Abstract
We discuss new trends in Layer-by-Layer (LbL) encapsulation of spherical and tubular cores of 50–150 nm diameter and loaded with drugs. This core size decrease (from few micrometers to a hundred of nanometers) for LbL encapsulation required development of sonication assistant non-washing technique and shell PEGylation to reach high colloidal stability of drug nanocarriers at 2–3 mg/mL concentration in isotonic buffers and serum. For 120–170 nm spherical LbL nanocapsules of low soluble anticancer drugs, polyelectrolyte shell thickness controls drug dissolution. As for nanotube carriers, we concentrated on natural halloysite clay nanotubes as cores for LbL encapsulation that allows high drug loading and sustains its release over tens and hundreds hours. Further drug release prolongation was reached with formation of the tube-end stoppers.
Polyelectrolyte coated drug nanocarriers
Spherical nanocarriers, especially emulsion and polymer based systems, due to low toxicity of used materials and easiness of preparation are under intensive investigation for encasing therapeutic compounds. A wide range of drugs and reporting molecules have been incorporated into the nanovehicles that release the drugs in a prolonged or stimuli-responsive manner. Several nanocrystalline drug formulations have also been approved for medical usage or are in clinical trials [1,2].
A state of art in the field is layer-by-layer (LbL) coated nanocarriers since this approach allows for combining drug-loaded core matrix with a shell of variable architecture that can consist of polyelectrolytes, proteins, nanoparticles, and selected molecules of low molecular weight. Based on electrostatic interaction between adsorbing layers, LbL shell preparation does not require harsh conditions to interlock different components together in the same coating. As an advantage, its architecture in the direction normal to the core surface is decided beforehand [3–5]. The possibility of scaling-down and combining with different core preparation techniques makes LbL assembly a highly promising approach for nanomedicine applications.
Nevertheless, only within the recent five years the design of the LbL nanovehicle systems reaches the level of properties desirable for efficient systemic delivery: minimum toxicity due to biodegradable materials, stability of nanocolloid as an isotonic formulation, prolonged release of encased drug in vitro and in vivo, effective endosomal escape, and extended serum half-life that allows for prolonged exposure of cells to the treatment, active targeting and tumor cell uptake [1,6]. In a proof-of-concept study, Hammond et al [7] recently demonstrated a systemic co-delivery of doxorubicin and siRNA in NCr nude mice bearing subcutaneous xenograft tumors of luciferase-expressing MDA-MB-468 cells via 120 nm LbL coated liposomes. Liposomes containing 5.5 wt.% of doxorubicin were used as a core. By assembling MRP1 siRNA in alternation with poly-L-arginine as a multilayer shell, a high loading of the gene silencing RNA per nanoparticle was reached. A layer of hyaluronic acid deposited atop the shell efficiently shields it from recognition by immune system and enhances the nanocapsules uptake by tumor tissue through the interaction of hyaluronic acid with CD4 glycoprotein. In another study, LbL-coated paclitaxel (PTX) nanoparticles with attached tumor-specific mAb 2C5 antibody effectively target the nucleosome monolayer in the ELISA assay. Attached antibody enhances in vitro toxicity of the nanocapsules towards MCF-7 and BT-20 cancer cell lines [8]. Yet in another work, bovine serum albumin (BSA) nanoparticles of a 200 nm diameter modified with polyallylamine hydrochloride/polystyrene sulfonate (PAH/PSS) multilayers were used for encapsulation of doxorubicin and covalent modification with aptamer AS1411 to target the over-expressed nucleolin on cancer cell membrane [9].
Apart from preset architecture of the coating as above, the polyelectrolyte shell stabilizes nanocrystals of poorly water-soluble drugs prepared by top-down disintegration or nanoprecipitation technique (Fig. 1). By applying constant ultrasonication and step-wise addition of polyelectrolytes, 200–250 nm nanocrystals of PTX, tamoxifen, curcumin, resveratrol were successfully coated with two bilayer shells of biopolyelectrolytes (alginic acid, chitosan, heparin (Hep), poly-L-lysine (PLL), and proteins (protamine sulfate, BSA) [8, 10–13]. A coating consisted of 1.5–2 chitosan/chondroitin sulfate bilayers was required to produce stable 130–200 nm colloids of furosemide, PTX, isoxyl, and nefidipine and suppress for at least 3 months their growth in suspension due to the Ostwald ripening [14]. The current LbL approach has an advantage as compared with nano-encapsulation through direct ultrasonication of low soluble drugs microparticles suggested earlier [15].
Fig. 1.
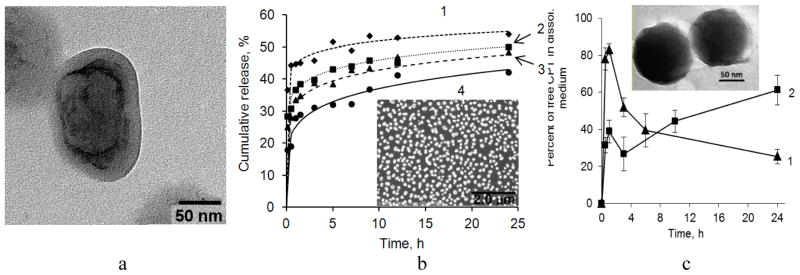
(a) SEM image of nano-paclitaxel coated with a (PLB16-5/Hep)3/PLG65[4.5]5 shell. The core/shell structure of the nanocapsule is visualized by staining with (NH4)2Mo2O7). b) PTX release from 300 nm nanocolloids prepared using top-down sonication assisted LbL method and coated with a (PLL/Hep)n shell. Number of bilayer in shell, n: 1- 0.5, 2–4, 3–8, 4–12. 0.2% Polysorbate 80 in water at 37 °C. Insert: PTX nanocapsules with a (PLL/Hep)4 shell. Reprinted with permission from [16]. Copyright (2014) The Royal Society of Chemistry. c) Percentage of free CPT found in FBS as a function of time of CPT release from (Hep/PLB16-5)5 (1) and (Hep/PLB16-5)5/mPEG5kDa (2) nanocapsules. C(CPT) = 2.27 μg/mL. Insert: TEM image of CPT nanoparticles with (Hep/PLB16-5)7.0/PEG5kDa shell. The hazy irregular outer periphery of particles indicates the presence of the shells with attached hydrophilic PEG5kDa layer. Reprinted with permission from [17].
The non-washing LbL assembly technique was suggested to form polyelectrolyte coating on semistable drug nanocrystals prepared in the presence of high concentrations of uncharged stabilizers [16–18]. The obtained 150–200 nm diameter capsules with a shell consisted of 4.5–7.5 bilayers of low molecular weight block-copolymers of PLL with polyethylene glycol (PLB) in combination with Hep and BSA can be further separated from the supernatant without compromising colloid stability. The optimization of capsule shell architecture and thickness allowed for concentrated up to 3–5 mg/mL colloids that are stable in isotonic salt buffers. To enhance stability of the colloid and add antifouling properties to the polyelectrolyte capsules, their outer surface was further modified by polyethylene glycol (PEG) chains [17].
The kinetic profile of drug dissolution from the LbL nanocapsules is largely affected by the design of release system. In vitro release medium often contains surfactants, albumin, and other additives to increase solubility of poorly water-soluble substances and mimic biological fluids. These facts must be taken into account while considering dissolution of nanoformulations.
Drug release from nanoparticulates is usually faster than from micronized powders due to smaller particles size [14, 16]. De Villeirs et al [14] found that the dissolution rate of poorly water soluble drugs from nanocapsules in PBS buffer decreases almost linearly with increasing shell thickness. For a 10 bilayer coating (thickness of ~25 nm), the release rate was comparable to the micronized suspensions. The effect of LbL shell thickness on dissolution suppression was less for more soluble drugs, furosemide and nifedipine, compared to the less soluble drugs, PTX and isoxyl. Usually three polyelectrolyte bilayers, that is, only one bilayer more than needed to stabilize nanoparticles in colloid are already effective in prolongation of drug release in a medium without solubility enhancer. For example, under sink conditions in PBS buffer, pH 7.2 in eight hours only 40 % of PTX was release from a (PAH/BSA)3 coated sample as compared with 80 % for one layer coated sample [11]. Similarly, non-coated tamoxifen nanocrystals were completely solubilized within approx. 2 h, while a three bilayer polyelectrolyte shell extended this time to approx. 10 h [8]. The drug release profiles fit into the exponential Peppas’ model.
For LbL-coated PTX colloids, a fast initial dissolution (more than 20 %) of the drug was observed after addition of the nanoparticles to a PBS - Polysorbate 80 release medium (Fig. 1b). The rest of the drug was released very slowly (within 65–70 h). No influence on PTX release rate in the medium with solubility enhancer was found for shells thinner than 3.5 PLB/Hep bilayers. With larger number of polyelectrolyte layers (4, 8 and 12 bilayers of PLL and Hep), a slight decrease in the drug release rate with increasing shell thickness was detected [16].
Grafting PEG chains of 5 kDa on the outer surface of camptothecin (CPT) nanocapsules with a (Hep/PLB)n shell decreases the initial drug burst in fetal bovine serum (FBS) down to 40 % (versus 80% for unPEGylated counterpart) and sustains linear release of the drug for at least 24 h (Fig. 1c). The additional PEG layer is approximately 6–7 nm thick, the thickness is comparable with that of nanocapsule shell wall itself. Moreover, this highly hydrophilic layer shields nanocapsules from the FBS proteins, thus minimizing their attachment and stabilizing the colloid in FBS. Due to the prolonged character of release from the PEGylated nanocapsules, a new portion of active lactone CPT is supplied into FBS during a long period of time; a high fraction of the active drug form is maintained [17]. An improvement of sustained release performance upon grafting PEG 4 kDa on the surface was previously reported for polyelectrolyte hybrid microspheres consisted of chitosan and magnetite nanoparticles [18].
Thicker capsule wall efficiently resists to protease-induced degradation, the kinetics of degradation can be further slowed down by inserting synthetic polyelectrolyte layers into the shell comprised of biodegradable polymers [19]. Polyelectrolyte-coated nanoparticles gain increased stability in vivo with larger numbers of deposited layers, thus circulation time of the nanocapsules is effectively increased, and the final layer adsorbed generates a critical surface cascade, which dictates the biological properties of the nanoparticle [20].
Soft gel nanoparticles is another example of spherical carrier that while coated with a LbL shell exhibits modified release properties. LbL coating in such systems suppresses the degree of core swelling under stimuli (temperature, pH, ionic strength, etc.), thus reducing fast initial release of encapsulate associated with nanoparticulated gel delivery systems [21,22]. PAH/PSS shells assembled on 200 nm acrylate nanogels slow release of procaine hydrochloride loaded in the nanocapsules [21]. Similarly, polyphenols (tannic acid, theaflavin, (−)-epigallocatechin gallate) were encased into soft 100–250 nm gelatin nanoparticles in high concentrations irrespectively to polyelectrolyte coating, but their release was decelerated by LbL shells [22].
The general methods of release under external stimuli such as temperature, light, magnetic fields, ultrasound, pH and ionic strength changes that are well developed for polyelectrolyte capsules with a diameter of several micrometers can be used for fine tunning of the nanocapsule shell permeability or for immediate release of the load [23,24].
Thus, multilayer polyelectrolyte coating is more suitable for the encapsulation of poorly soluble anticancer drugs. The composite core/shell structure improves colloidal and chemical stability of encapsulated drugs and reporting nanoparticles in biologically relevant buffers and their resistivity to serum protein adsorption. Another aspect is preset architecture of the polyelectrolyte shell that converges components of different nature in one coating and related with this fact possibility of co-delivery of two or more therapeutic agents in one nanovehicle and active targeting of such composite nanocapsule to damaged cells and tissues. Simultaneously thick LbL shell wall may prolong drug release from the nanocapsules.
Nanotubule mediated drug encapsulation
Tubular nanosystems emerged recently as a part of novel approaches to design of drug delivery nanoparticles. The nanocarrier aspect ratio has been recognized as a factor affecting cellular interaction and biodistribution of drug vehicle [25]. For fabrication of polyelectrolyte nanotubes, sacrificial nanoporous anodic aluminum oxide and track-etched polymer membranes with a pore diameter of 200–400 nm and pore lengths from hundreds of nanometers to tens of microns are used [26]. Arrays of tubules incorporating cytochrome C, collagen, peroxidase, HSA, ferritin, myoglobin, were fabricated using synthetic polyelectrolytes as alternated building blocks [27]. Li et al [28] reported on the first preparation of phospholipid/protein nanotubes based on negatively charged L-α-dimyristoylphosphatidic acid and HSA as a counterpart.
The open-end structure of the tubular nanocariers prepared by membrane template synthesis does not allow for effectively loading pharmaceutically relevant substances in their interior space. Rubner et al [29] demonstrated that selected probe molecules (Rose Bengal, acid red 8, and ibuprofen) can be trapped by nanotubes wall material after acid activation. Another example is binding of zinc (II) protoporphyrin IX to HSA in the walls of (poly-L-arginine/HSA)3 nanotubules and its release in vitro by competitive binding of miristic acid to HAS [30].
We describe here new biocompatible nanotube system based on halloysite clay. Halloysite is chemically identical to kaolin although usually contains minor amount of iron. Typically, 10–15 alumosilicate layers roll into a cylinder of a 40–60 nm diameter with inner lumen diameter of 10–15 nm (Fig. 2a) [31–37]. Outer surface of halloysite tubes is silica providing a strong negative zeta-potential to the nanocarrier in aqueous dispersions at normal pH (Fig. 2b).
Fig. 2.
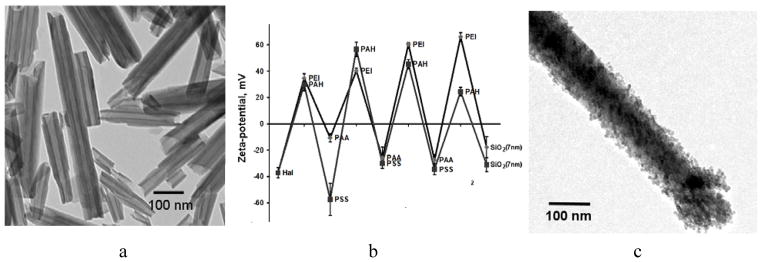
(a) TEM images of pristine halloysite from Dragon Mine, Utah, Applied Minerals Inc. Scale bar is 1 μm. b) Zeta-potential alternation in the processes of LbL assembly of multilayer shells on halloysite clay nanotube (PAA – polyacrylic acid, PSS – polystyrene sulfonate, PEI – polyethyleneimine, PAH – polyallylamine hydrochloride; (c) TEM image of (PAH/PSS/(PAH/silica)2 tube coating.
Using halloysite for drug loading and slow release was first suggested by Price, Gaber and Lvov [34] followed with papers by Lewis, Kelly, Deasy, Geckeler, and others [38–42]. Drug, proteins or other chemicals may be loaded into the clay tubes at 10–15 wt.%, kept for a long time, and then released in aqueous media during 10–50 h controlled by lumen opening and concentration gradient.
For loading, halloysite is mixed with a highly concentrated solution of selected drug and the solvent is slowly evaporated (melted compounds could be also used). In the first work on halloysite drug loading, the release of oxytetracycline (a water soluble antibiotic) was completed in 20 h; nicotinamide adenine dinucleotide, an important enzymatic co-factor (NAD) was released in 5 h. For khellin loaded from melt, the dissolution time extended over 200 h due to its low solubility in water. Loading into halloysite extends furosemide, nifedipine, and dexamethasone release to 10–15 h versus 10–15 min for corresponding powders (Fig. 3a) [34]. Some of the cosmetic applications of halloysite are encapsulations of skin moisturizing agent glycerol, vitamin C (ascorbic acid) and antiseptics. Glycerol-halloysite loading efficiency was 15 wt. % and its slow release from clay nanotube followed Peppas’ law (30 % of the glycerol was released within 10 hours) [36].
Fig. 3.
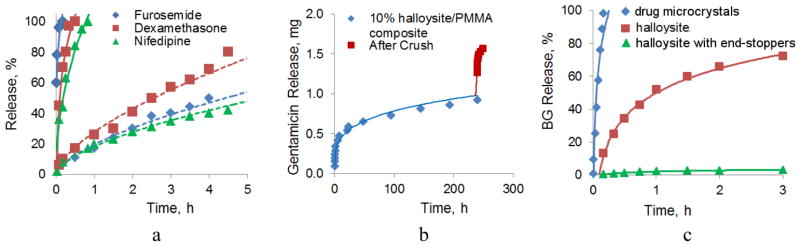
a) Furosemide, dexamethasone and nifedipine sustained release from halloysite nanotubes; almost vertical curves close to Y-axe are drug powder dissolution. b) Normal (initial) and stress induced (jump) antibiotic gentamicin release from halloysite doped into PMMA bone cement, total sample loading was 1.8 mg; c) Kinetic of brilliant green (BG) dissolution from microcrystal (close to Y-axe), from pristine halloysite nanotubes and from BTA-Cu encapsulated halloysite with end-stoppers. Reprinted with permission from [43]. Copyright 2014 RSC Publishing.
Halloysite-drug formulations are suggested either for direct biomedical applications or may be admixed to different polymers (from polar polysaccharides, polymethylmethacrylate (PMMA), polyamides and epoxy resins to low polar plastics such as polypropylene) producing smart composites with antimicrobial or healing properties [37–39]. Thus, tetracycline loaded halloysite in complex with cationic chitosan was proposed for dog teeth fillings with sustained antimicrobial properties [38,39]. In another preparation, antibiotic gentamicin is released from halloysite nanotubes doped into PMMA used as bone cement for 250–300 h (Fig 3b), much longer than the release from free halloysite or PMMA with directly admixed gentamicin. This time corresponds to standard orthopedic medicine practice demanding two weeks antibacterial protection of the bone surgery zone [37]. Under external stress, the sample cracks and additionally burst-releases the antibiotic from the sample internal layers. Halloysite mixes well with the most of polar or low polarity polymers and even with wax, thus, opening a perspective for halloysite-antiseptics containing plastics (for example, to prevent spread of infections in hospitals).
Halloysite-based polymeric composites provide also a significant enhancement in mechanical properties (5 % halloysite doping gives 30–50 % tensile strength increase). This enables the design of smart composite materials with synergistically improved strength and adhesivity combined with controlled release of antimicrobial, antifouling, flame-retardant, self-healing, and other agents [31, 42–45].
A way to extend the halloysite formulation release time is based on its additional encapsulation through formation of surface complex of benzotriazole (BTA) and copper ions or with LbL polyelectrolyte coating [43]. Loading a commonly used antiseptic agent brilliant green (BG) within halloysite lumen provided extended control over release rate, while microcrystals of BR were giving fast dissolution (Fig. 3c). Further sealing the tube with BTA-Cu complex allowed only 4 % of brillant green release within 3 h and 90 % release in 250 h.
Further, we combined LbL encapsulation with clay nanotube cores to get additional control over drug release rate due to polyelectrolyte coating. Fig. 2b demonstrates LbL assembly of polyelectrolyte multilayer coating on halloysite with finishing with 7-nm diameter silica. One can see a regular alternation of zeta-potential between −40 and +6 mV and even tube coating with enhancement at the tube ends (Fig. 2c). This kind of a stopper allowed to prolong release rate of dexamethasone loaded into halloysite nanotubes from 6 h for pristine halloysite to 15 h for a halloysite carrier coated with three PAH/PSS bilayers [31].
One of the big advantages of halloysites is a possibility of directional release through tube ends. In the case of polyelectrolyte multilayer capsules, directional release has been achieved by a very different approach: targeting a specific area on a capsule [46].
Halloysite toxicity
The pharmaceutical applications of halloysite-based materials (such as drug carriers [47], gene delivery [48], cells adhesion amplifying coatings [49, 50], scaffolds for tissue engineering [51] imply the high level of biocompatibility of these nanotubes. Nevertheless, just a few recently published papers directly address the toxicity of halloysite nanotubes. Majority of the studies are focused on structural properties of halloysite-based materials, while employing just a limited number of toxicity tests on biological objects, such as selected human cell lines and several microbial species. To the best of our knowledge, there is no published data on halloysite nanotubes toxicity towards model multicellular organisms.
The first direct investigation of halloysite nanotubes toxicity and cellular uptake was performed by Vergaro et al [47]. The researchers studied the influence of pristine (surface-modified with fluorescein-labelled silane molecules) and functionalised (coated with several layers of fluorescently-labelled polyelectrolytes) halloysite nanotubes on human epithelial adenocarcinoma cells (HeLa) and human breast cancer cells (MCF-7). FITC-labelled halloysite readily penetrates into both types of cells and can be seen as the green area around blue cell nuclei (Fig. 4a, insert). The cell viability was well preserved (up to 70% of viable cells) within the wide range of pristine and modified halloysite nanotubes concentrations (up to 75 μg/mL) (Fig. 4a). The increased nanotubes concentration (up to 1000 μg/mL) induced a higher mortality rate in both HeLa and MCF-7 cells. The study suggests that the toxicity of halloysite nanotubes is comparable with that of spherical silica nanoparticles, and postulates the biocompatibility of biomaterials designed using halloysite nanotubes [47]. For many biological cells and tissues, halloysite is safe up to 0.2 mg/mL which is very high safe concentration for inorganic inclusions [52]. All these properties justify using halloysite as nanocontainers for loading and sustained release of biological agents (drugs, proteins, DNA, antibacterials, marine biocide and anti-fouling agents). However, the secondary effects of halloysite taken up by cells on tissues and organs may be essential.
Fig. 4.
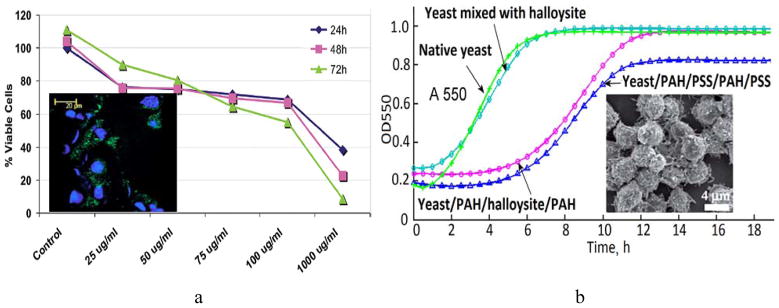
a) MTT viability test curves demonstrating the cellular viability after subjecting the MCF-7 cells to increasing concentrations of halloysite nanotubes. Insert: laser scanning confocal microscopy image of FITC-polyelectrolyte coated halloysite nanotubes (green) located near cellular nuclei (blue) of cultured MCF-7 cells. Reproduced with permission from [47]; b) growth curves of native yeast cells, yeast mixed with halloysite nanotubes, yeast coated with PAH/PSS/PAH/PSS and yeast coated with PAH/HNTs/PAH/PSS. Insert: SEM image of yeast cells coated with halloysite-doped polymer shells. Reprinted with permission from [45]. Copyright (2013) The Royal Society of Chemistry.
The major halloysite applications are attributed either to fabrication of novel drug delivery vehicles based on nanotubes [47], or to usage of the nanotubes to attenuate mechanical or adhesive properties of biomaterials [53]. As a result, in many cases the toxicity of halloysite modified with other materials rather than pristine halloysite is investigated. Liu et al [51] shows that apart from significantly enhanced compressive strength, compressive modulus, and thermal stability of the chitosan-based scaffolds for tissue engineering, its doping with halloysite nanotubes had no significant effect on the mouse fibroblast attachment and development on the nanocomposites. This is attributed to the high Si content in the scaffold and also to the increased surface roughness of the halloysite-chitosan composite. The cell proliferation assay (MTT-test) suggests that up to 80% of halloysite nanotubes in the scaffold is non-toxic to cells. Another example of biocompatibility of halloysite-based scaffolds was demonstrated by Bottino et al [54] who employed electrospinning to fabricate a dental nanocomposite composed of polydioxanone and halloysite nanotubes. The scaffolds were used to cultivate dental pulp fibroblast cells, which were found to growth even better on halloysite-doped surfaces. One may presume that halloysite nanotubes significantly increase the adhesion of cells onto surfaces, which was also demonstrated in microfluidic chambers [49].
Apart from mammalian cells, microorganisms have also been used in halloysite toxicity studies. Zhang et al [55] demonstrated that the halloysite nanotubes lack any inhibiting effect on two bacterial species – Escherichia coli and Staphilococcus aureus. Therefore, it was suggested to dope nanotubes with Ag nanoparticles [43,55] or fill the lumen with Cu ions [56] to provide them with antibacterial functionality. A similar approach was realised by Wei et al [43] by employing halloysite nanotubes as nanoscale vessels filled with an antimicrobial drug brilliant green demonstrating the extended release profiles.
As a functional nano-block, halloysite tubes may be used for fabricating hybrid microparticles using single yeast cells as templates for deposition of halloysite via the layer-by-layer assembly [45], providing microorganisms with additional functions, for example, spore-like microbial shells (Fig. 4b). Neither pristine halloysite nor polyelectrolyte-embedded halloysite significantly affected the viability and vitality of yeast. Moreover, the polymer nanoshells doped with halloysite slightly decrease the budding in yeast, which require piercing the shells, resulting in 2–3 hours growth delay. Loading clay nanotubes with enzymes and DNA and coating them onto microorganisms was also demonstrated [41,42].
The results obtained so far suggest that halloysite nanotubes are not toxic within the reasonable range of concentrations. We envisage the following major directions of future work: 1) the detailed investigation of uptake pathways and mechanisms of interaction of nanotubes with human cells and biomacromolecules in cytoplasm and 2) the investigation of halloysite effects on whole organisms, starting from relatively simple models, such as nematodes and crustaceans, and then moving to mammals.
Halloysite clay is natural environmentally friendly inexpensive nanomaterial which may serve as a new type of core nanocontainer of high loading capacity releasing drugs in a sustained manner from tens hours to months. Unlike multilayer polyelectrolyte nanocapsule, halloysite nanotubules prolong release not only enzymes and DNA but also low molecular weight and relatively soluble therapeutic compounds. Additional encapsulation of halloysite nanotubes through formation of surface complex of benzotriazole and copper ions or with LbL polyelectrolyte coating and associated with it formation of end–tube stoppers prolongs time of release many-fold.
Unlike polyelectrolyte-encapsulated formulation, halloysite is not biodegradable and hardy injectable, but non-toxic and at certain conditions even improve the adhesivity and proliferation of human cells. Simple one step processing, biocompatibility of the clay and its low price makes halloysite based formulation very promising. It is perspective for scalable production of functional tubule biocomposites useful for oral and transdermal drug formulations, skin care, medical implants and dentistry.
Highlights.
Layer-by-Layer nanocapsules for efficient systemic delivery of drugs are designed.
Halloysite is a natural “green” material of low toxicity towards biological systems.
Halloysite nanotubes serve as containers for loading and sustained release of drugs.
Shell formation on halloysite prolongs drug release over tens and hundreds hours.
Acknowledgments
This work was supported by award 1R01CA134951 from the National Cancer Institute (NCI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI or National Institute of Health. The work is performed according to the Russian Government Program of Competitive Growth of Kazan Federal University. This work was funded by the subsidy allocated to Kazan Federal University for the state assignment in the sphere of scientific activities. This study was supported by Russian Scientific Fund grant No 14-14-00924 (RFF). This work was supported by grant 4.10 from the State Program for Scientific Research “Chemical Technologies and Materials. Natural Resource Potential”, subprogram “ChemPharmSynthesis” for 2013-2015, the Republic of Belarus.
Footnotes
Conflict of interest
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Torchilin VP. Multifunctional pharmaceutical nanocarriers: Promises and problems. Nanotechnologies for the Life Sciences Online. 2012 doi: 10.1002/9783527610419.ntls0249. [DOI] [Google Scholar]
- 2.Mora-Huertas CE, Fessi H, Elaissari A. Polymer-based nanocapsules for drug delivery. Int J Pharmaceutics. 2010;385:113–142. doi: 10.1016/j.ijpharm.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Sukhorukov GB. Multilayer hollow microspheres. In: Arshady R, Guyot A, editors. Dendrimers, Assemblies, Nanocomposites. Vol. 5. Citrus Books; 2002. pp. 111–147. MML Series. [Google Scholar]
- 4.De Geest BG, Sukhorukov GB, Möhwald H. The pros and cons of polyelectrolyte capsules in drug delivery. Expert Opin Drug Deliv. 2009;6:613–624. doi: 10.1517/17425240902980162. [DOI] [PubMed] [Google Scholar]
- 5.DeVilliers M, Lvov Y. Nanocapsules for Drug Delivery. In: Kumar C, editor. Nanotechnologies for Life Sciences. Vol. 10. Wiley-VCH; 2007. pp. 527–556. [Google Scholar]
- 6.Hammond PT. Polyelectrolyte multilayered nanoparticles: Using nanolayers for controlled and targeted systemic release. Nanomedicine. 2012;7:619–622. doi: 10.2217/nnm.12.47. [DOI] [PubMed] [Google Scholar]
- 7•.Deng ZJ, Morton SW, Ben-Akiva E, Dreaden EC, Shopsowitz KE, Hammond PT. Layer-by-layer nanoparticles for systemic codelivery of an anticancer drug and siRNA for potential triple-negative breast cancer treatment. ASC Nano. 2013;7:9571–9584. doi: 10.1021/nn4047925. This study describes the design of LbL coated liposomes for efficient systemic co-delivery of doxorubicin and siRNA in NCr nude mice bearing subcutaneous xenograft tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal A, Lvov Y, Sawant R, Torchilin V. Stable nanocolloids of poorly soluble drugs with high drug content prepared using sonicated layer-by-layer technology. J Control Release. 2008;128:255–260. doi: 10.1016/j.jconrel.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Xie L, Tong W, Yu D, Xu J, Li J, Gao C. Bovine serum albumin nanoparticles with multilayers and aptamers for pH-responsive and targeted anti-cancer drug delivery. J Mater Chem. 2012;22:6053–6060. [Google Scholar]
- 10.Zheng Z, Zhang X, Carbo D, Clark C, Nathan C, Lvov Y. Sonication assisted synthesis of polyelectrolyte coated curcumin nanoparticles. Langmuir. 2010;26:7679–7681. doi: 10.1021/la101246a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pattekari P, Zheng Z, Zhang X, Levchenko T, Torchilin V, Lvov Y. Top-down and bottom-up approaches in production aqueous nanocolloids of paclitaxel. Phys Chem Chem Phys. 2011;13:9014–9019. doi: 10.1039/c0cp02549f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lvov Y, Pattekari P, Zhang X, Torchilin V. Converting poorly soluble materials into stable aqueous nanocolloids. Langmuir. 2011;27:1212–1217. doi: 10.1021/la1041635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lvov Y, Pattekari P, Shutava T. Making aqueous nanocolloids from low soluble materials: LbL shells on nanocores. In: Decher G, Schlenoff J, editors. Multilayer Thin Films: Sequential Assembly of Nanocomposite Materials. Wiley-VCH; 2012. pp. 151–170. [Google Scholar]
- 14.Strydom SJ, Otto DP, Stieger N, Aucamp ME, Liebenberg W, de Villiers MM. Self-assembled macromolecular nanocoatings to stabilize and control drug release from nanoparticles. Powder Technology. 2014;256:470–476. [Google Scholar]
- 15.Pargaonkar N, Lvov YM, Li N, Steenekamp JH, de Villiers MM. Controlled release of dexamethasone from microcapsules produced by polyelectrolyte layer-by-layer nanoassembly. Pharmaceutical Research. 2005;22:826–835. doi: 10.1007/s11095-005-2600-0. [DOI] [PubMed] [Google Scholar]
- 16.Shutava TG, Pattekari PP, Arapov KA, Torchilin VP, Lvov YM. Architectural layer-by-layer assembly of drug nanocapsules with PEGylated polyelectrolytes. Soft Matter. 2012;8:9418–9427. doi: 10.1039/C2SM25683E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17•.Parekh G, Pattekari P, Joshi C, Shutava T, DeCoster M, Levchenko T, Torchilin V, Lvov Y. Layer-by-layer nanoencapsulation of camptothecin with improved activity. Int J Pharm. 2014;465:218–227. doi: 10.1016/j.ijpharm.2014.01.041. This study describes polyelectrolyte nanocapsules with enhanced colloidal stability prepared using the non-washing Layer-by-Layer assembly technique and external surface PEGylation that preserve the active lactone form of camptothecin, sustains drug release in serum, and increase drug activity in vitro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao XB, Du PC, Liu P. Preparation of aggregation-resistant biocompatible superparamagnetic noncovalent hybrid multilayer hollow microspheres for controlled drug release. Mol Pharmaceutics. 2012;9:3330–3339. doi: 10.1021/mp300363g. [DOI] [PubMed] [Google Scholar]
- 19.Marchenko I, Yashchenok A, Borodina T, Bukreeva T, Konrad M, Möhwald H, Skirtach A. Controlled enzyme-catalyzed degradation of polymeric capsules template on CaCO3: Influence of the number of LbL layers, conditions of degradation, and disassembly of multicompartments. J Controlled Release. 2012;162:599–60. doi: 10.1016/j.jconrel.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Poon Z, Lee JB, Morton SW, Hammond PT. Controlling in Vivo Stability and Biodistribution in Electrostatically Assembled Nanoparticles for Systemic Delivery. Nano Lett. 2011;11:2096–2103. doi: 10.1021/nl200636r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan JP, Wang Q, Tam KC. Control of burst release from nanogels via layer by layer assembly. J Control Release. 2008;128:248–254. doi: 10.1016/j.jconrel.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Shutava TG, Balkundi SS, Vangala P, Steffan JJ, Bigelow RL, Cardelli JA, O’Neal DP, Lvov YM. Layer-by-layer coated gelatin nanoparticles as a vehicle for delivery of natural polyphenols. ACS Nano. 2009;3:1877–1885. doi: 10.1021/nn900451a. [DOI] [PubMed] [Google Scholar]
- 23.Skirtach AG, Yashchenok AM, Möhwald H. Encapsulation, release and applications of LbL polyelectrolyte multilayer capsules. Chem Commun. 2011;47:12736–12746. doi: 10.1039/c1cc13453a. [DOI] [PubMed] [Google Scholar]
- 24.Antipina MN, Sukhorukov GB. Remote control over guidance and release properties of composite polyelectrolyte based capsules. Adv Drug Delivery Rev. 2011;63:716–729. doi: 10.1016/j.addr.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Shimoni O, Yan Y, Wang Y, Caruso F. Shape-dependent cellular processing of polyelectrolyte capsules. ACS Nano. 2013;7:522–530. doi: 10.1021/nn3046117. [DOI] [PubMed] [Google Scholar]
- 26.Azzaroni O, Lau KHA. Layer-by-layer assemblies in nanoporous templates: Nano-organized design and application of soft nanotechnology. Soft Matter. 2011;7:8709–8724. doi: 10.1039/C1SM05561E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landoulsi J, Roy CJ, Dupont-Gillain C, Demoustier-Champagne S. Synthesis of collagen nanotubes with highly regular dimensions through membrane-templated layer-by-layer assembly. Biomacromolecules. 2009;10:1011–1024. doi: 10.1021/bm900245h. [DOI] [PubMed] [Google Scholar]
- 28.Lu G, Ai S, Li J. Layer-by-Layer assembly of human serum albumin and phospholipid nanotubes based on a template. Langmuir. 2005;21:1679–1682. doi: 10.1021/la047771r. [DOI] [PubMed] [Google Scholar]
- 29.Lee D, Cohen RE, Rubner MF. Heterostructured magnetic nanotubes. Langmuir. 2007;23:123–129. doi: 10.1021/la0612926. [DOI] [PubMed] [Google Scholar]
- 30.Qu X, Komatsu T. Molecular capture in protein nanotubes. ACS Nano. 2010;4:563–573. doi: 10.1021/nn901474y. [DOI] [PubMed] [Google Scholar]
- 31••.Lvov Y, Abdullayev E. Functional polymer - clay nanotube composites with sustained release of chemical agents. Prog Polym Sci. 2013;38:1690–1719. A review of the latest results on clay nanotube composites with sustained drug release. [Google Scholar]
- 32.Abdullayev E, Joshi A, Wei W, Zhao Y, Lvov Y. Enlargement of clay nanotube lumen by selective etching of aluminum oxide. ACS Nano. 2012;6:7216–7226. doi: 10.1021/nn302328x. [DOI] [PubMed] [Google Scholar]
- 33.Yah W-O, Takahara A, Lvov Y. Selective modification of halloysite lumen with octadecyl phosphonic acid: New inorganic tubular micelle. J Am Chem Soc. 2012;134:1853–1859. doi: 10.1021/ja210258y. [DOI] [PubMed] [Google Scholar]
- 34.Price R, Gaber B, Lvov Y. In-vitro release characteristics of tetracycline, khellin and nicotinamide adenine dinucleotide from halloysite; a cylindrical mineral for delivery of biologically active agents. J Microencap. 2001;18:713–23. doi: 10.1080/02652040010019532. [DOI] [PubMed] [Google Scholar]
- 35.Veerabadran N, Price R, Lvov Y. Clay nanotubes for encapsulation and sustained release of drugs. NANO. 2007;2:215–22. [Google Scholar]
- 36.Suh Y, Kil D, Chung K, Abdullayev E, Lvov Y, Mongayt D. Natural nanocontainer for the controlled delivery of glycerol as a moisturizing agent. J Nanosci Nanotechol. 2011;11:661–665. doi: 10.1166/jnn.2011.3194. [DOI] [PubMed] [Google Scholar]
- 37.Wei W, Abdullayev E, Hollister A, Lvov Y, Mills D. Clay nanotube/poly(methylmethacrylate) bone cement composite with sustained antibiotic release. Macromolec Materials Engineering. 2012;297:645–653. [Google Scholar]
- 38.Kelly H, Deasy P, Ziaka E, Claffey N. Formulation and preliminary in vivo dog studies of a novel drug delivery system for the treatment of periodontitis. Int J Pharm. 2004;274:167–183. doi: 10.1016/j.ijpharm.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 39.Ward C, Song S, Davis E. Controlled release of tetracycline–HCl from halloysite–polymer composite films. J Nanosci Nanotechnol. 2010;10:6641–6649. doi: 10.1166/jnn.2010.2647. [DOI] [PubMed] [Google Scholar]
- 40.Forsgren J, Jämstorp E, Bredenberg S, Engqvist H, Strømme M. A ceramic drug delivery vehicle for oral administration of highly potent opioids. J Pharm Sci. 2010;99:219–226. doi: 10.1002/jps.21814. [DOI] [PubMed] [Google Scholar]
- 41.Shami M, Geckeler K. The first biopolymer-wrapped non-carbon nanotubes. Nanotechnology. 2008:19–075604. doi: 10.1088/0957-4484/19/7/075604. [DOI] [PubMed] [Google Scholar]
- 42.Lvov Y, Aerov A, Fakhrullin R. Clay nanotubes encapsulation for functional biocomposites. Adv Colloid Interface Sci. 2014;205 doi: 10.1016/j.cis.2013.10.006. web public. [DOI] [PubMed] [Google Scholar]
- 43.Wei W, Minullina R, Fakhrullin R, Abdullayev E, Mills D, Lvov Y. Enhanced efficiency of antiseptics with sustained release from clay nanotubes. RCS Advances. 2014;4:488–495. [Google Scholar]
- 44.Abdullayev E, Sakakibara K, Okamoto K, Wei W, Ariga K, Lvov Y. Natural tubule clay template synthesis of silver nanorods for antibacterial composite coating. ACS Appl Mater Interf. 2011;3:4040–4048. doi: 10.1021/am200896d. [DOI] [PubMed] [Google Scholar]
- 45•.Konnova S, Sharipova I, Demina T, Osin Y, Yarullina D, Zelenikhin P, Ishmuchametova D, Ilinskaya O, Lvov Y, Fakhrullin R. Cell-mediated three-dimensional assembly of halloysite nanotubes. Chem Comm. 2013;49:4208–4212. doi: 10.1039/c2cc38254g. This study descibes building functional clay nanotube shells on microbes. [DOI] [PubMed] [Google Scholar]
- 46.Bedard MF, De Geest BG, Möhwald H, Sukhorukov GB, Skirtach AG. Direction specific release from giant microgel-templated polyelectrolyte microcontainers. Soft Matter. 2009;5:3927–3931. [Google Scholar]
- 47.Vergaro V, Abdullayev E, Cingolani R, Lvov Y, Leporatti S. Cytocompatibility and uptake of halloysite clay nanotubes. Biomacromolecules. 2010;11:820–828. doi: 10.1021/bm9014446. [DOI] [PubMed] [Google Scholar]
- 48.Shi Y, Tian Z, Zhang Y, Shen H, Jia N. *Functionalized halloysite nanotube-based carrier for intracellular delivery of antisense oligonucleotides. Nanoscale Research Letters. 2011;6:608. doi: 10.1186/1556-276X-6-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hughes AD, King MR. Naturally occurring halloysite nanotubes for enhanced capture of flowing cells. Langmuir. 2010;26:12155–12164. doi: 10.1021/la101179y. [DOI] [PubMed] [Google Scholar]
- 50.Mitchell MJ, Chen CS, Ponmudi V, Hughes AD, King MR. E-selectin liposomal and nanotube-targeted delivery of doxorubicin to circulating tumor cells. J Control Release. 2012;160:609–617. doi: 10.1016/j.jconrel.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu M, Wu C, Jiao Y, Xiong S, Zhou C. Chitosan–halloysite nanotubes nanocomposite scaffolds for tissue engineering. J Mater Chem B. 2013;1:2078–2089. doi: 10.1039/c3tb20084a. [DOI] [PubMed] [Google Scholar]
- 52.Lai X, Agarwal M, Lvov Y, Pachpande C, Varahramyan K, Witzmann F. Proteomic profiling of halloysite clay nanotube exposure in intestinal cell co-culture. J Appl Toxicity. 2013;33:1316–1329. doi: 10.1002/jat.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu M, Zhang Y, Wu C, Xiong S, Zhou C. Chitosan/halloysite nanotubes bionanocomposites: Structure, mechanical properties and biocompatibility. Int J Biol Macrom. 2012;51:566–575. doi: 10.1016/j.ijbiomac.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 54.Bottino MC, Yassen GH, Platt JA, Labban N, Windsor J, Spolnik KJ, Bressiani AHA. A novel three-dimensional scaffold for regenerative endodontics: materials and biological characterizations. J Tissue Eng Regen Med. 2013 doi: 10.1002/term.1712. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Chen Y, Zhang H, Zhang B, Liu J. Potent antibacterial activity of a novel silver nanoparticle-halloysite nanotube nanocomposite powder. J Inorg Biochem. 2013;118:59–64. doi: 10.1016/j.jinorgbio.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y, Zhang Y, Liu J, Zhang H, Wang K. Preparation and antibacterial property of polyethersulfone ultrafiltration hybrid membrane containing halloysite nanotubes loaded with copper ions. Chem Eng J. 2012;210:298–308. [Google Scholar]


