Abstract
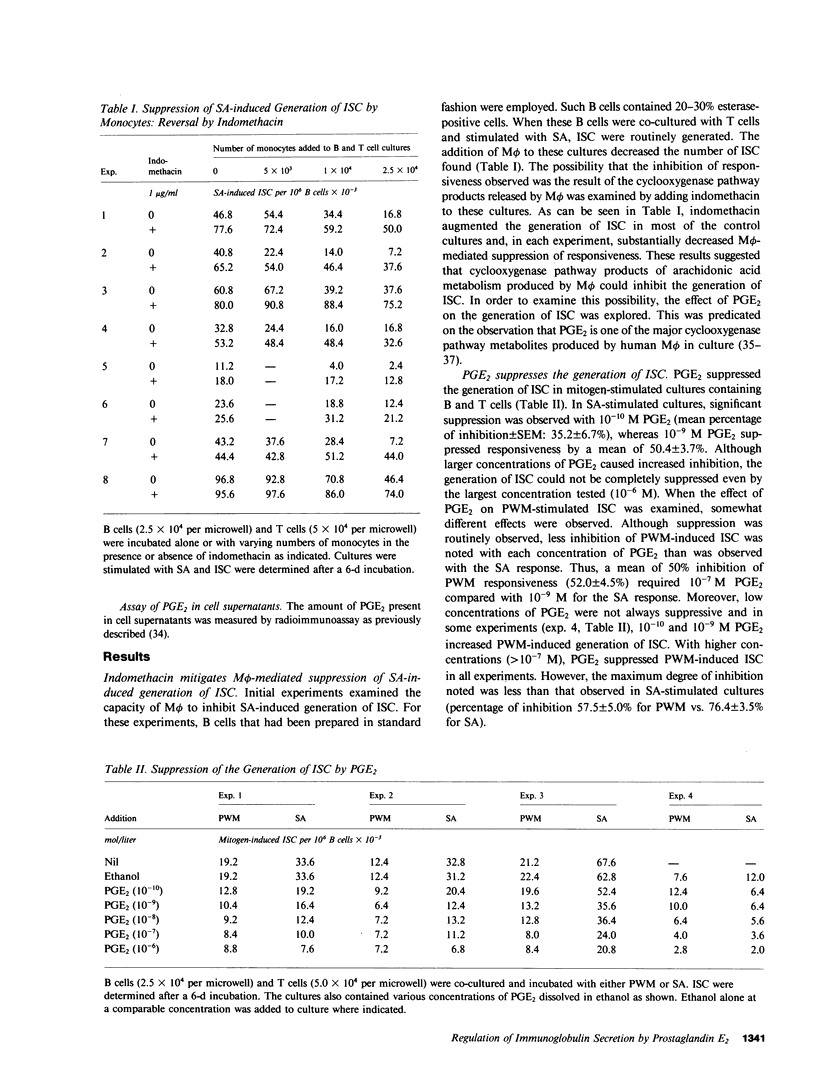
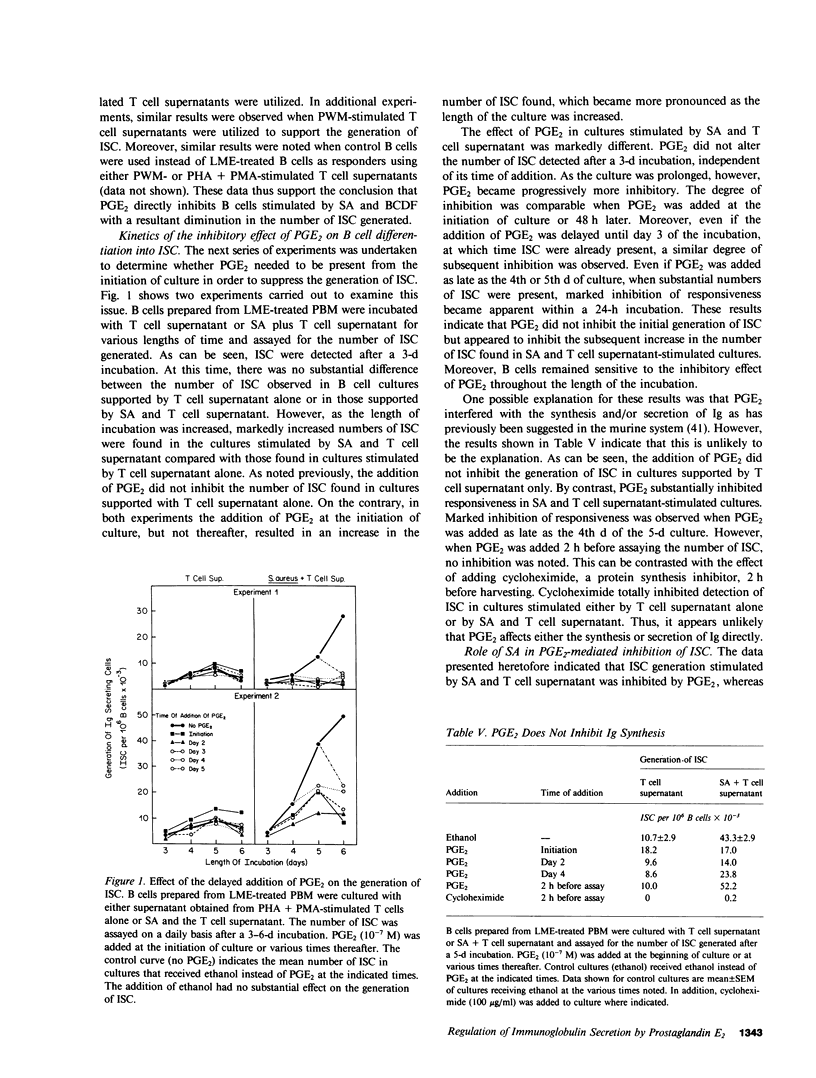
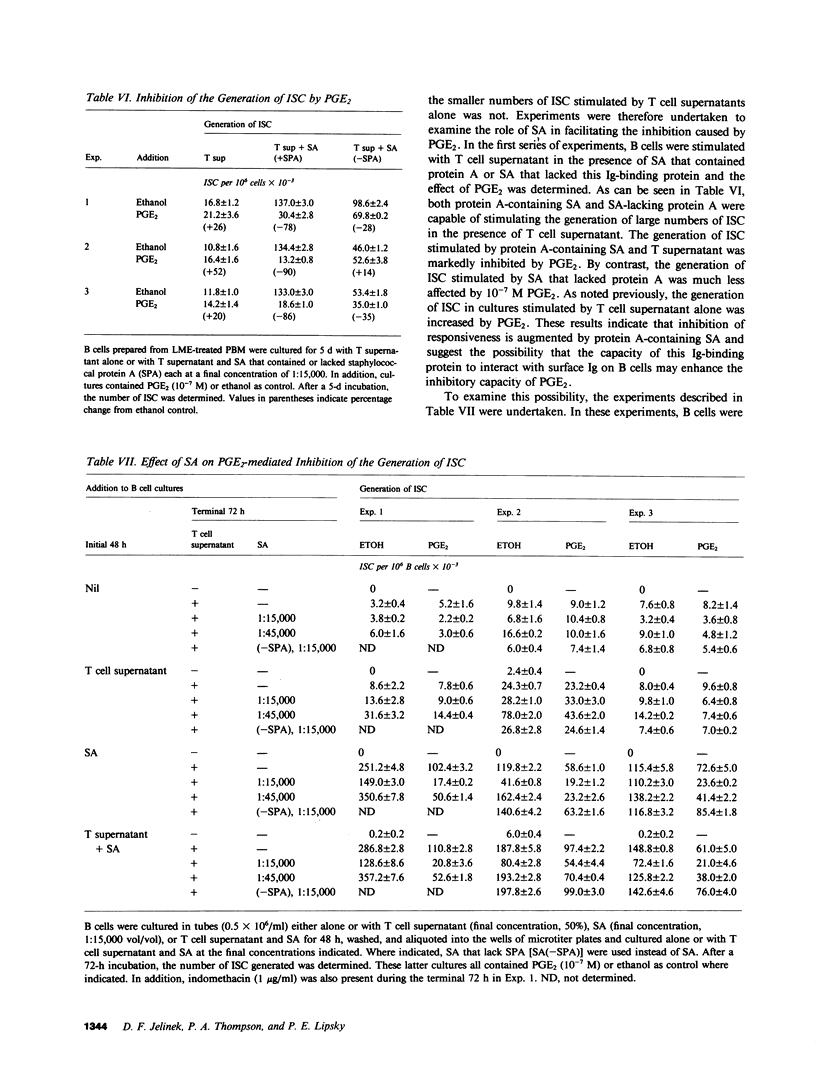
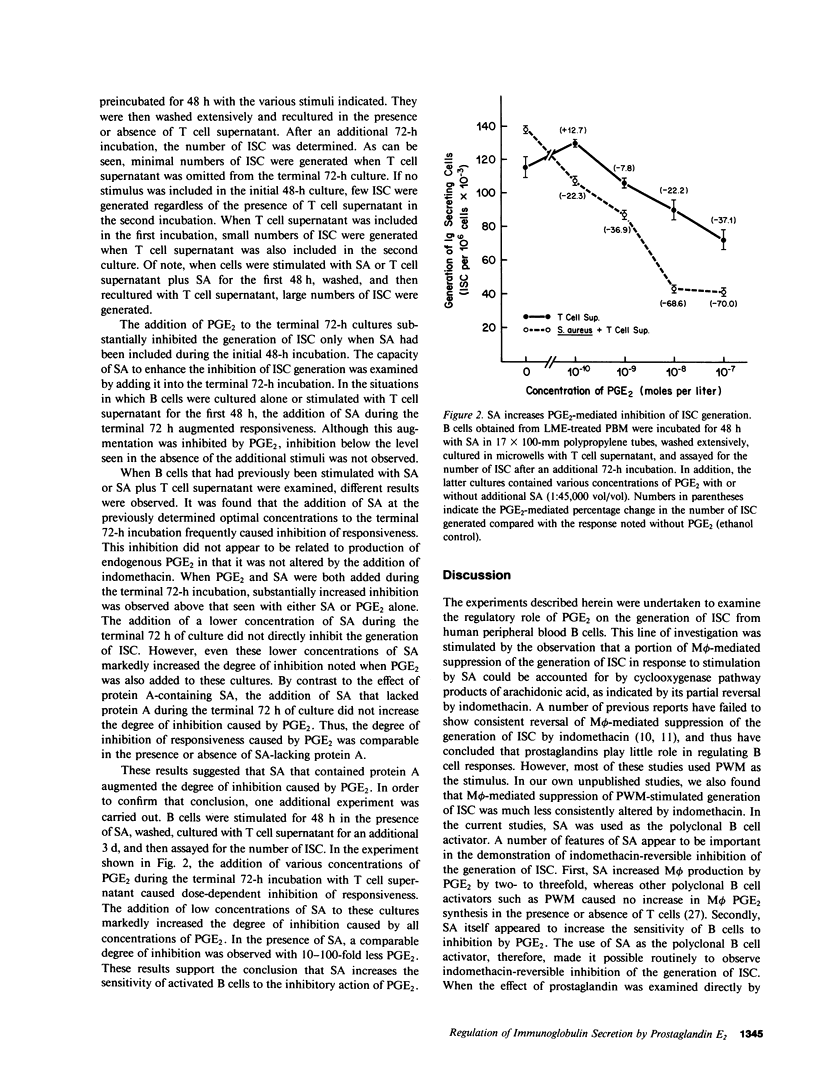
The role of prostaglandin E2 (PGE2) in the generation of immunoglobulin-secreting cells (ISC) from human peripheral blood B cells was examined. Initial studies demonstrated that monocyte (M phi)-mediated suppression of the generation of ISC in Staphylococcus aureus (SA)-stimulated cultures was mitigated by indomethacin, and thus suggested that the cyclooxygenase pathway products of arachidonic acid played a role in the regulation of B cell activation. The possibility that PGE2, one of the major products of this pathway generated by M phi-affected human B cell responses, was therefore investigated. PGE2 was found to cause concentration-dependent inhibition of the generation of ISC in pokeweed mitogen- or SA-stimulated B cell cultures supported by T cells. Studies were therefore carried out to determine whether PGE2 inhibited the production of necessary T cell factors or directly altered B cell responsiveness. Initially, the effect of PGE2 on the capacity of mitogen-stimulated cells to secrete a factor that supported the differentiation of B cells into ISC was investigated. Excessive numbers of M phi or PGE2 inhibited the production of B cell differentiation factor from mitogen-stimulated T cells. The effect of PGE2 on the capacity of B cells to differentiate into ISC was more complex. PGE2 inhibited the generation of ISC when B cells were stimulated with SA and B cell differentiation factor-containing T cell supernatants. PGE2-mediated inhibition of ISC generation was observed even when addition of PGE2 was delayed until after ISC first were detected in culture. By contrast, PGE2 caused only minimal inhibition of the generation of ISC cultures stimulated by T cell supernatants alone or protein A-free SA and T cell supernatants. These results suggested that SA-responsive B cells were particularly sensitive to inhibition by PGE2. Additional experiments supported the conclusion that B cell sensitivity to inhibition by PGE2 is augmented by the immunoglobulin cross-linking effects of protein A-containing SA. Overall, the results support the conclusion that PGE2 at physiologically relevant concentrations can influence human antibody responses by means of a direct inhibitory action on the responding B cell or an indirect one on the production of necessary T cell factors.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blotman F., Chaintreuil J., Poubelle P., Flandre O., Crastes de Paulet A., Simon L. PGE2, PGF2 alpha, and TXB2 biosynthesis by human rheumatoid synovia. Adv Prostaglandin Thromboxane Res. 1980;8:1705–1708. [PubMed] [Google Scholar]
- Bockman R. S. Prostaglandin production by human blood monocytes and mouse peritoneal macrophages: synthesis dependent on in vitro culture conditions. Prostaglandins. 1981 Jan;21(1):9–31. doi: 10.1016/0090-6980(81)90192-1. [DOI] [PubMed] [Google Scholar]
- Burchiel S. W., Warner N. L. Cyclic AMP modulation of Fc receptor expression on a pre-B cell lymphoma. J Immunol. 1980 Mar;124(3):1016–1021. [PubMed] [Google Scholar]
- Ceuppens J. L., Goodwin J. S. Endogenous prostaglandin E2 enhances polyclonal immunoglobulin production by tonically inhibiting T suppressor cell activity. Cell Immunol. 1982 Jun;70(1):41–54. doi: 10.1016/0008-8749(82)90131-9. [DOI] [PubMed] [Google Scholar]
- Chouaib S., Fradelizi D. The mechanism of inhibition of human IL 2 production. J Immunol. 1982 Dec;129(6):2463–2468. [PubMed] [Google Scholar]
- Delfraissy J. F., Galanaud P., Wallon C., Balavoine J. F., Dormont J. Abolished in vitro antibody response in elderly: exclusive involvement of prostaglandin-induced T suppressor cells. Clin Immunol Immunopathol. 1982 Sep;24(3):377–385. doi: 10.1016/0090-1229(82)90008-3. [DOI] [PubMed] [Google Scholar]
- Dray F., Charbonnel B., Maclouf J. Radioimmunoassay of prostaglandins Falpha, E1 and E2 in human plasma. Eur J Clin Invest. 1975 Jul 29;5(4):311–318. doi: 10.1111/j.1365-2362.1975.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Dunne J. V., Foss B., Leung T., McKendry R. J. Effects of prostaglandins E1 and E2 on the in vitro production of immunoglobulin by human peripheral blood lymphocytes. Prostaglandins Med. 1981 Apr;6(4):419–425. doi: 10.1016/0161-4630(81)90074-4. [DOI] [PubMed] [Google Scholar]
- Ellner J. J., Spagnuolo P. J. Suppression of antigen and mitogen induced human T lymphocyte DNA synthesis by bacterial lipopolysaccharide: mediation by monocyte activation and production of prostaglandins. J Immunol. 1979 Dec;123(6):2689–2695. [PubMed] [Google Scholar]
- Falkoff R. J., Zhu L. P., Fauci A. S. Separate signals for human B cell proliferation and differentiation in response to Staphylococcus aureus: evidence for a two-signal model of B cell activation. J Immunol. 1982 Jul;129(1):97–102. [PubMed] [Google Scholar]
- Fischer A., Durandy A., Griscelli C. Role of prostaglandin E2 in the induction of nonspecific T lymphocyte suppressor activity. J Immunol. 1981 Apr;126(4):1452–1455. [PubMed] [Google Scholar]
- Ginsburg W. W., Finkelman F. D., Lipsky P. E. Circulating and mitogen-induced immunoglobulin-secreting cells in human peripheral blood: evaluation by a modified reverse hemolytic plaque assay. J Immunol. 1978 Jan;120(1):33–39. [PubMed] [Google Scholar]
- Gmelig-Meyling F., Waldmann T. A. Human B cell activation in vitro: augmentation and suppression by monocytes of the immunoglobulin production induced by various B cell stimulants. J Immunol. 1981 Feb;126(2):529–537. [PubMed] [Google Scholar]
- Goldyne M. E., Stobo J. D. Synthesis of prostaglandins by subpopulations of human peripheral blood monocytes. Prostaglandins. 1979 Nov;18(5):687–695. doi: 10.1016/0090-6980(79)90089-3. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Bankhurst A. D., Messner R. P. Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J Exp Med. 1977 Dec 1;146(6):1719–1734. doi: 10.1084/jem.146.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Ceuppens J. L., Rodriguez M. A. Administration of nonsteroidal anti-inflammatory agents in patients with rheumatoid arthritis. Effects on indexes of cellular immune status and serum rheumatoid factor levels. JAMA. 1983 Nov 11;250(18):2485–2488. [PubMed] [Google Scholar]
- Goodwin J. S., Ceuppens J. Regulation of the immune response by prostaglandins. J Clin Immunol. 1983 Oct;3(4):295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S. Modulation of conconavalin-A-induced suppressor cell activation by prostaglandin E2. Cell Immunol. 1980 Feb;49(2):421–425. doi: 10.1016/0008-8749(80)90046-5. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Selinger D. S., Messner R. P., Reed W. P. Effect of indomethacin in vivo on humoral and cellular immunity in humans. Infect Immun. 1978 Feb;19(2):430–433. doi: 10.1128/iai.19.2.430-433.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Webb D. R. Regulation of the immune response by prostaglandins. Clin Immunol Immunopathol. 1980 Jan;15(1):106–122. doi: 10.1016/0090-1229(80)90024-0. [DOI] [PubMed] [Google Scholar]
- Hasler F., Bluestein H. G., Zvaifler N. J., Epstein L. B. Analysis of the defects responsible for the impaired regulation of Epstein-Barr virus-induced B cell proliferation by rheumatoid arthritis lymphocytes. I. Diminished gamma interferon production in response to autologous stimulation. J Exp Med. 1983 Jan 1;157(1):173–188. doi: 10.1084/jem.157.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedfors E., Holm G., Pettersson D. Activation of human peripheral blood lymphocytes by concanavalin A dependence of monocytes. Clin Exp Immunol. 1975 Nov;22(2):223–229. [PMC free article] [PubMed] [Google Scholar]
- Hersh E. M., Harris J. E. Macrophage-lymphocyte interaction in the antigen-induced blastogenic response of human peripheral blood leukocytes. J Immunol. 1968 Jun;100(6):1184–1194. [PubMed] [Google Scholar]
- Jelinek D. F., Lipsky P. E. The role of B cell proliferation in the generation of immunoglobulin-secreting cells in man. J Immunol. 1983 Jun;130(6):2597–2604. [PubMed] [Google Scholar]
- Keightley R. G., Cooper M. D., Lawton A. R. The T cell dependence of B cell differentiation induced by pokeweed mitogen. J Immunol. 1976 Nov;117(5 Pt 1):1538–1544. [PubMed] [Google Scholar]
- Kelly R. H., Miller D. H., Rodnan G. P., Hagmann J. Prostaglandin-mediated inhibition of lymphokine secretion in normal individuals and patients with progressive systemic sclerosis (scleroderma, PSS). Agents Actions. 1982 Oct;12(4):471–477. doi: 10.1007/BF01965929. [DOI] [PubMed] [Google Scholar]
- Kennedy M. S., Stobo J. D., Goldyne M. E. In vitro synthesis of prostaglandins and related lipids by populations of human peripheral blood mononuclear cells. Prostaglandins. 1980 Jul;20(1):135–145. doi: 10.1016/0090-6980(80)90013-1. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Ishizaka K. Regulation of antibody response in vitro. X. Biphasic effect of cyclic AMP on the secondary anti-hapten antibody response to anti-immunoglobulin and enhancing soluble factor. J Immunol. 1976 Feb;116(2):534–541. [PubMed] [Google Scholar]
- Knapp W., Baumgartner G. Monocyte-mediated suppression of human B lymphocyte differentiation in vitro. J Immunol. 1978 Sep;121(3):1177–1183. [PubMed] [Google Scholar]
- Kurland J. I., Kincade P. W., Moore M. A. Regulation of B-lymphocyte clonal proliferation by stimulatory and inhibitory macrophage-derived factors. J Exp Med. 1977 Nov 1;146(5):1420–1435. doi: 10.1084/jem.146.5.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky P. E., Thompson P. A., Rosenwasser L. J., Dinarello C. A. The role of interleukin 1 in human B cell activation: inhibition of B cell proliferation and the generation of immunoglobulin-secreting cells by an antibody against human leukocytic pyrogen. J Immunol. 1983 Jun;130(6):2708–2714. [PubMed] [Google Scholar]
- Melmon K. L., Bourne H. R., Weinstein Y., Shearer G. M., Kram J., Bauminger S. Hemolytic plaque formation by leukocytes in vitro. Control by vasoactive hormones. J Clin Invest. 1974 Jan;53(1):13–21. doi: 10.1172/JCI107530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morito T., Bankhurst A. D., Williams R. C. Studies on the modulation of immunoglobulin production by prostaglandins. Prostaglandins. 1980 Aug;20(2):383–390. doi: 10.1016/s0090-6980(80)80055-4. [DOI] [PubMed] [Google Scholar]
- Ohara J., Kishimoto T., Yamamura Y. In vitro immune response of human peripheral lymphocytes. III. Effect of anti-mu or anti-delta antibody on PWM-induced increase of cyclic nucleotides in human B lymphocytes. J Immunol. 1978 Nov;121(5):2088–2096. [PubMed] [Google Scholar]
- Pelus L. M., Strausser H. R. Prostaglandins and the immune response. Life Sci. 1977 Mar 15;20(6):903–913. doi: 10.1016/0024-3205(77)90274-0. [DOI] [PubMed] [Google Scholar]
- Potter M. R., Moore M. The effect of adherent and phagocytic cells on human lymphocyte PHA responsiveness. Clin Exp Immunol. 1977 Jan;27(1):159–164. [PMC free article] [PubMed] [Google Scholar]
- Ptak W., Naidorf K. F., Gershon R. K. Interference with the transmission of T cell-derived messages by macrophage membranes. J Immunol. 1977 Aug;119(2):444–449. [PubMed] [Google Scholar]
- Radnay J., Goldman J., Weiss E., Rozenszajn L. A. Regulation of human B-cell colony growth. Cell Immunol. 1984 Apr 15;85(1):179–190. doi: 10.1016/0008-8749(84)90288-0. [DOI] [PubMed] [Google Scholar]
- Rappaport R. S., Dodge G. R. Prostaglandin E inhibits the production of human interleukin 2. J Exp Med. 1982 Mar 1;155(3):943–948. doi: 10.1084/jem.155.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart J. J., Orser M., Kaplan M. E. Human monocyte and macrophage modulation of lymphocyte proliferation. Cell Immunol. 1979 Apr;44(1):131–143. doi: 10.1016/0008-8749(79)90034-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez M. A., Ceuppens J. L., Goodwin J. S. Regulation of IgM rheumatoid factor production in lymphocyte cultures from young and old subjects. J Immunol. 1982 Jun;128(6):2422–2428. [PubMed] [Google Scholar]
- Romagnani S., Giudizi M. G., Biagiotti R., Almerigogna F., Maggi E., Del Prete G., Ricci M. Surface immunoglobulins are involved in the interaction of protein A with human B cells and in the triggering of B cell proliferation induced by protein A-containing Staphylococcus aureus. J Immunol. 1981 Oct;127(4):1307–1313. [PubMed] [Google Scholar]
- Rosenberg S. A., Lipsky P. E. Monocyte dependence of pokeweed mitogen-induced differentiation of immunoglobulin-secreting cells from human peripheral blood mononuclear cells. J Immunol. 1979 Mar;122(3):926–931. [PubMed] [Google Scholar]
- Rosenberg S. A., Lipsky P. E. The role of monocytes in pokeweed mitogen-stimulated human B cell activation: separate requirements for intact monocytes and a soluble monocyte factor. J Immunol. 1981 Apr;126(4):1341–1345. [PubMed] [Google Scholar]
- Saiki O., Ralph P. Induction of human immunoglobulin secretion. I. Synergistic effect of B cell mitogen Cowan I plus T cell mitogens or factors. J Immunol. 1981 Sep;127(3):1044–1047. [PubMed] [Google Scholar]
- Scheid M. P., Goldstein G., Boyse E. A. The generation and regulation of lymphocyte populations: evidence from differentiative induction systems in vitro. J Exp Med. 1978 Jun 1;147(6):1727–1743. doi: 10.1084/jem.147.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staite N. D., Panayi G. S. Regulation of human immunoglobulin production in vitro by prostaglandin E2. Clin Exp Immunol. 1982 Jul;49(1):115–122. [PMC free article] [PubMed] [Google Scholar]
- Stenson W. F., Parker C. W. Prostaglandins, macrophages, and immunity. J Immunol. 1980 Jul;125(1):1–5. [PubMed] [Google Scholar]
- Thiele D. L., Kurosaka M., Lipsky P. E. Phenotype of the accessory cell necessary for mitogen-stimulated T and B cell responses in human peripheral blood: delineation by its sensitivity to the lysosomotropic agent, L-leucine methyl ester. J Immunol. 1983 Nov;131(5):2282–2290. [PubMed] [Google Scholar]
- Thiele D. L., Lipsky P. E. The accessory function of phagocytic cells in human T cell and B cell responses. J Immunol. 1982 Sep;129(3):1033–1040. [PubMed] [Google Scholar]
- Thompson P. A., Jelinek D. F., Lipsky P. E. Regulation of human B cell proliferation by prostaglandin E2. J Immunol. 1984 Nov;133(5):2446–2453. [PubMed] [Google Scholar]
- Tilden A. B., Balch C. M. A comparison of PGE2 effects on human suppressor cell function and on interleukin 2 function. J Immunol. 1982 Dec;129(6):2469–2473. [PubMed] [Google Scholar]
- Unsgaard G., Lamvik J. Inhibitory effect of human mononuclear phagocytes on DNA synthesis in stimulated lymphocytes. Acta Pathol Microbiol Scand C. 1977 Oct;85(5):373–380. doi: 10.1111/j.1699-0463.1977.tb03656.x. [DOI] [PubMed] [Google Scholar]
- Webb D. R., Osheroff P. L. Antigen stimulation of prostaglandin synthesis and control of immune responses. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1300–1304. doi: 10.1073/pnas.73.4.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisler R. L., Newhouse Y. G. Inhibition of human B lymphocyte colony responses by endogenous synthesized hydrogen peroxide and prostaglandins. Cell Immunol. 1982 May 1;69(1):34–45. doi: 10.1016/0008-8749(82)90048-x. [DOI] [PubMed] [Google Scholar]
- Zabala C., Lipsky P. E. Immunomodulatory effects of bacterial lipopolysaccharide on human B lymphocyte activation in vitro. J Immunol. 1982 Dec;129(6):2496–2503. [PubMed] [Google Scholar]
- de Vries J. E., Caviles A. P., Jr, Bont W. S., Mendelsohn J. The role of monocytes in human lymphocyte activation by mitogens. J Immunol. 1979 Mar;122(3):1099–1107. [PubMed] [Google Scholar]



