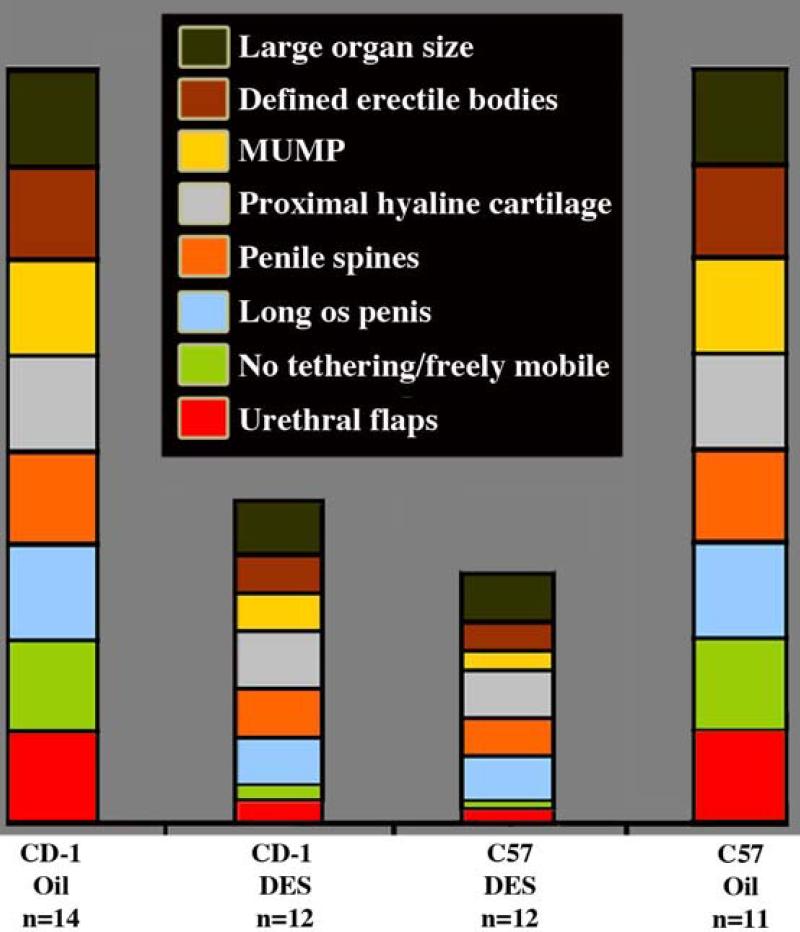
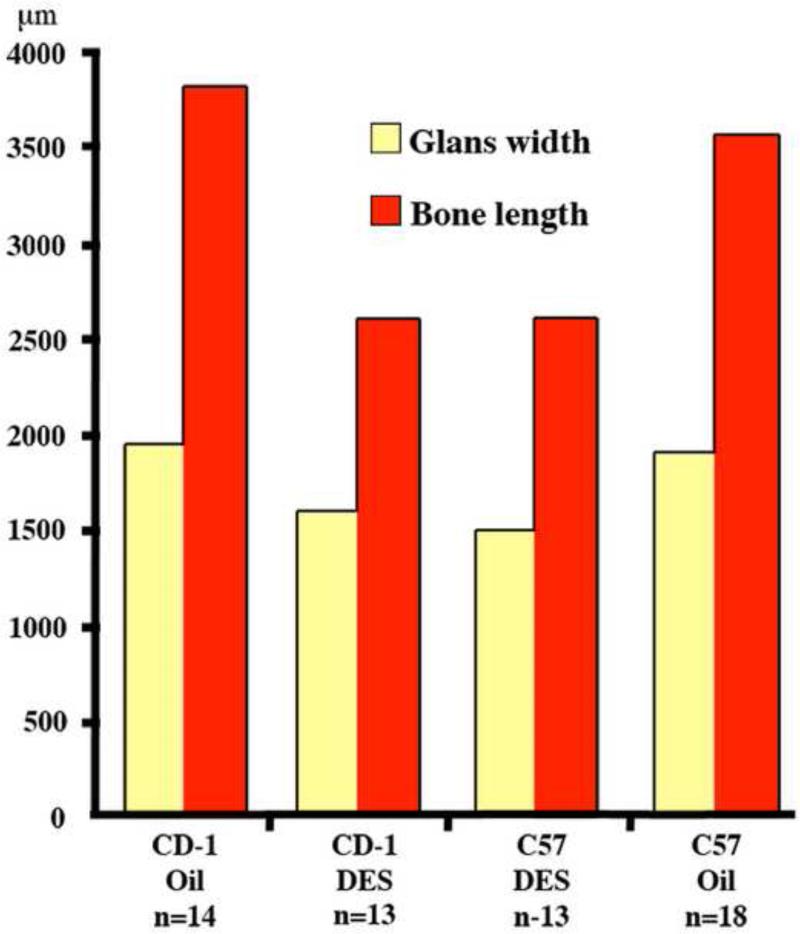
The effects of neonatal oil and DES treatment on male ExG are summarized in Table 1 for both CD-1 and C57Bl/6 mice. All neonatally oil-treated male mice (14 CD-1 and 18 C57BL/6) exhibited normal male ExG morphology. The external prepuce was clefted distally in oil-treated mice (Figs. 2A & 2D), and the tip of the glans penis (the tip of the MUMP) was located within the preputial space (Fig. 1E and see Figure 10 in accompanying paper). The distally bifid MUMP (Fig. 1F, 7A) is composed of fibrocartilage (Figs. 1C-1E, 3A & 3D), which dorsally overlaps the os penis (Figs. 1C, 4A) and extends distally to the tip of the penis (Figs. 1E, 3A, 3D, 13A1 & 13D1) (Rodriguez et al., 2011). The os penis consists of hyaline cartilage proximally (the growth plate) and bone distally (Rodriguez et al., 2011) (Figs. 1C, 3A & D & 4A). The average width of the glans penis did not differ statistically between the oil-treated CD-1 (n=3 for this measure) and oil-treated C57BL/6 (n=4 for this measure) mice (1942μm vs. 1899μm, p=0.164). Average length of the os penis of oil-treated CD-1 (n=3) and C57BL/6 (n=4) males also did not differ between strains (3807μm vs. 3559μm, p=0.245). The glans penis was untethered (Figs. 1C-1E, 3A, 3D, 4A, 8A, 8C, 13A1-A4 and 13D1-D4), freely mobile, and contained well-defined erectile bodies (corpus cavernosum glandis, MUMP corpora cavernosa and corpus cavernosum urethrae [Figs. 1C, 4A, 8A-8D & 9A]). The surface of the penis consisted of a stratified squamous cornified surface epithelium adorned with keratinized penile spines (Figs. 1C, 1F, 8C, 8D) as described previously (Blaschko et al., 2013; Rodriguez et al., 2011; Weiss et al., 2012; Yang et al., 2010). The urethra, with urethral flaps attached to its ventral wall, was situated ventral to the MUMP cartilage and os penis (Figs. 1C, 3, 4A, 8A-8D & 13). The normal “Y” shaped urethral meatus is formed by the MUMP fused to the MUMP ridge (Figs. 1G, 7A, 9A). All parameters were remarkably similar between both strains of oil-treated mice.
Table 1.
Effect of neonatal oil and DES treatment on penile development
| CD-1 | C57BL/6 | |||
|---|---|---|---|---|
| Treatment | Oil | DES | Oil | DES |
| Number of dams | 4 | 4 | 6 | 6 |
| Number of adult males | 26 | 28 | 47 | 23 |
| Malformations | ||||
| Abnormal urethral meatus | 0/14 (0%) | 11/13 (84.6%) | 0/18 (0%) | 13/13 (100%) |
| Ventral tethering | Absent 14/14 (100%) | Present 11/13 (85%) | Absent 18/18 (100%) | Present 13/13 (100%) |
| Hypoplastic prepuce | 0/10 (0%) | 7/10 (70%) | 0/10 (0%) | 10/10 (100%) |
| Urethral flaps | Present 14/14 (100%) | Absent 10/12 (83%) | Present 12/12 (100%) | Absent 11/11 (100%) |
| Impaired corpus cavernosum urethrae | 0/14 (0%) | 10/12 (83%) | 0/18 (0%) | 11/11 (100%) |
| Os penis length* | 3807 μn ± 100 (N=3) | 2587μm ± 885 (N=8) | 3559μm ± 157 (N=4) | 2594μm ± 144 (N=7) |
| Impaired MUMP cartilage differentiation | 0/14 (0%) | 10/12 (83%) | 0/12 (0%) | 11/11 (100%) |
| Preputial stones | 0/10 (0%) | 3/10 (30%) | 0/10 (0%) | 2/10 (20%) |
None of the os penis lengths of the DES-treated specimens overlapped with any of the lengths of the oil-treated specimens.
Figure 10.
Graphical representation of penile features of neonatally oil- and DES-treated adult CD-1 and C57BL/6 mice. Reduction in the size of the boxes in the DES-treated specimens means reduction in size or incidence.
The response of ExG to neonatal DES treatment was similar in CD-1 and C57BL/6 mice even though effects were consistently more severe in C57BL/6 mice (Table 1). The external prepuce of DES-treated males was markedly hypoplastic, a condition that was more severe in C57BL/6 versus CD-1 mice (Figs 2B & C). Indeed, in some neonatally DES-treated adult C57BL/6 mice the external prepuce was almost completely obliterated (Fig. 2C). Penises of neonatally DES-treated adult mice were also markedly reduced in size and exhibited an abnormal urethral meatus (Figs. 3, 7 & 9B). This generalized ExG truncation was observed developmentally in mice treated from birth to day 5 with DES (Fig. 5) as well as in neonatally DES-treated adult mice in so far as internal penile structures were seen at lower section numbers (Fig. 13). One of the obvious effects of neonatal DES treatment was truncation of the MUMP and impaired differentiation of the MUMP cartilage seen developmentally at day 5 as will as in adulthood (Figs. 3B, 3C, 5, 6B, 7D, & 10). Additionally, the external appearance of penises of many neonatally DES-treated mice were severely distorted (Figs. 6C & 7C), with effects consistently more severe in C57BL/6 versus CD-1 mice (Fig. 10). While neonatally oil-treated mice exhibited the characteristic ventral cleft in the MUMP ridge (Figs. 1F, 1G, 7A, 9A), in neonatally DES-treated mice the ventral cleft in the MUMP ridge was absent, and instead bilateral dorsal clefts were present between the MUMP and MUMP ridge (Figs. 7C, 9B, 13B2 & 13C2). In addition, penises of most C57BL/6 and CD-1 mice (Table 1) were ventrally tethered to the inner preputial wall by a frenulum-like structure that could be observed grossly (Figs, 6B-6C), as well as in SEMs (Figs, 7C-7D) and in histological sections (Figs. 3B-3C, 8E-8H, 9B, 13B2, 13B4, 13C2-13C4). While these profound distortions in penile morphology were evident in SEMs and in histological sections, they were more difficult to discern in wholemount photos.
Figure 5.
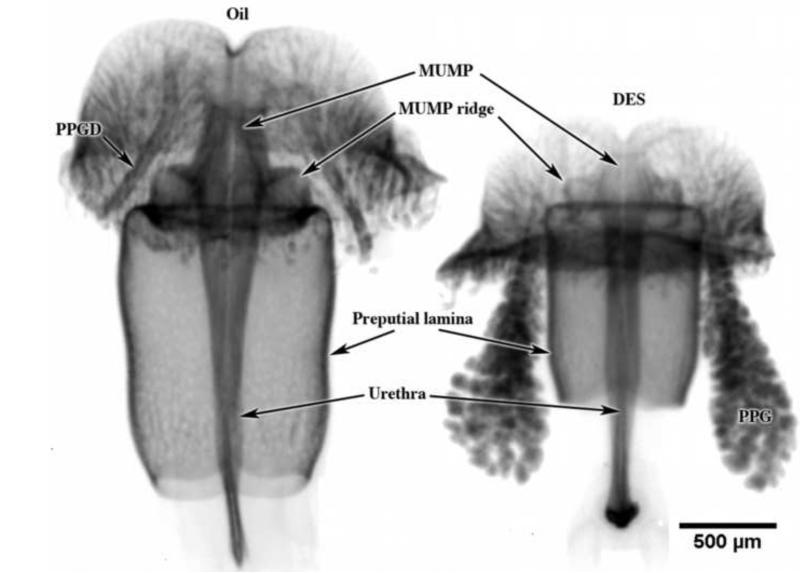
Optical projection tomography images stained with anti-E-cadherin of day 5 postnatal CD-1 ExG derived from male mice injected on days 1 and 3 with oil or DES. Note overall reduction in size of all structures, specifically reduction in overall length of the preputial lamina and truncation of distal structures destined to form the penile urethral meatus. The urethra is enclosed within the preputial lamina as indicated in Figure 14. PPG = preputial gland, PPGD = preputial gland duct.
Penises of neonatally DES-treated adult mice of both strains exhibited impaired morphogenesis/differentiation of erectile bodies, MUMP cartilage, proximal hyaline cartilage, and penile spines, as well as reductions in organ diameter and os penis length with effects more severe in C57BL/6 versus CD-1 mice (Table 1 and Fig. 10). Erectile bodies (MUMP corpus cavernosa, corpus cavernosum glandis and corpus cavernosa urethrae), normally well demarcated by a thin layer of smooth muscle in oil-treated mice (Figs. 1C-1D, 4A, 8B, 8D & 9A), were indistinct and poorly organized in neonatally DES-treated adult mice (Figs. 4B, 8F, & 8H). The MUMP cartilage of neonatally DES-treated adult mice was consistently smaller and poorly differentiated relative to oil-treated controls (Figs. 4, 9, 13B2). Average widths of the glans penis of neonatally DES-treated mice were reduced relative to the respective oil-treated controls (1599μm [DES] vs. 1942μm [Oil] for CD-1, p=0.027; 1490μm [DES] vs. 1899μm [Oil] for C57, p<0.001 for both comparisons) (Fig. 11). Likewise, the average length of the os penis of neonatally DES-treated CD-1 and C57BL/6 males was significantly shorter than those of the respective oil-treated males (2587μm [DES] vs. 3807μm [Oil] for CD-1, p=0.008; 2594μm [DES] vs. 3559μm [Oil] for C57BL/6, p<0.001 for both comparisons) (Fig. 11).
Figure 11.
Organ dimensions of the adult mouse penis of neonatally oil- or DES-treated CD-1 and C57BL/6 (C57) male mice assessed at age 60 days. The two strains did not differ from each other when comparing controls to controls or DES-treated animals to DES-treated animals. OS penis length was significantly reduced by DES treatment in both CD-1 and C57BL/6 mice (p<0.001 for both). See Result text for statistical analysis.
The high incidence of ventral tethering (Table 1) of penises in DES-treated C57BL/6 and CD-1 mice represents a defect in the ventral surface of the penis. To understand why this malformation developed, we examined normal penile development from birth to sexual maturity at 30 days postnatal and showed that the penile surface is not revealed until 30 days postnatal when the preputial lamina is fully canalized (Fig. 17, accompanying paper). Formation of the preputial lamina begins in the last few days of gestation as the genital tubercle is enveloped by the preputial folds (Perriton et al., 2002; Petiot et al., 2005; Baskin et al., 2004). At birth the genital tubercle (the protuberance in the perineum) is clefted ventrally where the preputial/urethral folds are approaching each other in the ventral midline (Fig 12A-D). More proximally this ventral preputial cleft closes (Fig. 12E) to form the preputial space distally and the tubular urethra proximally. In so doing the urethra is progressively incorporated within penile mesenchyme defined peripherally by the preputial lamina (Figs. 12E-12I). During the morphologic remodeling associated with the distal incorporation of the urethra into the substance of the glans penis, a ventral gap develops in the preputial lamina representing mesenchymal confluence between penile and preputial mesenchyme (Figs. 12G-12I). In untreated/oil-treated mice this midline gap in the preputial lamina is present from birth to day 24 (See Fig. 17C3 in the accompanying paper). By day 30 when delamination of preputial lamina is complete (See Fig. 17 in the accompanying paper), the ventral edges of the preputial lamina fuse across the midline, and thus a continuous 360-degree penile surface is constituted (Figs. 1C, 4A, 8A-8B & 13 and Figs. 17D3 & 17D4 in the accompanying paper). Persistence of stromal confluence between the penis and prepuce through this ventral gap in the preputial lamina results in the ventral tether seen in neonatally DES-treated mice (Figs. 4B, 7C-D, 8E-8H, 13B2, 13B4, 13C2-4). Figure 13 summarizes and compares the effects of neonatal DES in adult CD-1 and C57BL/6 mice demonstrating the following: (a) Normal MUMP cartilage differentiation in oil-treated specimens (Figs. 13A1, 13A2, 13D1, 13D2) versus impaired MUMP cartilage differentiation in neonatally DES-treated specimens (Figs. 13B1, 13C1 & 13C2). (b) Absence of a ventral tether in oil-treated specimens (Figs. 13A1-4 & 13D1-4) versus presence of a ventral tether in DES-treated specimens (Figs. 13B2, 13B4, & 13C2-4). (c) Open dorsal clefts between the MUMP and MUMP ridge in DES-treated specimens (Figs. 13B2 & 13C2) versus their absence in oil-treated specimens (Figs. 13A2 & 13D2). (d) A normal Y-shaped urethral meatus in oil-treated specimens (Figs. 13A2 & 13D2) versus an abnormal urethral meatus in DES-treated specimens (Figs. 13B2 & 13C2). Section numbers (starting at the distal tip of the MUMP) placed on each section of Figure 13 provide additional evidence of overall truncation of the penis as a result of neonatal DES treatment.
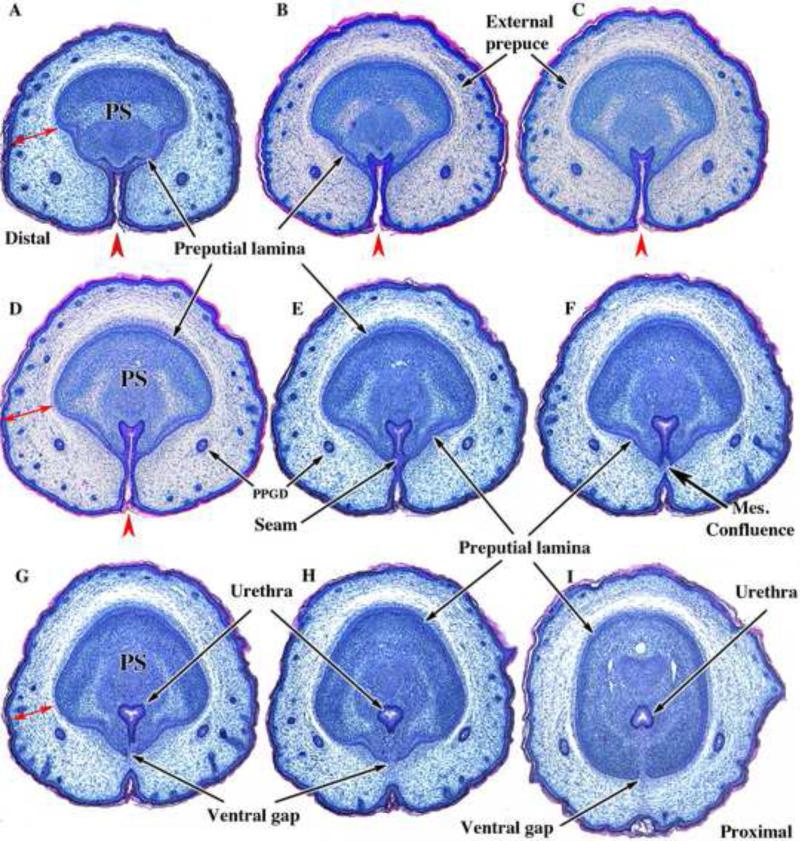
Figure 12.
Transverse sections of newborn male CD-1 external genitalia. Sections are arranged from distal (A) to proximal (I). In (A) note the thick wall of the prepuce (doubleheaded red arrows) containing the preputial gland ducts (PPGD). The developing penis at this distal location consists of dense penile stroma (PS) surrounded by epithelium of the preputial lamina. Note the open ventral cleft (red arrowheads) and the absence of a tubular urethra in (A-D). In (E), a slightly more proximal section, note that the ventral cleft has become subdivided into a tubular urethra dorsally via midline fusion of epithelial layers to create a midline epithelial seam. The tubular urethra thus formed now lies within penile stroma encompassed by the preputial lamina, but the epithelium of the tubular urethra is attached to the preputial lamina (E & F). In (F) note that the epithelial seam has disappeared with mesenchymal confluence across the midline. As the urethral epithelium becomes detached from the preputial lamina (G-I), a ventral gap appears in the preputial lamina (G-I). The mesenchyme-filled ventral gap in the preputial lamina represents confluence between penile stroma and preputial stroma.
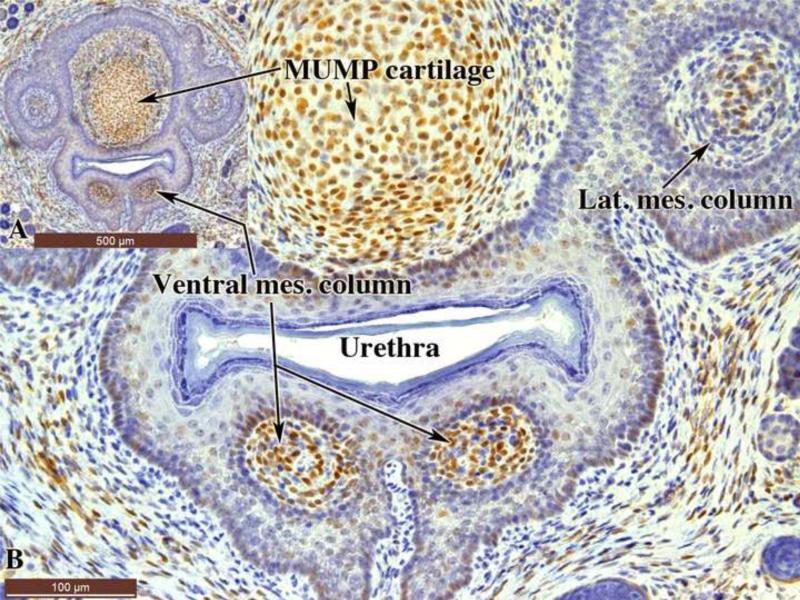
The urethral flaps and corpus cavernosum urethrae develop from the ventral mesenchymal columns, which during the neonatal period are surrounded by epithelium (Fig. 14)(Schlomer et al., 2013). Since neonatal DES treatment elicited malformations of the urethral flaps and corpus cavernosum urethrae (Table 1 and figure 4), we examined the expression of ERα in the ventral mesenchymal columns of 10-day-old mice. Intense ERα staining was detected within the ventral mesenchymal columns with less intense ERα staining in the epithelium surrounding the ventral mesenchymal columns (Fig. 14). ERα was also detected in the MUMP cartilage and in the lateral mesenchymal columns destined to form the MUMP corpora cavernosa.
Figure 14.
Sections of 10-day-old untreated mouse penis stained with anti-ERα. (A) is a low power over view indicating that the section is take through the ventral mesenchymal columns destined to form the corpus cavernosum urethrae and the urethral flaps, whose development is adversely affected by neonatal DES. Note ERα staining in the mesenchyme of the ventral and lateral mesenchymal columns and the MUMP cartilage. Much lower levels of ERα staining are seen in the surrounding epithelium.
DISCUSSION
The most profound effect on ExG development in neonatally DES treated mice was severe truncation of both the prepuce and penis, an effect which was more severe in C57BL/6 mice presumably due to increased estrogen sensitivity of C57BL/6 mice (relative to CD-1 mice) (Spearow et al., 1999). Truncation of the ExG, and especially that of the MUMP, was observed at day 5 following DES injections on days 1 and 3, but may be evident even earlier. Examination of neonatally DES-treated mice at day 5 (Fig. 5) demonstrates a reduction in overall ExG length estimated at ~40%. These developmental growth retardations seen at day 5 persisted into adulthood as overall reduction in penile length (Fig. 3) and it constituent parts. While DNA synthesis was not examined in the current paper, DES-induced inhibition of DNA synthesis is a possible explanation for the marked truncation in ExG length. Several possible mediators of growth of the developing external genitalia have been reported in prenatal mice (Haraguchi et al., 2001; Miyagawa et al., 2009; Ogino et al., 2001; Petiot et al., 2005; Seifert et al., 2009). Whether these reports are relevant to postnatal development of the external genitalia remains to be determined. Alternately, enhanced apoptosis may be at play. However, given that apoptotic bodies were not evident in histologic sections, we favor the former possibility.
While reduction in overall ExG length was evident developmentally (Fig. 5) and in adulthood (Fig. 3), it is evident that the various elements (MUMP, MUMP ridge, erectile bodies, etc.) forming the penis exhibit differences in the degree of severity of DES effects. In this regard, the developing MUMP clearly exhibited a profound inhibition in size (Figs. 3 & 5), which was also associated with impaired cartilage differentiation (as judged by H&E histology) (Figs. 4 & 9). Presumably, examination of molecular markers of cartilage differentiation would reveal corresponding perturbations. The profound truncation of the MUMP corresponds spatially with the expression of ERα (Fig. 15) and ERβ in the developing MUMP (Blaschko et al., 2013; Rodriguez et al., 2012). In earlier publications (Cunha et al., 2014; Mahawong et al., 2014), we have raised the possibility that certain effects of prenatal exposure to estrogen are reversible, that is, estrogen-induced “embryonic hypospadias” seen at the end of gestation as an open preputial/urethral groove (Kim et al., 2004) can resolve to normality by adulthood (Mahawong et al., 2014). Clearly, the profound truncation of the ExG seen at day 5 after DES injections on days 1 and 3 represents an irreversible defect that persists into adulthood as substantial truncation of both the penis and prepuce.
Since the various precursor elements within the developing penis (MUMP, MUMP ridge, erectile body precursors, urethral meatus) respond differentially to neonatal estrogen treatment, the result of neonatal DES treatment is an overall distortion of penile patterning, which is particularly evident in regard to the structures forming the urethral meatus, namely the MUMP and the MUMP ridge. The mouse urethral meatus in untreated or neonatally oil-treated mice exhibits a consistent reproducible pattern consisting of the MUMP dorsally and the ventral-lateral MUMP ridge (partially bisected by the ventral cleft). The MUMP ridge also has a characteristic pattern of shallow clefts (Blaschko et al., 2013; Rodriguez et al., 2011). We have interpreted this pattern of clefts as reflecting a series of fusion events that form the MUMP ridge and join the MUMP with the MUMP ridge to constitute the urethral meatus. Neonatal (this report) as well as prenatal exposure to DES (Mahawong et al., 2014) elicit profound distortions in the pattern of the urethral meatus. Neonatal DES treatment typically eliminates the ventral cleft in the MUMP ridge, truncates the MUMP, and profoundly distorts the pattern of processes and clefts within the MUMP ridge resulting in deep bilateral dorsal clefts between the MUMP and MUMP ridge. Taken together these malformations constitute mouse hypospadias.
Hypospadias is fundamentally a perturbation in penile morphologic patterning that affects location of the urethral meatus, which in humans can be present in the perineum, at mid-shaft or distally within the glans (Baskin and Ebbers, 2006). In humans, hypospadias involves a ventrally defective prepuce, abnormal positioning and morphology of the urethral meatus with an associated defect in the corpus spongiosum (which normally forms the ventral stromal wall of the penile urethra) as well as chordee, an abnormal curvature of the penis. It is notable that as a result of neonatal treatment of mice with DES, the urethral meatus is profoundly distorted, the prepuce is hypoplastic and defective ventrally, the corpus cavernosum urethrae (the homologue of the corpus spongiosum) is abnormal, ventral penile skin is abnormal with retention of a ventral tether, and ventral urethral epithelium is abnormal (absence of the urethral flaps). Thus, these abnormalities observed in adult mice treated neonatally (as well as prenatally) with DES exhibit remarkable similarity with the homologous elements of human hypospadias and thus meet the definition of hypospadias.
Penile urethral development in the mouse appears to occur via two separate mechanisms. Prenatally, penile urethral development within the shaft of the penis appears to occur via canalization of the urethral plate (Hynes and Fraher, 2004a; b; Seifert et al., 2008). However, the distal aspect of the penile urethra, and especially the urethral meatus, forms postnatally and appears to develop as a result of fusion of the elements constituting the urethral meatus (Blaschko et al., 2013; Kim et al., 2004; Rodriguez et al., 2012; Schlomer et al., 2013). Accordingly, the form of hypospadias reported herein in mice treated postnatally with DES involves the distal most portion of the urethra, which coincides with the most common form of human hypospadias (Baskin and Ebbers, 2006).
Full comprehension of developmentally induced adult malformations requires detailed analysis of the adult malformations as well as developmental perturbations observed in the first few days after initiation of DES treatment. As mentioned above, overall reduction in adult penile size in neonatally DES-treated mice is associated with profound ExG size reductions seen at day 5, and thus will allow further analysis of the molecular mechanism(s) of this developmental growth inhibition. Another striking endpoint of neonatal DES treatment is ventral tethering of the penis to the ventral wall of the prepuce. This unusual malformation, seen in adult neonatally DES-treated but not prenatally DES-treated mice, is clearly understandable in the context of normal development of the preputial lamina. From birth to sexual maturity at day 30 the preputial lamina has a ventral gap, which represents ventral mesenchymal/stromal confluence between the penis and prepuce. Formation of the ventral gap in the preputial lamina occurs at birth during the process of incorporation of the urethra into the substance of the glans penis (Fig. 12). The presence of this ventral gap in the preputial lamina is maintained following neonatal DES treatment as ventral tethering of the penis. The corresponding absence of ventral tethering in prenatally DES-treated mice is presumably due to the fact that formation of the preputial lamina is not advanced enough to be perturbed in a similar way by prenatal DES treatment. Clearly there is a “window of sensitivity” for this malformation that corresponds with postnatal (and not prenatal) developmental events. During the course of normal postnatal development of the ExG (untreated or oil-treated), this stromal confluence between the penis and prepuce disappears as the two sides of the preputial lamina fuse in the ventral midline at 24 to 30 days postnatal, thus forming a complete 360-degree penile surface. Failure of this midline fusion results in retention of a frenulum-like ventral tether, thus restricting penile extrusion during urination and mating. We speculate that this restriction in penile mobility may result in urination into the preputial space leading to urine stasis within the preputial space and formation of preputial stones that in time can become quite large with very adverse consequences (Warner et al., 1979).
In the accompanying paper strain differences in response to prenatal DES were noted both in terms of incidence and degree of severity with C57BL/6 mice being substantially more sensitive to DES. In the current study employing neonatal DES treatment, the incidence of various malformations was similar in both CD-1 and C57BL/6 mice even though in all instances the severity of malformations was higher in C57BL/6 mice. The clear difference in outcome in the two strains in response to prenatal versus neonatal DES-treatment may be attributed to strain differences in “windows of sensitivity” or differences in the ontogeny of estrogen receptors. Strain differences in the ontogeny of estrogen receptors have been reported previously. Uterine epithelial ERα is expressed at days 3 to 4 postnatal in CD-1 mice and at days 6 to 7 postnatal in Balb/c mice (Bigsby et al., 1990). Thus, direct effects of estrogens on uterine epithelium occur at different times in these two mouse strains. A similar strain difference in the ontogeny of epithelial or mesenchymal ERα may be at play in developing mouse ExG.
The teratogenic effects of neonatal DES treatment provide clues to the normal developmental processes involved in the morphogenesis of the urethral meatus, which we suggest to be due to multiple fusion events. We have noted 3 pairs of bilateral mesenchymal columns (dorsal, lateral and ventral) initially surrounded by epithelium in the glans penis of the mouse (Schlomer et al., 2013). In the course of development these 3 pairs of mesenchymal columns fuse in a characteristic fashion. The distally located dorsal mesenchymal columns fuse with each other to form the MUMP. This fusion is incomplete resulting in the characteristic bifid tip of the adult MUMP. The lateral and ventral mesenchymal columns are incorporated into the penis to form erectile bodies. The lateral mesenchymal columns become associated with the MUMP to form the MUMP corpus cavernosa. The ventral mesenchymal columns fuse with each other ventral to the urethra to form the corpus cavernosum urethrae, with the distal portions of the ventral mesenchymal columns remaining as projections into the urethral lumen forming the urethral flaps. Perturbation in development of the ventral mesenchymal columns by neonatal DES can be attributed to the presence of ERα in the cells of the ventral mesenchymal columns and/or the low level of ERα in the surrounding epithelial cells (Fig. 15). An associated defect in the corpus cavernosum urethrae involves the absence of the smooth muscle capsules demarcating this erectile body. Exogenous estrogen administrated during ExG development is known to impair smooth muscle cell differentiation (Okumu et al., 2012). Impaired development/differentiation of all of the ExG elements (Fig. 10) elicited by prenatal or neonatal DES is presumably mediated via ERα and/or ERβ, which are expressed globally throughout the developing mouse ExG (Blaschko et al., 2013; Rodriguez et al., 2012).
Development of the human as well as the mouse ExG involves multiple fusion events. In both species the bilateral halves of the prepuce fuse in the ventral midline. Another example involves the ventral gap in the preputial lamina (Fig. 14) and its subsequent fusion at 24 to 30 days postnatal to complete the ventral epithelial surface of the mouse penis. Truncation and distortion of the MUMP in both pre- and neonatally DES treated mice can be attributed to perturbation in fusion of the bilateral dorsal mesenchymal columns that form the MUMP (Schlomer et al., 2013). Based upon the presence of the normal ventral cleft in the MUMP ridge as well as a characteristic pattern of processes and clefts in the MUMP ridge, we have suggested that the urethral meatus forms as a consequence of fusion of the various elements that constitute the urethral meatus, namely the elements of the MUMP and MUMP ridge. The pattern of processes and clefts in the urethral meatus are profoundly distorted in neonatally as well as prenatally DES-treated mice. Most notable in neonatally DES-treated mice are the deep dorsal clefts between the MUMP and the MUMP ridge, which we interpret as failure of fusion of the MUMP with the MUMP ridge. Thus, multiple fusion events appear to be involved in development of the urethral meatus, which are profoundly impaired/distorted as a result of perinatal DES treatment. In this sense, development of the urethral meatus in mice may share many developmental features with development of the palate and face, which occurs as a result of fusion of bilateral elements (Bush and Jiang, 2012; Som and Naidich, 2013; 2014; Som et al., 2014).
Different adverse outcomes were observed in mice treated prenatally (E12 to E18) versus neonatally (P1 to P9) with DES. These differences (Table 2) presumably are due to differences in the state of ExG development during these periods. Prenatally, the main developmental event is formation of the ambisexual ExG with minimal sex differentiation taking place. Postnatally, sex differentiation of the ExG occurs with morphogenesis of distinct male and female forms (Rodriguez et al., 2012). Of course, for mice treated prenatally with DES, it is likely that residual DES is still present after delivery, and thus DES injected into the pregnant dams is surely present and acting for a short period postnatally as well. In this respect, while adverse effects of DES differed somewhat following pre- versus neonatal treatment, a consistent similarity for both pre- and neonatal DES-treated groups was profound distortion of the urethral meatus and the elements (MUMP and MUMP ridge) constituting the urethral meatus with effects being more severe in the neonatally DES-treated group. Comparing ExG from pre- versus neonatally DES treated mice, we noted respectively subtle preputial malformations versus severe preputial hypoplasia, exposed urethral flaps versus absence of urethral flaps, an elongated ventral cleft in the MUMP ridge versus absence of the ventral cleft, MUMP cartilage present versus absent/defective, absence versus presence of ventral tethering in the prenatal versus neonatal DES-treatment groups. These differences in response to pre-versus neonatal DES implies differences in “windows of sensitivity” for each element. For example, the mesenchymal condensation (the precursor of the MUMP cartilage and os penis) is readily apparent after birth with the chondrogenic and osteogenic process occurring postnatally (Murakami, 1987; Rodriguez et al., 2012). In contrast, the os penis precursor (mesenchymal condensation) is poorly organized prenatally (Baskin et al., 2001a). Given that development and differentiation of the MUMP cartilage and os penis are postnatal events, it is reasonable that the effect of DES on these elements is more severe in neonatally versus prenatally treated mice. Likewise, the abnormal ventral tethering seen exclusively in the neonatally DES-treated group correlates with postnatal development of the ventral gap in the preputial lamina as discussed above. The corpus cavernosum urethrae and urethral flaps develop from ventral mesenchymal columns that appear on days 0 to 1 postnatal (Schlomer et al., 2013). Accordingly, urethral flaps were normally retained in prenatally DES-treated mice, and completely obliterated in neonatally DES-treated mice, with more profound disruption of the corpus cavernosum urethrae in neonatally versus prenatally DES-treated mice. Finally, for each malformation induced by DES the incidence and severity of malformations was consistently higher for C57BL/6 versus CD-1 mice. Prenatal DES disruption of formation of the ambisexual pattern has profound global consequences, whereas neonatal DES treatment individually targets and disrupts specific developmental events such as development/differentiation of the MUMP cartilage and os penis as well as morphogenesis of erectile bodies. Thus, we attribute the differences in DES induction of ExG malformations as due to differences in development status and differences in “windows of sensitivity” for individual development processes. Alternatively, differential effects of pre- versus neonatal DES treatment could be due to the ontogeny of estrogen receptor expression, which has not been adequately examined to date.
Table 2.
Comparison of the effects of prenatal versus neonatal DES treatment
| Prenatal DES treatment | Neonatal DES treatment |
|---|---|
| Subtle preputial malformations | Severe preputial hypoplasia |
| Preputial stones = absent | Preputial stones = absent |
| “Exposed” urethral flaps | Urethral flaps = absent |
| “Exposed” os penis | N/A |
| MUMP = mild truncation | MUMP = severe truncation |
| MUMP ridge -Ventral cleft elongated |
MUMP ridge -Ventral cleft = absent -Abnormal dorsal clefts |
| MUMP cartilage differentiation = normal | MUMP cartilage differentiation = abnormal |
| Os penis = normal | Os penis = truncated |
| No ventral tethering | Ventral tethering |
| Abnormal urethral meatus | Abnormal urethral meatus |
CONCLUSIONS
Short-term neonatal exposure to exogenous estrogens during development can exert adverse influences that persist into adulthood, altering morphologic patterning and cell differentiation in male ExG in mice. In this study, we compared DES-induced ExG outcomes in two mouse strains (CD-1 and C57BL/6) differing in their sensitivity to estrogen. Compared to oil-treated controls, neonatal DES elicited a plethora of ExG abnormalities mice in both strains of mice that included the following: (a) severe truncation of the prepuce and glans penis, (b) an abnormal urethral meatus, (c) ventral tethering of the penis, (d) reduced os penis length and glans width, (e) impaired differentiation of the MUMP cartilage, (f) absence of urethral flaps, and (g) impaired differentiation of erectile bodies. While the effects were similar in the more estrogen-sensitive C57BL/6 mice versus the less estrogen-sensitive CD-1 mice, the severity of DES effects was consistently more severe in C57BL/6 mice. We suggest that many of the effects of DES, including the induction of hypospadias, are due to impaired fusion events during development.
HIGHLIGHTS.
Prenatal DES treatment of C57BL/6 and CD-1 mice elicited a broad spectrum of penile and preputial malformations that were consistently more severed in C57BL/6 mice having enhanced estrogen sensitivity.
Adverse effects of DES correlated with the expression of estrogen receptors within the affected tissues.
The developmental basis of several adult DES-induced malformations was presented.
Neonatally DES-induced penile and preputial hypospadias are suggested to be due to impaired growth and tissue fusion events during development.
ACKNOWLEDGMENTS
This work was supported by NSF Grant IOS-0920793 and NIH grant RO1 DK0581050.
ABBREVIATIONS
- ExG
external genitalia
- DES
diethylstilbestrol
- MUMP
male urogenital mating protuberance
- SEM
scanning electron micrograph
- PFA
paraformaldehyde
- DMSO
dimethyl sulfoxide
- ERα
estrogen receptor alpha
- ERβ
estrogen receptor beta
- gbw
gram body weight
- OPT
optical projection tomography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baskin LS. Hypospadias and urethral development. J Urol. 2000;163:951–956. [PubMed] [Google Scholar]
- Baskin LS, Ebbers MB. Hypospadias: anatomy, etiology, and technique. J Pediatr Surg. 2006;41:463–472. doi: 10.1016/j.jpedsurg.2005.11.059. [DOI] [PubMed] [Google Scholar]
- Baskin LS, Erol A, Jegatheesan P, Li Y, Liu W, Cunha GR. Urethral seam formation and hypospadias. Cell Tissue Res. 2001a;305:379–387. doi: 10.1007/s004410000345. [DOI] [PubMed] [Google Scholar]
- Baskin LS, Himes K, Colborn T. Hypospadias and endocrine disruption: is there a connection? Environ Health Perspect. 2001b;109:1175–1183. doi: 10.1289/ehp.011091175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin LS, Liu W, Bastacky J, Yucel S. Anatomical Studies of the Mouse Genital Tubercle. In: Baskin LS, editor. Hypospadias and Genital Development. Plenum Publisher; NewYork: 2004. pp. 103–120. [DOI] [PubMed] [Google Scholar]
- Bern HA, Talamantes FJ. Neonatal mouse models and their relation to disease in the humal female. In: Herbst A, Bern HA, editors. Developmental Effects of Diethylstilbestrol (DES) in Pregnancy. Thieme Stratton Inc.; New York: 1981. pp. 129–147. [Google Scholar]
- Bigsby RM, Li AX, Luo K, Cunha GR. Strain differences in the ontogeny of estrogen receptors in murine uterine epithelium. Endocrinology. 1990;126:2592–2596. doi: 10.1210/endo-126-5-2592. [DOI] [PubMed] [Google Scholar]
- Blaschko SD, Mahawong P, Ferretti M, Cunha TJ, Sinclair A, Wang H, Schlomer BJ, Risbridger G, Baskin LS, Cunha GR. Analysis of the effect of estrogen/androgen perturbation on penile development in transgenic and diethylstilbestrol-treated mice. Anat Rec (Hoboken) 2013;296:1127–1141. doi: 10.1002/ar.22708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush JO, Jiang R. Palatogenesis: morphogenetic and molecular mechanisms of secondary palate development. Development. 2012;139:231–243. doi: 10.1242/dev.067082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Sinclair A, Risbridger G, Hutson J, Baskin LS. Current Understanding of Hypospadias, Relevance of Mouse Models and Future Preventive Efforts. Nature Medicine. 2014 doi: 10.1038/nrurol.2015.57. submitted. [DOI] [PubMed] [Google Scholar]
- Gardner WU, Argyris BF. Differences in vaginal sensitivity of mice of inbred strain; localization in the vagina. Endocrinology. 1957;60:532–546. doi: 10.1210/endo-60-4-532. [DOI] [PubMed] [Google Scholar]
- Goyal HO, Braden TD, Williams CS, Dalvi P, Mansour M, Williams JW. Estrogen-induced abnormal accumulation of fat cells in the rat penis and associated loss of fertility depends upon estrogen exposure during critical period of penile development. Toxicol Sci. 2005;87:242–254. doi: 10.1093/toxsci/kfi233. [DOI] [PubMed] [Google Scholar]
- Goyal HO, Braden TD, Williams CS, Williams JW. Role of estrogen in induction of penile dysmorphogenesis: a review. Reproduction. 2007;134:199–208. doi: 10.1530/REP-07-0207. [DOI] [PubMed] [Google Scholar]
- Haraguchi R, Mo R, Hui C, Motoyama J, Makino S, Shiroishi T, Gaffield W, Yamada G. Unique functions of Sonic hedgehog signaling during external genitalia development. Development. 2001;128:4241–4250. doi: 10.1242/dev.128.21.4241. [DOI] [PubMed] [Google Scholar]
- Hynes PJ, Fraher JP. The development of the male genitourinary system: II. The origin and formation of the urethral plate. Br J Plast Surg. 2004a;57:112–121. doi: 10.1016/j.bjps.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Hynes PJ, Fraher JP. The development of the male genitourinary system: III. The formation of the spongiose and glandar urethra. Br J Plast Surg. 2004b;57:203–214. doi: 10.1016/j.bjps.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Jirasek JE. Genital ducts and external genitalia: development and anomalies. Birth Defects Orig Artic Ser. 1971;7:131–139. [PubMed] [Google Scholar]
- Kim KS, Torres CR, Jr., Yucel S, Raimondo K, Cunha GR, Baskin LS. Induction of hypospadias in a murine model by maternal exposure to synthetic estrogens. Environ Res. 2004;94:267–275. doi: 10.1016/S0013-9351(03)00085-9. [DOI] [PubMed] [Google Scholar]
- Ma L. Endocrine disruptors in female reproductive tract development and carcinogenesis. Trends Endocrinol Metab. 2009;20:357–363. doi: 10.1016/j.tem.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahawong P, Sinclair A, Li Y, Schlomer B, Rodriguez E, Ferretti M, Liu B, Baskin LS, Cunha GR. Prenatal diethylstilbestrol induces malformation of the external genitalia of male and female mice and persistent second-generation developmental abnormalities of the external genitalia in two mouse strains. Differentiation. 2014 doi: 10.1016/j.diff.2014.09.005. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan JA, Newbold RR, Bullock B. Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Res. 1980;40:3988–3999. [PubMed] [Google Scholar]
- McLachlan JA, Newbold RR, Bullock BC. Reproductive tract lesions in male mice exposed prenatally to diethylstilbestrol. Science. 1975;190:991–992. doi: 10.1126/science.242076. [DOI] [PubMed] [Google Scholar]
- Miyagawa S, Satoh Y, Haraguchi R, Suzuki K, Iguchi T, Taketo MM, Nakagata N, Matsumoto T, Takeyama K, Kato S, Yamada G. Genetic interactions of the androgen and Wnt/beta-catenin pathways for the masculinization of external genitalia. Mol Endocrinol. 2009;23:871–880. doi: 10.1210/me.2008-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Nagasawa H, Bern HA. Long-term effects of perinatal exposure to hormones on normal and neoplastic mammary growth in rodents: a review. Journal of environmental pathology and toxicology. 1979;3:191–205. [PubMed] [Google Scholar]
- Murakami R. A histological study of the development of the penis of wild-type and androgen-insensitive mice. J Anat. 1987;153:223–231. [PMC free article] [PubMed] [Google Scholar]
- Ogino Y, Suzuki K, Haraguchi R, Satoh Y, Dolle P, Yamada G. External genitalia formation: role of fibroblast growth factor, retinoic acid signaling, and distal urethral epithelium. Ann N Y Acad Sci. 2001;948:13–31. [PubMed] [Google Scholar]
- Okumu LA, Bruinton S, Braden TD, Simon L, Goyal HO. Estrogen-induced maldevelopment of the penis involves down-regulation of myosin heavy chain 11 (MYH11) expression, a biomarker for smooth muscle cell differentiation. Biol Reprod. 2012;87:109. doi: 10.1095/biolreprod.112.103556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perriton CL, Powles N, Chiang C, Maconochie MK, Cohn MJ. Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev Biol. 2002;247:26–46. doi: 10.1006/dbio.2002.0668. [DOI] [PubMed] [Google Scholar]
- Petiot A, Perriton CL, Dickson C, Cohn MJ. Development of the mammalian urethra is controlled by Fgfr2-IIIb. Development. 2005;132:2441–2450. doi: 10.1242/dev.01778. [DOI] [PubMed] [Google Scholar]
- Rodriguez E, Jr., Weiss DA, Ferretti M, Wang H, Menshenia J, Risbridger G, Handelsman D, Cunha G, Baskin L. Specific morphogenetic events in mouse external genitalia sex differentiation are responsive/dependent upon androgens and/or estrogens. Differentiation. 2012;84:269–279. doi: 10.1016/j.diff.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez E, Jr., Weiss DA, Yang JH, Menshenina J, Ferretti M, Cunha TJ, Barcellos D, Chan LY, Risbridger G, Cunha GR, Baskin LS. New insights on the morphology of adult mouse penis. Biol Reprod. 2011;85:1216–1221. doi: 10.1095/biolreprod.111.091504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlomer BJ, Feretti M, Rodriguez E, Jr., Blaschko S, Cunha G, Baskin L. Sexual differentiation in the male and female mouse from days 0 to 21: a detailed and novel morphometric description. J Urol. 2013;190:1610–1617. doi: 10.1016/j.juro.2013.02.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Harfe BD, Cohn MJ. Cell lineage analysis demonstrates an endodermal origin of the distal urethra and perineum. Dev Biol. 2008;318:143–152. doi: 10.1016/j.ydbio.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Yamaguchi T, Cohn MJ. Functional and phylogenetic analysis shows that Fgf8 is a marker of genital induction in mammals but is not required for external genital development. Development. 2009;136:2643–2651. doi: 10.1242/dev.036830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Som PM, Naidich TP. Illustrated review of the embryology and development of the facial region, part 1: Early face and lateral nasal cavities. AJNR. American journal of neuroradiology. 2013;34:2233–2240. doi: 10.3174/ajnr.A3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Som PM, Naidich TP. Illustrated review of the embryology and development of the facial region, part 2: Late development of the fetal face and changes in the face from the newborn to adulthood. AJNR. American journal of neuroradiology. 2014;35:10–18. doi: 10.3174/ajnr.A3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Som PM, Streit A, Naidich TP. Illustrated review of the embryology and development of the facial region, part 3: an overview of the molecular interactions responsible for facial development. AJNR. American journal of neuroradiology. 2014;35:223–229. doi: 10.3174/ajnr.A3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearow JL, Barkley M. Genetic control of hormone-induced ovulation rate in mice. Biol Reprod. 1999;61:851–856. doi: 10.1095/biolreprod61.4.851. [DOI] [PubMed] [Google Scholar]
- Spearow JL, Doemeny P, Sera R, Leffler R, Barkley M. Genetic variation in susceptibility to endocrine disruption by estrogen in mice. Science. 1999;285:1259–1261. doi: 10.1126/science.285.5431.1259. [DOI] [PubMed] [Google Scholar]
- Spearow JL, O'Henley P, Doemeny P, Sera R, Leffler R, Sofos T, Barkley M. Genetic variation in physiological sensitivity to estrogen in mice. Apmis. 2001;109:356–364. doi: 10.1034/j.1600-0463.2001.090504.x. [DOI] [PubMed] [Google Scholar]
- Vorherr H, Messer RH, Vorherr UF, Jordan SW, Kornfeld M. Teratogenesis and carcinogenesis in rat offspring after transplacental and transmammary exposure to diethylstilbestrol. Biochem Pharmacol. 1979;28:1865–1877. doi: 10.1016/0006-2952(79)90638-5. [DOI] [PubMed] [Google Scholar]
- Warner MR, Warner RL, Clinton CW. Reproductive tract calculi, their induction, age incidence, composition and biological effects in Balb/c crgl mice injected as newborns with estradiol-17β. Biol. Reprod. 1979;20:310–322. doi: 10.1095/biolreprod20.2.310. [DOI] [PubMed] [Google Scholar]
- Weiss DA, Rodriguez E, Jr., Cunha T, Menshenina J, Barcellos D, Chan LY, Risbridger G, Baskin L, Cunha G. Morphology of the external genitalia of the adult male and female mice as an endpoint of sex differentiation. Mol Cell Endocrinol. 2012;354:94–102. doi: 10.1016/j.mce.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada G, Satoh Y, Baskin LS, Cunha GR. Cellular and molecular mechanisms of development of the external genitalia. Differentiation. 2003;71:445–460. doi: 10.1046/j.1432-0436.2003.7108001.x. [DOI] [PubMed] [Google Scholar]
- Yang JH, Menshenina J, Cunha GR, Place N, Baskin LS. Morphology of mouse external genitalia: implications for a role of estrogen in sexual dimorphism of the mouse genital tubercle. J Urol. 2010;184:1604–1609. doi: 10.1016/j.juro.2010.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]